Abstract
Background
Mycosis fungoides (MF) has a wide range of clinical presentations and it has been reported rarely to involve the nail apparatus.
Objective
We intended to evaluate the frequency and characteristics of nail changes in patients with biopsy-proven MF.
Methods
A retrospective analysis of 60 patients with MF who were evaluated at our cancer center from 2013 to 2014 was performed to identify patients with nail changes. Histological examinations of the skin around the nail apparatus were obtained from 10 patients with periungual skin erythema and scaling.
Results
In 45 patients out of 60 cases, the skin around the nail apparatus was normal, and only in 5 patients of these 45 cases, nail changes were detected. These changes included leukonychia, longitudinal ridging, nail thickening, and opacity. In the remaining 15 patients, erythema and scaling was observed in periungual skin, and 13 of them demonstrated nail changes including longitudinal ridging, nail thickening, fragility of the nail plate, subungual hyperkeratosis, pigmented nail band, Beau's lines, onychomadesis, koilonychia, nail thinning, distal notching, subungual debris, leukonychia, and pitting. In biopsies of periungual skin, none of 10 cases revealed histological findings consistent with MF.
Conclusions
Evidence of nail changes was observed in 18 cases (30%). The most common nail changes detected in MF patients included longitudinal ridging, nail thickening, nail fragility, and leukonychia.
Keywords: Mycosis fungoides, Nail changes, Histology
Introduction
Primary cutaneous T-cell lymphomas are a heterogeneous group of skin-homing T-lymphocyte malignancies. Mycosis fungoides (MF) accounts for nearly 50% of all cases. The classic type of MF is characterized by infiltration of atypical T lymphocytes with cerebriform nuclei in the papillary dermis and evidence of epidermotropism (i.e., presence of atypical T-cell lymphocytes in the epidermis without significant spongiosis) [1]. Classic MF typically exhibits slow progression in the first years after diagnosis and rarely progresses to extracutaneous involvement or disease-related death. MF has a wide range of clinical presentations, which may vary from erythematous patches, plaques, and tumors to hypopigmented macules and pustules [2].
Despite the fact that nail involvement has been infrequently described, MF has been reported to involve the nail apparatus. The clinical presentations of previously reported cases included subungual hyperkeratosis [3], onycholysis [4], onychomadesis [5], a yellow nail discoloration [6,7,8], thinning of the nail plate, splinter hemorrhage, and 20-nail dystrophy [9]. The nail findings tend to involve most if not all of the nails [10]. Nail changes that occur in association with MF are sometimes related to medical therapy, for example, chemotherapy-induced Beau's lines and nail pigmentation due to PUVA therapy [11].
To the best of our knowledge, nail involvement has been rarely reported in only a limited number of MF patients in the literature. This is the first study on the frequency and characteristics of nail changes in patients with biopsy-proven MF who were treated in the tumor clinic of Razi Hospital, Tehran, Iran.
Materials and Methods
The study protocol was reviewed and approved by the Tehran University of Medical Sciences Research Center. We conducted a cross-sectional analysis of 60 patients with biopsy-proven MF who were evaluated at our cancer center from 2013 to 2014. Clinical data collected included age of patients, sex, family history of MF or other skin diseases, personal history of skin disorders, smoking, disease duration, type and duration of treatment, characteristics of nail changes, and stage of MF at the time of diagnosis. Patients with nail changes due to other skin diseases or traumatic injury or any other situation that may contribute to nail change were excluded from our study. In the case of clinical suspicion, laboratory examination was performed for the detection of dermatophyte, and if it was positive, patients were excluded from the study.
Histological examination from 10 patients was obtained, and they were stained by hematoxylin and eosin and were evaluated by our dermatopathologists. The presence of atypical T lymphocytes within the epithelium (i.e., epidermotropism) and the presence of other diagnostic features of MF were evaluated in biopsy specimens of the nail apparatus.
Results
A total of 60 MF patients (28 men and 32 women) were evaluated during the study period (2013–2014). Evidence of nail changes was observed in 18 cases (30%). The median age of all patients was 49.7 years (with a range from 10 to 87 years), and the median age of MF patients with nail changes was 54 years (with a range from 42 to 73 years). Most of the patients were in stage IIA and stage IA (51.7 and 35%, respectively). Demographic findings and other features of patients are summarized in Table 1.
Table 1.
Basic information of all patients with mycosis fungoides (MF) and patients with nail changes
| Variables | All patients with MF (n = 60) | MF patients with nail changes (n = 18) |
|---|---|---|
| Sex | ||
| Male | 28 (46.6%) | 12 (66.6%) |
| Female | 32 (53.4%) | 6 (33.4%) |
| Age (range), years | 49.7 (10–87) | 54 (42–73) |
| Disease duration, years | 7.4 | 7.6 |
| Treatment duration, years | 3.9 | 5.2 |
| Treatment | ||
| Phototherapy | 39 (65%) | 12 (66.7%) |
| Photochemotherapy | 15 (25%) | 6 (27.8%) |
| Photochemotherapy + chemotherapy | 3 (5%) | - |
| Retinoid alone | 3 (5%) | - |
| Disease stage | ||
| IA | 21 (35%) | 4 (22.2%) |
| IIA | 31 (51.7%) | 12 (66.7%) |
| IB | 3 (5%) | - |
| IIB | 4 (6.7%) | 1 (5.6%) |
| III | - | 1 (5.6%) |
| IV | 1 (1.7%) | - |
The range of disease duration was between 3 and 16 years with a median duration of 7.4 and 7.6 years in all patients and MF patients with nail changes, respectively. Family history of the patients was negative for MF or other skin diseases. History of smoking was positive in 10 MF patients (16.7%) and also in 4 MF patients with nail changes (22.2%). Ninety-five percent of all MF patients were undergoing phototherapy alone or in combination with topical steroids, chemotherapy, or systemic retinoids.
Among 60 patients, in 45, the skin around the nail apparatus was normal, whereas only in 5 patients of these 45 cases, nail changes were detected (Fig. 1, 2). These changes included leukonychia (2 cases), longitudinal ridging (2 cases), and nail thickening and opacity (1 case). In the remaining 15 patients, erythema and scaling was observed in periungual skin, and 13 of them demonstrated nail changes including longitudinal ridging (6 cases), nail thickening (3 cases), fragility of the nail plate (3 cases), subungual hyperkeratosis, pigmented nail band, Beau's lines, onychomadesis, koilonychia, nail thinning, distal notching, subungual debris, leukonychia, and pitting (each in 1 patient). Ten of these patients with skin changes consented to biopsy from scaly and erythematous periungual skin, none of which revealed histological findings consistent with MF or other skin disorders. Only nonspecific findings including hyperkeratosis, parakeratosis, acanthosis, and spongiosis were found (Fig. 1, 2).
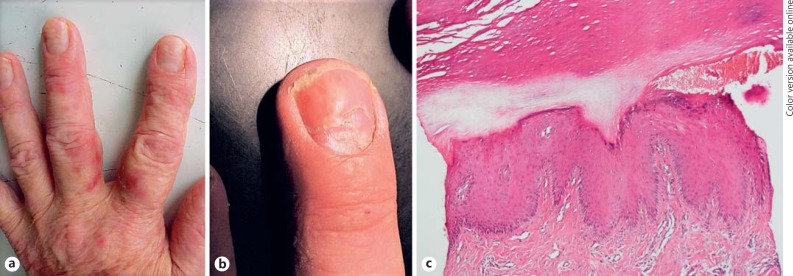
Fig. 1.
Beau's line (a) and onychomadesis with erythema and scaling of periungual skin (b) in a patient with stage III mycosis fungoides. c Acral-type hyperkeratosis, hypergranulosis, and psoriasiform acanthosis in histological examination of periungual skin (hematoxylin and eosin, ×40).
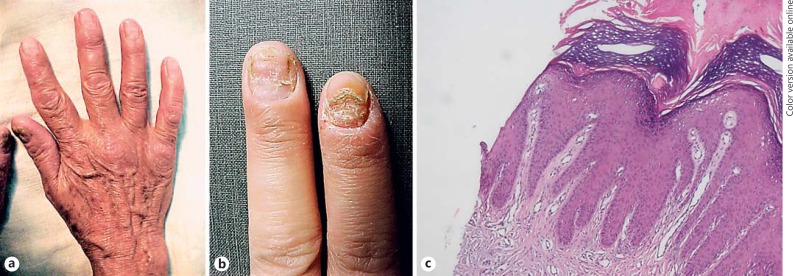
Fig. 2.
Dystrophy of thumb nails (a) in a patient with patch- and plaque-type mycosis fungoides lesions, and nail dystrophy and fragility of two nails (b) without evidence of tumoral cell infiltration in biopsy specimens (c) (hematoxylin and eosin, ×100).
Totally, the most common nail findings were longitudinal ridging (8 cases) and nail thickening (4 cases). The type of the nail changes and disease stage are presented in Table 2.
Table 2.
Frequency of nail changes according to disease stage
| Stage |
||||
|---|---|---|---|---|
| IA | IIA | IIB | III | |
| Longitudinal ridging | 3 | 4 | 1 | |
| Nail thickening | 4 | |||
| Nail fragility and dystrophy | 1 | 2 | ||
| Leukonychia | 1 | 2 | ||
| Subungual hyperkeratosis | 1 | |||
| Onychomadesis | 1 | |||
| Pigmented nail band | 1 | |||
| Nail thinning | 1 | |||
| Distal notching | 1 | |||
| Koilonychia | 1 | |||
| Subungual debris | 1 | |||
| Pitting | 1 | |||
| Beau's line | 1 | |||
| Opacity | 1 | |||
Discussion
MF has a wide range of clinical presentations and rarely has been reported to involve the nail apparatus [1]. The clinical presentations of nail involvement in previous case reports included subungual hyperkeratosis, onycholysis, onychomadesis, yellow discoloration, thinning of the nail plate, splinter hemorrhage, and 20-nail dystrophy [2,3,4,5,6,7,8,9,10].
In our study, 18 patients (30%) out of 60 cases showed nail changes ranging from nail pitting to onychomadesis. The most prevalent nail changes were longitudinal ridging (8 cases), nail thickening (4 cases), nail fragility (3 cases), and leukonychia (3 cases). Longitudinal ridging, especially when associated with nail thinning, is a common manifestation of lichen planus; a mild form of nail plate thinning may be a physiological finding in the elderly. Nail thickening and subungual hyperkeratosis are commonly due to psoriasis and onychomycosis. Tomsick [3] reported a case of patch- and plaque-stage MF with hyperkeratosis of all 20 nails with subungual debris, which was associated with crumbling of the nail plate. Nail fragility was another finding in our study, which was manifested with nail plate destruction and fragility. In the literature, we have found one case report by Dalziel et al. [9] of 20-nail dystrophy in a patient with progressive cutaneous T-cell lymphomas.
Toritsugi et al. [7] in 2004 reported a vesiculopustular variant of palmaris et plantaris MF with nail changes including thickening, roughness, and yellow discoloration of the nail plate. Interestingly, in a case series of 4 patients with palmaris et plantaris MF, involvement of the nails, despite extensive lesions of the hands and feet, was not observed [12].
In our patients, 3 of them presented with punctate leukonychia in finger nails without history of similar nail findings before the diagnosis of MF.
Biopsy specimens were taken from 10 patients in our study. Surprisingly, none of them revealed histopathological evidence of MF. It seems that nail findings in our patients are more likely due to the chronic inflammatory process of MF rather than atypical lymphocyte infiltration in the nail apparatus. Also, in 5 patients, besides the absence of skin lesions around the nail apparatus, nail changes including leukonychia, longitudinal ridging, and nail thickening and opacity have been found, and in 2 patients with erythema and scaling of periungual skin, the nails were normal. Although our biopsies did not reveal any findings in favor of MF, they were taken from the skin with erythema and scaling around the nail apparatus, not the nail bed or nail matrix, which might affect our results.
In a case series comparing the nail findings in patients with Sezary syndrome and pityriasis rubra pilaris patients, splinter hemorrhage, onycholysis, and yellow brown nail plate discoloration were reported to affect both groups of patients. They believed that these findings are more likely due to erythroderma rather than a specific disease process, because the nail changes were identical to those of pityriasis rubra pilaris [8].
Onychomadesis, another reported nail change in MF in the study by Fleming et al. [5], occurred in the absence of erythrodermia in a patient with progressive plaque stage of MF. One of our patients, in stage III with progressive MF, also demonstrated nail changes including onychomadesis and Beau's lines similar to this report.
Another main issue is that our patients were under different treatment modalities, and nearly 95% of patients were under phototherapy. The occurrence of pigmentary changes, especially lentigines and pigmented nail band, nail dystrophy, and nail shedding, is well documented in patients during PUVA therapy and electron-beam radiation [11,13,14].
Conclusion
This study illustrates that nail findings were observed in 30% of early MF patients, and not only can it be due to the nail involvement of the MF, but also the disease process itself can induce nail changes, even if there is not any skin lesion around the nail apparatus. The most common nail changes detected in MF patients included longitudinal ridging, nail thickening, nail fragility, and leukonychia. In our study, patients were under different treatment modalities that can cause various nail abnormalities. As a result, nail changes observed in our patients can be attributed to heterogeneous factors.
Limitations
Studies with a higher number of patients along with histological examination of all patients with nail changes from the nail bed or nail matrix are required to estimate the exact incidence and pathogenesis of nail changes in MF patients.
Statement of Ethics
The study protocol was reviewed and approved by the Tehran University of Medical Sciences Research Center.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276–285. doi: 10.1016/j.jaad.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 3.Tomsick RS. Hyperkeratosis in mycosis fungoides. Cutis. 1982;29:621–623. [PubMed] [Google Scholar]
- 4.Harland E, Dalle S, Balme B, Dumontet C, Thomas L. Ungueotropic T-cell lymphoma. Arch Dermatol. 2006;142:1071–1073. doi: 10.1001/archderm.142.8.1071. [DOI] [PubMed] [Google Scholar]
- 5.Fleming CJ, Hunt MJ, Barnetson RS. Mycosis fungoides with onychomadesis. Br J Dermatol. 1996;135:1012–1013. doi: 10.1046/j.1365-2133.1996.d01-1118.x. [DOI] [PubMed] [Google Scholar]
- 6.Stosiek N, Peters KP, Hiller D, Riedl B, Hornstein OP. Yellow nail syndrome in a patient with mycosis fungoides. J Am Acad Dermatol. 1993;28:792–794. doi: 10.1016/s0190-9622(09)80277-6. [DOI] [PubMed] [Google Scholar]
- 7.Toritsugi M, Satoh T, Higuchi T, Yokozeki H, Nishioka K. A vesiculopustular variant of mycosis fungoides palmaris et plantaris masquerading as palmoplantar pustulosis with nail involvement. J Am Acad Dermatol. 2004;51:139–141. doi: 10.1016/j.jaad.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Sonnex TS, Dawber RP, Zachary CB, Millard PR, Griffiths AD. The nails in adult type 1 pityriasis rubra pilaris. J Am Acad Dermatol. 1986;15:956–960. doi: 10.1016/s0190-9622(86)70256-9. [DOI] [PubMed] [Google Scholar]
- 9.Dalziel KL, Telfer NR, Dawber RPR. Nail dystrophy in cutaneous T-cell lymphoma. Br J Dermatol. 1989;120:571–574. doi: 10.1111/j.1365-2133.1989.tb01333.x. [DOI] [PubMed] [Google Scholar]
- 10.Grande-Sarpa H, Callis Duffin KP, Florell SR. Onychodystrophy and tumor-stage mycosis fungoides confined to a single digit: report of a case and review of nail findings in cutaneous T cell lymphoma. J Am Acad Dermatol. 2008;59:154–157. doi: 10.1016/j.jaad.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Parkins GJ, Burden AD, Makrygeorgou A. Psoralen ultraviolet A-induced melanonychia. Clin Exp Dermatol. 2015;40:331–332. doi: 10.1111/ced.12481. [DOI] [PubMed] [Google Scholar]
- 12.Resnik KS, Kantor GR, Lessin SR, Kadin ME, Chooback L, Cooper HS, et al. Mycosis fungoides palmaris et plantaris. J Am Acad Dermatol. 1995;131:1052–1056. [PubMed] [Google Scholar]
- 13.Ledbetter LS, Hsu S. Melanonychia associated with PUVA therapy. J Am Acad Dermatol. 2003;48:S31–S32. doi: 10.1067/mjd.2003.117. [DOI] [PubMed] [Google Scholar]
- 14.Desai KR, Pezner RD, Lipsett JA, Vora NL, Luk KH, Wong JY, et al. Total skin electron irradiation for mycosis fungoides: relationship between acute toxicities and measured dose at different anatomic sites. Int J Radiat Oncol Biol Phys. 1988;15:641–645. doi: 10.1016/0360-3016(88)90306-9. [DOI] [PubMed] [Google Scholar]