Abstract
Introduction:
Mirror therapy suggested to help relieve phantom limb pain (PLP) by resolving the visual- proprioceptive dissociation in the brain, but studies so far either had shorter follow-up or smaller sample size.
Materials and Methods:
In this randomized single crossover trial, 64 amputees with PLP in the age group of 15–75 years of age were distributed into test and control groups by simple randomization method. Of these 28 in control and 32 in test groups, respectively, completed the 4 weeks of mirror therapy and 12 weeks of follow-up assessments. A standardized set of exercises for 15 min/day for 4 and 8 weeks in test and control groups (in the first 4 weeks, the mirror was covered), respectively, was administered under supervision of one of the authors. All were assessed using the visual analog scale and Short-Form McGill Pain Questionnaire on day 0 and at 4, 8, and 12 weeks after therapy. In control group for the initial 4 weeks, the mirror was covered. The assessing author was blinded to the group to which the participants belonged.
Results:
Significant reduction in PLP was noted in the test group at 4 weeks compared to the control group (P < 0.0001). Significant reduction was seen in control group also after the switchover and sustained for 12 weeks in both. No harm was reported.
Conclusion:
Mirror therapy is effective in relieving the intensity, duration, frequency, and overall PLP, and improvement is maintained up to 12 weeks’ posttherapy.
Keywords: Mirror Therapy, Phantom sensation, Phantom pain
INTRODUCTION
While amputations have been recorded since time immemorial, the first mention of phantom limb phenomenon was in 1551 by Ambroise Paré (1510–1590), a French Military surgeon, who is called the father of the modern-day amputation surgery.[1] In 1830, Charles Bell, a British physician, described phantom limb sensations in his monograph “The Nervous System of the Human Body.”[2]
Phantom limb sensation, residual limb pain, and phantom limb pain (PLP) are not uncommon in amputees. PLP may be present in up to 72% of patients soon after surgery, may persist for years, and can be very bothersome.[3,4,5] PLP can affect the individual's quality of life by the distress, physical limitation, and the disability that it may cause.[5]
Surgical and pharmacological modalities such as preemptive, preoperative, and immediate postoperative analgesia,[6,7,8] acetaminophen and nonsteroidal anti-inflammatory drugs,[9] opioids,[10] antidepressants,[11] anticonvulsants, and other medications[12,13] have been used to relieve PLP. Nonpharmacological treatments such as transcutaneous electrical nerve stimulation have also been used. Of the several modalities studied, few have been found to be helpful in reducing PLP. However, the effects are neither lasting nor gratifying.[1,14,15,16]
Mirror therapy was first reported by Ramachandran and Rogers in 1996 and is purported to relieve PLP by resolving visual-proprioceptive dissociation in the brain.[17] Studies have revealed the effectiveness of mirror therapy for PLP in both clinical and home settings, but all had either only few weeks of follow-up or had a small number of patients.[18,19,20,21,22,23] One study did not find it useful.[24] Studies on large sample sizes over longer periods are lacking. This area being important for the intervention in cases of amputation, a need was felt to analyze this method of managing PLP. Hence, the present study was planned to assess the nature and severity of PLP in a larger group of amputees and to evaluate the effectiveness of mirror therapy in alleviating the PLP over a longer period.
MATERIALS AND METHODS
A pilot study was conducted at a tertiary Artificial Limb Centre (ALC) in Pune, Maharashtra. Amputees admitted at ALC, Pune, during August 1, 2014, to January 31, 2015, were evaluated for PLP and followed up with a single crossover, single-blind study design.
Selection of patients
Patients were evaluated using “Limb Deficiency and Phantom Limb Questionnaire (Questionnaire 2008, Version 2)” and visual analog scale (VAS) for the presence and extent of PLP. Patients having PLP were selected based on the following inclusion and exclusion criteria.
Inclusion criteria
Amputees with PLP
Age above 15 years and below 75 years
Ability to communicate in English/Hindi.
Exclusion criteria
Age below 15 years and above 75 years of age
Amputees with traumatic brain injury/major psychiatric illness.
One of the authors enrolled 64 patients with PLP over the 6-month period. They were drafted into two groups (test and control) of 32 amputees each, using a random number table by the second author. The patients were coded by the third author and details were kept in a sealed envelope.
Assessment
All were assessed using the VAS and Short-Form McGill Pain Questionnaire (SF-MPQ) on day 0 (baseline) and at 4th, 8th, and 12th week after the mirror therapy (i.e., 16 weeks from baseline). For control group, the assessment was till 20th week, as they underwent 4 weeks of covered mirror therapy prior to commencement of actual mirror therapy. The assessing author (author 1) was blinded to which group the patient belonged.
The visual analog scale
The VAS evaluates pain subjectively. It consists of a 100-mm line, with two end points representing “no pain” to “worst pain imaginable.” Patients are instructed to mark on the line according to the level of pain and the same is measured. Follow-up scores after therapeutic intervention provide an indication of whether the pain is reducing or not. VAS is reliable to be used to assess acute pain, has excellent test–retest reliability, and has a good validity and reliability.[25,26] The second author, who was blinded to which group the patient belonged, applied this scale.
Short-form version of the McGill Pain Questionnaire
SF-MPQ was developed in 1987 by Ronald Melzack. This contains 15 descriptors (4 affective and 11 sensory) which are rated on an intensity scale: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. SF-MPQ has been used in more than 250 published studies.[27] This was applied by the first author at all stages. He was blind as to which group the participants belonged.
Equipment
Mirrors:
A standing mirror (130 cm × 46 cm) with wooden frame and base (62 cm × 65 cm)
A mirror (44 cm × 46 cm) placed on one side of partition at the center of a plywood box (65 cm × 48 cm × 46 cm).
Procedure
Mirror therapy uses a flat mirror placed parasagittally in front of the patient's body with the reflective surface toward the sound limb so that the amputee sees the reflection of the sound limb in the mirror [Figure 1 & 2]. This reflection mimics the amputated limb, and with movement of the intact limb, the mirror provides an optical illusion that the phantom limb is moving simultaneously. Patients in the test arm were told to move both their intact and phantom limbs while seeing the image of the moving limb. Patients in control arm were told to move both their intact and phantom limbs in a similar manner, but in their case, the mirror was covered so that no reflection is seen. The therapy was conducted for both groups for 15 min a day, 7 days a week, over a period of 4 weeks. The control group underwent the routine with the mirror covered for the first 4 weeks, and thereafter crossed over to doing it without the cover for another 4 weeks.
Figure 1.
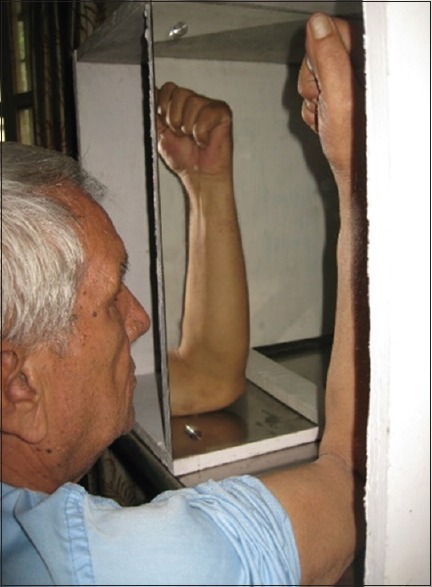
Upper-limb amputee on mirror therapy
Figure 2.
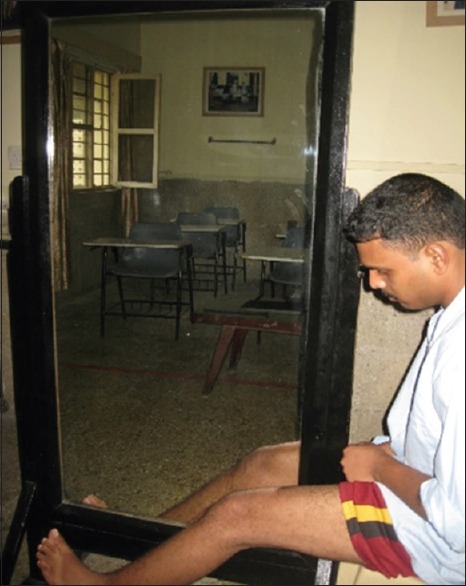
Lower-limb amputee on mirror therapy
Mirror therapy involved performing the following set of movements on both sound and phantom limb sides at the same time.
Slowly straighten and then bend the knee/elbow at the knee or elbow
Point feet/palm upward and downward
Move feet/wrist around half in a circle to the left and to the right
Point toes/fingers upward and then downward while trying to keep foot/wrist still
Clench and unclench toes/fingers.
The therapy was administered by the third author. He also coded their form to blind the assessors of their status in the therapy. The data acquisition was done by the second author using SF-MPQ and VAS and he was unaware of group allocation.
Statistical analysis
The statistical analysis was done by the third and fourth authors, who were blinded to the identity of patients. Analysis was done using repeated measures of analysis of variance for baseline PLP score, and two independent sample t-test was also used for analysis. Pearson's correlation coefficient was used to calculate correlations. The level of significance was set at P < 0.05 and at 95% confidence interval.
Ethical consideration
The study was conducted after getting approval from the Institutional Ethics Committee. An informed written consent was taken from the participants included in the study.
OBSERVATIONS AND RESULTS
Majority of the patients were serving soldiers/ex-servicemen (52) and the rest were civilians (8). Both the test and control groups did not differ significantly in terms of distribution of these two categories, in terms of cause of amputation, laterality of amputation, and duration of PLP prior to intervention. These and other issues shall be elaborated in articles to be published soon. The age of patients ranged from 17 to 62 years. There was no record of any other significant injuries in the patients. Of the 32 participants in control group (covered group), 3 left the session due to surgical procedures required for revision of stump and 1 participant discontinued as he was transferred to another center for management of fever, both due to causes unrelated to PLP. The total 28 amputees in control group completed the mirror therapy. A total of 60 participants, 32 in test group and 28 in control, were available for analysis until 12th week after therapy. All were males, as there is no inpatient facility for females at this center. No harm or adverse effect of therapy was reported by any of the participants.
Thirty-seven (61.67%) of them had left-sided and 23 (38.33%) had right-sided amputation. However, there was no significant association between laterality of amputation and baseline PLP (P < 0.393 in cases and P < 0.88 in controls). Majority of them had lower-limb amputation (84%). The distribution of the site is summarized in Table 1. One-third of the patients reported several days’ duration of PLP, with mean pain intensity score of 3 with standard deviation (SD) 1.7.
Table 1.
Location of amputation and relation to baseline phantom limb pain
For both case and control groups comparing the baseline to 4, 8, 12, and 16 weeks regarding the duration of pain, there was a significant reduction in pain at 4 and 8 weeks after completion of mirror therapy.
Similarly, duration of pain also reduced in case and control groups from baseline to end of 16th week. Continuous and intermittent pain disappeared by the end of 8th week, and at the end of 16th week, only brief pain remained in a few amputees.
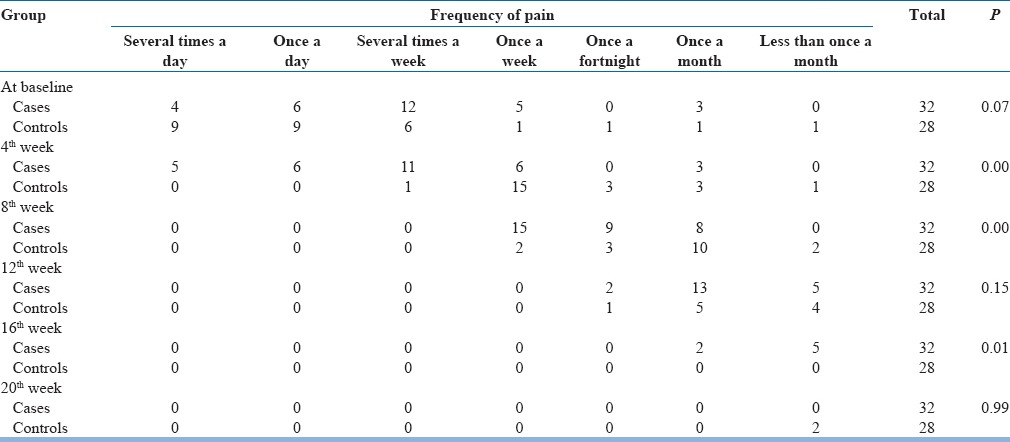
As regard frequency of pain, at the beginning of the study, a majority of participants had experienced PLP several times a week. This was followed by once a day, once a week, several times a day, permanent, and once a month in that order. Frequency gradually reduced. At the conclusion of the study, a few amputees experienced the pain once a month and less than once a month.
On evaluation of pain by SF-MPQ, the score reduced gradually from mean baseline score (3.65) to lowest level (0.15) at the end of 16th week in test group. In the control group, mean pain score in control group was 2.37 initially, and at the end of 16th week after therapy, it reduced to 0.33.
The result showed that there was a significant decline in mean pain score in cases (test group). However in control group, there was no significant change in mean pain score during the initial 4 weeks of covered mirror therapy. However, when control group switched to uncovered mirror therapy after 4 weeks, there was a significant decline in the mean pain score (8 weeks). This indicates that mirror therapy definitely reduced the PLP. The decline in PLP score was maintained at significantly low levels even 12 weeks after therapy has been stopped (16 and 20 weeks in test and control groups, respectively).
The commonest site of amputation was below knee [Figure 3].
Figure 3.
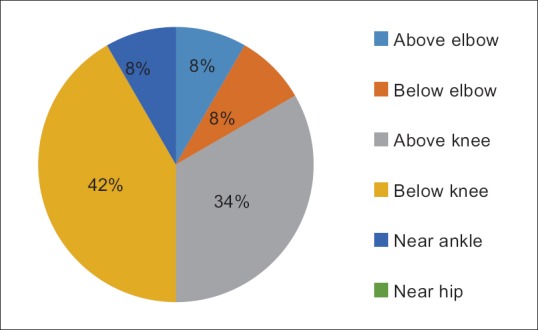
Location of amputation
There is reduction in phantom limb pain by fourth week of mirror therapy compared to control group where mirror was covered, and it is maintained untill twelve weeks after therapy ended. When control group was subjected to Mirrror therapy after four weeks similar result was seen [Figure 4].
Figure 4.
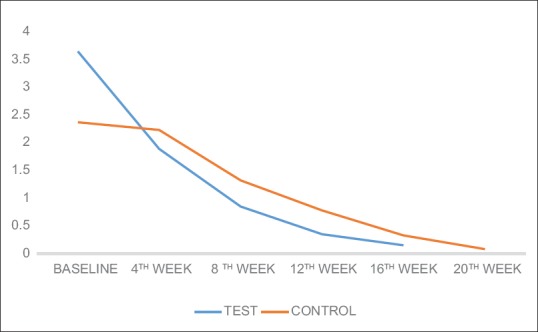
Change in mean phantom limb pain in test and control groups
DISCUSSION
This study is one of the largest controlled prospective studies from India on this subject. Sixty-four amputees were included initially and four dropped out. The remaining participants were followed up for 16 weeks after receiving 4 weeks of mirror therapy. The only other study to have a larger sample (eighty) had only one session of mirror therapy with no follow-up.[24]
Majority of our participants had lower-limb and right-sided (mostly dominant side) amputation [Table 1], similar to a study by Bosmans et al.[28] Studies have also shown that a significant relationship exists between the frequency of their experiences and the level of amputation. Those with higher levels of amputation on their dominant side were more likely to frequently experience phantom limb pain (PLP) than those with amputation at a lower level or of the nondominant.[29,30]
It is interesting to note that while the most common type of pain experienced by the amputees in our study (sickening type – 66.6%) is much different from other studies (pins and needles or sharp pain), the experience of other types of pain in amputees [exhausting – 61.6%, heavy – 61.6%, shooting – 61.6%, stabbing – 58.3%, cramping – 56.6%, aching – 56.6%, sharp – 53.33%, tender – 50%, splitting – 45% and hot burning – 45%; Table 2] is not much different across various studies.[29,31]
Table 2.
Characteristics of phantom limb pain
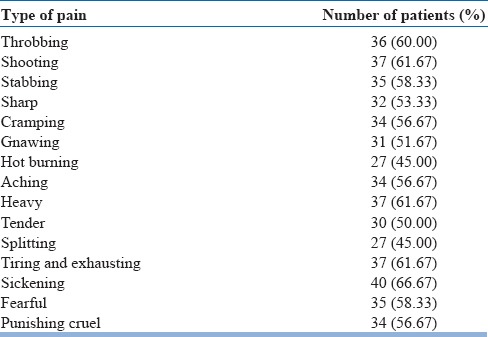
At the beginning, one-third of patients reported several days’ duration of PLP, with a mean pain intensity score of 3 (SD: 1.7). This mean intensity of pain as measured on VAS is much lower than reported by others (5.1 [SD: 2.6]) and (5.5 [SD: 2.6]).[31,32] In this study, a significant change has been seen in the intensity of PLP (as subjectively described in the SF-MPQ) from distressing, discomforting to mild and no pain. There is a continuous shift of pain from a distressing intensity to no pain even much after the cessation of mirror therapy [Table 3]. It has also been observed that a majority of participants had the duration decreased from either continuous/intermittent to either brief or no pain at all. At 12 weeks’ postmirror therapy, most of them had it only briefly while at 16 weeks’ postmirror therapy, it disappeared [Table 4], thereby implying that changes continue to occur even after the mirror therapy ended. Similarly, the frequency of PLP diminished gradually from almost several times a day to no pain [Table 5]. Hanling et al. also found that preamputation mirror therapy for 2 weeks helps reduce PLP, but only one in four had no pain, while the rest had diminished frequency.[33] This is the first study in India to evaluate all these aspects (duration/intensity/frequency) of PLP.
Table 3.
Change in the intensity of phantom limb pain with mirror therapy
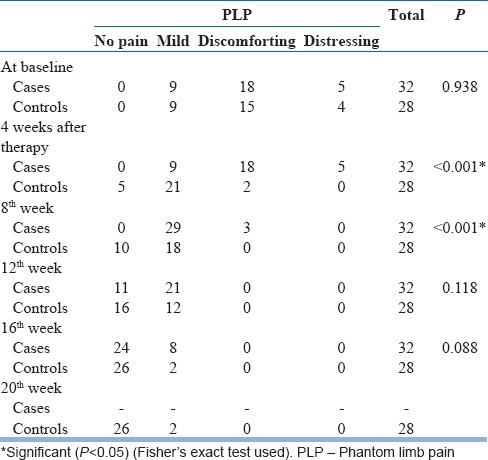
Table 4.
Change in the duration of phantom limb pain
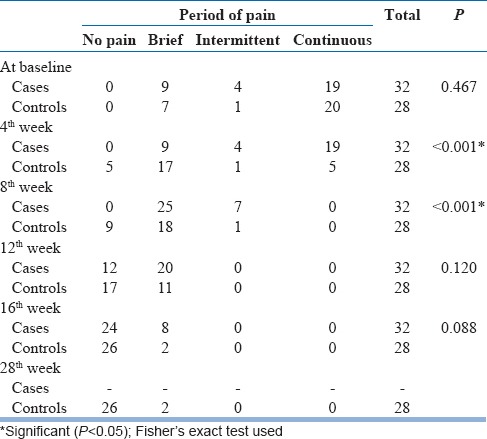
Table 5.
Change in the frequency of phantom limb pain with mirror therapy
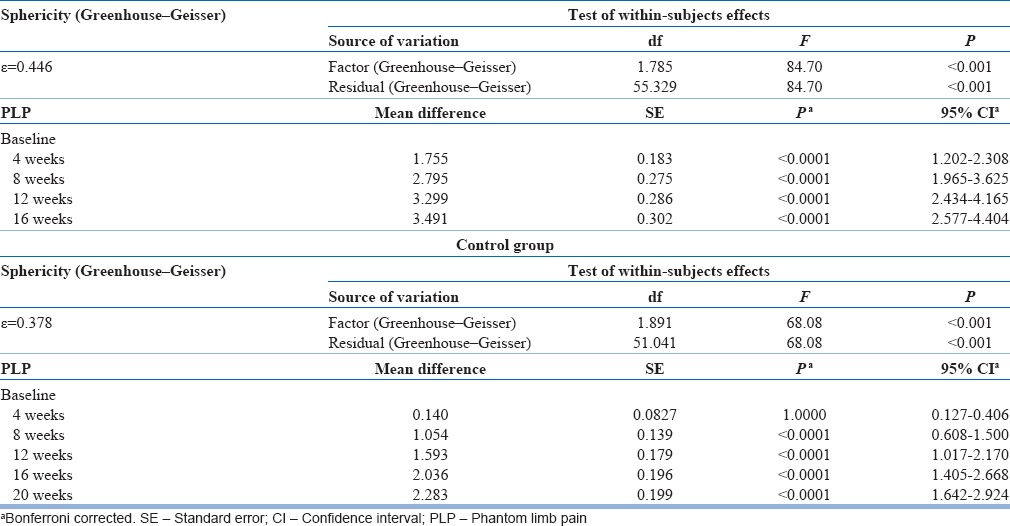
Our study showed a significant reduction in PLP in the test group, with mean pain score at 4th week of 1.89 (SD: 1.5, P < 0.001), at 8th week of 0.85 (SD: 0.91, P < 0.001), at 12th week of 0.35 (SD: 0.91, P < 0.001), and at 16th week being 0.15 (SD: 0.29, P < 0.001) [Table 6]. However, in the control group, there was no reduction in PLP from baseline to 4th week (when mirror was covered), with the mean pain score at 2.23 (SD: 1.28, P = 0.103). However, when the control group crossed to mirror therapy, evaluation at 8th week showed a mean phantom pain score of 1.32 (SD: 0.93, P < 0.001), that at 12th week being 0.78 (SD: 0.66 P < 0.001), at 16th week being 0.33 (SD: 0.39, P < 0.001), and at 20th week reduced to 0.08 (SD: 0.143, P < 0.001) [Table 6 and Figure 4].
Table 6.
Change in phantom limb pain score (repeated measures analysis of variance)
Chan et al. conducted a three-way crossover study in 18 participants. He had six participants in each group, the first group observed mirror movements (by watching the reflected image of their intact foot in a mirror), while the second group underwent covered mirror movements (participants performed the same movements, but the mirror was covered) and the third group undertook the same by imagined movements (participants mentally imagined with closing eyes). The PLP level was rated on the 100-mm VAS. After 4 weeks of exercise in front of the mirror, 17% of the control group, who completed exercises in front of an opaque covered mirror (covered mirror), reported a decrease in pain. About 50% of the control group reported an increase in pain after 4-week exercises. The comparison group completed mental visualization techniques. In this group, 33% of the participants reported a decrease in pain after 4 weeks while 67% of the participants reported an increase in PLP. PLP decreased in eight of the nine (89%) participants who crossed to mirror therapy after either a covered mirror or mental visualization group. On comparison of changes in the score on the VAS at 4 weeks, the mirror group differed significantly from the covered mirror group (P = 0.04).[18] In another randomized controlled study involving eighty amputees, Brodie et al. did not find virtual mirror therapy as being useful.[24]
Our study also did not show reduction in PLP after 4 weeks in control group; however, subsequent evaluation at 8th, 12th, 16th, and 20th weeks revealed a significant reduction in PLP.
Complete resolution of PLP was reported by Darnall, following a month-long self-delivered home-based mirror therapy in amputees with lower-limb PLP,[19] while others reported improvement even within 2 and 3 weeks of mirror therapy.[34,33] On the other hand, Catherine Mercier and Angela Sirigu in 2009 reported 38% reduction in PLP after 4 weeks of virtual visual feedback.[20] However, none of these studies had a follow-up period after end of the therapy, so it is not known whether the relief was maintained.
Though adverse effects of mirror therapy such as confusion, dizziness, sensation of irritation, and grief have been reported by some studies, none of our participants reported such effects.[18,12] The questionnaires in our study did not evaluate for this, but one of the authors interacted with the participants during the therapy and followed up to ascertain such adverse effects.
Several mechanisms have been put forth to explain how mirror therapy helps reduce PLP. Mirror therapy effect is connected to cortical reorganization: as PLP decreased, using visual input of an intact limb as a substitute for the now-missing proprioceptive feedback from the amputated limb, the representation in the S1 (somatosensory cortices) of both cerebral hemispheres becomes more similar, partially recreating their presumed normal state. It is suggested that the mismatch between what the limb feels like and how it looks during mirror therapy is the likely reason for pain relief. The primary motor cortex is also activated by observation of mirror image. Furthermore, treatment effectiveness depends on an ability to relate the mirror image to one's phantom (i.e., the feeling of observing one's own hand). This finding is supported by a decrease in cortical activity in inferior parietal cortex, an area connected to a feeling of agency and pain generation.[35,36,37]
Like mirror neurons exist for motor commands, there are “pain” mirror neurons in the anterior cingulate gyrus that fire when participants are hurt with a needle or watching someone else being hurt. Touch receptors from the skin send signals which after relay in the thalamus project to the somatosensory cortex (S1) and eventually to the superior parietal lobe. The presence of mirror neurons in the brain is also supported by the phenomenon of tactile sensation in the phantom limb elicited by touching the virtual image of the limb in the mirror. When an amputee watches his/her intact limb mirror image during the mirror therapy, the “touch mirror neurons” fire, but the receptors in the skin (of the image) are not stimulated and this lack of activity (the “null signal”) informs regular large but not special touch neurons (i.e., nonmirror touch neurons) that amputated limb is not being touched. They in turn partially veto the output of mirror touch neurons and modulate the somatosensory inputs. This activation probably blocks the protopathic pain perception in the PLP, thereby leading to relief of PLP.[18,38,39]
All these studies evaluated the changes over a very short period of time. The diminution of PLP for a prolonged period of time as shown in this study suggests that probably some of these changes may be permanent. This needs to be evaluated in further studies. Limitations of the study have been lack of female patients and no assessment tool being used for evaluation of emotional status of patient. Since the findings were in a wide variety of PLP patients, the findings could be generalized to at least male amputees.
Findings of the study revealed some important indications in the intervention after amputation. Mental health needs of the patients can be addressed by mitigating the pain after amputation. It is worth mentioning here that mirror therapy is effective in PLP irrespective of its intensity, duration, and frequency. It has been observed that improvement is maintained up to 12 weeks’ posttherapy. It cannot be overemphasized that the present study is by far the longest follow-up study to date not only in India but also in the literature available on the subject.
The impact of therapy is heartening and future studies may include female patients. It is recommended that further multicentric studies with larger number of participants and longer follow-up period are required to establish the long-term effectiveness of mirror therapy to relieve PLP.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The research work was funded by the Director General of Armed Forces Medical Services, Ministry of Defense, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank all our patients for their eager participation and completion of the study. The contribution of the physiotherapists in supervising and helping the actual therapeutic sessions has been of immense help.
REFERENCES
- 1.Black LM, Persons RK, Jamieson B. Clinical inquiries. What is the best way to manage phantom limb pain? J Fam Pract. 2009;58:155–8. [PubMed] [Google Scholar]
- 2.Berger IH, Bacon DR. Historical notes on amputation and phantom limb pain: “All Quiet on the Western Front?”. Gunderson Lutheran Med J. 2009;6:26–9. [Google Scholar]
- 3.Houghton AD, Nicholls G, Houghton AL, Saadah E, McColl L. Phantom pain: Natural history and association with rehabilitation. Ann R Coll Surg Engl. 1994;76:22–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Flor H. Maladaptive plasticity, memory for pain and phantom limb pain: Review and suggestions for new therapies. Expert Rev Neurother. 2008;8:809–18. doi: 10.1586/14737175.8.5.809. [DOI] [PubMed] [Google Scholar]
- 5.Ashley C. There is Evidence Showing Mirror Therapy to Reduce Phantom Limb Pain in Adult Clients with Lower Limb Amputations. Physical Function CATs. Paper 6. 2009. [Last accessed on 2014 Sep 14]. Available from: http://www.commons.pacificu.edu/otpf/6 .
- 6.Reuben SS, Buvanendran A. Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. J Bone Joint Surg Am. 2007;89:1343–58. doi: 10.2106/JBJS.F.00906. [DOI] [PubMed] [Google Scholar]
- 7.Wilson JA, Nimmo AF, Fleetwood-Walker SM, Colvin LA. A randomised double blind trial of the effect of pre-emptive epidural ketamine on persistent pain after lower limb amputation. Pain. 2008;135:108–18. doi: 10.1016/j.pain.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Karanikolas M, Aretha D, Tsolakis I, Monantera G, Kiekkas P, Papadoulas S, et al. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: A prospective, randomized, clinical trial. Anesthesiology. 2011;114:1144–54. doi: 10.1097/ALN.0b013e31820fc7d2. [DOI] [PubMed] [Google Scholar]
- 9.Hanling SR, Wallace SC, Hollenbeck KJ, Belnap BD, Tulis MR. Preamputation mirror therapy may prevent development of phantom limb pain: A case series. Anesth Analg. 2010;110:611–4. doi: 10.1213/ANE.0b013e3181b845b0. [DOI] [PubMed] [Google Scholar]
- 10.Nathanson M. Phantom limbs as reported by S. Weir Mitchell. Neurology. 1988;38:504–5. doi: 10.1212/wnl.38.3.504. [DOI] [PubMed] [Google Scholar]
- 11.Verdu B, Decosterd I, Buclin T, Stiefel F, Berney A. Antidepressants for the treatment of chronic pain. Drugs. 2008;68:2611–32. doi: 10.2165/0003495-200868180-00007. [DOI] [PubMed] [Google Scholar]
- 12.Casale R, Alaa L, Mallick M, Ring H. Phantom limb related phenomena and their rehabilitation after lower limb amputation. Eur J Phys Rehabil Med. 2009;45:559–66. [PubMed] [Google Scholar]
- 13.Hackworth RJ, Tokarz KA, Fowler IM, Wallace SC, Stedje-Larsen ET. Profound pain reduction after induction of memantine treatment in two patients with severe phantom limb pain. Anesth Analg. 2008;107:1377–9. doi: 10.1213/ane.0b013e31817f90f1. [DOI] [PubMed] [Google Scholar]
- 14.Bittar RG, Otero S, Carter H, Aziz TZ. Deep brain stimulation for phantom limb pain. J Clin Neurosci. 2005;12:399–404. doi: 10.1016/j.jocn.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Z, Hu Y, Tao W, Zhang X, Li Y. Dorsal root entry zone lesions for phantom limb pain with brachial plexus avulsion: A study of pain and phantom limb sensation. Stereotact Funct Neurosurg. 2009;87:249–55. doi: 10.1159/000225978. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan A, Phan PC, Burton AW. Use of spinal cord stimulation in the treatment of phantom limb pain: Case series and review of the literature. Pain Pract. 2010;10:479–84. doi: 10.1111/j.1533-2500.2010.00374.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377–86. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 18.Chan BL, Witt R, Charrow AP, Magee A, Howard R, Pasquina PF, et al. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357:2206–7. doi: 10.1056/NEJMc071927. [DOI] [PubMed] [Google Scholar]
- 19.Darnall BD. Self-delivered home-based mirror therapy for lower limb phantom pain. Am J Phys Med Rehabil. 2009;88:78–81. doi: 10.1097/PHM.0b013e318191105b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercier C, Sirigu A. Training with virtual visual feedback to alleviate phantom limb pain. Neurorehabil Neural Repair. 2009;23:587–94. doi: 10.1177/1545968308328717. [DOI] [PubMed] [Google Scholar]
- 21.Seidel S, Kasprian G, Furtner J, Schöpf V, Essmeister M, Sycha T, et al. Mirror therapy in lower limb amputees – A look beyond primary motor cortex reorganization. Rofo. 2011;183:1051–7. doi: 10.1055/s-0031-1281768. [DOI] [PubMed] [Google Scholar]
- 22.Tsao J. Navy Doctor's Work in ‘Mirror Therapy’ Benefits Military Amputees. Adaptive Therapy Devices. 2011. [Last accessed on 2014 Sep 14]. Available from: http://www.usmedicine.com/agencies/department-of-defense-dod/military-medicine-comes-upwith-novel-treatments-for-phantom-limb-pain-persists-after-amputation/
- 23.Kim SY, Kim YY. Mirror therapy for phantom limb pain. Korean J Pain. 2012;25:272–4. doi: 10.3344/kjp.2012.25.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodie EE, Whyte A, Niven CA. Analgesia through the looking-glass? A randomized controlled trial investigating the effect of viewing a ‘virtual’ limb upon phantom limb pain, sensation and movement. Eur J Pain. 2007;11:428–36. doi: 10.1016/j.ejpain.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8:1153–7. doi: 10.1111/j.1553-2712.2001.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 26.Brokelman RB, Haverkamp D, van Loon C, Hol A, van Kampen A, Veth R, et al. The validation of the visual analogue scale for patient satisfaction after total hip arthroplasty. Eur Orthop Traumatol. 2012;3:101–5. doi: 10.1007/s12570-012-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason ST, Arceneaux LL, Abouhassan W, Lauterbach D, Seebach C, Fauerbach JA, et al. Confirmatory factor analysis of the short form McGill pain questionnaire with burn patients. Eplasty. 2008;8:e54. [PMC free article] [PubMed] [Google Scholar]
- 28.Bosmans JC, Geertzen JH, Post WJ, van der Schans CP, Dijkstra PU. Factors associated with phantom limb pain: A 31/2.year prospective study. Clin Rehabil. 2010;24:444–53. doi: 10.1177/0269215509360645. [DOI] [PubMed] [Google Scholar]
- 29.Fraser CM, Halligan PW, Robertson IH, Kirker SG. Characterising phantom limb phenomena in upper limb amputees. Prosthet Orthot Int. 2001;25:235–42. doi: 10.1080/03093640108726607. [DOI] [PubMed] [Google Scholar]
- 30.Flor H. Phantom-limb pain: Characteristics, causes, and treatment. Lancet Neurol. 2002;1:182–9. doi: 10.1016/s1474-4422(02)00074-1. [DOI] [PubMed] [Google Scholar]
- 31.Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81:1039–44. doi: 10.1053/apmr.2000.7583. [DOI] [PubMed] [Google Scholar]
- 32.Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–9. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Hanley MA, Ehde DM, Campbell KM, Osborn B, Smith DG. Arch Phys Med Rehabil. 2006. Self-reported treatments used for lower-limb phantom pain Descriptive findings; pp. 270–7. [DOI] [PubMed] [Google Scholar]
- 34.MacLachlan M, McDonald D, Waloch J. Mirror treatment of lower limb phantom pain: A case study. Disabil Rehabil. 2004;26:901–4. doi: 10.1080/09638280410001708913. [DOI] [PubMed] [Google Scholar]
- 35.Knecht S, Henningsen H, Elbert T, Flor H, Höhling C, Pantev C, et al. Reorganizational and perceptional changes after amputation. Brain. 1996;119(Pt 4):1213–9. doi: 10.1093/brain/119.4.1213. [DOI] [PubMed] [Google Scholar]
- 36.Garry MI, Loftus A, Summers JJ. Mirror, mirror on the wall: Viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res. 2005;163:118–22. doi: 10.1007/s00221-005-2226-9. [DOI] [PubMed] [Google Scholar]
- 37.Foell J, Bekrater-Bodmann R, Diers M, Flor H. Mirror therapy for phantom limb pain: Brain changes and the role of body representation. Eur J Pain. 2014;18:729–39. doi: 10.1002/j.1532-2149.2013.00433.x. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- 39.Subedi B, Grossberg GT. Phantom limb pain: Mechanisms and treatment approaches. Pain Res Treat 2011. 2011:864605. doi: 10.1155/2011/864605. [DOI] [PMC free article] [PubMed] [Google Scholar]


