Abstract
Metastasis can be generalized as a linear sequence of events whereby halting one or more steps in the cascade may reduce tumor cell dissemination and ultimately improve patient outcome. However, metastasis is a complex process with multiple parallel mechanisms of dissemination. Clinical strategies focus on removing the primary tumor and/or treating distant metastases through chemo- or immunotherapies. Successful strategies for blocking metastasis will need to address the parallel mechanisms of dissemination and identify common bottlenecks. Here, we review the current understanding of common pathways for tumors to disseminate. Understanding of the complexities of metastasis will guide the design of new therapies that halt dissemination.
Keywords: metastasis, dissemination, intravasation, extravasation, tumor vasculature, tumor microenvironment
TUMOR DISSEMINATION
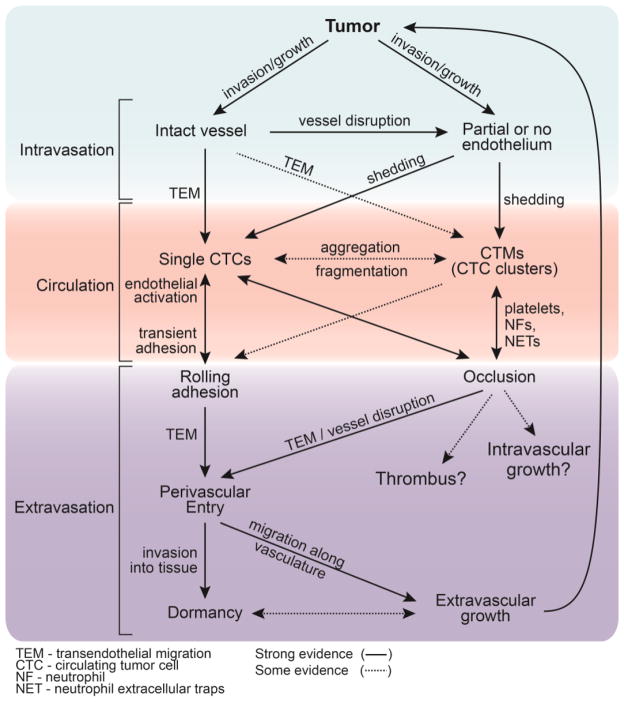
Dissemination of tumor cells to distant organs ultimately leads to the majority of cancer related deaths [1, 2]. The steps in the metastatic cascade are often described in terms of a linear sequence of events involving a single tumor cell, however, accumulating evidence suggests that there are multiple parallel pathways for tumor cell dissemination and colonization (Fig. 1, Key Figure) that depend on the local microenvironment and highlights the important roles of the endothelium and immune system (TextBox 1).
Figure 1, Key Figure. Multiple parallel pathways for cancer cell dissemination from a primary tumor.
Key steps in the metastatic cascade - intravasation, circulation, extravasation- often exhibit parallel routes. Characteristics of the tumor vasculature and the role of the immune system dictate the mechanism of dissemination and the transition of tumor cells from different microenvironments and states. Cancer cells at the primary site may encounter intact or partially lined vasculature, which may affect the mode of intravasation (i.e. single cells or microemboli). Microemboli exhibit shorter half-lives in circulation than single tumor cells and interact to a larger degree with blood constituents. These interactions determine the mode of arrest in downstream vessels (i.e. rolling adhesion or vessel occlusion) and organs, and ultimately impact extravasation and possibly dormancy and/or extravascular growth. Supporting clinical and pre-clinical evidence for the transition of tumor cells between states is represented by the dashed or solid lines, respectively.
Text Box 1. The metastatic cascade Models.
Linear sequence of events model
A single tumor cell undergoes a sequence of steps resulting in formation of a secondary tumor: invasion → intravasation → circulation → extravasation → colonization
Blocking any step can reduce the probability of metastasis
Multiple parallel pathways model
Multiple pathways exist for intravasation of tumor cells at intact, defective, or non-existent endothelial barriers (mosaic vessels) depending on the location within a solid tumor
Circulating tumor cells (CTCs) or circulating tumor microemboli (CTMs) modulate cell survival by association with platelets, neutrophils, natural killer cells, and other soluble factors in circulation
Multiple mechanisms exist for arrest and extravasation at multiple sites throughout the body
Intervention strategies should consider the wide range of dissemination mechanisms and the corresponding diversity in microenvironments
Tumor cells are able to invade short distances, either individually or collectively, through the local microenvironment (e.g. tissues, lymphatics, or vascular interfaces), but generally gain widespread dissemination by intravasating into local microvessels and detaching when exposed to high shear flow. Tumor cells enter circulation as either single cells or aggregates (herein referred to as microemboli). The intravasation of single tumor cells or microemboli is dependent on the tumor phenotype and the local microenvironment, which play key roles in determining the degree of endothelial activation and dysfunction. Intravasation can occur across intact, defective, or non-existent endothelial barriers, and is dependent on vessel size and location in a tumor. After entering circulation, tumor cells can interact with blood components and other cell types (i.e. platelets, neutrophils, red blood cells (RBCs)) resulting in the formation of complex microemboli. The wide range of mechanisms for arrest, and the corresponding diversity in microenvironments, results in many possible pathways for extravasation and colonization at a secondary site.
A consequence of the linear-sequence-of-events model is the widely-held view that intervention at any one step can stop metastasis. In contrast, the multiple parallel pathways viewpoint underscores that intervening in the dissemination of tumor cells is extremely challenging. Here we evaluate the biological and physiological factors that control these pathways, and highlight the involvement of the vascular and immune systems during the steps of intravasation, circulation, and extravasation. We examine tumor cell circulation and extravasation in the context of microthrombi formation and the resulting impact on the growth and/or dormancy of a secondary tumor site. A broader perspective on the multiple steps in the metastatic cascade will provide a framework for developing strategies that decrease metastatic efficiency and extend the time before cancer relapse.
INTRAVASATION
Intravasation is one of the first steps in the metastatic cascade and involves tumor cell migration from tissue across and into an endothelial or epithelial vessel [1, 2] (TextBox 2). Factors that contribute to the rate of intravasation include the phenotype of the tumor cell, the local state of the endothelium, and the local microenvironment [3]. Tumor vessels exhibit vast heterogeneity in size, permeability, and endothelial coverage [4, 5]. Physical parameters such as vessel diameter and shear forces may play a role in the frequency or success of tumor cell escape into vascular flow [6]. The existence of an intact endothelium separating tumor tissue from vascular flow necessitates transendothelial migration (TEM) or vessel disruption prior to intravasation; however, an incomplete endothelial lining may directly expose tumor cells to vessel flow. The the magnitude of vascular shear stress and intercellular and/or focal adhesion also regulate the release of individual tumor cells or microemboli. Both single tumor cells and microemboli have been discovered in circulation; however, the physical and biological factors regulating their entry into circulation remain poorly understood [6]. Although the immune system is known to attack the tumor microenvironment, certain immune cells have been shown to directly enhance intravasation through activation of the tumor vasculature [7]. Here, we explore the role of the tumor vasculature on dictating the parallel pathways of tumor cell intravasation.
Box 2. Intravasation & Extravasation.
Intravasation
Associated with endothelium activation and/or downregulation of cell-cell junctions
The tumor vasculature within a solid tumor is spatially heterogeneous with intact, defective, or non-existent endothelial barriers, all of which can sustain different mechanisms of intravasation
Extravasation
Arrest in circulation can occur by occlusion of circulating tumor cells (CTCs) in capillary beds, circulating tumor microemboli (CTMs) in capillary beds or small vessels, or by capture and adhesion of CTCs or CTMs in larger vessels
Occlusion can lead to a successful metastatic event, hypoxia and tissue necrosis, formation of neurophil extracellular traps (NETs), and patient death from a thromboembolic event
Occlusion by circulating tumor microemboli is difficult to distinguish from venous thromboembolism
The complex microenvironments established around arrested CTCs and CTMs results in multiple pathways for extravasation at intact (e.g. transendothelial migration), defective endothelium, and non-existent (e.g. vessel co-option) endothelium
Heterogeneous tumor vasculature: intact, partial, or non-existent endothelium
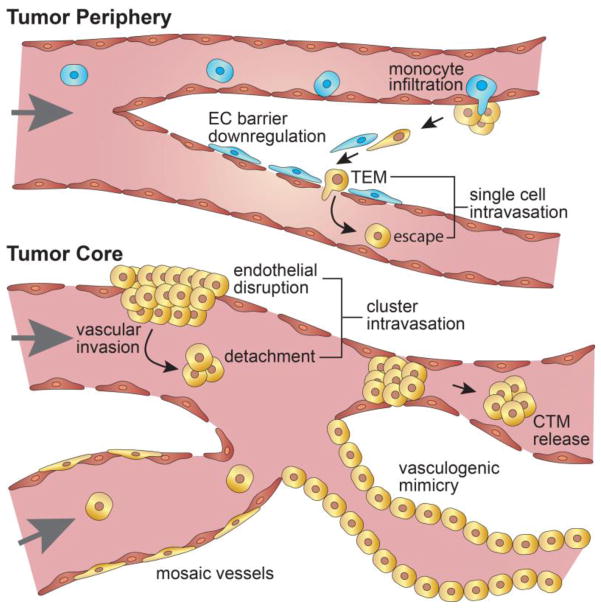
Tumor vasculature exhibits a spectrum of barrier properties ranging from functional to leaky and/or to non-existent (Fig. 2) In solid tumors, vessel functionality varies based on location within the tumor. For example, replicas (i.e. corrosion casts) of tumor vasculature from human colorectal cancer show that the intervessel spacing, tortuosity, and diameter become more aberrant in going from the periphery to the core of the tumor [5]. Tumor vasculature is typically less hierarchical than normal vasculature and often exhibits loops, dead-ends, shunts, and other defects [5]. Although normal vasculature also exhibits large functional heterogeneity, ranging from gaps and fenestrae in the liver sinuses to tight-junctions of the blood-brain barrier [4], these characteristics are tailored to the functional role of distinct organs, whereas tumor vasculature is leaky due to rapidly and irregularly formed architecture that lacks lymphatic drainage, resulting in dysregulated endothelium with reduced barrier function [8].
Figure 2. Tumor vessel heterogeneity influences intravasation.
Vascular integrity and functionality varies from the tumor core to its periphery. An intact endothelium, often observed at the tumor periphery, necessitates the disruption of endothelial cell-cell junctions prior to intravasation possibly through activation by tumor associated macrophages (TAMs) and/or transendothelial migration (TEM) of tumor cells. Partially lined (i.e. mosaic vessels) or disrupted vasculature directly exposes tumor cells to shear forces and facilitates vascular invasion and the release of tumor microemboli that may enter systemic circulation or occlude the primary tumor vasculature depending on cluster size and vessel diameter. Functional tumor vasculature that lacks an endothelium but is lined by endothelial-like tumor cells, known as vasculogenic mimicry, may also contribute to tumor progression by further enabling nutrient transport and conduits for intravasation.
In animal models, vessel functionality can be directly determined by measuring the retention of fluorescent tracer molecules perfused through the vessel lumen. Tumor vasculature in animal models shows a range of low-to-moderate permeability (on the order of 10−6 – 10−7 cm s−1 for albumin and 70 kDa dextran) [9]; however, these studies are often limited to peripheral/ectopic tumor vasculature where the vessel endothelium is largely intact. Nevertheless, intact tumor vasculature has been shown to be leakier than normal vasculature with defect cut-off sizes of 400 – 600 nm [10]. This leakiness, known as the enhanced permeation and retention (EPR) effect, is widely exploited in the delivery of cancer therapeutics [8]. Histological sections of resected tumor xenografts reveal that certain tumor vasculature exhibits intercellular defects up to 5 μm in size that enable the local extravasation of nanoparticles and red blood cells [10]. In addition to intercellular gaps, tumor vasculature may exhibit partial lining by tumor cells. For example, it has been reported that up to 4% of the surface of area of tumor vessels in melanoma tumor xenografts was lacking endothelial coverage [3]. In the extreme case, there is evidence of perfusable networks comprised entirely of tumor cells, termed vascular mimicry, and characterized by deposition of basement membrane (e.g. collagen IV), expression endothelium-associated genes (e.g. vascular endothelial (VE) cadherin, ephrin type-A receptor 2 (EPHA2), laminin 5γ2, neuropilin-2), and formation of connections with existing tumor vasculature [11, 12].
Pathways for tumor cell entry: transendothelial migration and vessel disruption
The wide range of vasculature function dictates the mode and frequency of intravasation. In intact tumor vessels, evidence suggests that disruption of the endothelium is required for tumor cell transendothelial migration (TEM) and escape into the vascular system. For example, focal leaks and transient increases in vascular permeability have been observed during intravasation in mouse models of spontaneous mammary tumors [13]. The expression of growth factors (e.g. vascular endothelial growth factor (VEGF), heparin binding endothelial growth factor (HBEGF)) by tumor cells and tumor associated macrophages (TAMs) have been shown to locally down-regulate endothelial barrier properties, which may facilitate transendothelial migration and enable the intravasation of single tumor cells that is observed in mouse models [13].
In addition to disruption of endothelial junctions, the loss of endothelial cells in vessels has been suggested as a mechanism that exposes tumor cells directly to flow [3], thus facilitating intravasation by removing the necessity for TEM. In patient biopsy samples, the presence of vessels where the endothelial lining is completely absent [12], such as those that have been coopted by tumor cells or formed by tumor vasculogenesis (i.e. vascular mimicry), is associated with poor patient survival in many cancer types including melanoma, gastric cancer, or sarcomas [14]. Vascular mimicry may also improve tumor perfusion, thus promoting tumor growth [11], and although it is significantly associated with the formation of distant metastases [14], the contribution to intravasation is unclear. Nevertheless, endothelial disruption has been suggested as a means for the bulk or high-rate intravasation of tumor cells [3], and the absence or lack of endothelial integrity precludes the necessity for TEM and gives tumor cells greater access to circulation.
After TEM or endothelial disruption, the size of tumor cells or tumor microemboli entering circulation from a primary tumor is likely regulated by the diameter of the tumor vessels. For example, a microembolus larger than the vessel diameter may become lodged in the vessel lumen and form a clot that disrupts local flow and further prevents upstream tumor cells from entering circulation (Fig. 2). In mouse models, the distribution of tumor vessel diameters has been correlated to the frequency and size of individual tumor cells and microemboli released from in situ perfused tumors [15]. Furthermore, it has been suggested that only vessels greater than 30 μm in diameter contribute to the concentration of circulating tumor cells [15]. In tissue sections from the primary tumors of patients, tumor microemboli are often observed in vessels, a phenomenon termed vascular invasion [16] (Fig. 2). Although it has been shown in vitro that circulating tumor cells (CTCs) exhibit significant deformability that enables collective squeezing through tight constrictions [17], impeded or irregular flow within the tumor vasculature may prevent cells from immediately disseminating beyond the local vasculature. Nevertheless, the prevalence of vascular invasion, in particular within larger diameter venous vasculature (> 200 μm), has been correlated with increased risk for metastasis and poor patient survival [16] and suggests that large microemboli occluding the primary tumor vasculature may eventually be released into circulation. The detection of lumen fragmentation or disruption surrounding tumor-occluded vasculature also suggests that collective tumor invasion can overrun vessels [16], thus bypassing TEM.
Mechanisms of tumor cell escape: shedding of single tumor cells and microemboli
Single tumor cell entry into circulation has been directly observed across functional vessels in mouse models [13]. These observations show that intact tumor vasculature can regulate tumor cell intravasation by necessitating TEM and disruption of vessel barrier function. However, as tumors grow, vessel disruption can be induced by proteolytic degradation of basement membrane proteins and endothelial displacement [3, 16], and may eventually lead to the invasion of tumor cell clusters that frequently occlude the venous vessels in primary pancreatic and rectal tumors [16]. While the shedding of tumor cell clusters has not been directly observed in vivo, triggered release and collection of clusters has been studied after palpation or massaging of tumor xenografts in mice [15].
Involvement of the immune system
The immune system is involved in the development of primary tumors and metastases [24]. Primary tumors often recruit immune cells that can either attack the tumor or protect the microenvironment from further immune targeting [24, 25]. Immunotherapy strategies typically attempt to enhance immune cell recognition and targeting of tumor presenting antigens using vaccines and/or by reducing immunosuppressive cues (i.e. immune checkpoint blockades) expressed by tumor cells or within the local microenvironment [26]. Tumors where immunotherapy is effective are recognized by circulating activated C8+ T lymphocytes that induce apoptosis of targeted cells [26]. Systemic treatment with immune checkpoint blockers has shown increased patient survival, however, side-effects often include autoimmune disorders, such as colitis and dermatitis [26], and patients without an underlying immune response tend not to benefit from this treatment [27], while others may experience tumor growth [9]. An improved understanding of the recruitment and protective role of immune cells within the tumor microenvironment may aid in the development of enhanced immunotherapy strategies.
Three main cell types are involved in the intravasation of cancer cells: Breg cells, Treg cells, and tumor associated macrophages (TAMs). Breg cells have the primary role of suppressing T cell proliferation and inducing phenotypic conversion of T cells into Treg cells [24]. Treg cells lack cytotoxic functions and are recruited to the tumor microenvironment by CCL12, CCL5, or prostaglandin 2 [24]. Infiltration of Treg cells promotes metastasis [24]. Treg cells have been shown to suppress natural killer (NK) cell cytotoxicity by direct cell contact and through TGFβ secretion, creating an immunosuppressive environment [8].
TAMs may comprise up to 50% of a solid tumor’s mass and play a significant role in tumor progression [28]. Blood monocytes are recruited to the tumor microenvironment by chemoattractants produced by the tumor stroma and neoplastic tissue (e.g. CCL2, M-CSF, VEGF), and differentiate into mature TAMs when exposed to IL-3 and cerebrospinal fluid (CSF) [28]. Pro-inflammatory TAMs express VEGF-A, which increases vasculature leakiness [7, 13]. TAMs also increase tumor cell invasion through a CSF-EGF signaling loop that chemotactically directs tumor cell migration towards blood vessels [7]. Tumor cell intravasation in mice is mediated by the presence of TAMs in close proximity to blood vessels, which is often the site of tumor cell TEM [7, 13]. TAM-induced loss of endothelial junctional integrity facilitates tumor cell TEM and intravasation [13].
In summary, intravasation can occur across intact, defective, or non-existent endothelial barriers as single cells or clusters. The local microenvironment plays a key role in determining the degree of endothelial activation and dysfunction. Both single tumor cells and clusters require TEM to intravasate across intact microvessels and may benefit from increased vascular permeability. In addition, the vessel size influences whether tumor cells or clusters are released into circulation or remain arrested in the primary tumor vasculature. In contrast to intravasation in functional vasculature, intravasation in leaky or partially lined vasculature can occur without downregulation of endothelial cell-cell junctions or migration through confined regions that require significant cytoskeletal reorganization. Due to the parallel mechanisms of tumor cell entry and escape into the vasculature, blocking intravasation is likely to be difficult. Furthermore, tumor neovasculature develops early in disease progression, and thus leaves little opportunity between clinical detection of a primary tumor and first-line treatment. In a pooled study of breast cancer, 30% of patients exhibited micro-metastases detected in bone marrow aspirates at the time of diagnosis [29]. However, since intravasation is likely to occur from secondary and even tertiary tumor sites [7], anti-intravasation strategies may be effective at limiting the continued spread of cancer from distant metastases and/or dormant tumors.
CIRCULATION
Circulating tumor cells (CTCs) and circulating tumor microemboli (CTMs)
The dissemination of tumor cells through the circulatory system was proposed as the cause of metastasis more than 100 years ago [30]. Tumor cells in circulation can be generated by the intravasation mechanisms described in the previous section (Fig. 1), or as a result of cancer surgery or other trauma that causes disruption of the vasculature or induces shedding of tumor cells from mosaic vessels [23].
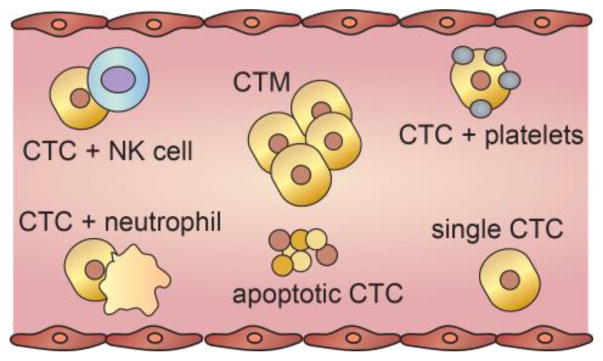
Tumor cells in circulation exist as both individual cells and clusters of cells (Fig. 3). Circulating tumor cells (CTCs) are defined as single cells or aggregates of less than 3 cells; a definition used to minimize enumeration errors due to the presence of mitotic cells in circulation [17, 34]. Larger clusters are referred to as circulating tumor microemboli (CTMs), and have been detected in the blood of mouse models and cancer patients [15, 18, 19]. Recent evidence suggests that both CTCs and CTMs are prevalent in patients with metastatic cancer [32].
Figure 3. Tumor cell populations in circulation.
Cancer cells circulate as individual cells (CTCs), as microemboli (CTMs), or in combination with other blood components such as platelets, neutrophils, or natural killer cells. The predominant form found in circulation is cell fragments from apoptotic CTCs.
CTMs can, in principle, originate from aggregation or proliferation of CTCs in circulation, or intravasation of tumor cell clusters. Evidence from labeled breast cancer cells in mouse models suggests that CTMs are derived from intravasating clusters, rather than the aggregation or proliferation of single CTCs [22, 32]. CTMs are held together by plakglobin-dependent intercellular adhesion proteins [20]. The presence of these adhesion proteins enhances survival since single tumor cells are also more likely to undergo anoikis, or programmed cell death of anchorage dependent cells after detachment from the surrounding extracellular matrix (ECM) [21]. Furthermore, CTMs exhibit an increased likelihood of occlusion in downstream vessels, and thus may contribute more to metastasis [22].
The concentration of CTCs in blood is negatively correlated with prognosis in multiple cancer types including breast, prostate, and colorectal cancers [31]. Understanding the specific role that CTCs play in cancer progression and metastasis is key to developing therapeutic strategies [31]. Clinically, CTC enumeration is being explored as a method for monitoring disease progression [31, 32], and harvested CTCs are being used to identify effective chemotherapies for individualized treatment [31]. Analysis of isolated CTCs showed that they are often more resistant to hypoxia, more invasive, form larger tumors than cell line controls, and express different markers than the primary tumor [33]. Therefore, identifying the phenotypical differences of CTCs and establishing the correlation to survival and metastatic potential remain key questions.
CTC enumeration remains a subject of debate and has been reviewed extensively [35]. Enumeration is usually performed by isolation from blood samples using immunoseparation [35]. A challenge in CTC assays is in distinguishing cells and cell-like objects. In circulation, there are healthy single CTCs, apoptotic CTCs or CTC fragments, healthy CTMs, and CTCs in clusters with other cell types (e.g. platelets, neutrophils, natural killer cells). Biomarkers such as endothelial cell adhesion molecule precursor (EpCAM), CD44, and cytokeratin are highly expressed on CTCs of epithelial origin compared to other cell types (e.g. neutrophils), and are commonly used to select for CTCs [35]. Evidence suggests that the reliance on the expression of epithelial markers results in an underestimation of CTCs and CTMs, as not all cancer cells have the same phenotype [36]. Since blood samples are typically on the order of a few milliliters, the resolution of this method is on the order of 1 CTC mL−1 blood [35]. The concentration of CTCs in cancer patients is typically 1 – 10 mL−1 blood, although values up to 10,000 mL−1 have been reported in patients with small-cell lung cancer [34]. The concentration of cancer cells in blood is a small fraction of the total cell concentration of around 109 mL−1, making isolation of individual CTCs extremely demanding. The intrinsic limitation in resolution (1 CTC) imposed by the volume of blood samples (e.g. 1 mL) corresponds to a total number of CTCs in circulation of about 5,000 (assuming a total blood volume of 5 L). To achieve a higher resolution (i.e. < 1 mL−1) will require new assays or in vivo monitoring methods.
Various strategies have been developed to study tumor cells in circulation. For example, fluorescently labeled cells in an implanted tumor can be photo-switched to a different color in circulation to track cell fate [37]. This technique has been used to observe cells returning to a primary tumor, colonization, dormancy, and growth [37]. In vivo flow cytometry studies on mice with labeled CTCs and CTMs indicate a half-life of 30 minutes for CTCs and 10 minutes for CTMs [18]. These results are consistent with the observation that the concentration of CTCs decreases significantly 24 hours following surgical resection in prostate, colorectal, and breast cancer [18, 38]. From enumeration of CTCs, the half-life in human breast cancer was estimated to be 1 – 2 hours [18]. The short half-life highlights the limited survival of CTCs and CTMs, but also implies that the rate of intravasation is relatively fast to maintain a measurable number of CTCs in circulation.
The role of immune system in survival of CTCs and CTMs
CTC viability in circulation is dependent on evasion of the immune system (particularly NK cells), surviving shear flow, and resistance to other apoptotic cues. In vitro studies have shown that NK cells induce death in 50% of cancer cells in 4 hours [39], and that macrophages induce death in 50% of cancer cells in 72 hours [40]. The survival of CTCs in NK cell deficient mice is increased 20-fold compared to controls [41]. The binding of platelets cloaks CTCs and CTMs from the immune system and increases survival [25].
Another pathway for immune protection is the formation of microthrombi. Evidence suggests that some CTCs and CTMs express tissue factor (TF), promoting thrombin generation and ultimately leading to fibrin formation and thrombosis, thereby providing a physical barrier and protection from NK cells [24]. These thrombi are formed rapidly and typically are cleared after 24 – 48 hours [42]. Neutrophils can become trapped in microthrombi, or recruited to them, through the release of CXCL8 by cancer cells. Neutrophils also induce upregulation of adhesion molecules such as ICAM in cancer cells, which has been implicated in enhanced extravasation [24]. TF has been shown to be important for progression of the primary tumor as well as formation of metastases, independent of the role of NK cells in mice with a severe NK deficiency [43]. The formation of these thrombi can be minimized with anticoagulants, which have decreased the incidence of metastasis in mouse models [25]. In the 1960s it was observed that decreased platelet count correlated with a decrease in metastatic potential, and anticoagulants have been used to mimic this effect [44]. Anticoagulants downregulate adhesion between CTCs and platelets which enables lysis by NK cells [44].
Not all CTCs or CTMs isolated from cancer patients are alive [45]. The fraction of viable cells in circulation is highly variable across cancer types, and as low as 17% in patients with prostate cancer [46]. Other cell-like objects, such as damaged cells or cell fragments, are often captured in CTC assays and vary in number between patients [46]. Surviving CTCs often express bcl-2 or Ki67, markers that protect against apoptosis through the M30 pathway [45].
Clinical relevance: CTCs and CTMs in metastatic progression
The correlation between CTC count and clinical outcome is a subject of intense research [31]. In many cases CTC burden was shown to be predictive of survival in metastatic breast, colorectal, prostate, and lung cancers [47]. Following chemotherapy, the average CTC count decreased in patients with small cell lung, colorectal, and breast cancer [34]. A decrease in CTCs is often associated with improved outcome following therapy [47]. In many prostate cancer patients, the CTC count was below the detection limit (< 1 mL−1) after tumor resection [38]. When the CTC count is decreased following treatment, the outcome of patients is improved [38, 47].
As described above, the protocols for isolating and enumerating CTCs often involve dissociation of clusters and hence much less is known about CTMs. In studies where both CTCs and CTMs have been enumerated, the detection of CTMs is correlated with poor prognosis and rapid disease progression [34]. For patients with breast, prostate, and lung cancer, prognosis for patients with CTMs was significantly worse than those without [22, 32, 34]. CTMs have been shown to be more metastatic than single CTCs [22], although less is known about their specific involvement in metastasis. CTMs are more likely to survive in circulation, form a secondary tumor, are more adhesive and invasive than CTCs [34].
In summary, tumor cells can enter circulation as CTCs or as CTMs, and CTMs may include multiple cell types. Intravasation into circulation results in a dramatic change in the microenvironment for tumor cells. Successful tumor cell dissemination depends on the cell’s ability to survive the high shear flow environment, evade the immune system, and to find a way to arrest and obtain nutrients. Disrupting the ability of the cell to arrest and undergo transendothelial migration at a secondary site will maintain exposure to apoptotic forces and immune surveillance in circulation, and ultimately reduce metastatic events.
EXTRAVASATION
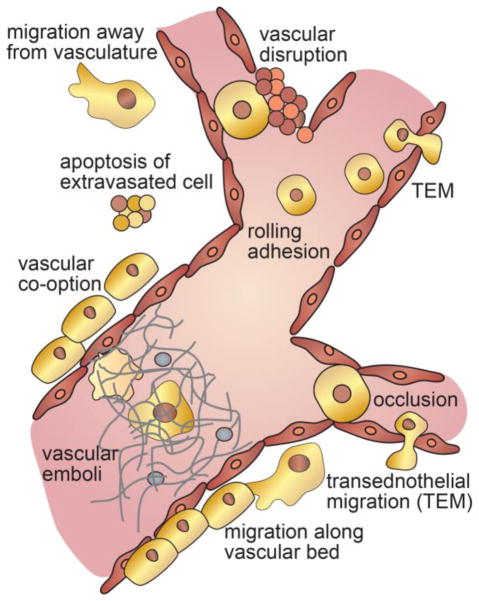
Extravasation of CTCs or CTMs and the formation of a secondary tumor can occur by multiple pathways (TextBox 2). Classically, extravasation and colonization involves three sequential steps: (1) arrest in a blood vessel, (2) migration across the endothelium, and (3) survival, proliferation and colonization in the peripheral tissue. However, accumulating evidence suggests that there are multiple pathways for these processes (Fig. 4). Arrest from circulation can occur by occlusion of CTCs or small CTMs in capillary beds, occlusion of tumor-associated thrombi, or by capture and adhesion – usually in the venous system where the shear flow is relatively low [48]. Entry into the perivascular space can occur by transendothelial migration across intact or disrupted endothelium. While proliferation and colonization can proceed without the tumor cells entering the perivascular space, there is little evidence supporting this pathway [49]. Following extravasation, a cell or cluster of cells can grow into a micrometastasis, die, or become dormant. Migration may be important in locating a niche that promotes proliferation and growth and hence may determine the fate of an extravasated tumor cell. Proximity to a vascular bed provides a source of nutrients for proliferation and growth, while locations farther from the vasculature may promote dormancy [50]. Understanding the cues and pathways that modulate these processes will inform and improve methods of cancer treatment.
Figure 4. Mechanisms of extravasation.
Extravasation can occur by occlusion in a small capillary or bifurcation, or by rolling adhesion in a larger vessel. CTCs may also be trapped in vascular emboli formed by NETs or TF-expressing CTCs. Following arrest the cells may cross the endothelium by downregulation of cell-cell junctions and transendothelial migration, or by vascular disruption and apoptosis of surrounding vasculature. The fate of the extravasated tumor cells depends largely on its proximity to nutrients. An extravasated cell may migrate along the vasculature or away from the vasculature resulting in varying fates.
Arrest: occlusion
The first step in extravasation is the arrest of a CTC or CTM. In functional vasculature, arrest can occur by occlusion or adhesion to the endothelium. Evidence for both mechanisms comes from autopsy data in humans and animal studies [1, 19, 50] Occlusion is thought to occur in the. first capillary bed that the CTC or CTM encounters [1], and support for this mechanism comes from analysis of autopsy data where as many as two thirds of all metastases occur in the organ immediately downstream of the primary tumor [51, 52]. Occlusion has also been observed by intravital microscopy, often in the sinusoids of the liver [50, 53] where imaging is performed at the organ just downstream of the injection site: commonly cells are injected into the spleen and imaged in the liver [53, 54]. Occlusion is also seen at branch points where flow is turbulent [19]. In mouse models, more than 80% of injected cells remain lodged in the liver three days after injection and more than 33% after thirteen days, consistent with the observation that metastases are often found in the next downstream organ [54]. In mouse models or acute or surgical trauma, metastasis has been observed in non-privileged organs, likely due to CTC entry across defective vasculature [55–57].
Occlusion may also occur via formation of tumor-associated thrombi, as described above. This type of occlusion can result in a successful metastatic event, hypoxia and tissue necrosis, or in patient death from a thromboembolic event. In addition, there is an increased incidence of neutrophil extracellular traps (NETs), which contribute to thrombus formation, in cancer patients [58]. The presence of thrombi initiates endothelial activation, upregulating adhesive proteins that promote adhesion of cancer cells to the endothelium, providing a mechanism for the CTCs to extravasate before the thrombus clears [24].
Occlusion of a CTM can lead to cancer-related death, an outcome that is difficult to distinguish from a venous thromboembolism [38]. Patients with metastatic cancer have a significantly increased risk of thrombosis since chemotherapy, radiation therapy, and surgery increase clotting factors circulating in blood [48]. As a result, thrombosis is the second leading cause of death for cancer patients after cancer progression [59]. Increasingly, “incidental” or “unsuspected” venous thromboembolisms are identified on CT scans, and incidental pulmonary embolisms are also increasingly diagnosed in parallel with cancer progression [59]. An increase in thrombosis is associated with both common treatments for cancer and disease progression, making it difficult to determine if the increase is due to disease progression [48]. Despite the fact that thrombus formation is implicated in cancer progression, and that cancer patients have an increased risk for thrombosis, there is no direct connection between thrombosis and extravasation after the initial arrest of the cancer cell.
Arrest: rolling adhesion
Evidence of tumor cell arrest by adhesion is largely indirect from in vitro and in vivo studies in mouse models [19]. Due to the difficulties in predicting the location of arrest events, very few intravital microscopy studies have visualized adhesion and extravasation of CTCs in larger vessels [19]. Following arrest and adhesion to the endothelium, cells can migrate along the lumen against the direction of flow and squeeze through capillaries [60, 61].
Numerous in vitro studies have highlighted the similarity in the arrest and extravasation of tumor cells and leukocytes [62]. Leukocyte extravasation has been studied extensively and involves three distinct steps: rolling, adhesion, and transendothelial migration [63]. Rolling is mediated by upregulation of adhesion molecules (selectins) on the endothelium; adhesion by activation of leukocytes and integrin binding; and transendothelial migration involves adhesion to junctional proteins. While the steps involved in tumor cell extravasation are similar, the mechanisms may be somewhat different [64]. Studies using parallel plate flow chambers (PPFC) and other assays identified several binding partners for tumor cell capture and adhesion, including E- and P-selectin on ECs binding to sLe(x) and sLe(a) on tumor cells, MUC1 on endothelial cells binding to Galectin 3 and ICAM on tumor cells, and CD44 on endothelial cells binding to VCAM on tumor cells [1]. Furthermore, leukocyte adhesion in extravasation is a transient process, whereas tumor cell adhesion and extravasation is thought to induce irreversible damage to the endothelium [64].
Entry into the perivascular space: transendothelial migration
There are multiple pathways for tumor cell arrest resulting in extravasation into many different microenvironments. For example, a cell could be trapped in a narrow capillary, bound to the walls of a larger vessel, or lodged in a thrombus or NET (Fig. 4). Therefore, the possible pathways for transendothelial migration are more diverse than the classical case of migration across a healthy endothelium. The majority of transendothelial migration studies were performed on healthy vasculature in vivo and on healthy or inflamed endothelium in vitro using established cancer cell lines. Therefore, the role of transendothelial migration in metastatic progression is poorly understood.
Several pathways for transendothelial migration were observed following the arrest of CTCs in the vasculature in intravital microscopy studies in mice and chick embryos. Most commonly, protrusions extended by adherent tumor cells cross the endothelium at the site of arrest [61, 65, 66]. Tumor cells overexpressing Twist show enhanced extravasation suggesting an important role for the rho-associated protein kinase (ROCK) pathway in extravasation [61]. The mechanisms that govern transendothelial migration of tumor cells are not well known, but may be similar to that of leukocytes, which involve migration to endothelial cell-cell junctions, attachment to PECAM-1, and paracellular migration across the endothelium [67]. Boyden chamber assays [68] showed that a number of nutrient or chemokine gradients can increase or decrease transendothelial migration, including Rho-GTPase, IGF-1, CXCL7, CXCR4, CXCL12, and many others [69].
Entry into the perivascular space: vascular disruption
Following tumor cell arrest, the endothelium often becomes further activated resulting in vascular disruption [53]. Activation occurs in response to inflammatory cytokines or growth factors, such as VEGF, that promote downregulation of barrier function and angiogenesis [70]. In many cases down-regulation of barrier function occurs in a way that does not result in clear morphological changes, although intravital microscopy showed visible disruption of endothelium in the liver within 30 minutes following arrest [53]. Local disruption of barrier function can also occur as a result of the mechanical forces generated by division of a tumor cell arrested in a small vessel or capillary [49]. The pathways leading to the formation of tumor-associated thrombi likely also results in significant vascular disruption, typical of thrombotic events [15].
Fate of secondary tumors: growth and/or dormancy
In vivo studies of extravasation in mouse models usually involve intravenous injection of cancer cells into circulation and imaging of the vasculature downstream of the injection site. While intravital microscopy studies have captured adhesion and extravasation, colonization and growth are rare events and hence are not well understood. In these experiments less than 0.1% of the injected cancer cells successfully extravasate from circulation [71].
Following extravasation, migration along the vasculature to larger vessels often precedes proliferation and growth, whereas cells that migrate away from the vasculature typically do not develop into tumors, at least on the time scale of the experiment [50]. Growth along the vasculature results in vessel co-option, similar to that seen in mosaic vessels in the primary tumor [3]. Cells can migrate along smaller vessels toward a larger vessel where there is an increased supply of oxygen and nutrients and where they can then proliferate and form the initial secondary tumor. Vessel co-option is thought to be an important step in promoting growth at secondary sites in many cancers [50]. For example, a large fraction of early brain micrometastases show vessel co-option prior to angiogenesis [72]. A characteristic event in the formation of a secondary tumor is the remodeling of the existing vasculature [50, 65]. The first step in this process is the formation of a vascular loop through the main body of the tumor. This phenomena is well documented with intravital microscopy [50, 65].
Cells that survive extravasation but do not proliferate or form overt metastases may become dormant [73, 74]. After the initial diagnosis and treatment of a primary tumor, a long latency period before relapse is considered evidence for dormancy [74, 75]. Dormant tumor cells are considered either non-proliferative (i.e. quiescent) or exhibiting equal rates of proliferation and apoptosis [76]; in either case the tumor cell mass does not increase. For example, the discovery of CTCs in patients without overt metastases 7–22 years following surgical resection of the primary tumor suggests that dormant secondary tumors proliferate at approximately the same rate as cells shed into circulation [18]. Models of fast and slow growing tumors, based on data from breast cancer patients, predict that a tumor requires 1–50 years to reach 1 cm in diameter [75]. Due to the large variability in tumor growth rates, distinguishing between slow growing tumors and dormant tumor cells that suddenly exhibit a high proliferation rate is difficult [75]. In vivo evidence in mouse models suggests that cancer cells are more prone to dormancy when in close proximity to mature quiescent vasculature [77]. Various factors have been implicated with tumor cell (e.g. p38 MAPK) and angiogenic (e.g. TSP-1) dormancy [73, 78]. In a mouse model, inhibition of tumor cell-derived VEGF-A with bevacizumab inhibited angiogenesis and increased the dormancy of lung cancer micrometastases (P14-PE6 tumor cells) in the brain [50].
Immune system and the formation of the pre-metastatic niche
The role of the immune system in extravasation following escape from circulation is primarily in the formation of a pre-metastatic niche, an immuno-suppressive environment that promotes the growth of secondary tumors [79]. This is accomplished primarily through the recruitment of bone marrow derived myeloid cells (BDMCs) before metastasis occurs, possibly due to increased systemic inflammation [24]. BDMCs secrete MMP9, establish an immunosuppressive environment, and promote adherence and growth of tumor cells that may extravasate [25]. Studies of early stage lung metastasis in a C57/BL6 mouse model showed sequential accumulation of distinct subsets of myeloid cells (neutrophils, monocytes, macrophages, as well as patrolling monocytes and dendritic cells) in lung capillaries following bolus injection of B16F10 melanoma cells [80]. The early recruitment of myeloid cells was associated with excretion of microscale blebs (termed cytoplasts) by arrested CTCs, many of which remained attached to the lung vasculature. In addition to tumor cells and macrophages, some monocytes were also found to extravasate from circulation. Both pro-metastatic macrophages and rare resident anti-metastatic dendritic cells were found in the early metastatic niche [80]. The immune system is also thought to play a role in dormancy, similar to the role at the primary tumor, where the immune system directs cytotoxic attacks inducing arrest of tumor growth [75].
In summary, the wide range of mechanisms for arrest of CTCs or CTMs, and the corresponding diversity in microenvironments, results in many possible pathways for extravasation and colonization of a secondary site. Understanding the factors that control these pathways and the resultant cell fate could lead to improved therapies.
QUANTIFYING TUMOR CELL DISSEMINATION
Quantification of the steps in the metastatic cascade is challenging, but crucial to elucidating mechanisms and developing strategies for intervention. Measuring the time dependent concentration of CTCs and CTMs, and the rates of intravasation and extravasation associated with different mechanisms and different cancer types will be key to improving patient care. In part due to the difficulty in measuring these parameters, quantitative insight into tumor dissemination is very limited (TextBox 3).
Text Box 3. How Fast is Tumor Cell Dissemination.
Rate of CTC shedding into circulation: 1,000 – 10,000 cells h−1 g−1 (estimate from animal studies)
Number of CTCs in blood: 1 – 3,000 mL−1 or 5,000 – 15,000,000 in circulation (measured in cancer patients; detection limit is around 1 mL−1)
Half-life of CTCs: 1 – 2 h (estimates from mouse models and humans following tumor resection)
Clearance rate of CTCs: 2,500 – 7,500,000 cells h−1 (assuming a half-life of 1 h) (clearance by the mononuclear phagocyte system (MPS), apoptosis, or extravasation)
Extravasation of CTCs following arrest: 30 minutes (single cells injected into animal models)
Time for a tumor to reach 1 cm: 1 – 50 years (based on data from cancer patients)
The rate of single tumor cell intravasation was estimated to be 0.01 h−1 in mouse models [13], in particular at sites where both a tumor cell and TAM were in contact at the vessel interface, termed the “tumor microenvironment for metastasis” (TMEM) [13]. Assuming a density of 1 x 106 TMEMs g−1, we can approximate a global intravasation and shedding rate of 1,000 cells h−1 g−1 of tumor tissue. Global averages for the intravasation of both single and clusters of tumor cells were quantified using in situ perfused isolated tumor models [81, 82], and intravasation rates were reported to be 5,000 – 50,000 cells h−1 g−1 for human fibrosarcoma and colon cancer [3, 15, 82]. These rates are approximately 10-fold higher than the estimated rates from direct observations of single tumor cell intravasation events in mice, which suggest that the presence of an intact endothelium may significantly inhibit TEM and intravasation. The extent of tumor vascularization and aggressiveness is expected to impact the rate of tumor cell release [83].
Once within circulation, the half-life of tumor cells is around 1 – 2 hours in mice intravenously inoculated with a suspension of individual breast cancer cells [18], and in humans immediately following surgical resection of their primary tumors [18]. Typically, arrest of injected tumor cells in mice is observed within minutes [84], but CTCs are also capable of transiently adhering for up to 48 h after injection, and then re-entering circulation [84]. The average concentration of CTCs in patient blood samples varies widely across cancer cell types, but has been observed to range from approximately < 1 – 3,000 mL−1 for metastatic carcinomas, but the observation of ≥5 CTCs per 7.5 mL of blood is a poor prognostic indicator [83]. For patients with detectable CTCs, consistent concentrations are found in multiple blood samples over a 24 h period [83], which suggests a steady-state rate of tumor cell release and clearance from circulation, at least over a period of a few hours. Therefore, patients with metastatic cancer are expected to have approximately 5,000–15,000,000 cells in steady-state circulation (assuming a blood volume of 5 L with 1–3000 cells mL−1).
If the source of CTCs and CTMs is shedding from a primary tumor, and the sinks are clearance (e.g. mononuclear phagocyte system or apoptosis) and extravasation to a secondary site, then the difference in rates between the source and sink defines the time dependent number of cells in circulation. Assuming a half-life of 1 h, we can estimate that approximately 2,500–7,500,000 cells h−1 are cleared from the bloodstream, either through adhering/arresting to vessels, apoptosis, or clearance by the immune system. If a steady-state concentration of CTCs in patients is reached, then this rate of clearance is consistent with the rate of intravasation (i.e. 5,000 – 50,000 cells h−1 g−1) expected to be supplied from primary and/or secondary tumors with an aggregate 0.05–1,500 g in size. These numbers suggest that significant intravasation and metastasis may occur early in tumor growth and progression.
The extravasation of tumor cells occurs between 2 – 24 hours after arrest in animal models [61]. The formation of micrometastases have been observed within 3 days to 8 weeks, but is dependent on cancer cell type [54, 65, 66]. In a pooled study of breast cancer, up to 30% of patients exhibited micrometastases detected in bone marrow aspirates at the time of diagnosis; the presence of which was a significant prognostic indicator for poor overall and disease-free survival [29]. The bone marrow is common site for the metastasis of several cancers of epithelial origin, such as breast, lung, prostate, and colon [76]. Clusters of tumor cells have a higher metastatic potential than individual tumors released into circulation [20, 22], since they are better able to survive circulation, and seed and/or proliferate at secondary sites. While tumor cell dormancy is a possible cause for the sudden appearance of secondary tumors many years after surgery and removal of the primary tumor [75], the contribution of disseminated single cells and clusters to dormancy is still unknown.
Concluding Remarks
Tumor cells use a variety of pathways to cross biological interfaces and survive disparate microenvironments during cancer progression. Successful approaches for reducing the efficiency of cancer metastasis will need to target the multiple parallel mechanisms and/or consistent pathways/bottlenecks during intravasation, circulation, and extravasation.
The vascular endothelium is encountered during both intravasation and extravasation, and is consistently down-regulated, activated, disrupted, or non-existent during transendothelial migration. Our understanding of the microenvironment in which these processes occurs comes largely from analysis of tissue sections and a limited number of intravital microscopy studies, typically from the surface of xenografts. Improved in vivo imaging tools and models, and more physiological in vitro models will be essential to elucidate the details of these processes.
Attempts at normalizing the tumor vasculature through anti-angiogenic therapies and targeting and sequestering growth factors and their respective receptors, are expected to improve vascular function and the delivery of chemotherapeutics [85], and may also reduce the number of intravasating tumor cells. Bevacizumab decreases permeability and interstitial fluid pressure in rats [86], and was shown to improve progression-free survival in clinical trials for ovarian cancer and glioblastoma [87]; however, its direct impact on the mechanism of intravasation through the detection of CTCs has not been examined beyond providing prognostic information.
Quantification of tumor cell dissemination is important in elucidating mechanisms and developing strategies for intervention (see outstanding questions). The lack of quantitative insight into dissemination is a major barrier to progress in the field. For example, an improved understanding of the time-dependent number of CTCs and CTMs for patients across different cancer types and in response to different interventions would provide valuable insight into dissemination mechanisms. Enumeration is currently limited by the necessity to take a blood sample and the intrinsic detection limit of 1 mL−1 (assuming a 1 mL blood sample). New in vivo techniques may enable real-time measurement and enumeration of CTCs and CTMs with higher resolution. Similarly, new methods to quantify the rates of intravasation and extravasation will contribute to improved understanding of the sources and sinks for disseminated tumor cells and the efficiency of the steps in the metastatic cascade. Quantification of dissemination pathways will be important in gaining insight into the time dependence of the rates of different processes during growth of a primary tumor, and in establishing whether extravascular growth at a secondary site is dependent on specific pathways or exogenous factors. Improved quantification would enable the development of mathematical models for dissemination, similar to pharmacokinetic models, that could aid in treatment and clinical management.
Outstanding Questions Box.
How does the endothelium modulate intravasation and extravasation?
What are the rates of intravasation and extravasation, and how do they vary with time during the growth of a primary tumor?
Can in vivo imaging enable real-time measurement and enumeration of CTCs and CTMs?
Can real-time monitoring of the number of CTCs be used for clinical management?
Do the mechanisms of intravasation and extravasation determine the probability of growth at a secondary site?
Are there commonalities between dissemination of tumor cells and pathogens?
Treating metastasis is difficult due to deficiencies in early detection of primary tumors and limitations therapies that address the multiple parallel mechanisms of cancer dissemination. However, as early detection technologies improve, strategies that target the various steps in metastasis will become increasingly relevant in addressing the spread of secondary/tertiary tumors from dormant sites and postponing the relapse of patients showing early signs of tumor reoccurrence, such as CTCs and CTMs in blood.
Trends Box.
The wide range of mechanisms for dissemination of cells from a solid tumor along with the corresponding diversity in microenvironments result in multiple parallel pathways for colonization of a secondary site.
Shedding from a solid tumor can occur as single cells or clusters, and occurs across intact, defective, or non-existent endothelial barriers. Due to the heterogeneous nature of a tumor and the tumor vasculature, these processes may occur simultaneously at different locations within the tumor.
Tumor cells in circulation can form clusters with platelets, neutrophils, and natural killer cells that modulate their survival.
Extravasation can occur by occlusion of a CTC in a capillary bed, occlusion of tumor-associated thrombi in a capillary or microvessel, by capture and adhesion in a larger vessel, or by occlusion in defective vasculature.
Acknowledgments
Funding: The authors gratefully acknowledge support from the NIH (NIH R01CA170629).
GLOSSARY
- Vascular mimicry
the formation of channels lined with tumor cells lined with tumor cells that mimic blood vessels and supply nutrients to a growing tumor.
- Mosaic vessels
vessels where the lumen is formed from endothelial cells and tumor cells. Such vessels may result from rapid growth of tumor neovasculature without sufficient proliferation to form a complete monolayer, or may result from shedding or apoptosis of endothelial cells exposing underlying tumor cells.
- Neutrophil Extracellular Traps (NETs)
adhesion and arrest of neutrophils in capillaries can result in a cell death pathway known as NETosis, resulting in release of chromatin and granular material. These neutrophil extracellular traps can cause obstruction or “plugging” of the capillary.
- Circulating tumor cells (CTCs)
single tumor cells or aggregates of less than 3 cells in circulation.
- Circulating tumor microemboli (CTM)
clusters or aggregates of CTCs, often including other cell types, such as leukocytes and platelets, and proteins.
- Tumor microenvironment for metastasis (TMEM)
often defined as a site where both a tumor cell and tumor associated macrophage are in contact with the endothelium.
- Enhanced permeation and retention (EPR) effect
the defective or dysfunctional vasculature in a tumor results in increased permeability and preferential accumulation of a molecule or nanomedicine.
- Intravasation
entry of a tumor cell into circulation (or lymphatic vessel). Mechanisms include transendothelial transport of a tumor cell across the endothelium into a blood vessel, and shedding of a tumor cell or cluster from a mosaic vessel.
- Extravasation
exit of a tumor cell from circulation. The mechanism may include arrest, dissociation of tumor cells from CTCs or CTMs, transendothelial transport across the endothelium into a blood vessel, or direct entry into tissue through defective vasculature.
Footnotes
Conflicts of interest:none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chambers AF, et al. Metastasis: dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nature Medicine. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 3.Chang YS, et al. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97(26):14608–13. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174–90. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 5.Folarin AA, et al. Three-dimensional analysis of tumour vascular corrosion casts using stereoimaging and micro-computed tomography. Microvasc Res. 2010;80(1):89–98. doi: 10.1016/j.mvr.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirtz D, et al. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11(7):512–22. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roussos ET, et al. Chemotaxis in cancer. Nat Rev Cancer. 2011;11(8):573–87. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong AD, et al. Quantitative Analysis of the Enhanced Permeation and Retention (EPR) Effect. PloS one. 2015;10(5):e0123461. doi: 10.1371/journal.pone.0123461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan F, et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54(17):4564–8. [PubMed] [Google Scholar]
- 10.Yuan F, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–6. [PubMed] [Google Scholar]
- 11.Hendrix MJ, et al. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3(6):411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 12.El Hallani S, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133(Pt 4):973–82. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harney AS, et al. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015;5(9):932–43. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JP, et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: a meta-analysis. Angiogenesis. 2016;19(2):191–200. doi: 10.1007/s10456-016-9500-2. [DOI] [PubMed] [Google Scholar]
- 15.Liotta LA, et al. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36(3):889–94. [PubMed] [Google Scholar]
- 16.Nagakawa Y, et al. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas. 2002;24(2):169–78. doi: 10.1097/00006676-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Au SH, et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc Natl Acad Sci U S A. 2016;113(18):4947–52. doi: 10.1073/pnas.1524448113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng S, et al. Circulating tumor cells in patients with breast cancer dormancy. Clinical cancer research. 2004;10(24):8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi K, et al. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Research. 2006;66(8):4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]
- 20.Cheung KJ, Ewald AJ. A collective route to metastasis: Seeding by tumor cell clusters. Science. 2016;352(6282):167–9. doi: 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joosse SA, et al. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med. 2014;7(1):1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, et al. Laparoscopic surgery minimizes the release of circulating tumor cells compared to open surgery for hepatocellular carcinoma. Surg Endosc. 2015;29(11):3146–53. doi: 10.1007/s00464-014-4041-5. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura T, et al. Immune cell promotion of metastasis. Nature Reviews Immunology. 2015;15(2):73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature Reviews Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drake CG, et al. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11(1):24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulley JL, et al. Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment. JNCI: Journal of the National Cancer Institute. 2017;109(4) doi: 10.1093/jnci/djw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solinas G, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 29.Braun S, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 30.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70(14):5649–69. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potdar PD, Lotey NK. Role of circulating tumor cells in future diagnosis and therapy of cancer. Journal of Cancer Metastasis and Treatment¦ Volume. 2015;1(2):44. [Google Scholar]
- 32.Cho EH, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Physical biology. 2012;9(1):016001. doi: 10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banys M, et al. The influence of removal of primary tumor on incidence and phenotype of circulating tumor cells in primary breast cancer. Breast cancer research and treatment. 2012;132(1):121–129. doi: 10.1007/s10549-011-1569-0. [DOI] [PubMed] [Google Scholar]
- 34.Hou JM, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. Journal of Clinical Oncology. 2012;30(5):525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 35.Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Current opinion in genetics & development. 2010;20(1):96–99. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.den Toonder J. Circulating tumor cells: the Grand Challenge. Lab on a chip. 2011;11(3):375–377. doi: 10.1039/c0lc90100h. [DOI] [PubMed] [Google Scholar]
- 37.Galanzha EI, et al. In vivo acoustic and photoacoustic focusing of circulating cells. Scientific reports. 2016:6. doi: 10.1038/srep21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stott SL, et al. Isolation and Characterization of Circulating Tumor Cells from Patients with Localized and Metastatic Prostate Cancer. Science Translational Medicine. 2010;2(25) doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanna N. Role of natural killer cells in control of cancer metastasis. Cancer and Metastasis Reviews. 1982;1(1):45–64. doi: 10.1007/BF00049480. [DOI] [PubMed] [Google Scholar]
- 40.Fidler IJ, Poste G. Springer Seminars in immunopathology. Springer; 1982. Macrophage-mediated destruction of malignant tumor cells and new strategies for the therapy of metastatic disease; pp. 161–174. [DOI] [PubMed] [Google Scholar]
- 41.Gorelik E, et al. Role of NK cells in the control of metastatic spread and growth of tumor cells in mice. International journal of cancer. 1982;30(1):107–112. doi: 10.1002/ijc.2910300118. [DOI] [PubMed] [Google Scholar]
- 42.Menter DG, et al. Tumor cell-platelet interactions in vitro and their relationship to in vivo arrest of hematogenously circulating tumor cells. Clinical & experimental metastasis. 1987;5(1):65–78. doi: 10.1007/BF00116627. [DOI] [PubMed] [Google Scholar]
- 43.Palumbo JS, et al. Tumor cell–associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell–dependent–and independent mechanisms. Blood. 2007;110(1):133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nash G, et al. Platelets and cancer. The lancet oncology. 2002;3(7):425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 45.Barradas A, Terstappen LW. Towards the biological understanding of CTC: capture technologies, definitions and potential to create metastasis. Cancers. 2013;5(4):1619–1642. doi: 10.3390/cancers5041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larson CJ, et al. Apoptosis of circulating tumor cells in prostate cancer patients. Cytometry Part A. 2004;62(1):46–53. doi: 10.1002/cyto.a.20073. [DOI] [PubMed] [Google Scholar]
- 47.Cristofanilli M, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 48.Falanga A, et al. The mechanisms of cancer-associated thrombosis. Thrombosis research. 2015;135:S8–S11. doi: 10.1016/S0049-3848(15)50432-5. [DOI] [PubMed] [Google Scholar]
- 49.Lu W, et al. Pathogenesis and vascular integrity of breast cancer brain metastasis. International journal of cancer. 2007;120(5):1023–1026. doi: 10.1002/ijc.22388. [DOI] [PubMed] [Google Scholar]
- 50.Kienast Y, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nature medicine. 2010;16(1):116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 51.Weiss L. Comments on Hematogenous Metastatic Patterns in Humans as Revealed by Autopsy. Clinical & Experimental Metastasis. 1992;10(3):191–199. doi: 10.1007/BF00132751. [DOI] [PubMed] [Google Scholar]
- 52.Weiss L. Patterns of metastasis. Cancer and Metastasis Reviews. 2000;19(3):281–301. [PubMed] [Google Scholar]
- 53.Mook OR, et al. Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology. 2003;38(2):295–304. doi: 10.1053/jhep.2003.50297. [DOI] [PubMed] [Google Scholar]
- 54.Luzzi KJ, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. The American journal of pathology. 1998;153(3):865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agostino D, Cliffton EE. Organ localization and the effect of trauma on the fate of circulating cancer cells. Cancer research. 1965;25(10):1728–1732. [PubMed] [Google Scholar]
- 56.Murthy SM, et al. The influence of surgical trauma on experimental metastasis. Cancer. 1989;64(10):2035–44. doi: 10.1002/1097-0142(19891115)64:10<2035::aid-cncr2820641012>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 57.Tohme S, et al. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017;77(7):1548–1552. doi: 10.1158/0008-5472.CAN-16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demers M, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proceedings of the National Academy of Sciences. 2012;109(32):13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khorana AA. Cancer-associated thrombosis: updates and controversies. ASH Education Program Book. 2012;2012(1):626–630. doi: 10.1182/asheducation-2012.1.626. [DOI] [PubMed] [Google Scholar]
- 60.Zeidman I. The fate of circulating tumor cells I. Passage of cells through capillaries. Cancer research. 1961;21(1):38–39. [PubMed] [Google Scholar]
- 61.Stoletov K, et al. Visualizing extravasation dynamics of metastatic tumor cells. Journal of cell science. 2010;123(13):2332–2341. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tözeren A, et al. E-selectin-mediated dynamic interactions of breast-and colon-cancer cells with endothelial-cell monolayers. International Journal of Cancer. 1995;60(3):426–431. doi: 10.1002/ijc.2910600326. [DOI] [PubMed] [Google Scholar]
- 63.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 64.Strell C, Entschladen F. Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal. 2008;6:10. doi: 10.1186/1478-811X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naumov GN, et al. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. Journal of cell science. 1999;112(12):1835–1842. doi: 10.1242/jcs.112.12.1835. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka K, et al. In vivo time-course imaging of tumor angiogenesis in colorectal liver metastases in the same living mice using two-photon laser scanning microscopy. Journal of oncology. 2011:2012. doi: 10.1155/2012/265487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 68.Katt ME, et al. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Frontiers in bioengineering and biotechnology. 2016:4. doi: 10.3389/fbioe.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H-C. Boyden chamber assay. Cell Migration: Developmental Methods and Protocols. 2005:15–22. doi: 10.1385/1-59259-860-9:015. [DOI] [PubMed] [Google Scholar]
- 70.Weis S, et al. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. The Journal of cell biology. 2004;167(2):223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fidler IJ, Nicolson GL. Fate of recirculating B16 melanoma metastatic variant cells in parabiotic syngeneic recipients: brief communication. Journal of the National Cancer Institute. 1977;58(6):1867–1872. doi: 10.1093/jnci/58.6.1867. [DOI] [PubMed] [Google Scholar]
- 72.Carbonell WS, et al. The Vascular Basement Membrane as "Soil'' in Brain Metastasis. Plos One. 2009;4(6) doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol. 2016 doi: 10.1016/j.molonc.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sosa MS, et al. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–22. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. 2011;21(1):42–9. doi: 10.1016/j.gde.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 76.Pantel K, et al. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6(6):339–51. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 77.Ghajar CM, et al. The perivascular niche regulates breast tumour dormancy. Nature cell biology. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Páez D, et al. Cancer dormancy: a model of early dissemination and late cancer recurrence. Clinical Cancer Research. 2012;18(3):645–653. doi: 10.1158/1078-0432.CCR-11-2186. [DOI] [PubMed] [Google Scholar]
- 79.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Headley MB, et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016;531(7595):513–7. doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35(3):512–6. [PubMed] [Google Scholar]
- 82.Swartz MA, et al. Cells shed from tumours show reduced clonogenicity, resistance to apoptosis, and in vivo tumorigenicity. Br J Cancer. 1999;81(5):756–9. doi: 10.1038/sj.bjc.6690760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allard WJ, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 84.Morris VL, et al. Early interactions of cancer cells with the microvasculature in mouse liver and muscle during hematogenous metastasis: videomicroscopic analysis. Clinical & experimental metastasis. 1993;11(5):377–390. doi: 10.1007/BF00132981. [DOI] [PubMed] [Google Scholar]
- 85.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 86.Turley RS, et al. Bevacizumab-induced alterations in vascular permeability and drug delivery: a novel approach to augment regional chemotherapy for in-transit melanoma. Clin Cancer Res. 2012;18(12):3328–39. doi: 10.1158/1078-0432.CCR-11-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rossi L, et al. Bevacizumab in ovarian cancer: A critical review of phase III studies. Oncotarget. 2016 doi: 10.18632/oncotarget.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]