Abstract
Introduction
Microscopic colitis (MC) is a chronic inflammatory bowel disease with unclear etiology. Bile acid (BA) malabsorption has been described in MC patients. Farnesoid X receptor (FXR) is the main BA receptor; FXR-mediated mechanisms prevent the noxious effects of BA accumulation, preserving the integrity of the intestinal epithelial barrier and preventing intestinal inflammation.
Aim
Our aim was to describe the expression of FXR in patients with MC.
Methods
Archival formalin-fixed paraffin-embedded samples from the terminal ileum, right and left colon were obtained from patients with MC and matched controls. Immunohistochemistry was performed and nuclear FXR expression scored in a semi-quantitative way.
Results
169 formalin-fixed paraffin-embedded samples from 35 patients with MC and 31 controls were retrieved. There was a significant reduction of FXR expression in patients with MC versus controls both in the right colon (moderate-strong FXR expression: 21.1 vs. 64.3%; p = 0.003) and left colon (moderate-strong FXR expression: 8.3 vs. 38.7%; p = 0.027). No significant differences in FXR expression were observed in the ileum of patients with MC (moderate-strong FXR expression: 76.9 vs. 90.9%; p = 0.5). We found no difference in FXR expression between the two types of MC. No association between the degree of lymphocyte infiltration or the thickness of collagen band and FXR expression was found.
Conclusions
Patients with MC present a significantly lower expression of FXR in the colon. This could render colonic epithelial cells more susceptible to the deleterious effects of BA, contributing to disease pathogenesis and symptoms in MC.
Keywords: Microscopic colitis, Farnesoid X receptor, Immunohistochemistry, Bile salts, Intestinal inflammation
Resumo
Introdução
A colite microscópica (CM) é uma doença inflamatória do intestino com etiologia desconhecida. A má-absorção de ácidos biliares (AB) encontra-se descrita em doentes com CM. O Recetor Farnesoid X (FXR) é o principal recetor dos AB; mecanismos mediados pelo FXR previnem o efeito nocivo da acumulação dos AB, preservando a integridade da barreira epitelial intestinal e impedindo inflamação.
Objetivos
Avaliar a expressão do FXR em doentes com CM.
Métodos
Foram obtidas amostras em parafina do ileon terminal, cólon direito e esquerdo de doentes com CM e controlos emparelhados; foi realizada imunohistoquímica para FXR e a sua expressão nuclear quantificada de forma semi-quantitativa.
Resultados
Foram estudadas 169 amostras de 35 doentes com CM e 31 controlos. A expressão de FXR nos doentes com CM foi significativamente inferior à dos controlos no cólon direito (expressão FXR moderada-intensa: 21.1% versus 64.3%; p = 0.003) e esquerdo (expressão FXR moderada-intensa: 8.3% versus 38.7%; p = 0.027). Não observámos diferenças significativas na expressão do FXR no ileon em doentes com CM em comparação com controlos (expressão moderada-intensa em 76.9 versus 90.0%; p = 0.5). Não se verificaram diferenças na expressão FXR entre os dois tipos de CM. Não se encontrou associação entre o grau de infiltração linfocítica ou a espessura da banda de colagénio e a expressão de FXR.
Conclusões
os doentes com CM apresentaram uma expressão significativamente reduzida do FXR no cólon; isto pode tornar as células epiteliais mais suscetíveis aos efeitos deletérios dos AB, contribuindo para a patogénese da doença e a sintomatologia da CM.
Palavras Chave: Colite microscópica, Receptor Farnesóide X, Imunohistoquímica, Sais biliares, Colite
Introduction and Objectives
Microscopic colitis (MC) is a form of idiopathic inflammatory bowel disease that causes non-bloody chronic diarrhoea, especially in elderly patients, and that can impact quality of life due to increased stool frequency, urgency, incontinence, nocturnal bowel movements, and weight loss [1]. Studies have shown that most patients will respond to steroid therapy, but around 13% of patients present a relapsing course requiring immunosuppressive therapy and, rarely, surgery [2, 3]. MC encompasses two different disorders: lymphocytic and collagenous colitis. Both lymphocytic and collagenous colitis present with histologic evidence of chronic mucosal inflammation, in the absence of endoscopic or radiologic abnormalities of the colon [4]. Histological analysis from the colon is, therefore, necessary to make the diagnosis.
Previously considered to be a rare diagnosis, MC nowadays accounts for 4–13% of patients investigated for chronic diarrhoea [5, 6, 7]. The incidence of MC seems to be increasing, reaching levels comparable with the commonest forms of inflammatory bowel disease, Crohn's disease, and ulcerative colitis [3, 4, 8]. Research on MC has mainly focused on the incidence, clinical features, and natural history of disease through epidemiological studies [9]. However, there are still many unanswered questions regarding what causes this puzzling disorder. The aetiology of MC remains unknown, and it is likely multifactorial. Many hypotheses have been proposed, among which the association with drugs and with autoimmunity stands out [3]. However, there is also some evidence in the literature pointing to a possible role of bile acids (BAs) in MC. Patients refractory to medical therapy who have their faecal stream deviated by means of a temporary ileostomy have shown improvement and resolution of inflammation, suggesting that some noxious luminal factor contributes to the pathophysiology [10]. BA malabsorption measured by the 75Se-labelled homocholic acid-taurine (75SeHCAT) test has been found in approximately 44% of patients with MC [11]. Furthermore, those with an abnormal 75SeHCAT test often respond to cholestyramine, a BA-binding resin, with responses ranging from 59 to 86% in open-label studies [12]. The beneficial effects of budesonide, the only therapy that has been shown to be superior to placebo in randomized controlled trials [13, 14], may also be partly linked to changes in BA metabolism [15]. Budesonide is capable of activating the apical sodium BA transporter, which is the primary transporter responsible for BA absorption in the terminal ileum (TI) [16]. Other work has demonstrated that the 75SeHCAT values, which indirectly measure BA re-absorption in the TI, significantly increase during budesonide treatment, suggesting that the good clinical efficacy of budesonide may partially rely on modulatory effects on BA-induced diarrhoea or mucosal damage in MC [15].
BAs are important signalling molecules, acting in inflammation and metabolism, through activation of BA receptors such as the nuclear BA receptor farnesoid X receptor (FXR). FXR is a nuclear receptor recently discovered and characterized [17]. It acts as the main nuclear BA receptor, and therefore it is expressed at high levels in the liver and intestine, especially in the TI and proximal colon [18]. In the intestine, BA-dependent FXR activation results mainly in two events. First, FXR induces synthesis of fibroblast growth factor-19, which is then secreted into the portal circulation and acts on hepatocytes to suppress the rate-limiting enzyme responsible for BA synthesis [19]. Second, FXR activation is coupled with reduced apical sodium BA transporter expression, which results in decreased BA intestinal absorption and prevention of intracellular BA accumulation [20]. Therefore, FXR-mediated mechanisms prevent the noxious effects of BA accumulation on hepatocytes and on the cells lining the intestinal and biliary tract [21], playing a key role not only in the enterohepatic circulation, but also in the regulation of inflammatory responses in the liver and intestine [21]. Reduced FXR activation has been shown in experimental models of colitis [22, 23] and in samples from patients with Crohn's disease [24]; on the another hand, FXR knockout mice have been shown to be more susceptible to colitis, which resolves with the administration of a FXR agonist [21, 23]. No study has addressed or studied the expression of FXR in patients with MC. Herein we hypothesized that colonic inflammation, in part by inactivating FXR-mediated mechanisms, could exacerbate the toxic effects of secondary BA on colonic cells and therefore have a role in MC pathogenesis. Therefore, we sought to explore the expression of FXR in patients with MC across several colonic segments, as compared to healthy controls.
Material and Methods
Case Selection
Following approval by the Hospital Beatriz Ângelo and Hospital CUF Descobertas Ethics Commissions, patients were identified using each institution's pathology databases. Cases were patients with a diagnosis of collagenous colitis or lymphocytic colitis based on the usual histopathological criteria: (1) presence of an abnormal surface subepithelial collagen layer with an abnormal thickness (normal 5–7 μm), which entraps superficial capillaries and with an irregular lacy appearance of the lower edge of the basement membrane; (2) increased chronic inflammatory infiltrate (plasma cells, eosinophils, and lymphocytes) in the lamina propria; (3) increased number of intra-epithelial lymphocytes (normal <7 per 100 epithelial cells); and (4) damage of surface epithelium, with flattening of epithelial cells and/or epithelial loss and detachment, and minimal crypt architecture distortion [9, 25, 26, 27]. Gender- and age-matched (±5 years) individuals who performed colonoscopy for other reasons and from whom biopsies were available for retrieval and staining were selected to serve as controls. All samples had been collected during the past 5 years. Patients with a history of inflammatory bowel disease or any other inflammatory condition of the colon, including irritable bowel syndrome, were excluded. When available, biopsies were retrieved from the TI, right and left colon. Biopsies that were labelled as cecum, ascending or transverse colon were included as right colon biopsies, and biopsies that were labelled as descending, sigmoid colon or rectum were included as left colon samples. Biopsies that were labelled as right or left colon only were also included to each respective location. Cases or controls where biopsies were available but the location was not indicated were not included. Gender and age were recorded for all participants. All slides were reviewed prior to immunohistochemistry staining by a single pathologist with an expertise in gastrointestinal pathology (P.B.) to confirm the diagnosis of MC, and to exclude controls that presented any signs of microscopic inflammation in the colon.
Immunohistochemistry
Immunohistochemistry for FXR was performed manually on samples, using a mouse anti-human FXR monoclonal antibody (Perseus Proteomics, Tokyo, Japan). This antibody specifically recognizes human FXR and cross-reacts with mouse and rat FXR. From the formalin-fixed, paraffin-embedded tissue blocks of biopsy specimens, sequential sections were cut at 4-μm thickness and mounted on adhesive slides. Slides were deparaffinized in xylene and subsequently washed in graded ethanol (100%, followed by 95%) and rehydrated in distilled water. For antigen retrieval, sections were incubated in a microwave for 30 min using a 0.1% sodium citrate buffer and subsequently washed in PBS at room temperature. Endogenous peroxidase activity was blocked by incubating the slides with 3% H2O2 for 10 min and then rinsed three times with PBS. Sections were incubated for 60 min at room temperature in 2% BSA to avoid non-specific signal, and then overnight at 4°C with the primary anti-FXR antibody. Subsequently, slides were rinsed 3 times in PBS and treated for 30 min at room temperature with a polyclonal anti-mouse secondary antibody (EnVision+ System-HRP Labelled Polymer Anti-mouse, Dako, Denmark), and again washed three times with PBS. Slides were then incubated with diaminobenzidine using the peroxidase substrate diaminobenzidine kit™ (Vector Laboratories). After cleansing with water, slides were counterstained with Harris modified haematoxylin solution for 50 s, dipped in ethanol and in ammonia water, and rinsed in tap water in between. Finally, sections were consecutively dehydrated in 95 and 100% alcohol, washed with xylene and mounted with VectaMount ™ (Vector Laboratories). At least one section with normal small intestinal mucosa was included for each run as a positive control.
Evaluation of the Immunohistochemistry
FXR nuclear expression was scored by an experienced observer and expert gastrointestinal pathologist (P.B.) who was blinded to the clinical information and location of the biopsy. FXR nuclear staining intensity was scored as absent (0), weak (+), moderate (++), and strong (+++), and for the purposes of analysis grouped as absent to weak and moderate to strong.
Histologic Grading of Inflammation
Samples from MC patients were scored according to their degree of inflammation. In collagenous colitis cases, the thickness of the collagen band was measured in micrometres, by choosing the location in the biopsy with the thickest collagen deposition. For lymphocytic colitis, the degree of lymphocytic infiltration was assessed by the same gastrointestinal pathologist (P.B.) using a semi-quantitative scale evaluating the number of lymphocytes per 100 epithelial cells: mild (25–50 lymphocytes), moderate (50–75 lymphocytes), and severe (>75 lymphocytes) inflammation.
Statistical Analysis
Data analysis was performed using the computer software Statistical Package for Social Sciences (SPSS) for Mac (version 19.0). When appropriate, the Student t test, logistic regression, the Fischer exact test, and χ2 tests were used for comparison between groups. Statistical significance was set at p < 0.05. For the purposes of analysis, FXR expression was grouped as absent-mild or moderate-strong. Correlations with disease state (MC vs. healthy), and specimen location (right vs. left colon) were performed. The degree of staining was also correlated with the degree of inflammation as measured by the thickness of the collagen band or the degree of lymphocyte infiltration in the lamina propria using χ2 tests.
Results
Patients and Samples
Following our inclusion and exclusion criteria, 35 cases of MC (27 with lymphocytic colitis and 8 with collagenous colitis) and 31 controls were included and their biopsies retrieved and stained. As expected, there were no significant differences in gender distribution (76% of females in cases vs. 71% in controls, p = 0.64) or in mean age (58.6 ± 17.3 years in cases vs. 53.6 ± 19.1 years in controls, p = 0.3) between study groups. A total of 169 formalin-fixed, paraffin-embedded samples (24 from the TI, 66 from the right colon, and 79 from the left colon) were retrieved. There were no significant differences in the distribution of samples by location and by study group (p = 0.8) (Table 1).
Table 1.
Description of the number of FFPE (formalin-fixed paraffin-embedded) samples by location and by disease group
| Location of FFPE samples | Microscopic colitis | Controls | Total |
|---|---|---|---|
| Ileum | 13 | 11 | 24 |
| Right colon | 7 | 7 | 14 |
| Left colon | 7 | 6 | 13 |
| Cecum | 7 | 2 | 9 |
| Ascending/transverse colon | 24 | 19 | 43 |
| Descending/sigmoid colon | 25 | 16 | 41 |
| Rectum | 16 | 9 | 25 |
| Total | 99 | 73 | 169 |
FXR Expression in the Colon of Cases and Controls
We observed a proximal to distal gradient of FXR expression in the ileo-colon: overall, 83% of samples from the TI, 40% of samples from the right colon, and 30% of the samples from the left colon demonstrated a moderate to strong FXR expression (p < 0.001). There were no differences in FXR expression by patient sex: 30.3% of samples from female patients and 30.4% of samples from male patients displayed moderate-strong expression (p = 0.66); this observation was identical when results were analysed by study group (data not shown). Likewise, we did not observe differences in FXR expression by age of the patient, either treating age as a continuous variable (p = 0.10, logistic regression) or as a categorical variable (age < or >50 years, χ2 test, p = 0.45).
When we stratified by study group, we observed a reduced expression of FXR in MC cases as compared to controls in all locations. A non-statistical reduction of FXR expression was observed in the TI: 90.9% of samples from healthy controls displayed moderate-strong FXR expression versus 76.9% of cases (p = 0.5). Furthermore, the proximal-distal gradient observed in the colon (from the right colon to the left colon) remained statistically significant only for the healthy controls samples. In healthy controls, 64.3% of the samples from the right colon versus 38.7% of the left colon samples presented strong FXR expression (p = 0.04). While there was also a trend towards stronger FXR expression in the proximal colon from MC patients (strong FXR expression in 21.1% of samples from right colon versus 8.3% from left colon), this did not reach significance (p = 0.09) (Fig. 1). The description of FXR staining by location and by disease group is presented in Table 2. Because there were cases and controls in whom multiple samples were available throughout the colon, we conducted a sensitivity analysis including only one sample from the right colon and one sample from the left colon from each individual. This analysis, conducted in 116 samples (54 from the right and 62 from the left colon), revealed overlapping results: 64.3% of samples from the right colon and 44.8% of samples from the left colon from controls presented moderate-strong FXR expression versus 23 and 12.1% of samples from the right colon and left colon of MC patients, respectively (p = 0.003 for both comparisons).
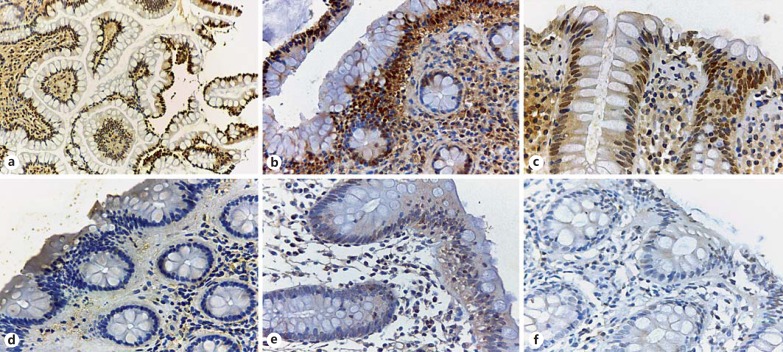
Fig. 1.
FXR immunohistochemical staining of patients with microscopic colitis and controls. a Normal terminal ileum from a control patient: there is strong nuclear staining in the epithelial cells in the villi (×40). b Right colon biopsy from a control patient; a strong nuclear positivity at the surface and upper part of the colonic glands with a gradual loss of expression in the crypts is seen (×200). c Left colon biopsy of a control patient displaying strong FXR nuclear expression (×400). d Right colon of a patient with collagenous colitis: absent FXR nuclear expression is observed (×200). Right colon (e) and left colon (f) in a patient with lymphocytic colitis displaying only minimal or no FXR nuclear staining (×400).
Table 2.
Relationship between degree of FXR expression according to colonic location and disease type
| Location of samples | Study group | Absent-weak FXR expression, n (%) | Moderate-strong FXR expression, n (%) | p value |
|---|---|---|---|---|
| TI | HC | 1 (9.1) | 10 (90.9) | 0.59 |
| MC | 3 (23.1) | 10 (76.9) | ||
| RC | HC | 10 (35.7) | 18 (64.3) | <0.001 |
| MC | 30 (78.9) | 8 (21.1) | ||
| LC | HC | 19 (38.7) | 12 (38.7) | 0.001 |
| MC | 44 (91.7) | 4 (8.3) |
FXR, farnesoid X receptor; TI, terminal ileum; RC, right colon; LC, left colon; HC, healthy controls; MC, microscopic colitis.
FXR Expression in MC Subtypes
There were no differences in FXR expression between collagenous and lymphocytic colitis. Overall, 25% of samples from patients with collagenous colitis and 22.5% of samples from lymphocytic colitis showed moderate-strong FXR expression (when samples from TI were included; p = 0.7). Samples from the TI displayed moderate-strong FXR expression in 75 and 78% of collagenous and lymphocytic colitis cases (p = 0.8). Samples from patients with collagenous colitis presented moderate-strong FXR expression in 37.5% of samples from the right colon and in 0% of samples from the left colon; in lymphocytic colitis, these figures were 18.5% for the right colon and 11.4% for the lymphocytic colitis, respectively (p = 0.45).
Correlation of FXR Expression with the Degree of Inflammatory Infiltrate in MC
The mean thickness of the collagen band in collagenous colitis samples was 40 ± 15.4 μm. The degree of lymphocyte infiltration was mild in 41.5%, moderate in 32.1%, and severe in 26.4% of samples from lymphocytic colitis. No association was found between the thickness of collagen band and FXR expression: samples displaying absent-mild FXR expression had a mean collagen band thickness of 40.4 ± 16.2 μm, compared to 36.5 ± 4.9 μm in those samples with moderate-strong FXR expression (p = 0.7). Likewise, no trend was observed for FXR expression in patients with lymphocytic colitis according to their degree of inflammation: 81.2% of those with mild inflammation, 94.1% of those with moderate inflammation, and 78.57 of those with severe inflammatory infiltrated displayed absent-mild FXR expression (p = 0.4).
Discussion
Herein, we have for the first time described the expression of FXR, the main BA receptor, in patients with MC, and compared it to age- and gender-matched controls. We observed that patients with MC present a significantly lower expression of this marker, both in the right and left colon, as compared to controls. Furthermore, the proximal-distal gradient of FXR expression observed in controls was lost in MC patients. Finally, no differences were observed between patients with collagenous and lymphocytic colitis and no association was found between the intensity of inflammation (as measured by the degree of lymphocyte infiltration or thickness of the collagen band) and FXR expression.
The potential involvement of BAs in MC pathogenesis, coupled with the increasing evidence showing how the BA-FXR axis is crucial in the maintenance of epithelial barrier and the involvement of FXR in intestinal inflammation, makes FXR an interesting marker to study in MC. Indeed, the BA-FXR axis has been implicated in intestinal barrier function and antibacterial defence [21, 28]. At the intestinal level, BA metabolites have demonstrated important roles in regulating intestinal homeostasis by preventing pathogen invasion [29], inhibiting inflammation [22] and maintaining cell integrity [29, 30]. It has been demonstrated that intestinal inflammation decreases FXR cellular expression in an experimental colitis model, and in the reverse way, FXR knockout mice have been shown to be more susceptible to intestinal inflammation [23, 31]. Patients with MC have shown to present impaired mucosal barrier function [32]. Recent work has demonstrated that secondary BAs can exacerbate this mucosal barrier dysfunction in patients with collagenous colitis in remission, by increasing bacterial uptake [32, 33]. Although nothing is known about the faecal BA concentrations in MC patients, the reports of a pathological 75SeHCAT in 44% of collagenous colitis patients suggest higher BA loss via the colon [11]. An excessive level of BAs in the intestine, resulting from defects in BA reabsorption, can result in chronic diarrhoea and inflammation in the bowel. Holmquist et al. [34] reported that patients with ulcerative colitis who had BA malabsorption showed a high degree of mucosal inflammation in the right colon at colonoscopy. The right colon is also the main site of the classical histological findings of collagenous colitis [35], and where FXR is most strongly expressed. In healthy controls, we observed a distal decrease in FXR expression, with a stronger expression in the right colon samples as compared to the left colon samples. This pattern of distribution had already been described in normal subjects, and proposed to occur in parallel with the proximal-distal gradient of BA flow along the colon [36, 37]. However, even if there was a trend for this gradient in samples from MC patients, this did not reach statistical significance; indeed, samples from patients with MC presented an overall reduction in the expression of FXR both in right and in left colon.
MC remains an understudied disease. Despite its increasing incidence, it is still a relatively rare disease. Importantly, once diagnosis is made there is no formal recommendation to follow-up with new biopsies, and, therefore, it is difficult to get fresh biopsies to pursue molecular studies, and most studies looking at other tissue markers or cytokines in MC, normally rely on a very small number of samples [38]. Here, having access to a large number of samples in several locations among the colon, from more than 30 patients and 30 controls, we were able to describe in detail the expression and gradient of FXR in MC. All samples from participants (cases and controls) had been collected in the past 5 years, and therefore no bias by aging and degradation of the samples was introduced. Furthermore, all our cases and controls were reviewed by an expert gastrointestinal pathologist who confirmed diagnosis and excluded controls where inflammation could have been present in biopsies, even if minimal or non-specific. Although our findings require further confirmation and validation, this is to our knowledge, the first study examining the expression of BA receptors in MC. This is surprising considering that BA malabsorption has been described in patients with MC, and that cholestyramine has been used for years to treat patients. Our study also has limitations. Our main limitation relies on the fact that being a descriptive, retrospective immunohistochemical study on archival tissue, it does not allow to draw any mechanistic conclusions, such as whether low FXR expression results from FXR down-regulation or from post-transcriptional events modulating FXR expression. Furthermore, despite the relatively large number of samples available and analysed, the number of patients included was relatively small, which could have especially limited the sub-analysis comparing lymphocytic with collagenous colitis. Additionally, being retrospective, and based mostly on the selection of samples from the pathology departments, no clinical information was possible to retrieve from cases, and therefore no information on medications, nor in other clinical factors that could potentially impact entero-hepatic circulation (e.g., history of hepato-biliary surgery) could be assessed.
It is evident that further research is required to elucidate the underlying mechanisms for FXR down-regulation in MC patients. We can hypothesize that BA malabsorption that has been described to occur in MC could lead to a secondary feedback down-regulation of colonic FXR, or that alternatively inflammation in the colon leading to FXR down-regulation could render the epithelial cells more susceptible to the noxious effects of BAs. Even if preliminary, our results open the possibility of studying the use of the new FXR agonist, obeticholic acid [39, 40], in patients with refractory MC, and open avenues for a new line of research in this puzzling cause of intestinal inflammation.
Statement of Ethics
This study was approved by the appropriate ethics committee at each institution.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
This work was funded by the NGHD (Núcleo de Gastrenterologia dos Hospitais Distritais).
References
- 1.Gentile NM, Yen EF. The incidence of microscopic colitis: microscopic no more. Dig Dis Sci. 2017;62:1394–1395. doi: 10.1007/s10620-017-4484-3. [DOI] [PubMed] [Google Scholar]
- 2.Jarnerot G, Tysk C, Bohr J, Eriksson S. Collagenous colitis and fecal stream diversion. Gastroenterology. 1995;109:449–455. doi: 10.1016/0016-5085(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 3.Fumery M, Kohut M, Gower-Rousseau C, et al. Incidence, clinical presentation, and associated factors of microscopic colitis in Northern France: a population-based study. Dig Dis Sci. 2017;62:1571–1579. doi: 10.1007/s10620-016-4306-z. [DOI] [PubMed] [Google Scholar]
- 4.Yen EF, Pardi DS. Review article: microscopic colitis – lymphocytic, collagenous and “mast cell” colitis. Aliment Pharmacol Ther. 2011;34:21–32. doi: 10.1111/j.1365-2036.2011.04686.x. [DOI] [PubMed] [Google Scholar]
- 5.Shah RJ, Fenoglio-Preiser C, Bleau BL, Giannella RA. Usefulness of colonoscopy with biopsy in the evaluation of patients with chronic diarrhea. Am J Gastroenterol. 2001;96:1091–1095. doi: 10.1111/j.1572-0241.2001.03745.x. [DOI] [PubMed] [Google Scholar]
- 6.Fine KD, Seidel RH, Do K. The prevalence, anatomic distribution, and diagnosis of colonic causes of chronic diarrhea. Gastrointest Endosc. 2000;51:318–326. doi: 10.1016/s0016-5107(00)70362-2. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Banares F, Salas A, Forne M, Esteve M, Espinos J, Viver JM. Incidence of collagenous and lymphocytic colitis: a 5-year population-based study. Am J Gastroenterol. 1999;94:418–423. doi: 10.1111/j.1572-0241.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 8.Pardi DS, Loftus EV, Jr, Smyrk TC, et al. The epidemiology of microscopic colitis: a population-based study in Olmsted County, Minnesota. Gut. 2007;56:504–508. doi: 10.1136/gut.2006.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munch A, Aust D, Bohr J, et al. Microscopic colitis: current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis. 2012;6:932–945. doi: 10.1016/j.crohns.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Jarnerot G, Tysk C, Bohr J, Eriksson S. Collagenous colitis and fecal stream diversion. Gastroenterology. 1995;109:449–455. doi: 10.1016/0016-5085(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Banares F, Esteve M, Salas A, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci. 2001;46:2231–2238. doi: 10.1023/a:1011927302076. [DOI] [PubMed] [Google Scholar]
- 12.Ung KA, Gillberg R, Kilander A, Abrahamsson H. Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut. 2000;46:170–175. doi: 10.1136/gut.46.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miehlke S, Heymer P, Bethke B, et al. Budesonide treatment for collagenous colitis: a randomized, double-blind, placebo-controlled, multicenter trial. Gastroenterology. 2002;123:978–984. doi: 10.1053/gast.2002.36042. [DOI] [PubMed] [Google Scholar]
- 14.Miehlke S, Madisch A, Karimi D, et al. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology. 2009;136:2092–2100. doi: 10.1053/j.gastro.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 15.Bajor A, Kilander A, Galman C, Rudling M, Ung KA. Budesonide treatment is associated with increased bile acid absorption in collagenous colitis. Aliment Pharmacol Ther. 2006;24:1643–1649. doi: 10.1111/j.1365-2036.2006.03168.x. [DOI] [PubMed] [Google Scholar]
- 16.Jung D, Fantin AC, Scheurer U, Fried M, Kullak-Ublick GA. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 2004;53:78–84. doi: 10.1136/gut.53.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chignard N, Poupon R. Targeting farnesoid x receptor in hepatic and biliary inflammatory diseases. Gastroenterology. 2009;137:734–735. doi: 10.1053/j.gastro.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabolism. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Augustin O, Sanchez de Medina F. Intestinal bile acid physiology and pathophysiology. World J Gastroenterol. 2008;14:5630–5640. doi: 10.3748/wjg.14.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010;1801:683–692. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 23.Gadaleta RM, van Erpecum KJ, Oldenburg B, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 24.Nijmeijer RM, Gadaleta RM, van Mil SW, et al. Farnesoid X receptor (FXR) activation and FXR genetic variation in inflammatory bowel disease. PLoS One. 2011;6:e23745. doi: 10.1371/journal.pone.0023745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren BF, Edwards CM, Travis SP. “Microscopic colitis:” classification and terminology. Histopathology. 2002;40:374–376. doi: 10.1046/j.1365-2559.2002.01341.x. [DOI] [PubMed] [Google Scholar]
- 26.Jawhari A, Talbot IC. Microscopic, lymphocytic and collagenous colitis. Histopathology. 1996;29:101–110. doi: 10.1046/j.1365-2559.1996.d01-498.x. [DOI] [PubMed] [Google Scholar]
- 27.Lazenby AJ, Yardley JH, Giardiello FM, Jessurun J, Bayless TM. Lymphocytic (“microscopic”) colitis: a comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol. 1989;20:18–28. doi: 10.1016/0046-8177(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki T, Moschetta A, Lee Y-K. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiorucci S, Cipriani S, Mencarelli A, Renga B, Distrutti E, Baldelli F. Counter-regulatory role of bile acid activated receptors in immunity and inflammation. Curr Mol Med. 2010;10:579–595. doi: 10.2174/1566524011009060579. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 32.Munch A, Soderholm JD, Ost A, Strom M. Increased transmucosal uptake of E. coli K12 in collagenous colitis persists after budesonide treatment. Am J Gastroenterol. 2009;104:679–685. doi: 10.1038/ajg.2008.95. [DOI] [PubMed] [Google Scholar]
- 33.Münch A, Söderholm JD, Öst Å, Carlsson AH, Magnusson KE, Ström M. Low levels of bile acids increase bacterial uptake in colonic biopsies from patients with collagenous colitis in remission. Aliment Pharmacol Ther. 2011;33:954–960. doi: 10.1111/j.1365-2036.2011.04611.x. [DOI] [PubMed] [Google Scholar]
- 34.Holmquist L, Andersson H, Rudic N, Ahren C, Fallstrom SP. Bile acid malabsorption in children and adolescents with chronic colitis. Scand J Gastroenterol. 1986;21:87–92. doi: 10.3109/00365528609034628. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Mazzoleni G, Riddell RH. Distribution of collagenous colitis: utility of flexible sigmoidoscopy. Gut. 1992;33:65–70. doi: 10.1136/gut.33.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lax S, Schauer G, Prein K, et al. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int J Cancer. 2012;130:2232–2239. doi: 10.1002/ijc.26293. [DOI] [PubMed] [Google Scholar]
- 37.Torres J, Bao X, Iuga AC, et al. Farnesoid X receptor expression is decreased in colonic mucosa of patients with primary sclerosing cholangitis and colitis-associated neoplasia. Inflamm Bowel Dis. 2013;19:275–282. doi: 10.1097/MIB.0b013e318286ff2e. [DOI] [PubMed] [Google Scholar]
- 38.Carrasco A, Esteve M, Salas A, et al. Immunological differences between lymphocytic and collagenous colitis. J Crohns Colitis. 2016;10:1055–1066. doi: 10.1093/ecco-jcc/jjw058. [DOI] [PubMed] [Google Scholar]
- 39.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41:54–64. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 40.Keely SJ, Walters JR. The farnesoid X receptor: good for BAD. Cell Mol Gastroenterol Hepatol. 2016;2:725–732. doi: 10.1016/j.jcmgh.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]