Abstract
BACKGROUND
The amygdala is a central component of the neural circuitry that underlies fear learning. N-methyl-D-aspartate receptor (NMDAR)-dependent plasticity in the amygdala is required for Pavlovian fear conditioning and extinction. NMDAR activation requires the binding of a co-agonist, D-serine, which is synthesized from L-serine by the neuronal enzyme serine racemase (SR). However, little is known about SR and D-serine function in the amygdala.
METHODS
We used immunohistochemical methods to characterize the cellular localization of SR and D-serine in the mouse and human amygdala. Using biochemical and molecular techniques, we determined whether trace fear conditioning and extinction engages the SR and D-serine in the brain. D-serine was administered systemically to mice evaluate its effect on fear extinction. Finally, we investigated whether a functional SNP (rs4523957), which is an expression-linked quantitative trait locus (eQTL) of the human serine racemase (SRR) gene, was associated with fear-related phenotypes in a highly traumatized human cohort.
RESULTS
We demonstrate that approximately half of the neurons in the amygdala express SR, including both excitatory and inhibitory neurons. We find that the acquisition and extinction of fear memory engages the SR/D-serine system in the mouse amygdala and that D-serine administration facilitates fear extinction. We also demonstrate that the SRR SNP, rs4523957, is associated with post-traumatic stress disorder (PTSD) in humans, consistent with the facilitatory effect of D-serine on fear extinction.
CONCLUSIONS
These new findings have important implications for understanding D-serine mediated NMDAR plasticity in the amygdala and how this system could contribute to disorders with maladaptive fear circuitry.
Keywords: Serine racemase, D-serine, NMDA receptor, amygdala, fear conditioning, post-traumatic stress disorder
INTRODUCTION
N-methyl-D-aspartate receptors (NMDARs) belong to the class of ionotropic glutamate receptors that are essential mediators of synaptic plasticity, learning and memory (1). These receptors are unique, in that their activation requires the concomitant binding of glutamate and a co-agonist, glycine or D-serine (2, 3). D-serine is synthesized from L-serine by the enzyme serine racemase (SR) (4). Both SR and D-serine are enriched in cortico-limbic regions of the brain and are localized to the same areas as NMDARs (5). Although the endogenous co-agonists glycine and D-serine are present in the extracellular space (3), the glycine modulatory site (GMS) is not saturated in vivo (6). In the adult hippocampus, D-serine is important for proper NMDAR function, NMDAR-dependent long-term potentiation (LTP), and it is believed to be the primary co-agonist for synaptic, but not extra-synaptic NMDARs (7–11). From studies with mice with a constitutive deletion in SR (SR−/−) mice, D-serine was shown to also be important for maintaining glutamatergic dendritic integrity in the adult cortex and hippocampus (8, 12, 13). However, outside of these cortical regions, little is known about the function and cellular localization of SR and D-serine. Thus, we investigated the distribution of SR and D-serine in the murine and human amygdala.
The amygdala is a central hub in the emotional learning circuit, integrating sensory information from both cortical and subcortical brain regions related to the conditioning experience (14). LTP in the amygdala is NMDAR-dependent (15, 16). Furthermore, NMDAR activation in the amygdala is necessary for fear conditioning and fear extinction (17). Using SR−/− mice, and enzymatic degradation of D-serine with D-amino acid oxidase in brain slices from control mice, we found that the induction of NMDAR-dependent LTP at thalamo-LA synapses is dependent on D-serine (11). Moreover, we demonstrated that the magnitude of LTP in thalamic inputs is directly determined by the level of NMDAR activation (11).
Pavlovian fear conditioning is one of the most widely used models for studying emotional memory and associative learning in rodents (18). In this form of conditioning, a neutral stimulus (conditioned stimulus; CS) acquires predictive value by pairing it with an aversive, unconditioned stimulus (US; foot shock) that has an intrinsic value to the subject. After training, exposure of the animal to the CS or context alone elicits conditioned fear responses such as freezing. Using SR−/− mice, we previously demonstrated that SR and D-serine are important for fear learning (19). Therefore, in the present study, we examined whether the D-serine system is dynamically involved in fear conditioned learning, specifically within the amygdala, and other brain regions known to be critical for this behavior.
There is abundant evidence that the amygdala is also dysfunctional in post-traumatic stress disorder (PTSD) and related anxiety disorders (20). Thus, we next demonstrated that a previously examined single nucleotide polymorphism (SNP), rs4523957, within the human serine racemase (SRR) gene previously associated with other disorders (21–23), is a functional eQTL at the level of regulating SR mRNA expression in post-mortem human brain. Finally, we found that this functional SRR SNP was associated with PTSD in a highly traumatized population (24, 25).
METHODS AND MATERIALS
Animals
Adult male mice (3–5 months old) were used for all the experiments. Animals were group housed in polycarbonate cages and maintained on a 12:12 h light/dark cycle in a temperature (22°C) and humidity controlled vivarium. Animals were given access to food and water ad libitum. All animal procedures were approved by the McLean Hospital Institutional Animal Care and Use Committee.
Immunohistochemistry
Brain fixation and immunohistochemistry/immunofluorescence in mouse and human brain tissue were performed as previously described (26), with modifications described in the Supplemental Material.
Stereological estimation of co-localization
Brain sections were visualized on a Zeiss Axio ImagerM2 equipped with StereoInvestigator software. The Optical Fractionator Workflow was used to stereologically sample neurons to estimate the percentage of neurons (NeuN) in the BLA and CeA that express SR or contain D-serine, as well as the number of inhibitory neurons that express SR or contain D-serine in the BLA.
Trace Fear Conditioning and Extinction
Behavioral procedures are described in the Supplemental Material. All testing was performed using the Near Infrared Fear Conditioning System (Med Associates, Inc.; St. Albans, VT). Freezing behavior was quantified using VideoFreeze software.
Western Blot Analysis
SDS-PAGE and immunoblotting was performed and analyzed as previously described (27).
qPCR
For relative quantification of mRNA expression (n = 6–8/group), geometric means were calculated using the comparative 2ΔCt method, with the housekeeping gene GAPDH used as the endogenous reference as previously described (12).
HPLC analysis of amino acids
D-serine levels in brain tissue were determined by HPLC analysis as previously described (28).
Human Genetic Analyses
The human subjects in this cohort analyzed for SRR genetic association with PTSD were part of the Grady Trauma Project (25). All procedures were approved by the Institutional Review Board of Emory University School of Medicine and the Grady Health Systems Research Oversight Committee. Genotyping was performed on DNA derived from saliva or blood using the Omni-Quad 1M or the Omni Express BeadChip (Illumina, San Diego, CA, USA), and genotypes were called in Illumina’s GenomeStudio (Illumina). Quality control measures were performed using PLINK (29). Previously, one SNP (rs4523957) within the human Serine Racemase gene had been associated as a potential functional variant, with multiple disorders related to NMDAR and D-serine function (21–23). The genotype calls for rs4523957 were determined from the Illumina GWAS platform to address whether this variant was associated with PTSD. Association with categorical PTSD diagnosis based on DSM-IV criteria from the mPSS was performed with chi-squared analyses based on rs4523957 GG, GT, or TT genotype.
Statistical Analyses
Unpaired t-tests were used to analyze Western blot and HPLC results when appropriate. Type I (fixed effect) one-way ANOVAs were used to analyze Western blot, HPLC, and fear conditioning results. Significant one-way ANOVA results were followed up with Tukey’s multiple comparisons test. Two-way repeated measures ANOVA was used to analyze the D-serine extinction results. Values of P < 0.05 were considered statistically significant.
RESULTS
Cellular Characterization of Serine Racemase in the Amygdala
Although the distribution and expression of SR in neurons of the murine hippocampus and cortex are well established (26, 30–32), there has been little research done to characterize SR expression in the amygdala. We found that SR protein is widely expressed in neurons, but not in astrocytes, throughout the amygdala, including the basolateral complex (BLA) and the central nucleus of the amygdala (CeA) (Figure 1A-B). We determined the percentage of neurons in the basolateral (BLA; Figure 1C-E) and central amygdala (CeA; Figure 1F-H) that express SR by using double label immunofluorescence for both neuronal nuclei (NeuN; a pan-neuronal marker) and SR, finding that 51% and 68% of neurons in BLA and CeA, respectively, express SR (Figure 1I).
Figure 1.
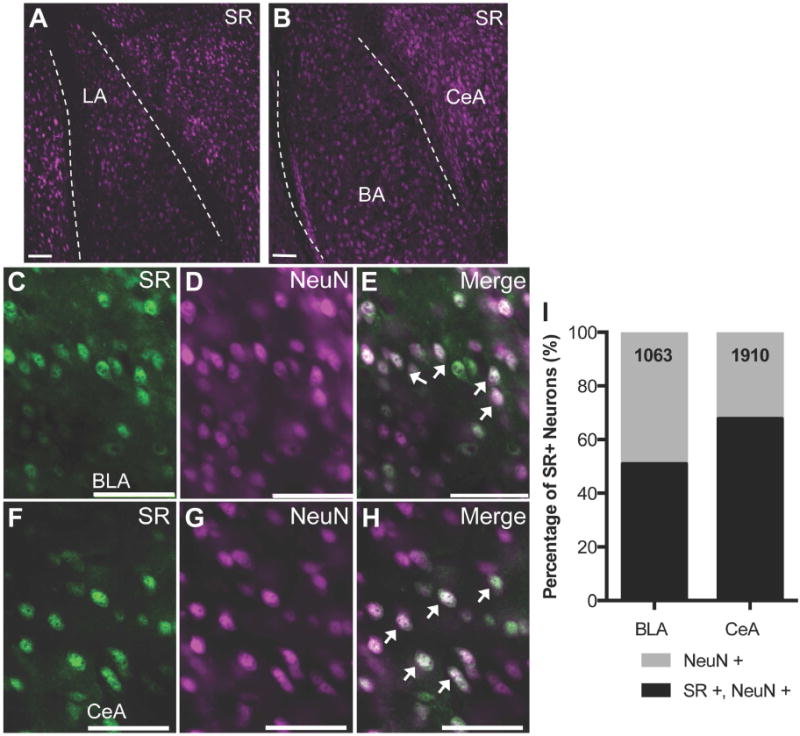
Serine racemase is widely expressed in neurons of the amygdala in mouse brain. (A-B) Representative images (10×) from coronal mouse brain sections using immunofluorescence to determine the cellular localization of serine racemase (SR). SR (magenta) is expressed throughout the mouse amygdala including the lateral (LA), basal (BA), and central (CeA) nuclei. The dashed lines demarcate the external capsule. SR (green) is expressed in neurons (NeuN; magenta) in both the basolateral amygdala (BLA; C-E) and CeA (F-H). (I) Stereology was used to estimate the percentage of neurons (NeuN) in the amygdala that express SR protein. The numbers in each bar represent the total number of NeuN+ cells that were counted to assess SR colocalization. Merged images are shown in (E) and (H). Arrows denote dual labeled neurons (white). Scale bars represent 5μm.
The BLA is a cortical-like structure comprised mainly of excitatory glutamatergic principal cells and inhibitory GABAergic interneurons. The CeA is a ventrocaudal extension of the striatum and consists of local and projecting GABAergic neurons. Using a GAD65 transgenic fluorescent reporter mouse (GAD65-T2a-NLS mCherry mouse), we did not find evidence of SR expression GAD65+ inhibitory neurons of the BLA (Figure 2A-C; 292 GAD65+ cells). Using an antibody against the other GABA synthetic enzyme, GAD67, we found that SR was expressed in only ~8% of BLA GAD67 neurons (44 out of 556 GAD67+ cells contained SR; Supplemental Figure S1A-C). On the other hand, SR was found in many inhibitory neurons of the CeA (Figure 2D-F). Moving to the human amygdala, we found that SR was expressed in excitatory neurons throughout the BLA, but not in parvalbumin positive inhibitory neurons (Figure 2G-I), similar to the mouse. SR was also widely expressed in the human CeA, co-localizing with somatostatin positive inhibitory neurons (Figure 2J-M).
Figure 2.
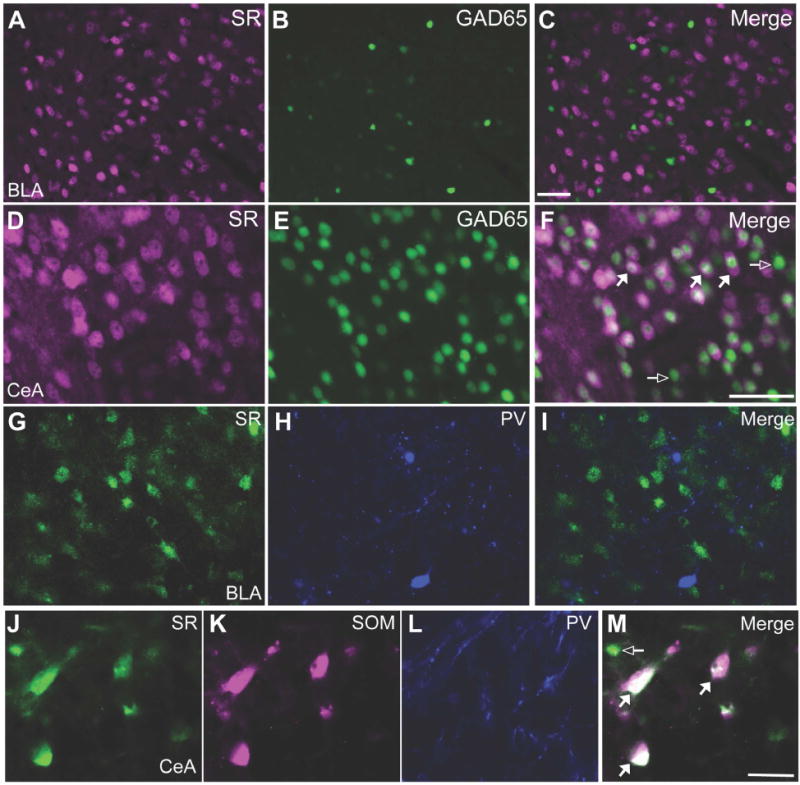
Serine racemase is expressed by excitatory and inhibitory neurons of the mouse and human amygdala. Glutamic acid decarboxylase 65 (GAD65; green) transgenic reporter mice were used to determine whether SR (magenta) is expressed in GABAergic inhibitory neurons (green) in the basolateral amygdala (A-C; BLA) and central amygdala (D-F; CeA). SR did not co-localize with GAD65 in the BLA (292 GAD65+ cells were interrogated for SR expression). However, SR was found in many GABAergic neurons of the CeA. Merged images are shown in (C) and (F). Representative coronal brain sections from human post-mortem tissue (20×): In the BLA, SR (G; green) is not found in parvalbumin (PV; H; blue) neurons, while in the CeA, SR (J; green) is found in a population of somatostatin (SOM; K; magenta) neurons. Merged images are shown in (I) and (M). Open arrows denote single-labeled neurons and white-filled arrows denote dual labeled neurons (white). Scale bars represent 50μm.
Cellular Characterization of D-serine in the Amygdala
D-serine is also widely distributed in neurons throughout the BLA and CeA (Figure 3A-C). We used brain tissue from SR−/− mice (containing about 90% less D-serine) to demonstrate that our immunostaining is specific to D-serine (Supplemental Figure S2A-D) (26). Using double-label immunofluorescence, we found that D-serine accounted for ~15% of the neurons in the BLA (Figure 3D-F; 173 out of 1148 NeuN+ neurons contained D-serine) and 39% of the neurons in the CeA (Figure 3G-I; 568 out of 1452 NeuN+ neurons contained D-serine). Interestingly, D-serine was found in ~90% of GAD67+ BLA neurons (Figure 3J-L; 485 out of 537 GAD67+ neurons contained D-serine), which do not express SR.
Figure 3.
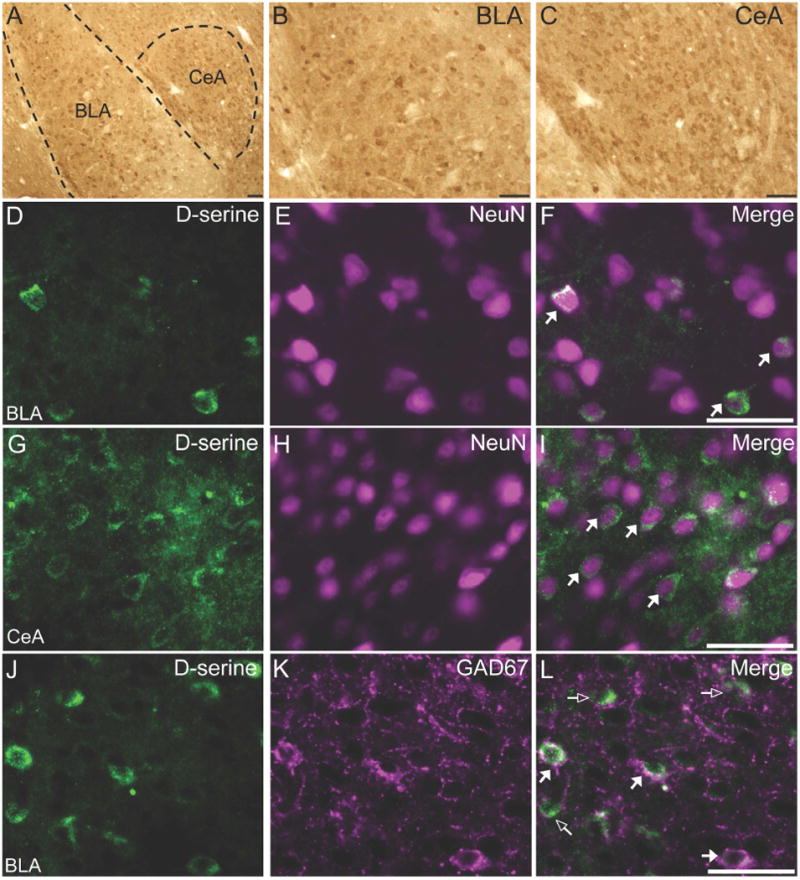
D-serine containing neurons are present widely throughout the mouse amygdala. (AC) D-serine is found throughout the amygdala, including the basolateral (BLA) and central (CeA) nuclei. The dashed lines demarcate the external capsule. D-serine (D, G; green) is localized to neurons (E, H) in the BLA and CeA. D-serine (J; green) is found in glutamic acid decarboxylase 67kDa (GAD67; K; magenta) inhibitory neurons of the BLA. Merged images are shown in panels (F, I, and L). Open arrows denote single-labeled neurons and white-filled arrows denote dual labeled neurons (white). Scale bars represent 50μm.
Trace-fear Conditioning Increase Serine Racemase and Arc Protein
Mice were trained in a trace-fear conditioning paradigm consisting of five tone-shock pairings and then were sacrificed 60 minutes after the conditioning session (Figure 4A). Only mice that were exposed to a foot shock during conditioning showed increased freezing during the conditioning session (Figure 4B). SR protein (Figure 4C) was increased in the amygdala (P< 0.05), but not in the prefrontal cortex (PFC; P= 0.54) or hippocampus (P= 0.25) of trace fear-conditioned mice. This increase in SR protein in the amygdala was accompanied by an increase at the transcript level (Supplemental Figure S2A; t13 = 2.56, P< 0.05). As a control, we also measured protein levels of activity-regulated cytoskeleton-associated protein (Arc; Arg3.1). Arc is a non-transcription factor immediate early gene that is positively regulated by NMDAR activity (33) and is up-regulated following fear conditioning (34–36). In agreement with previous studies (34, 37), Arc protein (Figure 4D) was robustly increased in the amygdala (P< 0.001), PFC (P< 0.0001) and hippocampus (P< 0.05) after trace-fear conditioning. The changes in SR (Supplemental Figure S3A; amygdala: P= 0.77; PFC: P= 0.29; hippocampus: P= 0.51) and Arc (Supplemental Figure S3B; amygdala: P = 0.12; hippocampus: P= 0.14) protein levels were due to fear conditioning, as they were unchanged in mice that were exposed to the sham protocol. Only in the PFC was there an increase in Arc under sham conditions (P< 0.05), which we believe was due to novelty.
Figure 4.
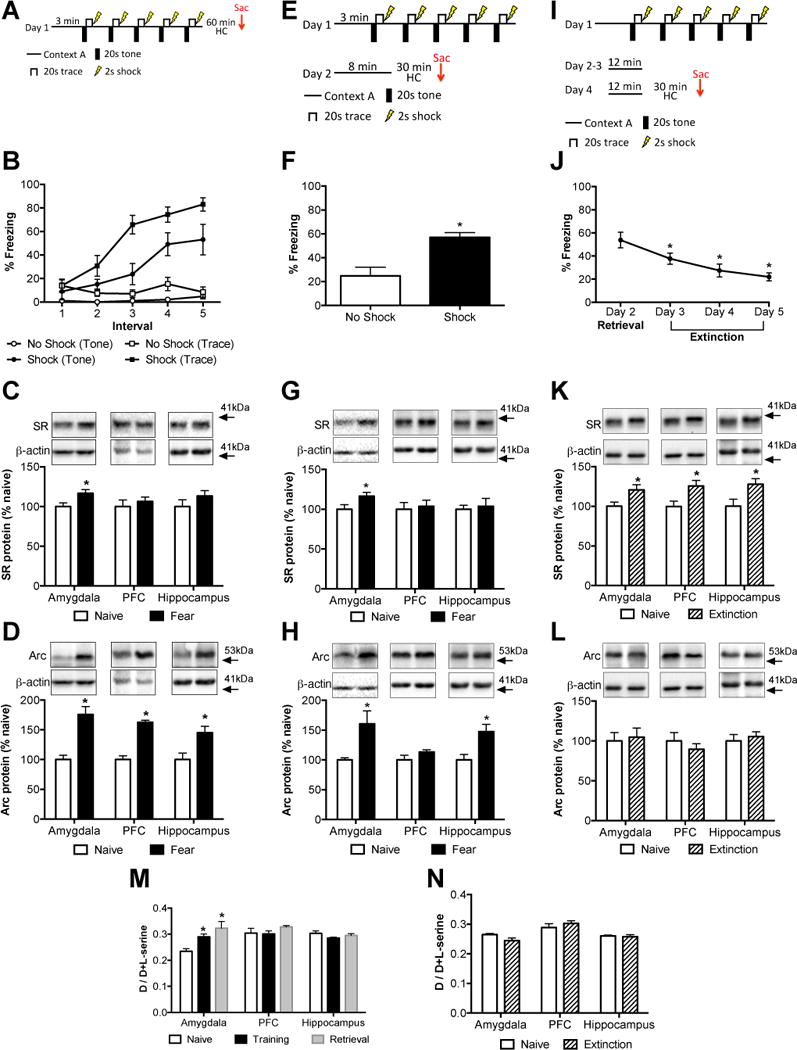
Serine racemase, D-serine, and Arc levels are differentially regulated by trace fear conditioning, retrieval, and extinction. (A) Mice were subjected to a trace fear-conditioning paradigm. One group did not receive foot shocks (open symbols; n = 5), while the other group did receive foot shocks (black symbols; n = 7). (B) The amount of freezing during each of the five tone presentations (circles) and trace intervals (squares) was measured for each group. Protein levels of serine racemase (SR; C) and Arc (D) were measured in the amygdala, prefrontal cortex (PFC), and hippocampus of naïve (white bars; n = 7) and fear-conditioned (black bars; n = 6) mice. Animals were sacrificed 60 minutes after training. (E) Mice were subjected to a trace fear-conditioning paradigm and were placed back in the same training context the next day to assess freezing behavior. (F) The average amount of freezing during the first three minutes of being placed in the training context on day 2 was measured for each group (no foot shock: black bars, n = 7; foot shock: white bars, n = 7). Protein levels of SR (G) and Arc (H) were measured in the amygdala, PFC, and hippocampus of naïve (white bars; n = 7) and fear-conditioned (black bars; n = 7) mice. (I) Mice were naïve or subjected to a trace fear-conditioning paradigm and then re-exposed to the training context for four days (n = 7/group). (J) The average freezing during the first three minutes of contextual re-exposure was measured for each extinction session. Asterisk (*) indicates significant differences from Day 2 retrieval (p < 0.001). Protein levels of SR (K) and Arc (L) were measured in the amygdala, PFC, and hippocampus of naïve (white bars; n = 6–7) and extinction (hatched bars; n =7) mice. (M) D-serine content was measured from brain tissue of mice that were naïve (white bars; n = 6–7), sacrificed 60 minutes after trace fear conditioning (black bars; n = 7–8), or 30 minutes after re-exposure to the training context on day 2 (retrieval; gray bars; n = 6). (N) D-serine content was measured in brain tissue from mice that were naïve (white bars; n = 6–7) or sacrificed after four days of contextual extinction (extinction; hatched bars; n = 10–12). Asterisk (*) indicates significant differences from the naïve group (P < 0.05). All values represent the mean ± SEM.
Re-exposure to the Training Context Increases Serine Racemase and Arc Protein
Mice were trained in a trace-fear conditioning paradigm and then placed back in the same conditioning context the next day (Figure 4E). Mice that underwent conditioning with foot shocks on Day 1 displayed significantly more freezing when placed in the conditioning context on Day 2 (Figure 4F; t12 = 3.85, P< 0.005). We found that SR mRNA (Supplemental Figure S4A; t12 = 2.48, P< 0.05) and protein (Figure 4G; t12 =2.26, P < 0.05) were again selectively up regulated in the amygdala after contextual retrieval. Arc protein was significantly elevated in the amygdala (Figure 4H; t12 = 2.76, P< 0.05) and hippocampus (Figure 4H; t12 = 3.12, P< 0.01). In the sham-conditioned animals, we did not find changes in either SR (Supplemental Figure S5A) or Arc (Supplemental Figure S5B) protein in any of the examined brain region after re-exposure to the context.
Differential Regulation of Serine Racemase and Arc Protein After Contextual Extinction
Mice were trained in a trace-fear conditioning paradigm and then re-exposed to the training context for four consecutive days (Figure 4I). Freezing behavior was significantly reduced each consecutive day with re-exposure to the context (Figure 4J; P< 0.0001). Notably, SR protein (Figure 4K) was now elevated in amygdala (t12 = 2.49, P< 0.05), PFC (t12 = 2.68, P< 0.05), and hippocampus (t12 = 2.44, P< 0.05), while SR mRNA remained significantly elevated only in the amygdala (Supplemental Figure S4B; t12 = 3.07, P< 0.01). Furthermore, Arc protein was unchanged in these brain regions (Figure 4L). Although the lack of increase in amygdalar Arc is contrast to previous work (38), our studies differ in that we measured total Arc protein and that it was measured after four days of extinction.
D-serine Content in the Amygdala is Increased After Fear Learning
The D-serine level in the amygdala was elevated after conditioning on Day 1 and after contextual retrieval on Day 2 (Figure 4M; F2,17 = 8.89, P< 0.005), without changes in L-serine level (naïve: 29.8 ± 2.6 nmol/mg protein, training: 29.8 ± 3.5 nmol/mg protein, retrieval: 29.5 ± 4.5 nmol/mg protein; F2,17 = 0.002, = 0.99). There were however, no differences in D-serine levels in the PFC (F2,16 = 1.12, P = 0.35) and hippocampus (F2,16 = 1.72, P= 0.21) after conditioning or retrieval. Tissue levels of glycine in the amygdala did not differ (F2,18 = 0.35, P = 0.71) between naïve mice (39.3 ± 6.5 nmol/mg protein) and mice that were sacrificed after conditioning (39.3 ± 3.9 nmol/mg protein) or retrieval (45.4 ± 7.0 nmol/mg protein). Following contextual extinction (Figure 4N), there were no changes in D-serine levels in the amygdala (t15 = 1.56, P = 0.14), PFC (t16 = 0.93, P = 0.37), and hippocampus (t16 = 0.22, P = 0.83).
D-serine Administration Facilitates Extinction
D-cycloserine has been shown to facilitate fear extinction in rodents and patients with PTSD (39). However, D-cycloserine is a partial agonist at the NMDAR GMS (40). Therefore, we investigated whether D-serine, which is a full GMS agonist, would facilitate extinction after trace-fear conditioning. After fear conditioning on day 1, mice received a single dose of D-serine [300 mg/kg, i.p.; (8)] thirty minutes prior to the first extinction session on day 2 (Figure 5A). This dose significantly increased (F (2, 9) = 7.1; P = 0.01) D-serine levels in the amygdala 60min (~50%), but not 24h (~20%), after injection (Supplemental Figure S6). On day 1, there was no difference in freezing behavior between groups either pre-CS (saline day 2: 4.4 ± 1.7s, D-serine day 2: 6.3 ± 2.0s; t15 = 0.72, P = 0.48) or during conditioning (Drug day 2: F(1, 15) = 2.09; P = 0.17; Trace: F(4, 60) = 22.3; P < 0.0001; Interaction: F(4, 60) = 1.07; P = 0.38). We found that mice treated with D-serine froze significantly less than saline-treated mice during all three extinction sessions (Figure 5B; Day 2: Drug: F(1, 15) = 5.26; P = 0.03; Bin: F(2, 30) = 15.9; P < 0.0001; Interaction: F(2, 30) = 1.15; P = 0.3; Day 3: Drug: F(1, 15) = 6.44; P = 0.02; Bin: F(2, 30) = 0.46; P = 0.63; Interaction: F(2, 30) = 0.56; P = 0.57; Day 4: Drug: F(1, 15) = 4.91; P = 0.04; Bin: F(2, 30) = 3.14; P = 0.06; Interaction: F(2, 30) = 0.22; P = 0.80). Therefore, a single dose of D-serine significantly facilitated the extinction of fear memory.
Figure 5.
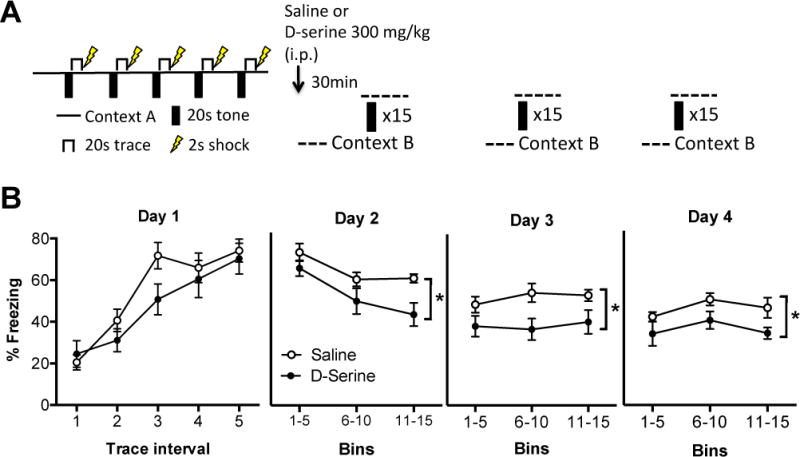
Acute D-serine administration facilitates fear extinction. (A) Mice were subjected to a trace fear-conditioning paradigm on day 1 in context A. On day 2, mice either received an intra-peritoneal injection of saline (n=8) or D-serine (300 mg/kg; n=9) 30min prior to an extinction session (15 tone presentations) in context B. Mice were then subjected to two more extinction sessions on consecutive days in context B. (B) The amount of freezing during each of the five trace intervals (20s) was measured for each group (open circles: saline day 2; black circles: D-serine day 2) during conditioning on day 1. The amount of freezing was analyzed during the 20s trace periods in 5 bin averages across the three extinction days (days 2–4).
Genetic Association of Serine Racemase and Post Traumatic Stress Disorder
Using a sample from a previously well-characterized dataset associated with civilian trauma and PTSD (~4000 subjects), we first analyzed whether there was a main effect association with the SRR polymorphism, rs4523957 and PTSD symptoms or symptom clusters. In those who had experienced significant trauma, we found an overall effect of the SRR rs4523957 genotype on PTSD diagnosis (n = 4094, x2=5.20, P<0.05) and PTSD symptoms (F(1,4028)=4.28; P<0.05), with the ‘T/T’ homozygous subjects at the rs4523957 SNP having increased PTSD symptoms.
As this cohort is better powered for females in sample size and rates of PTSD, further analyses of the SRR SNP demonstrated that females were carrying the majority of the signal within this cohort. Thus, we performed a number of additional analyses in females only to further understand the role of this SNP. First, we found that this effect was significant for predicting PTSD symptoms when accounting for comorbid diagnosis of schizophrenia, substance abuse and trauma history. We also found that in females, controlling for childhood trauma, race, and education levels, the rs4523957 SNP was associated with total PTSD symptoms (Figure 6A, F(1, 2886)=4.515, P=0.01), PTSD hyperarousal symptoms (Figure 6B, F(1,2881)=3.69, P=0.02), and PTSD avoidance/numbing symptoms (Figure 6C, F(1,2903)=5.63, P=0.004).
Figure 6.
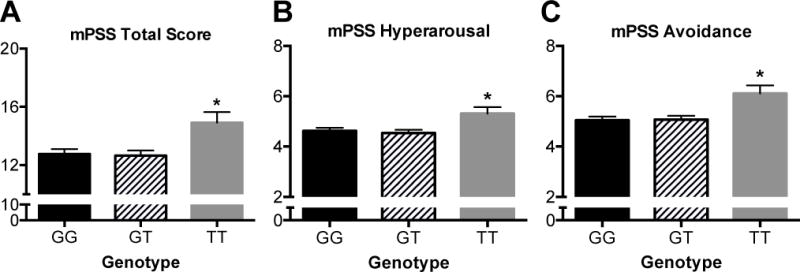
Genetic association of serine racemase and post-traumatic stress disorder. In subjects who had experienced significant trauma, there was an overall effect of the serine racemase (SRR) rs4523957 genotype on PTSD diagnosis and PTSD symptoms, with the ‘T/T’ homozygous subjects having increased PTSD symptoms. (A-C) As this cohort is most well powered for females in sample size and rates of PTSD, additional analyses were performed in females only to determine the effect of this SNP (G/G: black bar, G/T: hashed bars, T/T: gray bars) on continuous PTSD symptoms and symptom clusters. Controlling for childhood trauma, race, and education levels, the rs4523957 SNP was associated with (A) modified PTSD Symptom Scale (mPSS) total symptoms, (B) mPSS hyper-arousal symptoms, and (C) mPSS avoidance/numbing symptoms. Asterisk (*) indicates significant differences from G/G (P < 0.05). All values represent the mean ± SEM.
SNPs Linked to rs4523957 are Associated with Differential Brain SRR levels
We accessed the GTEx database (http://gtexportal.org) to determine whether this variant has been reported as an expression Quantitative Trait Locus (eQTL) in human brain tissue related to SRR mRNA expression levels. GTEx includes 13 RNA-seq brain regions, but no eQTL information is reported for rs4523957 for any brain regions. Of previous other SNPs associated with SRR function and in Linkage Disequilibrium (LD) with rs4523957, GTEx data were only available for SNP rs3744270. We found that rs3744270 is an eQTL for 11/13 brain regions, including frontal cortex, hippocampus, and basal ganglia, with P-values ranging between 5.1 × 10−7 (Hypothalamus) and 2.1 × 10−16 (Cerebellum). rs3744270 maps 1.5 Kb upstream and is in LD with rs4523957 in the PTSD association data described above (Grady civilian cohort) with D’> 0.99, and both are included in the same LD block. This evidence further supports that SNPs in this LD region might play a functional role within human brain in regulating SR levels, contributing to increased risk for fear-related disorders such as PTSD.
DISCUSSION
We have performed a detailed characterization of SR and D-serine localization in the amygdala and demonstrate that both the enzyme and the amino acid are found exclusively in neurons. Interestingly, depending on the amygdala subdivision, SR and D-serine are found in excitatory and/or inhibitory neurons in both the murine and human amygdala. Furthermore, we show that the levels of SR and D-serine are dynamically regulated in a learning-dependent manner. Finally, we demonstrate a genetic association of a SRR SNP, an eQTL associated with differential SRR levels in human brain, with PTSD and subsets of its symptom in a civilian trauma population.
The amygdala as a whole is composed of multiple interconnected nuclei. The BLA is a cortical-like structure consisting primarily of glutamatergic neurons (~80%) and GABAergic interneurons (~20%). Since SR was found in ~50% of all BLA neurons (NeuN+), but in almost no GAD65/67+ inhibitory neurons, we estimate that SR is expressed by ~63% of excitatory neurons in the BLA. Our D-serine staining revealed 15% of neurons (NeuN+) to be immune-positive. This number is most likely an underestimate because D-serine is diffusible, not appearing to be sequestered in storage vesicles (41). The more sensitive DAB immunohistochemical method revealed many glutamatergic neurons with low levels of perikaryal D-serine staining (Figure 3a-c). The glutamatergic cells in the lateral amygdala (LA) project to other excitatory neurons in the basal amygdala (BA), as well as to inhibitory neurons in the CeA and intercalated cell masses. Interestingly, we found that ~90% of GAD67+ inhibitory BLA neurons contain D-serine although they do not express SR. Thus, these GABAergic neurons appear to take up exogenous D-serine, presumably from neighboring glutamatergic neurons.
The CeA contains local and projecting GABAergic neurons and is divided into lateral (CeL) and medial (CeM) sectors (42). The CeL gates fear expression by tonically inhibiting the CeM (43), the major amygdala output to downstream fear effectors that control defensive responses, such as freezing (44). We found that SR and D-serine were found in 68% and 39%, respectively, of neurons in the CeA. There are at least two subpopulations of neurons within the CeL that express distinct markers and have opposing functions (14, 45, 46). Protein kinase C delta (PKCδ) neurons are referred to as CeLOFF because they inhibit output CeM neurons (47). SOM+ neurons (CeLON), which express SR, promote fear expression, in part, by inhibiting PKCδ neurons, thereby disinhibiting CeM (47, 48). These SOM+ neurons also send long-range projections to brain areas implicated in defensive behaviors (49). Future research will be needed to determine if D-serine is concentrated in any particular interneuron subpopulation.
Although there have been studies on neuronal cultures to identify regulatory mechanisms of SR activity (50–54), there is still very little known as to what regulates brain SR expression and D-serine production in vivo. We show here for the first time that a fear-based learning task increases the mRNA and protein expression of SR, as well as the amount of D-serine in the amygdala. Specifically, trace fear conditioning and contextual retrieval increased SR expression and D-serine levels selectively in the amygdala. The increases in amygdala SR and tissue D-serine were detected 60 minutes after conditioning, demonstrating the rapid nature over the control of its transcription and translation. While additional brain structures, including the hippocampus and mPFC, are important for trace conditioning (55, 56), the amygdala is still a critical brain structure required for learning this task (57–60).
We next examined fear extinction, which from a pathophysiological perspective, is critical for recovery in a number of trauma, fear, and anxiety-related disorders. Importantly, a single dose of D-serine prior to the first extinction session facilitated the acquisition and retention of extinction, which is similar to what has been observed in delay conditioning with D-cycloserine (39) and D-serine (61), and also comports with the ability of D-serine to facilitate the extinction of drug seeking behavior (62). Future studies will be needed to determine the circuits important for this D-serine-mediated enhanced extinction. After four days of extinction, we found that SR protein levels were elevated in the amygdala, hippocampus and PFC, but tissue D-serine content was unaltered. The reason for this discrepancy between SR and D-serine levels is unclear but may be due to increased release of D-serine during a period of high presynaptic activity, consistent with the induction of SR. Similar discrepancies are well documented in the catecholaminergic systems where chronic stress results in elevated tyrosine hydroxylase, increased amine turnover, but stable amine levels (63). Furthermore, we have observed a discrepancy between extracellular D-serine levels measured by in vivo microdialysis and tissue D-serine levels, in mice where SR was conditionally eliminated from neurons (64). In addition, the increase in D-serine mediated NMDAR transmission might be most important during the initial extinction session because brain D-serine returned to near baseline levels 24h after injection (Supplemental Figure S6) and SR/D-serine were increased after memory retrieval, which is essentially an extinction session since there were no shock presentations.
Finally, in a large civilian cohort (~4000 subjects), who had a high prevalence of adverse life experiences and PTSD, we found an overall effect of the SRR rs4523957 genotype on PTSD diagnosis and PTSD symptoms, with the ‘T/T’ homozygous subjects having increased PTSD symptoms. Notably, the most robust association in PTSD (P< 0.004) was with symptoms of avoidance and numbing, perhaps similar to prior reports that NMDAR blockade in humans results in emotional blunting (65). Additionally, D-cycloserine has previously been associated with decreased avoidance/numbing subscales in PTSD patients (66) and multiple studies have demonstrated the role of extinction as a critical aspect of recovery for avoidance and numbing symptoms in successful exposure therapy (39, 67, 68).
Regarding the functional role of this SNP, the T/T variant of rs4523957 has been associated with decreased brain SR mRNA levels, in a post-mortem RNA sequencing analysis of the dorsolateral prefrontal cortex of subjects with schizophrenia (Birnbaum, R. et al., ACNP 2014 Meeting; T181). The role of rs4523957 as a potential eQTL regulating SRR mRNA levels was further explored in our study with the GTEx postmortem database. These data suggest that T/T carriers of the rs4523957 variant may be associated with decreased endogenous D-serine.
Interestingly, PTSD is frequently associated with a deficit in extinction learning, and D-cycloserine has previously been shown to enhance fear extinction (39, 69). Our current data from both mice and humans suggest that SR deficits may be associated with a fear extinction deficit, increasing risk for PTSD development or maintenance.
In summary, we found that SR is widely distributed throughout the mouse and human amygdala, involving ~50–65% of neurons in this region. It is expressed by glutamatergic neurons in the BLA and GABAergic neurons in the CeA. D-serine is also found in neurons throughout the amygdala, being particularly concentrated in GABAergic neurons. Localization of SR and D-serine in GABAergic neurons suggests that D-serine is not acting as a co-transmitter but in an autocrine capacity, rendering their post-synaptic NMDARs “primed” to respond to glutamate. Fear learning and extinction dynamically regulate both the enzyme and its amino acid product in brain regions important for these behavioral processes. Furthermore, D-serine administration enhances fear extinction. Our findings have important implications for understanding D-serine mediated NMDAR-dependent synaptic plasticity in the amygdala and how this system could contribute to disorders with maladaptive fear circuitry, such as PTSD.
Supplementary Material
Acknowledgments
This research was supported by a 1K99MH099252-01A1 and 5R00MH099252-04 (DTB), R01MH05190 (JTC), Israel Science Foundation, the Allen and Jewel Prince Center for Neurodegenerative Disorders of the Brain (H.W.), and R01MH096764, R01MH071537 (KJR). We thank the Harvard Brain Tissue Resource Center and the brain donors and their families for the tissue samples used in these studies. We thank Drs. Uwe Rudolph and Sabina Berretta for the generous use of their equipment.
JTC has served as a consultant to Forum Pharmaceuticals in the last 2 years. A patent owned by Massachusetts General Hospital for the use of D-serine as a treatment for serious mental illness could yield royalties for Dr. Coyle.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The remaining authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 2.Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 4.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, et al. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci U S A. 2013;110:E2400–2409. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Bail M, Martineau M, Sacchi S, Yatsenko N, Radzishevsky I, Conrod S, et al. Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc Natl Acad Sci U S A. 2015;112:E204–213. doi: 10.1073/pnas.1416668112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Sacchi S, Pollegioni L, Basu AC, Coyle JT, Bolshakov VY. Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nature communications. 2013;4:1760. doi: 10.1038/ncomms2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balu DT, Basu AC, Corradi JP, Cacace AM, Coyle JT. The NMDA receptor co-agonists, d-serine and glycine, regulate neuronal dendritic architecture in the somatosensory cortex. Neurobiol Dis. 2012;45:671–682. doi: 10.1016/j.nbd.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balu DT, Coyle JT. Chronic D-serine reverses arc expression and partially rescues dendritic abnormalities in a mouse model of NMDA receptor hypofunction. Neurochem Int. 2014;75C:76–78. doi: 10.1016/j.neuint.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Balu DT, Li Y, Takagi S, Presti KT, Ramikie TS, Rook JM, et al. An mGlu5-Positive Allosteric Modulator Rescues the Neuroplasticity Deficits in a Genetic Model of NMDA Receptor Hypofunction in Schizophrenia. Neuropsychopharmacology. 2016;41:2052–2061. doi: 10.1038/npp.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimasaki A, Kondo K, Saito T, Esaki K, Otsuka Y, Mano K, et al. A genetic variant in 12q13, a possible risk factor for bipolar disorder, is associated with depressive state, accounting for stressful life events. PLoS One. 2014;9:e115135. doi: 10.1371/journal.pone.0115135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Auwera S, Teumer A, Hertel J, Homuth G, Volker U, Lucht MJ, et al. The inverse link between genetic risk for schizophrenia and migraine through NMDA (N-methyl-D-aspartate) receptor activation via D-serine. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2016;26:1507–1515. doi: 10.1016/j.euroneuro.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Xiao J, Ren Q, Han X, Tang Y, Yang W, et al. Association of serine racemase gene variants with type 2 diabetes in the Chinese Han population. J Diabetes Investig. 2014;5:286–289. doi: 10.1111/jdi.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, et al. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med. 2013;5:188ra173. doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balu DT, Takagi S, Puhl MD, Benneyworth MA, Coyle JT. D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell Mol Neurobiol. 2014;34:419–435. doi: 10.1007/s10571-014-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balu DT, Coyle JT. Glutamate receptor composition of the post-synaptic density is altered in genetic mouse models of NMDA receptor hypo- and hyperfunction. Brain Res. 2011;1392:1–7. doi: 10.1016/j.brainres.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto A, Nishikawa T, Oka T, Takahashi K, Hayashi T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert.-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. Journal of chromatography. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehmsen JT, Ma TM, Sason H, Rosenberg D, Ogo T, Furuya S, et al. D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J Neurosci. 2013;33:12464–12469. doi: 10.1523/JNEUROSCI.4914-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, et al. Serine racemase is predominantly localized in neurons in mouse brain. The Journal of comparative neurology. 2008;510:641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- 32.Wolosker H, Balu DT, Coyle JT. The Rise and Fall of the d-Serine-Mediated Gliotransmission Hypothesis. Trends Neurosci. 2016;39:712–721. doi: 10.1016/j.tins.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011;34:591–598. doi: 10.1016/j.tins.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czerniawski J, Ree F, Chia C, Ramamoorthi K, Kumata Y, Otto TA. The importance of having Arc: expression of the immediate-early gene Arc is required for hippocampus-dependent fear conditioning and blocked by NMDA receptor antagonism. J Neurosci. 2011;31:11200–11207. doi: 10.1523/JNEUROSCI.2211-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maddox SA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for reconsolidation of a Pavlovian fear memory. J Neurosci. 2011;31:7073–7082. doi: 10.1523/JNEUROSCI.1120-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chau LS, Prakapenka A, Fleming SA, Davis AS, Galvez R. Elevated Arc/Arg 3.1 protein expression in the basolateral amygdala following auditory trace-cued fear conditioning. Neurobiology of learning and memory. 2013;106:127–133. doi: 10.1016/j.nlm.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Onoue K, Nakayama D, Ikegaya Y, Matsuki N, Nomura H. Fear extinction requires Arc/Arg3.1 expression in the basolateral amygdala. Mol Brain. 2014;7:30. doi: 10.1186/1756-6606-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McBain CJ, Kleckner NW, Wyrick S, Dingledine R. Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol. 1989;36:556–565. [PubMed] [Google Scholar]
- 41.Lin H, Jacobi AA, Anderson SA, Lynch DR. D-Serine and Serine Racemase Are Associated with PSD-95 and Glutamatergic Synapse Stability. Frontiers in cellular neuroscience. 2016;10:34. doi: 10.3389/fncel.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 44.Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, et al. Midbrain circuits for defensive behaviour. Nature. 2016;534:206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- 45.McCullough KM, Morrison FG, Ressler KJ. Bridging the Gap: Towards a cell-type specific understanding of neural circuits underlying fear behaviors. Neurobiology of learning and memory. 2016;135:27–39. doi: 10.1016/j.nlm.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gafford GM, Ressler KJ. Mouse models of fear-related disorders: Cell-type-specific manipulations in amygdala. Neuroscience. 2016;321:108–120. doi: 10.1016/j.neuroscience.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci. 2014;34:2432–2437. doi: 10.1523/JNEUROSCI.4166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balan L, Foltyn VN, Zehl M, Dumin E, Dikopoltsev E, Knoh D, et al. Feedback inactivation of D-serine synthesis by NMDA receptor-elicited translocation of serine racemase to the membrane. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0809442106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dikopoltsev E, Foltyn VN, Zehl M, Jensen ON, Mori H, Radzishevsky I, et al. FBXO22 is Required for Optimal Synthesis of the NMDA Receptor Co-agonist D-Serine. J Biol Chem. 2014 doi: 10.1074/jbc.M114.618405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dumin E, Bendikov I, Foltyn VN, Misumi Y, Ikehara Y, Kartvelishvily E, et al. Modulation of D-serine levels via ubiquitin-dependent proteasomal degradation of serine racemase. J Biol Chem. 2006;281:20291–20302. doi: 10.1074/jbc.M601971200. [DOI] [PubMed] [Google Scholar]
- 53.Ma TM, Paul BD, Fu C, Hu S, Zhu H, Blackshaw S, et al. Serine Racemase Regulated by Binding to Stargazin and PSD-95: POTENTIAL N-METHYL-d-ASPARTATE-alpha-AMINO-3-HYDROXY-5-METHYL-4-ISOXAZOLEPROPIONIC ACID (NMDA-AMPA) GLUTAMATE NEUROTRANSMISSION CROSS-TALK. J Biol Chem. 2014;289:29631–29641. doi: 10.1074/jbc.M114.571604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mustafa AK, van Rossum DB, Patterson RL, Maag D, Ehmsen JT, Gazi SK, et al. Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc Natl Acad Sci U S A. 2009;106:2921–2926. doi: 10.1073/pnas.0813105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17:289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- 57.Gilmartin MR, Kwapis JL, Helmstetter FJ. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiology of learning and memory. 2012;97:452–464. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guimarais M, Gregorio A, Cruz A, Guyon N, Moita MA. Time determines the neural circuit underlying associative fear learning. Frontiers in behavioral neuroscience. 2011;5:89. doi: 10.3389/fnbeh.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwapis JL, Jarome TJ, Schiff JC, Helmstetter FJ. Memory consolidation in both trace and delay fear conditioning is disrupted by intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Learn Mem. 2011;18:728–732. doi: 10.1101/lm.023945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kochli DE, Thompson EC, Fricke EA, Postle AF, Quinn JJ. The amygdala is critical for trace, delay, and contextual fear conditioning. Learn Mem. 2015;22:92–100. doi: 10.1101/lm.034918.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuda S, Matsuzawa D, Nakazawa K, Sutoh C, Ohtsuka H, Ishii D, et al. d-serine enhances extinction of auditory cued fear conditioning via ERK1/2 phosphorylation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:895–902. doi: 10.1016/j.pnpbp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 62.Hammond S, Seymour CM, Burger A, Wagner JJ. D-Serine facilitates the effectiveness of extinction to reduce drug-primed reinstatement of cocaine-induced conditioned place preference. Neuropharmacology. 2013;64:464–471. doi: 10.1016/j.neuropharm.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiological reviews. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 64.Ishiwata S, Umino A, Balu DT, Coyle JT, Nishikawa T. Neuronal serine racemase regulates extracellular D-serine levels in the adult mouse hippocampus. J Neural Transm. 2015;122:1099–1103. doi: 10.1007/s00702-015-1388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chambers RA, Bremner JD, Moghaddam B, Southwick SM, Charney DS, Krystal JH. Glutamate and post-traumatic stress disorder: toward a psychobiology of dissociation. Semin Clin Neuropsychiatry. 1999;4:274–281. doi: 10.153/SCNP00400274. [DOI] [PubMed] [Google Scholar]
- 66.Attari A, Rajabi F, Maracy MR. D-cycloserine for treatment of numbing and avoidance in chronic post traumatic stress disorder: A randomized, double blind, clinical trial. J Res Med Sci. 2014;19:592–598. [PMC free article] [PubMed] [Google Scholar]
- 67.Sripada RK, Garfinkel SN, Liberzon I. Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Front Hum Neurosci. 2013;7:672. doi: 10.3389/fnhum.2013.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wicking M, Steiger F, Nees F, Diener SJ, Grimm O, Ruttorf M, et al. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiology of learning and memory. 2016;136:116–126. doi: 10.1016/j.nlm.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 69.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


