Abstract
Mucosal-associated invariant T (MAIT) cells express a semi-invariant Vα7.2+ T cell receptor (TCR) that recognizes ligands from distinct bacterial and fungal species. In neonates, MAIT cells proliferate coincident with gastrointestinal (GI) bacterial colonization. In contrast, under non-inflammatory conditions adult MAIT cells remain quiescent due to acquired regulation of TCR signaling. Effects of inflammation and the altered GI microbiota after allogeneic hematopoietic cell transplantation (HCT) on MAIT cell reconstitution have not been described. We conducted an observational study of MAIT cell reconstitution in myeloablative (n=41) and non-myeloablative (n=66) allogeneic HCT recipients, and found that despite a rapid and early increase to a plateau at day 30 after HCT, MAIT cell numbers failed to normalize for at least one year. Cord blood transplant recipients and those who received post-HCT cyclophosphamide for graft versus host disease (GVHD) prophylaxis had profoundly impaired MAIT cell reconstitution. Sharing of TCRβ gene sequences between MAIT cells isolated from HCT grafts and blood of recipients after HCT showed early MAIT cell reconstitution was due at least in part to proliferation of MAIT cells transferred in the HCT graft. Inflammatory cytokines were required for TCR-dependent MAIT cell proliferation, suggesting that bacterial Vα7.2+ TCR ligands might promote MAIT cell reconstitution after HCT. Robust MAIT cell reconstitution was associated with an increased GI abundance of Blautia spp. MAIT cells suppressed proliferation of conventional T cells consistent with a possible regulatory role. Our data identify modifiable factors impacting MAIT cell reconstitution that could influence the risk of GVHD after HCT.
Keywords: MAIT cells, Hematopoietic stem cell transplantation, Intestinal Microbiota
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is complicated by perturbed immune reconstitution, resulting in an increased risk of infections and inflammatory complications, such as graft versus host disease (GVHD).1 Although the composition in the HCT graft of conventional αβ T cell subsets has been shown to influence the incidence of immune-mediated complications of transplant,2–4 the roles of non-conventional T cell subsets in immune reconstitution after HCT are poorly defined.
Mucosal-associated invariant T (MAIT) cells comprise a subset of non-conventional T cells that are characterized by high expression of CD161 and RORγ, and a T cell receptor (TCR) comprising an invariant Vα7.2+ TCRα chain paired with one of a diverse repertoire of TCRβ chains. MAIT cells recognize metabolite antigens derived from distinct gastrointestinal (GI) bacterial and fungal species that are presented by the MHC class 1-like molecule, MR1.5–9 Despite GI colonization with bacterial species capable of activating MAIT cells, MAIT cells in adults are remarkably quiescent due to acquired regulation of the TCR signaling pathway.10 However, in vitro studies show the regulation of proliferation and effector function of MAIT cells can be overcome by TCR stimulation in the presence of proinflammatory cytokines, including IL-12 and IL-18.10,11 These data suggest that inflammatory signals induced by conditioning chemotherapy, radiation, allogeneic cell infusion, and altered GI mucosal integrity, along with lymphopenia and antibiotic-induced changes in the composition of the GI microbiota might affect MAIT cell reconstitution and function after allogeneic HCT. 12,13
In this prospective observational study of recipients of allogeneic peripheral blood stem cell (PBSC) grafts, we observed that reconstitution of donor MAIT cells in blood was incomplete, and occurred with kinetics that were distinct from those of conventional lymphocytes and neutrophils. Early MAIT cell reconstitution was driven by proliferation of MAIT cells that were directly transferred in the HCT graft, suggesting that restraint of effector function in MAIT cells was overcome in vivo by the post-HCT inflammatory milieu. Studies of TCRβ chain gene (TRBV) sequence utilization in MAIT cells isolated at different times after HCT demonstrated expansion and contraction of distinct MAIT cell clones, suggesting that MAIT cell reconstitution may in part be governed by antigen stimulation. We established that robust MAIT cell reconstitution in blood correlated with abundance of distinct bacterial species in stool of HCT recipients, and a lower risk of subsequent development of grade ≥3 acute GVHD.
METHODS
Blood and HCT graft samples
Blood from healthy donors and HCT recipients, and GCSF-mobilized leukapheresis products from HCT donors were obtained after written informed consent. Blood and stool samples were collected from HCT recipients prior to conditioning and at approximately days 0, 10, 20, 30, 60, 100 and 365 after HCT. Studies were performed according to the guidelines of the Declaration of Helsinki and were approved by the Institutional Review Board of FHCRC.
Antibodies and cytokines
Fluorochrome-conjugated monoclonal antibodies (mAbs) are described in Supplementary Table 1. Recombinant human IL-1β IL-12 and IL-23 were obtained from R&D Systems (Minneapolis, USA), and IL-18 was obtained from MBL International (Massachusetts, USA). IL-1β IL-12, IL-18 and IL-23 were used in culture at 10 ng/mL.
Immunophenotyping
Peripheral blood mononuclear cells (PBMCs) were stained with Live/dead fixable violet stain (Thermofisher Scientific, Massachusetts, USA) and mAbs specific for surface antigens, followed by acquisition on an LSR-II flow cytometer (BD Biosciences) and analysis using FlowJo software v9.8 (Oregon, USA). MAIT cells were identified as viable CD45+/CD3+/CD161hi/Vα7.2+ events. Absolute MAIT cell counts in blood were determined by multiplying the percentage of MAIT cells in a CD45+ lymphoid forward scatter and side scatter gate by the absolute lymphocyte count performed on the same day. The absolute MAIT cell count in PBSC graft samples is reported as MAIT cells/kg recipient weight, and was determined by multiplying the MAIT cell percentage in a viable CD45+/CD3+ gate by the absolute graft CD3 count.
MAIT cell isolation
Healthy donor CD8+ cells were enriched from ficoll-separated peripheral blood mononuclear cells (PBMC) using the CD8+ T cell isolation kit (Miltenyi). MAIT cells (identified as CD3+/CD8+/CD161hi/Vα7.2+ events) and conventional T cells (CD3+/CD8+/CD161lo/Vα7.2− events) were sort purified from enriched CD8+ T cells using a FACS ARIA 2 flow sorter (BD Biosciences).
Activation and proliferation assays
Isolated MAIT and conventional T cell subsets were activated or not with plate-boud αCD3 (OKT3, Ortho Biotech), and cultured in 96 well plates at 1–2 × 104 cells/well in 200 μL RPMI 1640 medium with 10% human serum, penicillin/streptomycin, β-mercaptoethanol and L-glutamine with or without cytokine supplementation. To assess the immunophenotype in response to stimulation, isolated cells were cultured overnight before analysis by flow cytometry. Proliferation of isolated MAIT and conventional T cells after 4 days of stimulation in culture was evaluated by addition of tritiated thymidine (30 μCi/well) for the last 18 hours, followed by assessment of tritiated thymidine incorporation. The stimulation index was determined as the ratio of counts obtained in the presence of stimulation divided by counts obtained in the absence of stimulation.
Flow cytometry of TCR signaling pathway phosphoproteins
Whole blood was incubated with anti-CD62L and anti-CD161 for 10 minutes, followed by red cell lysis and αCD3 (OKT3 JanssenBiotech, Pennsylvania, USA) stimulation at 37°C for 10 minutes. Cells were immediately fixed and incubated with anti-CD45RA, then permeabilized and stained with anti-CD3, -CD4, -Vα7.2 and either Lck (pY505) CD3ζ (pY142) or ZAP70 (pY292) for 60 minutes at room temperature using an optimized BD Phosflow protocol for TCR stimulation.
Donor-recipient chimerism studies
Short tandem repeat (STR) PCR chimerism studies were used to determine comparative donor and HCT recipient chimerism status in sort purified MAIT, CD33+ myeloid, conventional CD3+ T cell, and CD56+ NK cell subsets from recipient blood samples using a modified Powerplex 16 System (Promega.com). STR fragments were PCR amplified from extracted DNA using the PowerPlex 16 Human Identity Kit (Promega, Wisconsin, USA) followed by separation of variable length amplified fragments by capillary gel electrophoresis using the ABI 3130×1–16 array capillary system (ThermoFisher Scientific, Massachusetts, USA) and analysis using GeneMapper ID software (Applied Biosystems).
TRBV sequencing
MAIT cells were sorted from PBSC grafts and from the peripheral blood of healthy donors and HCT recipients, and DNA was extracted for high throughput CDR3β region gene sequencing (Adaptive Biotechnologies, Seattle, WA).14 The CDR3β sequences from samples were amplified in a multiplex PCR system using forward and reverse primers for the Vβ and Jβ segments respectively generating 60bp amplicons that were sequenced using the Illumina HiSeq platform. Primer amplification bias was corrected using a suite of synthetic templates and the International Immunogenetics Database (www.imgt.org) was used to filter the raw sequence data for subsequent analysis by the ImmunoSEQ analyzer toolset (www.adaptivebiotech.com/immunoseq). TRBV sequence diversity was calculated as the productive entropy [summing the productive frequency times the log (base 2) of the same frequency over all productive rearrangements in a sample].
Microbiota profiling
Broad-range 16S rRNA gene PCR with pyrosequencing and phylogenetic assignment was used to profile the stool bacterial biota in HCT recipients. DNA was extracted from fecal swabs using the BiOstic Bacteremia DNA Isolation kit (MoBio, Carlsbad, CA), and eluted in 150 μL elution buffer (75 μL MoBio elution buffer, 75 μL 0.2× filtered 1 mM Tris, 0.1 mM EDTA buffer). Broad-range 16S rRNA gene quantitative PCR was used to measure total bacterial load15 and PCR inhibition was monitored using an internal amplification control.16 Conventional broad-range 16S rRNA gene PCR targeting the V3–V4 region of the 16S rRNA gene was coupled with pyrosequencing of the amplified fragments using the 454 Life Sciences Titanium technology (Roche, Branford, CT).15,17 No-template PCR and sham DNA extraction controls without human contact were run with each PCR assay to monitor for contamination. Samples were multiplexed with 6 bp barcodes. Sequence reads were classified using a curated reference set of GI bacteria and the pplacer phylogenetic placement tool.18 Sample identifiers and barcodes used for GI microbial species sequencing are shown in supplementary Table 2. Blood and stool samples used for correlation of MAIT cell counts and bacterial abundance, respectively were collected within a week of each other.
In vitro suppressive assay
MAIT cells, identified as Vα7.2+ CD161+ cells, and CD4+ CD25− responder T cells were sorted from the peripheral blood of 3 healthy donors. Responder T cells were labeled with 0.5 μM CFSE. 2.5×104 CFSE-labeled responder T cells were stimulated with Dynabeads CD3/CD28 T Cell Expander (Dynal Biotech) and cultured with MAIT cells or responder T cells at a ratio of 1:1, 1:2, 1:4, and 1:8 MAIT:responder in round-bottom 96-well plates for 4 days. After 4 days, CFSE dilution was evaluated by flow cytometry.
Statistical methods
Cox regression methods were used to assess the association between MAIT cell count and the cause-specific hazards of failure for the outcome of acute GVHD. The MAIT cell count was modeled as a linear, time-dependent covariate using SAS v9.4 (North Carolina, USA). An unpaired 2-tailed Mann-Whitney test was used to evaluate differences in MAIT cell counts after HCT. A paired 2-tailed Student’s t test was used to compare phosphorylated protein expression between MAIT and conventional T cells, as well as activation markers in stimulated and non-stimulated MAIT cells. Data are represented as mean ± SEM, except where indicated in the figure legends. P<0.05 is considered to represent a significant difference. Graphs and accompanying statistical analyses were generated using Prism v6.02 (GraphPad, California, USA).
RESULTS
Kinetics of MAIT cell recovery in blood after HCT
We conducted a prospective, observational study to evaluate the kinetics of MAIT cell recovery in HLA-matched or single HLA antigen mismatched peripheral blood stem cell transplant (PBSCT) recipients after myeloablative (MA, n=41) or non-myeloablative (NMA, n=66) conditioning. Patient and transplant characteristics are summarized in Supplementary Table 3. Absolute peripheral blood MAIT cell counts were measured before initiation of the conditioning regimen in pre-HCT patients and compared with MAIT cell counts in normal donors. There was variation in MAIT cell counts in healthy individuals, and this variation was more extreme in patients, who also had significantly lower MAIT cell numbers than healthy donors (Figure 1A). MA and NMA regimens differ in the severity of lymphopenia and mucosal injury, which are factors that could influence MAIT cell reconstitution, either from residual host or transplanted donor cells. After both MA and NMA conditioning therapy, absolute MAIT cell counts in blood decreased to a nadir on the day of PBSC infusion (Figure 1B, C). The MAIT cell nadir occurred earlier than the absolute neutrophil count (ANC) nadir and was followed by early and rapid recovery to a plateau from day 30 to day 100 after HCT. MAIT cell counts remained lower than those of healthy donors for at least one year after HCT (Figure 1D). We identified no differences in MAIT cell reconstitution at early or late times in patients receiving MA or NMA conditioning (Figure 1E), and at all time points after HCT we observed a similar marked variation in the magnitude of MAIT cell counts in blood between different individuals as observed before HCT (Figure 1F). Incorporation of total body irradiation (TBI) in conditioning, use of an HLA-matched related compared to unrelated PBSC donor, use of an HLA-matched compared to mismatched PBSC donor and the indication for transplant (acute leukemia versus other diagnoses) did not impact MAIT cell recovery (Supplementary Figure 1).
Figure 1. MAIT cell kinetics in blood after HLA-matched or single antigen HLA-mismatched PBSC transplant.
(A) Absolute MAIT cell counts in blood from healthy donors (n=33) and HCT recipients before starting conditioning (Pre HCT, n=87). (B–C) Absolute MAIT, neutrophil and lymphocyte counts in blood from MA (B; n=41) and NMA PBSCT (C; n=66) recipients. (D) MAIT cell counts in blood from healthy donors (n=33) and PBSCT recipients at one year after HCT (n=47). (E) Absolute MAIT cell counts in blood from MA and NMA recipients after PBSCT. (F) Absolute MAIT counts in blood after HCT. Horizontal bars represent the mean. In figures B, C, and E, the standard error of mean (SEM) is shown. *** p<0.001 using the Mann-Whitney test.
MAIT cells transferred in the HCT graft contribute to MAIT cell reconstitution
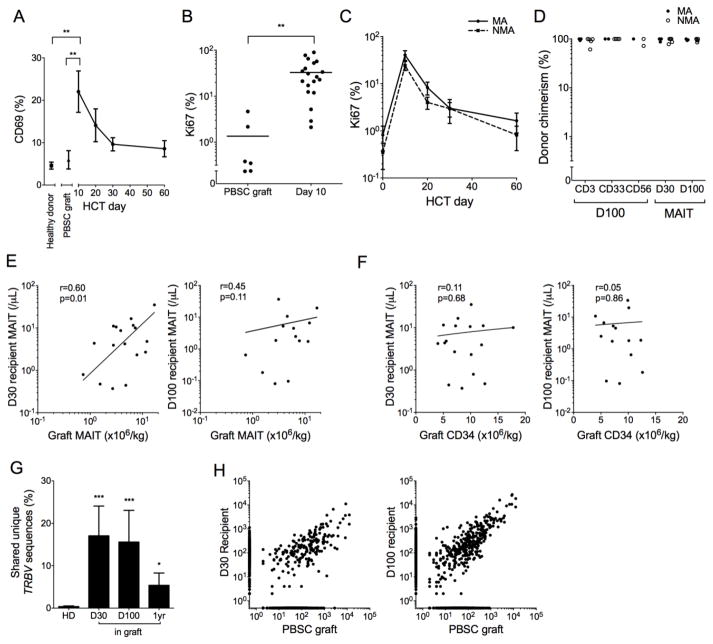
We considered that the variability in MAIT cell reconstitution between patients after HCT could in part be due to differences in the numbers of MAIT cells transferred in the HCT graft and that their in vivo activation and proliferation may account for the rapid and early increase in MAIT cell counts after HCT. We found that the frequency of MAIT cells that expressed CD69 was higher in PBSCT recipients at day 10 post HCT compared to that in healthy donors (4.6%, n=5) or in PBSC grafts (6.0%, n=6, Figure 2A), consistent with early MAIT cell activation in the post-HCT inflammatory milieu. This early activation of MAIT cells coincided with a greater fraction in cell cycle as measured by the frequency of cells expressing the proliferation marker Ki67. Although MAIT cells in HCT grafts expressed Ki67 at levels similar to those from healthy individuals (Ki67+, healthy donors, 0.38%, n=5; PBSC grafts, 1.30%, n=6; p=0.42); the fraction of Ki67+ MAIT cells had increased more than 10-fold in the recipient compared to the PBSC graft by day 10 after HCT (Figure 2B). We identified no difference in the fraction of Ki67+ MAIT cells after MA or NMA HCT (Figure 2C).
Figure 2. MAIT cells are donor derived and their recovery correlates with graft MAIT content.
(A) Percentage of CD69+ MAIT cells from healthy donors (n=5), PBSC grafts (n=6), and HCT recipients at the indicated days after transplant (n=15). (B) Percentage of Ki67+ MAIT cells from PBSC grafts and HCT recipients on day 10 after HCT. Horizontal bars represent the mean. (C) Percentage of Ki67+ MAIT cells in blood from MA (n=15) and NMA (n=11) HCT recipients. (D) Percent donor chimerism for isolated CD3, CD33, CD56, and MAIT cells after HCT (n=8). (E–F) The absolute numbers of MAIT cells (E) and CD34+ cells (F) in PBSC grafts and in PBSCT recipients on day 30 (left, n=17) or day 100 (right, n=14) after HCT. Log-log linear regression Pearson correlation coefficient r and p values are shown. (G) Shared TCRβ nucleotide sequences in MAIT cells from 42 pairs of healthy donors (HD, n=7 donors total) and the percentage of TCRβ sequences in MAIT cells from HCT recipients on days 30, 100 and 1 year after HCT that were also identified in the donor PBSC grafts (top panel, n=5). (H) Representative example of TCRβ nucleotide sequences in MAIT cells from a PBSC graft and the recipient on day 30 (middle) and day 100 (right) after HCT. Each point represents a unique nucleotide sequence and its abundance in each sample is shown on the axes. Points on the axes represent nucleotide sequences not shared between samples. In Figures A and C the mean +/− SEM shown, and in figure G the mean + SEM are shown. * p<0.05; ** p<0.01; *** p<0.001 using the Mann-Whitney test.
Because MAIT cells efflux ABCB1 substrate drugs,19 it was unknown if recipient MAIT cells could survive conditioning chemotherapy and contribute to early MAIT cell recovery or if early MAIT cell recovery was donor-derived. To address this question, we performed STR PCR chimerism studies on CD3+ T cells, CD33+ myeloid cells, CD56+ cells, and on sort-purified MAIT cells isolated from the blood of HCT recipients. We found that at 30 and 100 days after both NMA and MA HCT, MAIT cells were nearly exclusively of donor origin (Figure 2D). MAIT cell numbers in blood of HCT recipients on day 30 and day 100 after HCT correlated with the number of MAIT cells transferred in the PBSC graft (Figure 2E). In contrast, we did not observe an association between MAIT cell reconstitution and the number of CD34+ cells in the PBSC graft (Figure 2F) or with the number of graft CD19+ B cells, which are required for neonatal MAIT cell accumulation (Supplementary Figure 2).8 To determine whether MAIT cells that are transferred in the PBSC graft contribute to MAIT cell recovery in the recipient, we sequenced the TRBV genes in MAIT cells isolated from an aliquot of the PBSC graft and from the recipient’s blood (n=5) and evaluated the fraction of sequences in the recipient that were also found in the PBSC graft. Compared to the low level of sharing between pairs of unrelated individuals (0.4% ± 0.15%, n=42), a large fraction of TRBV sequences was shared between donor PBSC grafts and the blood of each graft recipient at day 30 (17.1%, p<0.001), day 100 (15.6%, p<0.001) and 1 year (5.4%, p=0.01) after PBSCT (Figure 2G, H). Together, the data indicate that direct transfer of MAIT cells and possibly MAIT cell precursors in the donor PBSC graft contributes to MAIT cell recovery in the recipient.
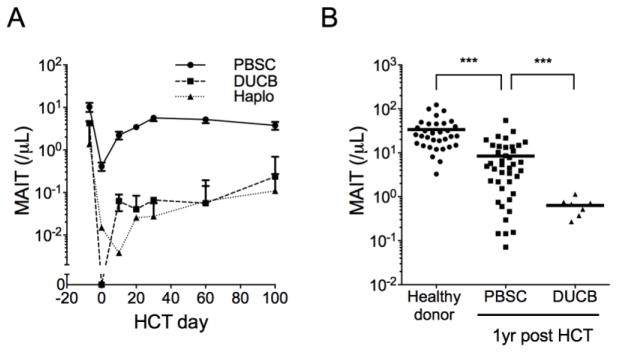
MAIT cell reconstitution is impaired after umbilical cord blood transplant and haploidentical PBSCT with post-HCT cyclophosphamide
We investigated the kinetics of MAIT cell recovery in recipients of umbilical cord blood (UCB) grafts, which contain markedly lower numbers of mature MAIT cells than adult PBSC grafts.10 We examined MAIT cell counts in blood of recipients of double UCB (DUCB) HCT (MA, n=3; NMA, n=3) and found that recipients of DUCB grafts had profoundly impaired recovery of MAIT cells compared to PBSCT recipients at all times up to and including 1 year after HCT (Figures 3A, 3B). Because MAIT cell reconstitution is dependent on proliferation of MAIT cells that are transferred in the HCT graft, we also investigated the impact of administration of post-HCT cyclophosphamide for GVHD prophylaxis to recipients of haploidentical PBSCT (n=6), and found that like DUCB HCT recipients, patients who received PBSCT with post-HCT cyclophosphamide had poor MAIT cell recovery compared to recipients of PBSC grafts without post-HCT cyclophosphamide (Figure 3A).
Figure 3. MAIT cell reconstitution is impaired in umbilical cord blood and haploidentical transplants receiving post-HCT cyclophosphamide.
(A) Absolute MAIT cell counts in recipients of HLA matched PBSC (n=107), double umbilical cord blood (DUCB, n=6), and haploidentical PBSC (haplo, n=6) grafts. Mean +/− SEM are shown. (B) MAIT counts in blood from healthy donors, and at one year after PBSC and DUCB transplant. Horizontal bars represent the mean. *** p<0.001 using the Mann-Whitney test.
MAIT cells are activated by inflammatory cytokines, but TCR stimulation is required for robust proliferation
After HCT, patients have a compromised GI mucosal barrier, altered microbiota, and release of inflammatory cytokines, which could activate MAIT cells.13,20,21 To determine whether inflammatory cytokines are sufficient to promote recipient MAIT cell reconstitution by inducing proliferation of MAIT cells transferred in the HCT graft or if TCR signaling is also required we established in vitro assays to study the effect of inflammatory cytokines alone or with TCR stimulation on activation and proliferation of adult healthy donor MAIT cells. MAIT cells stimulated with anti-CD3 (OKT3) mAb alone failed to upregulate Ki67 and proliferate, and exhibited reduced Lck, ZAP-70 and CD3ζ phosphorylation compared to naïve, central and effector memory subsets of conventional CD8+ T cells (Figure 4A; Supplementary Figure 3), consistent with our previous work that identified downregulation of genes encoding components of the proximal TCR signaling pathway in MAIT cells.10 Co-culture of MAIT cells with individual inflammatory cytokines at concentrations similar to those found in serum of HCT recipients22,23 was sufficient to induce CD69 expression in a small fraction of MAIT cells (Figure 4B), but was insufficient to completely overcome regulation of proximal TCR pathway signaling and induce MAIT cell Ki67 expression and proliferation (Figure 4C; Supplementary Figure 3), which was only induced by the combination of inflammatory cytokine stimulation in conjunction with TCR stimulation (Figure 4C). These data suggest that inflammatory cytokines released in the post-HCT milieu may prime MAIT cells, with robust activation and proliferation only occurring after additional stimulation through the semi-invariant MAIT cell TCR.
Figure 4. Inflammatory cytokines and TCR stimulation are required for full activation and proliferation of MAIT cells.
(A) Fold change in mean fluorescence intensity (MFI) of phosphorylated Lck (pY505) (left), ZAP-70 (pY292) (middle), and CD3ζ (pY142) (right) expression in MAIT, naïve, central and effector memory CD8+ T cells from healthy donors (n=10) after stimulation with the indicated concentrations of OKT3 mAb. Data represent the fold change in MFI of stimulated compared to unstimulated cells. *p<0.05, ** p<0.01, *** p<0.001 using paired Student’s t tests comparing phosphoprotein levels in MAIT versus conventional T cell subsets after Bonferroni correction for multiple comparisons. (B) Percentage of MAIT cells from healthy donors (n=6) expressing CD69 after overnight incubation with inflammatory cytokines (IL-1β, IL-12, IL-18 and IL-23) and/or OKT3 stimulation. p<0.01 using t test comparing OKT3 with cytokines versus cytokines alone. (C) Proliferation of MAIT cells and conventional T cells isolated from healthy donors (n=9) evaluated by tritiated thymidine incorporation after 4 days incubation with inflammatory cytokines. The stimulation index shows tritiated thymidine incorporation relative to unstimulated cells. Data represent the mean +/− SEM.
MAIT cell reconstitution correlates with the abundance of distinct bacterial species in the GI tract
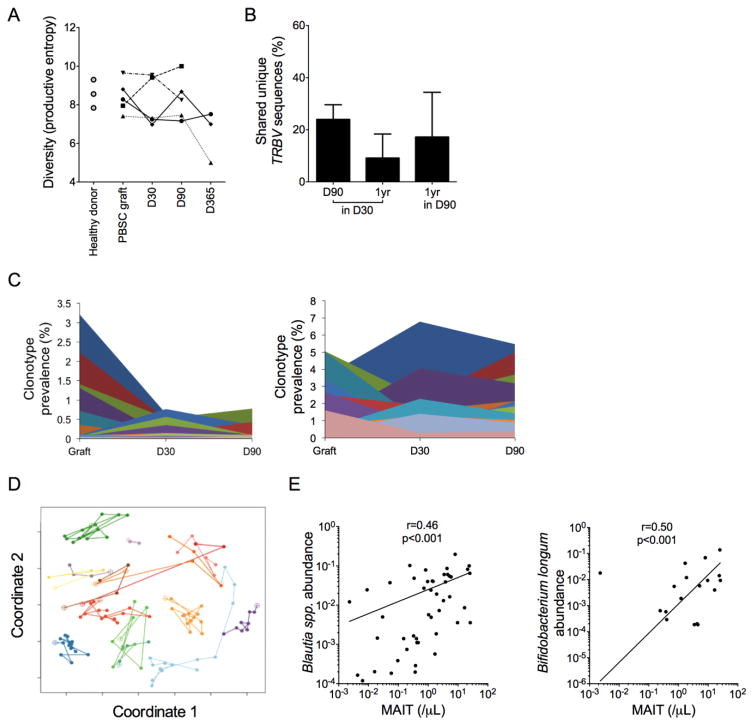
Our findings suggested that in addition to inflammatory cytokines in the post-HCT milieu, stimulation of the semi-invariant Vα7.2+ TCR might be required to induce proliferation in MAIT cells after transfer in the HCT graft. We examined the TRBV repertoire in MAIT cells that were isolated from the blood of HCT recipients and found that TRBV diversity was similar in healthy donors and PBSCT recipients; however, in contrast to the stability of the TCR repertoire in MAIT cells from healthy donors,24 there was variability in TRBV diversity (Figure 5A), sequence sharing (Figure 5B), and the contribution of distinct TRBV clonotypes (Figure 5C) in the MAIT cell repertoire at different times after HCT, indicative of alteration in the clonal composition of MAIT cells over time in HCT recipients. Alteration in the TRBV sequence composition of MAIT cells after stimulation with distinct bacterial species suggests that MAIT cells have the capacity to discriminate between TCR ligands derived from different bacterial species.25 Therefore, we considered that distinct bacterial species in the GI microbiota might contribute to TCR-mediated proliferation and reconstitution of MAIT cells after allogeneic HCT, as occurs after GI microbial colonization in neonates. We characterized the composition of the GI microbiota by performing 16S rRNA gene PCR on stool samples collected at distinct times after HCT followed by high throughput sequencing of the amplified fragments and phylogenetic assignment, and in parallel, collected blood samples for evaluation of MAIT cell recovery to determine if the abundance of distinct bacterial species correlated with MAIT cell numbers in blood. The GI microbiota after HCT was dynamic, consistent with the known effects of mucosal injury due to conditioning therapy, antibiotic use, and altered dietary intake on the microbiota (Figure 5D). Higher abundance of Blautia spp. and Bifidobacterium longum were each associated with higher MAIT cell counts in blood (Figure 5E).
Figure 5. MAIT cell reconstitution after HCT correlates with the abundance of distinct GI microbial species.
(A) TRBV nucleotide sequence diversity as measured by the productive entropy in MAIT cells from healthy donors (n=3), PBSC grafts (n=5) and paired HCT recipients (n=5) at the indicated days after HCT. Lines connect samples from each donor-recipient pair. (B) Percentage of TRBV nucleotide sequences from MAIT cells at day 90 and 1 year after HCT that were also identified at day 30 (left, middle), and of sequences identified at 1 year that were also present at day 90 (right, n=5). (C) Percentage of total unique TRBV sequences shared between the PBSC graft, and the recipient on day 30 or 90 after HCT. Data from two representative HCT recipients are shown. Each color represents a unique TRBV nucleotide sequence. (D) Principal components analysis (coordinates 1 and 2) of the composition of the GI microbiota. Each colored line connects samples from a single PBSC recipient at distinct times after HCT (n=15). Open circles indicate samples collected before HCT and closed circles are samples collected after HCT. A short distance between points indicates similarity between the composition of the microbiota at each timepoint. (E) Correlation between blood MAIT cell counts and the relative abundance in stool of Blautia spp. and Bifidobacterium longum (n=54 paired blood and stool samples). Log-log linear regression Pearson correlation coefficient r and p values are shown.
Impaired MAIT cell reconstitution may be associated with an increased risk of acute GVHD
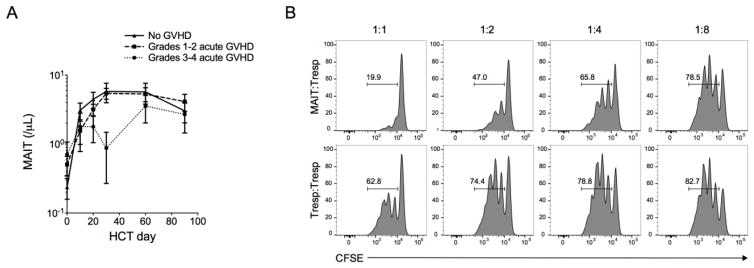
Higher abundance of Blautia spp. in the stool of HCT recipients was recently associated with a reduced risk of acute GVHD requiring systemic immunosuppression and reduced GVHD-related mortality.26 We therefore investigated whether MAIT cell reconstitution might also impact the risk of acute GVHD, and found that HCT recipients who developed severe (grade 3–4) acute GVHD had lower MAIT cell counts in blood compared to those without acute GVHD (Figure 6A). To confirm that the observed association between low MAIT cell counts and GVHD was not due to the effects of treatment for GVHD we performed multivariate Cox regression modeling with MAIT cell counts as a time dependent covariate, excluding MAIT cell counts after the diagnosis of GVHD. After adjusting for the recipient age, ANC, donor type (unrelated versus related), and conditioning regimen (MA versus NMA), we found that an increase in MAIT cell count in blood in the early post-HCT period was associated with reduced risk of subsequent development of GVHD (HR=0.76 for acute GVHD for every 10 cells/μL increase in MAIT counts, 95%CI 0.51–1.14, p=0.18). In vitro studies demonstrated that activated MAIT cells suppressed proliferation of CD4+ T cells in a co-culture assay (Figure 6B; Supplementary Figure 4). These data suggest that factors that impair MAIT cell recovery after HCT could increase the risk of acute GVHD.
Figure 6. MAIT counts are lower in HCT recipients who develop grade 3–4 acute GVHD and activated MAIT cells suppress CD4+ T cells in vitro.
(A) Absolute MAIT cell counts in blood of HCT recipients who did not develop acute GVHD (n=35) or who developed grade 1–2 (n=61) or grade 3–4 (n=8) acute GVHD. The mean +/− SEM are shown. (B) MAIT cells and CD4+ CD25− responder T cells were isolated from the peripheral blood of 3 healthy donors. CFSE-labeled CD4+ responder T cells were stimulated with aCD3/28 beads for 4 days in the presence of MAIT cells or CD4+ responder T cells at a ratio of 1:1, 1:2, 1:4, 1:8, and 1:16 MAIT:responder. Representative flow cytometric analysis of CFSE dilution in CD4+ responder T cells.
DISCUSSION
We present longitudinal data from 107 PBSCT recipients showing that early MAIT cell reconstitution in blood after allogeneic HCT is dependent on transfer of MAIT cells in the HCT graft and their subsequent activation and proliferation in the recipient after HCT. Our in vitro data demonstrate that MAIT cell proliferation was only induced by TCR stimulation in the presence of inflammatory cytokines and not by either TCR stimulation or inflammatory cytokines alone. These data suggest that innate cytokines that are elevated in HCT recipients after conditioning therapy and infusion of allogeneic cells may prime MAIT cells, but that TCR stimulation may be required for full activation, proliferation, and robust reconstitution. Stimulation of the MAIT cell TCR can be provided by bacterial ligands, including riboflavin metabolites,9 whose impact on MAIT cell proliferation may be modified after HCT by alteration in absorption and changes in the composition of the GI microbiota due to gastrointestinal mucosal injury after cytotoxic conditioning therapy, perturbed diet, parenteral feeding, and antibiotic use.27 We investigated whether distinct bacterial species in the GI tract were associated with MAIT cell reconstitution and found that Blautia spp. and Bifidobacterium longum abundance in stool after HCT correlated with MAIT cell counts in blood. While distinct Blautia species metabolize riboflavin28, additional studies will be required to determine if MAIT cell reconstitution is predominantly governed by riboflavin metabolites produced by Blautia spp. or other species, and if other ligands also stimulate the semi-invariant TCR and drive MAIT cell reconstitution.
Our finding that MAIT cell counts rapidly increased in the first 30 days after HCT but failed to reach normal levels up to one year after HCT was intriguing. Despite the ongoing presence of bacterial ligands in the GI tract, there was limited MAIT cell proliferation and accumulation beyond 30 days after HCT, which could in part be due to limitation of available innate cytokines after resolution of inflammation after hematopoietic engraftment, with subsequent restoration of TCR signaling pathway regulation. Alternatively, lack of bacterial diversity in the gut after HCT could limit availability of MAIT TCR ligands, suggesting that anti-microbial prophylaxis, therapy or gut decontamination practices might be important factors in governing MAIT cell reconstitution. MAIT cells may also be more prone to replicative senescence associated with short telomeres and expression of the pro-apoptotic gene, PLZF.29,30 Additional studies will be required to establish whether immunosuppressive agents used to prevent or treat GVHD impact MAIT cell recovery. We observed that MAIT cell reconstitution was markedly impaired in recipients of haploidentical transplantation who received post-HCT cyclophosphamide and in recipients of UCB, which contains few MAIT cells. While studies in murine models suggest that MAIT cells may contribute to mucosal immunity to bacterial infection, especially in the setting of impaired adaptive immunity,31 additional studies will be required to establish whether impaired MAIT recovery after haploidentical and UCB transplants contributes to the higher incidence of infections seen after these types of transplants compared to those who received PBSCT.32
The mechanisms by which alterations in MAIT cell counts in blood could impact the risk of GVHD are unclear. While previous studies showed that MAIT cells in blood were lower during acute exacerbation of autoimmune diseases33–37 and one study suggested this may also be true after diagnosis of acute GVHD,38 it was not determined in these studies whether the reduction in MAIT cell counts was a consequence of cytotoxic or immunosuppressive drugs used to treat inflammatory disease. We used a Cox regression model with MAIT cells as a time-dependent covariate to analyze the association of GVHD with MAIT cell counts collected at multiple times prior to the diagnosis of GVHD. The data indicate that low MAIT cell counts in blood precede the diagnosis of GVHD and are not a result of pharmacologic immunosuppression used to treat GVHD. Development of reproducible methods for detecting and quantitating MAIT cells in mucosal tissues will be pivotal in determining whether the increased risk of GVHD associated with low MAIT cell counts is due to their migration into inflamed sites before the presentation of clinically apparent GVHD or if MAIT cells possess regulatory activity that decreases the risk of GVHD. Examination of chemokine receptor expression on MAIT cells might provide additional insight into the mechanisms by which MAIT cell counts in blood fall prior to the onset of clinical acute GVHD. Our in vitro studies demonstrate that activated MAIT cells may have regulatory activity and suppress proliferation of CD4+ T cells, but additional studies will be required to determine the impact of MAIT cell suppression of GVHD in vivo. Human CD8αα T cells reside exclusively in the CD161hiVα7.2+ subset and a CD8αα T cell subset in mice may have regulatory activity39,40. Future investigation of the roles of CD8αα and CD8αβ CD161hi cells will provide insight into their contributions to the pathogenesis of acute GVHD. Of note, there is emerging evidence that robust reconstitution of another bacterial ligand-responsive subset, invariant natural killer T (iNKT) cells, is associated with a reduced risk of GVHD and better overall survival.2,3
This study provides new insights into MAIT cell reconstitution after HCT, providing a foundation for future efforts to determine the effects of altering the content of MAIT cells in stem cell grafts or promoting MAIT cell recovery in the recipient by modifications of the microbiome or microbial ligands on clinical outcomes after allogeneic HCT.
Supplementary Material
Highlights.
MAIT cell reconstitution after allogeneic HCT is due in part to proliferation of MAIT cells that are transferred in the HCT graft, and is impaired in cord blood and haploidentical transplant recipients.
Robust MAIT cell reconstitution correlates with stool abundance of distinct gastrointestinal bacterial species.
MAIT cells proliferate to TCR signaling with innate cytokine stimulation.
MAIT cells suppress T cell proliferation in vitro and may impact the risk of acute GVHD.
Acknowledgments
This work was funded by K99/R00 CA154608 and R01HL132350 (C.J.T.). A.B. is the recipient of an Investigator Scholarship from the Haematology Society of Australia and New Zealand. C.T. is a Damon Runyon Clinical Investigator.
Footnotes
AUTHORSHIP CONTRIBUTIONS
A.B. designed and performed experiments, analyzed data, prepared figures, and wrote the manuscript; D.N.F., S.S., J.G. and A.S. designed and performed experiments, and analyzed data; M.J.B., S.A.P., K.K.B, T.A.G, F.M., L.H. and S.M. analyzed data; S.R.R. edited the manuscript; C.J.T. developed the concept, designed experiments and wrote the manuscript.
CONFLICT OF INTEREST DISCLOSURES
C.J.T., S.R.R. have a licensed patent.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaidos A, Patterson S, Szydlo R, et al. Graft invariant natural killer T-cell dose predicts risk of acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Blood. 2012;119(21):5030–5036. doi: 10.1182/blood-2011-11-389304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubio MT, Moreira-Teixeira L, Bachy E, et al. Early posttransplantation donor-derived invariant natural killer T-cell recovery predicts the occurrence of acute graft-versus-host disease and overall survival. Blood. 2012;120(10):2144–2154. doi: 10.1182/blood-2012-01-404673. [DOI] [PubMed] [Google Scholar]
- 4.Malard F, Labopin M, Chevallier P, et al. Larger number of invariant natural killer T cells in PBSC allografts correlates with improved GVHD-free and progression-free survival. Blood. 2016;127(14):1828–1835. doi: 10.1182/blood-2015-12-688739. [DOI] [PubMed] [Google Scholar]
- 5.Billerbeck E, Kang YH, Walker L, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A. 2010;107(7):3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold MC, Cerri S, Smyk-Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 8.Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 9.Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 10.Turtle CJ, Delrow J, Joslyn RC, et al. Innate signals overcome acquired TCR signaling pathway regulation and govern the fate of human CD161(hi) CD8alpha(+) semi-invariant T cells. Blood. 2011;118(10):2752–2762. doi: 10.1182/blood-2011-02-334698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ussher JE, Bilton M, Attwod E, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204–3213. [PubMed] [Google Scholar]
- 13.Min CK, Lee WY, Min DJ, et al. The kinetics of circulating cytokines including IL-6, TNF-alpha, IL-8 and IL-10 following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28(10):935–940. doi: 10.1038/sj.bmt.1703258. [DOI] [PubMed] [Google Scholar]
- 14.Carlson CS, Emerson RO, Sherwood AM, et al. Using synthetic templates to design an unbiased multiplex PCR assay. Nat Commun. 2013;4:2680. doi: 10.1038/ncomms3680. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khot PD, Ko DL, Hackman RC, Fredricks DN. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infect Dis. 2008;8:73. doi: 10.1186/1471-2334-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan S, Morgan MT, Fiedler TL, et al. Metabolic signatures of bacterial vaginosis. MBio. 2015;6(2) doi: 10.1128/mBio.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsen FA, Kodner RB, Armbrust EV. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics. 2010;11:538. doi: 10.1186/1471-2105-11-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31(5):834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henden AS, Hill GR. Cytokines in Graft-versus-Host Disease. J Immunol. 2015;194(10):4604–4612. doi: 10.4049/jimmunol.1500117. [DOI] [PubMed] [Google Scholar]
- 21.Abhyankar S, Gilliland DG, Ferrara JL. Interleukin-1 is a critical effector molecule during cytokine dysregulation in graft versus host disease to minor histocompatibility antigens. Transplantation. 1993;56(6):1518–1523. doi: 10.1097/00007890-199312000-00045. [DOI] [PubMed] [Google Scholar]
- 22.Bonnotte B, Burdiles AM, Chehimi J, et al. Serum interleukin-12 levels in patients undergoing allogeneic or autologous bone marrow transplantation. Eur Cytokine Netw. 1996;7(3):389–394. [PubMed] [Google Scholar]
- 23.Fujimori Y, Takatsuka H, Takemoto Y, et al. Elevated interleukin (IL)-18 levels during acute graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2000;109(3):652–657. doi: 10.1046/j.1365-2141.2000.02095.x. [DOI] [PubMed] [Google Scholar]
- 24.Lepore M, Kalinichenko A, Colone A, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRbeta repertoire. Nat Commun. 2014;5:3866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- 25.Gold MC, McLaren JE, Reistetter JA, et al. MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. J Exp Med. 2014;211(8):1601–1610. doi: 10.1084/jem.20140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015;21(8):1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Xu S, Ren Z, Jiang J, Zheng S. Gut microbiota and allogeneic transplantation. J Transl Med. 2015;13:275. doi: 10.1186/s12967-015-0640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Doak TG, Ye Y. Subtractive assembly for comparative metagenomics, and its application to type 2 diabetes metagenomes. Genome Biol. 2015;16:243. doi: 10.1186/s13059-015-0804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerart S, Siberil S, Martin E, et al. Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood. 2013;121(4):614–623. doi: 10.1182/blood-2012-09-456095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havenith SH, Yong SL, Henson SM, et al. Analysis of stem-cell-like properties of human CD161++IL-18Ralpha+ memory CD8+ T cells. Int Immunol. 2012;24(10):625–636. doi: 10.1093/intimm/dxs069. [DOI] [PubMed] [Google Scholar]
- 31.Le Bourhis L, Martin E, Peguillet I, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 32.Parody R, Martino R, Rovira M, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12(7):734–748. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Lee SE, Lim JY, Yoon JH, et al. CD161(+) T cells as predictive markers for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(3):421–428. doi: 10.1016/j.bbmt.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Serriari NE, Eoche M, Lamotte L, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176(2):266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugimoto C, Konno T, Wakao R, Fujita H, Fujita H, Wakao H. Mucosal-associated invariant T cell is a potential marker to distinguish fibromyalgia syndrome from arthritis. J Korean Med Sci. 2015;10(4):e0121124. doi: 10.1371/journal.pone.0121124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willing A, Leach OA, Ufer F, et al. CD8(+) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol. 2014;44(10):3119–3128. doi: 10.1002/eji.201344160. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol. 2011;23(9):529–535. doi: 10.1093/intimm/dxr047. [DOI] [PubMed] [Google Scholar]
- 38.van der Waart AB, van der Velden WJ, van Halteren AG, et al. Decreased levels of circulating IL17-producing CD161+CCR6+ T cells are associated with graft-versus-host disease after allogeneic stem cell transplantation. PLoS One. 2012;7(12):e50896. doi: 10.1371/journal.pone.0050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker LJ, Kang YH, Smith MO, et al. Human MAIT and CD8alphaalpha cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119(2):422–433. doi: 10.1182/blood-2011-05-353789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang X, Maricic I, Purohit N, et al. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+TCRalphabeta+ T cells. J Immunol. 2006;177(11):7645–7655. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.