Abstract
Background and aims
A co-transplanted liver allograft has been thought to protect other organs from rejection-mediated injury; however, detailed analyses of co-transplanted liver on intestinal allograft outcomes have not been conducted to date. The aim of the study was to compare immune-mediated injury, causes of graft failure and clinical outcomes between recipients who underwent either a liver-inclusive intestinal transplant (LITx) or liver-exclusive intestinal transplant (LETx).
Methods
Between May 2000 and May 2010, 212 adult patients undergoing LITx (n =76) and LETx (n =136) were included. LITx underwent either liver combined intestinal or full multivisceral transplantation. LETx underwent either isolated intestinal or modified multivisceral transplantation.
Results
During 44.9 ± 31.4 months of follow-up, death-censored intestinal graft survival was significantly higher for LITx than LETx (96.9%, 93.2% and 89.9% vs 91.4%, 69.3% and 60.0% at 1, 3 and 5 years; p =0.0001). Incidence of graft loss due to rejection was higher in LETx than in LITx (30.9% vs 6.6%; p <0.0001), while infection was the leading cause of graft loss due to patient death in LITx (25.0% vs 5.1%; p <0.0001). Despite similar immunosuppression, the average number (0.87 vs 1.42, p =0.02) and severity of acute cellular rejection episode (severe grade: 7.9% vs 21.3%; p =0.01) were lower in LITx than in LETx. Incidence of acute antibody-mediated rejection was also significantly lower in LITx than in LETx (3.6% vs 15.2%; p =0.03). Incidence of chronic rejection was reduced in LITx (3.9% vs 24.3%; p =0.0002).
Conclusions
Intestinal allografts with a liver component appear to decrease risk of rejection but increase risk of infection. Our findings emphasize that LITx has characteristic immunologic and clinical features. Lower immunosuppression may need to be considered for patients who undergo LITx to attenuate increased risk of infection.
Keywords: Intestinal transplant, immunosuppression, rejection, infection
Introduction
Intestinal transplantation (ITx) has become an effective treatment option for patients with irreversible intestinal failure [1,2]. Due to the unique structure and immunologic properties of the intestine, it has been deemed one of the most difficult organs to transplant [3]. Despite improvements in short-term outcomes, long-term survival of both patient and graft after ITx is still poor compared to other solid-organ transplants, with a 5-year graft survival around 50–60% [1,4]. Rejection and infection continue to be the leading causes of graft loss after ITx [5,6]. Finding the delicate balance between rejection and infection remains one of the primary challenges of managing intestinal allograft recipients [7,8].
It is well known that liver allografts can spontaneously be accepted across the full major histocompatibility complex barrier in many animal models [9,10]. Clinically, a transplanted liver appears to be more resistant to alloimmune-mediated damage than other organ transplants and some liver allograft recipients who develop “operational tolerance” can successfully be withdrawn from chronic immunosuppressive medications [11,12]. Compelling evidence shows that, in the setting of highly sensitized recipients, a co-transplanted liver can provide immunoprotection for kidney or cardiac grafts [13–15]. Several mechanisms have been hypothesized to explain the better outcomes associated with a co-transplanted liver [16]. The immunoprotective property of a co-transplanted liver on intestinal allografts has been described earlier [17]. So far, the largest series of 500 cases of ITx have been reported regarding the evolution of surgical techniques, immunosuppression and management strategies but lack a detailed analysis of the effect of co-transplanted liver allografts on immune-mediated damage and outcomes [18].
Traditionally, ITx is divided into four main categories for clinical analyses: an intestinal transplant alone, a combined liver-intestinal transplant, a modified multivisceral transplant without a liver and a full multivisceral transplant [19,20]. Owing to the unique immunoprotective features of co-transplanted liver allografts, we hypothesize that different mechanisms and causes for intestinal graft loss exist between a liver-inclusive intestinal transplant (LITx) and liver-exclusive intestinal transplant (LETx). The purpose of this study was to retrospectively compare the incidence of immune-mediated injury, causes for graft failure and clinical outcomes between LETx and LITx to develop a better therapeutic strategy toward more balanced immunosuppression.
Methods
Patient selection
During a 10-year period from May 2000 to May 2010, a total of 236 consecutive adult ITx procedures were performed at the University of Pittsburgh Medical Center (UPMC). Of these, 212 patients with primary transplants were included in the final analysis; the results from 24 patients with secondary transplants were reported earlier [21]. Patient data were retrieved from computerized databases, flow-charts and medical records. Graft failure was defined as a patient returning to total parenteral nutrition (TPN), receiving an antrectomy or death. The average time of follow-up from date of transplant to date of graft failure or death was 44.9 ± 31.4 months.
All donors were cadaveric and ABO blood type-identical. Donor grafts were allocated based on blood group matching, size match and clinical urgency. Donor and recipient human leukocyte antigen (HLA) match were random. Immunological testing, including HLA tissue typing, panel reactive antibody (PRA), HLA antibodies and complement-dependent cytotoxicity cross-match (CDC-XM), was previously described in detail [18]. All 212 patients received a complete CDC-XM and PRA analysis; 154/212 (72.6%) patients underwent a complete HLA antibody assessment via either LAT Single Antigen ELISA or Lab Screen Single antigen bead Luminex assays.
Transplant types
Based on the unique immunoprotective features of the liver, intestinal graft recipients were divided into two categories in this study: liver-exclusive or liver-inclusive transplants. Liver-exclusive transplants underwent either isolated intestinal or modified multivisceral transplantation; liver-inclusive transplants underwent either liver combined intestinal or full multivisceral transplantation.
Post-transplant management
The immunosuppressive regimens used in our program have previously been described in detail [18]. In brief, 212 patients received either induction therapy including Daclizumab (Zenapax) (n=20), rabbit anti-thymocyte globulin (r-ATG) (n =43) and alemtuzumab (n =117) or no induction therapy (n =32). The maintenance immunosuppressive agents were mainly based on tacrolimus (Prograf; Astellas Pharma, Deerfield, IL) and steroids. Tacrolimus doses were targeted to achieve a trough level of 15–20 ng/ml within the first 3 post-operative months, 10–15 ng/ml between 4 and 12 months and 5–10 ng/ml thereafter. Similar immunosuppressive regimens and targeted tacrolimus levels were used between LETx and LITx.
For infection prophylaxis, the patient received piperacilline/tazobactam plus vancomycin, amphotericin B lipid complex (5 mg/kg/day for 1 week) and then fluconazole or voriconazole for 3 months, co-trimoxazole (80 mg every 2 days for 6–12 months) and ganciclovir (5 mg/kg/day q12hr for 2 weeks) and valganciclovir (900 mg for 3–6 months thereafter).
Diagnosis of rejection
Surveillance endoscopy was routinely performed twice per week for the first 2–3 weeks after transplantation and then weekly thereafter, with increased frequency as clinically indicated by increased stomal output, fever, abdominal pain or other symptoms. The histological criteria for a diagnosis of acute cellular rejection (ACR) were as described previously [22]. A diagnosis of acute antibody-mediated rejection (ABMR) is based on clinical evidence of graft dysfunction, high PRA levels with circulating donor-specific antibody (DSA) and pathological evidence of tissue injury and C4d deposition [23]. A new rejection episode is defined as the occurrence of new clinical symptoms with characteristic pathologies and at least one normal mucosal biopsy between two rejection events. A diagnosis of chronic rejection is based on clinical manifestations, confirmed with a full-thickness specimen of a totally or partially removed intestinal allograft to show evidence of vasculopathy and mesenteric lymphoid depletion with mesenteric sclerosis [24].
Statistical analysis
Data are presented with continuous variables as means ± standard deviation (SD) and with categorical variables as counts and proportions. Comparisons between groups were made using Student’s t-test and Fisher’s exact test for continuous and categorical variables, respectively. Time-to-event data (e.g. death, graft survival, rejection) are described using Kaplan–Meier curves and compared using log-rank test. Cox proportional hazards regression was used to assess univariate association of independent variables with chronic rejection. For graft survival analysis, all patient deaths were considered as graft failure regardless of graft functioning status at the time of patient death. For death-censored graft survival, all patient deaths were defined as lost to follow-up. Recipient variables (age, sex, cause of graft failure, positive CDC-XM, preformed DSA, de novo DSA, number of surgical procedures prior to transplant and duration of TPN), donor variables (age, sex, positive cytomegalovirus [CMV] status and cold ischemia time) and co-transplanted liver allograft were used for the analysis. Multivariate analyses were performed using a backward elimination method and a significance level of 0.10. Results of the Cox models are presented as odds ratios (ORs) and 95% confidence intervals (CIs). A p-value <0.05 was considered to be statistically significant.
Results
Baseline patient characteristics
A total of 212 adult patients undergoing primary ITx were included in this study. Of these, 136 (64.2%) received a liver-exclusive allograft including an isolated intestine (n =106) and a modified multivisceral allograft without a liver (n =30); 76 (35.8%) received a liver-inclusive allograft including combined liver-intestine (n =28) and a full multivisceral allograft (n =48).
At the time of transplant, LETx and LITx did not differ significantly in age, number of previous abdominal operations, durations of TPN or number of HLA mismatches. The proportion of female patients was higher in LETx than in LITx (65.4% vs 47.4%; p = 0.01). The rate of mesenteric thrombosis-related intestinal failure was significantly lower in LETx than in LITx (27.2% vs 27.3%; p =0.0001). The proportion of patients who had splenectomy was significantly higher in LITx than in LETx (5.9% vs 65.8%; p < 0.0001). The proportion of patients who received no induction therapy was significantly higher in LITx than in LETx (27.6% vs 8.1%; p =0.0001). The proportion of patients who underwent Campah-1H induction was higher in LETx than in LITx (61.7% vs 43.5%; p =0.01). The donor age was 25.3 ± 9.6 years in LETx and 26.2 ± 11.4 years in LITx. The cold ischemia times were shorter in LETx than in LITx (7.4 ± 1.4 vs 8.6 ± 1.5 hours; p <0.0001). The rate of positive CMV donors was significantly higher in LITx than in LETx (61.8% vs 44.1%; p =0.01) (Table 1).
Table 1.
Clinical characteristics of the liver-exclusive versus liver-inclusive allograft
| Clinical characteristics | All patients (n =212) | Liver-exclusive (n =136) | Liver-inclusive (n =76) | p-value |
|---|---|---|---|---|
| Mean age, years | 43.9 ± 12.1 | 42.1 ± 11.9 | 47.1 ± 11.9 | NS |
| Female sex, n (%) | 125 (59.0) | 89 (65.4) | 36 (47.4) | 0.01 |
| Prior abdominal operations | 5.6 ± 5.5 | 5.7 ± 5.2 | 5.5 ± 5.9 | NS |
| Durations of TPN, months | 30.3 ± 43.1 | 30.3 ± 42.1 | 30.3 ± 44.9 | NS |
| Primary diagnoses, n (%) | ||||
| Mesenteric thrombosis | 76 (35.8) | 37 (27.2) | 39 (51.3) | 0.0001 |
| Crohn’s disease | 38 (17.9) | 29 (21.4) | 9 (11.9) | NS |
| Motility disorders | 24 (11.3) | 17 (12.5) | 7 (9.2) | NS |
| Gardner’s syndrome | 14 (6.7) | 12 (8.8) | 2 (2.6) | NS |
| Radiation enteritis | 8 (3.8) | 7 (5.1) | 1 (1.3) | NS |
| Gastric bypass | 10 (4.7) | 8 (5.9) | 2 (2.6) | NS |
| Others | 42 (19.8) | 26 (19.1) | 16 (21.1) | NS |
| Cold-ischemic times, hours | 7.8 ± 1.5 | 7.4 ± 1.4 | 8.6 ± 1.5 | <0.0001 |
| Positive CMV donor, n (%) | 107 (50.5) | 60 (44.1) | 47 (61.8) | 0.01 |
| Splenectomy, n (%) | 58 (27.4) | 8 (5.9) | 50 (65.8) | <0.0001 |
| Donor BMTx, n (%) | 32 (15.1) | 22 (16.2) | 10 (13.2) | NS |
| Donor bowel irradiation, n (%) | 21 (9.9) | 17 (12.5) | 4 (5.3) | NS |
| Donor/recipient, n (%) | ||||
| Sex mismatch | 99 (46.7) | 65 (47.8) | 34 (44.7) | NS |
| Race mismatch | 40 (18.9) | 28 (20.5) | 12 (15.8) | NS |
| Induction, n (%) | ||||
| None | 32 (15.1) | 11 (8.1) | 21 (27.6) | 0.0001 |
| Zenapax | 20 (9.4) | 13 (9.6) | 7 (9.2) | NS |
| Thymoglobulin | 43 (20.3) | 28 (20.6) | 15 (19.7) | NS |
| Campath-1H | 117 (55.2) | 84 (61.7) | 33 (43.5) | 0.01 |
| Follow-up, months | 44.9 ± 31.4 | 45.7 ± 32.2 | 43.6 ± 29.9 | NS |
TPN, total parenteral nutrition; CMV, cytomegalovirus; BMTx, bone marrow transplantation; NS, not significant.
Patient and graft survival
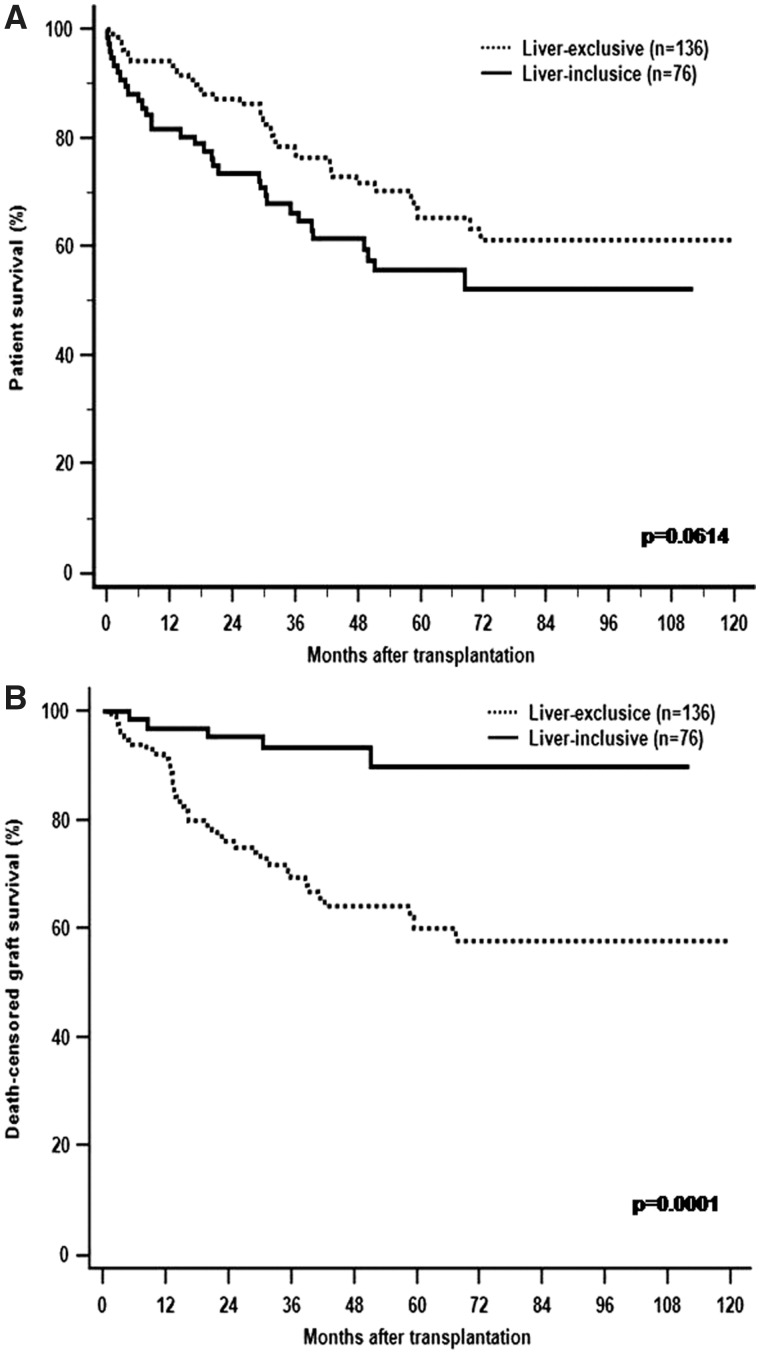
Overall survival rates at 1, 5 and 10 years were 94.0%, 65.2% and 61.3% for patients with LETx and 81.6%, 55.6% and 52.1% for those with LITx, respectively. Although patient survival was not significantly different between the two groups, it approached statistical significance (p =0.061; Figure 1A). The death-censored intestinal graft survival rate was significantly higher for LITx compared to LETx: 96.9% at 1 year, 93.2% at 3 years and 89.9% at 5 years vs 91.4% at 1 year, 69.3% at 3 years and 60.0% at 5 years (p =0.0001; Figure 1B).
Figure 1.
The Kaplan–Meier patient (A) and death-censored graft (B) survival for liver-exclusive transplants (dotted line) and liver-inclusive transplants (solid line). Although the trend of overall patient survival was lower in LITx than LETx, death-censored intestinal graft survival after LITx is superior to LET.
In LETx, a total of 56 recipients (41.2%) lost an intestinal graft with an average follow-up of 45.7 ± 32.2 months, and the leading cause of graft loss was irreversible rejection. Of the 136 LETx recipients, 42 (30.9%) developed uncontrolled rejection (ACR in 7, acute ABMR in 2 and chronic rejection in 33) (Table 2). Lethal infections occurred in seven (5.1%): bacterial in six and viral in one with no documented fungal-caused death. The locations of infection included intra-abdominal in three, respiratory in two and bloodstream in two. Three (42.9%) infections occurred within the first 6 months after transplantation and four (57.1%) occurred thereafter (Table 3). An opportunistic infection was documented in one case (EBV-related post-transplant lymphoproliferative disease [PTLD]). Other causes of graft failure due to patient death included technical failure by intra-abdominal bleeding (n =1), cardiovascular event (n =1), motor vehicle accident (n =1), intra-cranial bleeding (n =1) or undefined etiology (n =3). In this group, 22 (16.2%) patients underwent retransplantation. A total of eight (5.9%) patients died within the first year after transplantation (Table 2).
Table 2.
Immunological parameters and clinical outcomes of the liver-inclusive versus the liver-exclusive allograft
| Clinical characteristics | All patients (n =212) | Liver-exclusive (n =136) | Liver-inclusive (n =76) | p-value |
|---|---|---|---|---|
| Number of HLA mismatches | ||||
| Class I (A, B locus) | 2.9 ± 1.0 | 2.9 ± 1.0 | 2.8 ± 1.1 | NS |
| Class II (DR locus) | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.6 ± 0.5 | NS |
| PRA HLA class I (%) | 21.8 ± 32.1 | 20.7 ± 30.7 | 23.8 ± 34.6 | NS |
| PRA HLA class II (%) | 16.2 ± 29.6 | 16.8 ± 30.4 | 15.1 ± 28.3 | NS |
| Positive cross-match, n (%) | 55 (25.9) | 34 (25.0) | 21 (27.6) | NS |
| Preformed DSA, n (%) | 44/154 (28.6) | 30/99 (30.3) | 14/55 (25.5) | NS |
| De novo DSA, n (%) | 35/154 (22.7) | 28/99 (28.3) | 7/55 (12.9) | 0.03 |
| Acute rejection (≤ 360 days), n (%) | 133 (62.7) | 92 (67.6) | 41 (53.9) | 0.05 |
| Acute ABMR, n (%) | 17/154 (11.0) | 15/99 (15.2) | 2/55 (3.6) | 0.03 |
| ACR, n (%) | 116 (54.7) | 77 (56.6) | 39 (51.3) | NS |
| Mild | 91 (42.9) | 58 (42.6) | 33 (43.4) | 0.91 |
| Moderate | 54 (25.5) | 42 (30.9) | 12 (15.8) | 0.02 |
| Severe | 35 (16.5) | 29 (21.3) | 6 (7.9) | 0.01 |
| Median first acute rejection, months | 1.0 (0.1–35.4) | 0.8 (0.5–35.4) | 1.6 (0.1–31.3) | NS |
| Chronic rejection, n (%) | 36 (17.0) | 33 (24.3) | 3 (3.9) | 0.0002 |
| Causes of graft failure, n (%) | 89 (42.0) | 56 (41.2) | 33 (43.4) | NS |
| Rejection | 47 (22.2) | 42 (30.9) | 5 (6.6) | <0.0001 |
| Infection | 26 (12.3) | 7 (5.1) | 19 (25.0) | <0.0001 |
| Technical | 4 (1.9) | 1 (0.7) | 3 (3.9) | NS |
| Primary-non-function | 1 (0.5) | 0 | 1 (1.3) | NS |
| Graft-versus-host disease | 1 (0.5) | 0 | 1 (1.3) | NS |
| Neoplasm | 1 (0.5) | 0 | 1 (1.3) | NS |
| Others | 4 (1.9) | 3 (2.2) | 0 | NS |
| Unknown | 6 (2.8) | 3 (2.2) | 3 (3.9) | NS |
| Retransplantation, n (%) | 24 (11.3) | 22 (16.2) | 2 (2.6) | 0.003 |
| Mortality ≤360 days | 22 (10.4) | 8 (5.9) | 14 (18.4) | 0.004 |
PRA, panel reactive antibody; HLA, human leukocyte antigen; DSA, donor-specific antibody; ACR, acute cellular rejection; ABMR, antibody-mediated rejection; NS, not significant.
Table 3.
Type and timeline of post-transplant fatal infections in the liver-exclusive versus the liver-inclusive allograft
| Type of infections | Timeline of infections |
||
|---|---|---|---|
| <1 month | 2–6 months | >6 months | |
| Liver-exclusive | |||
| Bacterial | 1 | 2 | 3 |
| Fungal | 0 | 0 | 0 |
| Viral | 0 | 0 | 1 |
| Total | 1 | 2 | 4 |
| Liver-inclusive | |||
| Bacterial | 1 | 3 | 11 |
| Fungal | 0 | 1 | 2 |
| Viral | 0 | 0 | 1 |
| Total | 1 | 4 | 14 |
In LITx, 33 (43.4%) recipients lost grafts with an average follow-up of 43.6 ± 29.9 months. Five cases suffered irreversible rejection (ACR in two and chronic rejection in three). The rate of rejection-caused graft loss in LITx was significantly lower compared to LETx (6.6% vs 22.2%; p <0.0001) (Table 2). In contrast to LETx, lethal infection was the leading cause of graft loss in 19 LITx recipients: bacterial in 15, fungal in 3 and viral in 1. The locations of infection included nine respiratory infections, six abdominal abscesses (including two arterial graft pseudoaneurysms) and four bloodstream infections. Five (26.3%) infections occurred within the first 6 months after transplantation and 14 (73.7%) occurred after 6 months (Table 3). The lethal infection rate was significantly higher in LITx when compared to LETx (25.0% vs 5.1%; p <0.0001). Opportunistic infections were diagnosed in five cases including Nocardia (n =1), fungal (n =3) and EBV (n =1). The incidence of opportunistic infections was significantly higher in LITx than that in LETx (6.6% vs 0.7%; p =0.014). In this group, three patients died peri-operatively from technical complications, including intra-cardiac clot (n =1) and arterial graft rupture (n =2). Other causes of graft loss included primary graft non-function (n =1), lymphagiosarcoma (n =1), graft-versus-host disease (GVHD) (n =1) or undefined etiology (n =3). Two patients (2.6%) underwent retransplantation. Overall, a total of 14 (18.4%) patients died within the first year after transplantation and the mortality rate within the first year in LITx was significantly higher than in LETx (p =0.004) (Table 2).
Acute cellular rejection
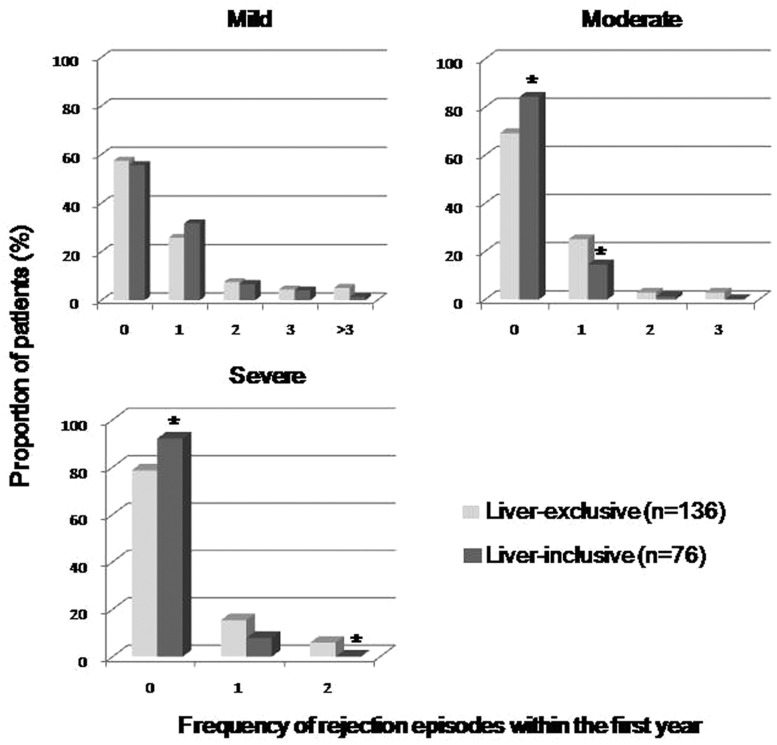
Of the 76 LITx patients, the incidence of ACR (≥mild grade) within the first year was 51.3%, which was similar to that seen in LETx patients (56.6%). The average number of episodes of ACR experienced per patient within the first year was significantly lower for LITx than LETx (0.87 [range 0–5] vs 1.42 [range 0–9]; p =0.02). In general, there were similar proportions of a mild grade ACR between LITx and LETx (43.4% vs 42.6%), whereas the LITx patients showed a less moderate (15.8% vs 30.9%; p =0.02) and severe grade of ACR (7.9% vs 21.3%; p =0.01) (Figure 2). The median onset of the first episode of ACR in LITx was 1.6 months (range 0.1–31.3 months) post transplantation versus 0.8 months (range 0.5–35.4 months) in LETx (p =0.58) (Table 2).
Figure 2.
The proportion of patients who experienced frequency of acute rejection episodes within the first year after transplantation. The LITx patients (heavy gray) showed a less moderate and severe grade of ACR than the LETx patients (light gray).
Acute ABMR and DSA
In the 154 patients (99 in the LETx and 55 in the LITx) with a complete HLA antibody assessment both before and after transplantation, we identified 17 cases (11.0%) that fulfilled all the criteria for a diagnosis of acute ABMR proposed by the National Conference. The incidence of acute ABMR in LITx was 3.6%—significantly less than 15.2% in LETx (p <0.03). A detailed description of acute ABMR patients was given in our previous publication [21].
A preformed DSA was present in 44/154 (28.6%) cases. Fourteen (25.5%) LITx patients had a preformed DSA at time of transplantation: 11 (78.5%) to HLA class I only, 1 (7.2%) to HLA class II only and 2 (14.3%) to both HLA class I and class II. Similarly to LITx, 30 (30.3%) LETx patients had a preformed DSA: 17 (56.7%) to HLA class I only, 2 (6.7%) to HLA class II only and 11 (36.6%) to both HLA class I and class II (Table 2). There was no difference in the proportion of positive CDC-XM between LITx and LETx (27.6% vs 25.0%).
Post-operatively, resolution of a preformed DSA occurred in 71.4% (10/14) of LITx but only 33.3% (10/30) of LETx (p =0.01). A newly formed DSA was observed in 7/55 (12.9%) of LITx and 28/99 (28.3%) of LETx (p =0.03) (Table 2). In both groups, these de novo DSAs were predominantly against class II HLA both in 6/7 (85.7%) for LITx and in 25/28 (89.3%) for LETx. The average time to detect a de novo DSA was 16.7 ± 15.1 months for LITx versus 18.9 ± 15.3 month for LETx.
Chronic rejection
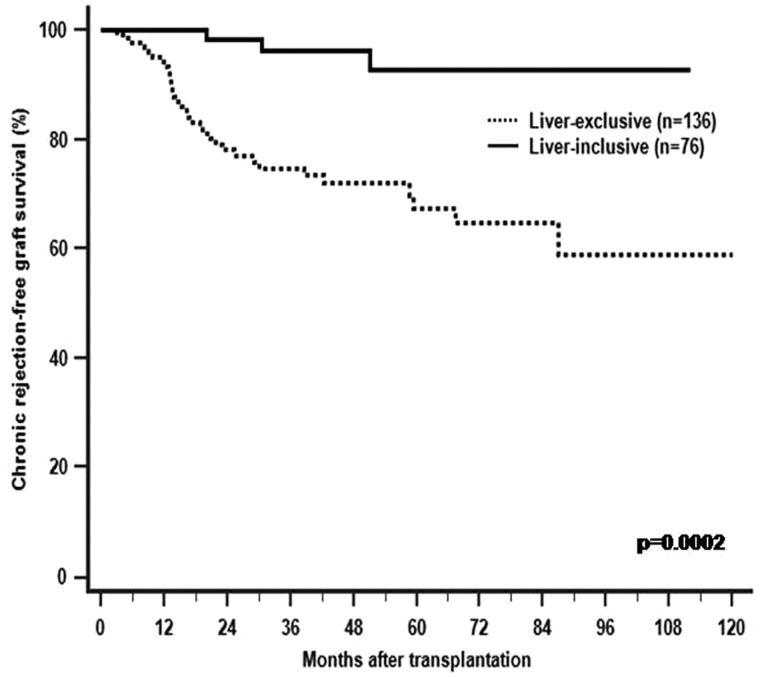
With a mean follow-up of 44.9 months, a total of 36 (16.9%) allografts developed pathology-confirmed chronic rejection. Of these, only 3/76 (3.9%) of LITx had chronic rejection (simultaneous liver and intestinal allograft chronic rejection in 2 and solitary intestinal chronic rejection in 1), which was significantly less frequent compared to 33 of 136 (24.3%) LETx (p =0.0002; Table 2). The time to develop chronic rejection for three LITx recipients was 16.1, 30.5 and 40.0 months, respectively. The average time to chronic rejection for LETx was 23.1 ± 19.5 months. Freedom from chronic rejection at 6 months, 1 year and 5 years was 95%, 90% and 30% for LITx and 60%, 50% and 30% for LETx, respectively (Figure 3).
Figure 3.
The Kaplan–Meier chronic rejection-free graft survival for liver-exclusive (dotted line) and liver-inclusive transplants (solid line). The incidence of chronic rejection is significantly lower after liver-inclusive transplantation.
The results of the univariate and multivariate analyses of risk factors associated with the development of chronic rejection are shown in Table 4. While a de novo DSA (OR 25.42, 95% CI: 7.72–89.73; p <0.0001), a higher number of acute rejection episodes (OR 1.93, 95% CI: 1.46–2.57; p =0.04) and a positive CMV donor (OR 2.72, 95% CI: 1.68–13.24; p =0.003) were independently associated with chronic rejection, simultaneous liver transplantation reduced this risk significantly (OR 0.21, 95% CI: 0.04–0.79; p =0.03).
Table 4.
Univariate and multivariate analysis of risk factors associated with chronic rejection
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Clinical characteristics | Odd ratio (95% CI) | p-value | Odd ratio (95% CI) | p-value |
| Recipient | ||||
| Age (years) | 1.08 (1.01–1.16) | 0.03 | 1.04 (0.99–1.09) | 0.09 |
| Male | 1.07 (0.13–8.63) | 0.95 | ||
| PRA HLA class I | 1.09 (0.96–1.03) | 0.93 | ||
| PRA HLA class II | 0.99 (0.97–1.02) | 0.78 | ||
| Positive cross-match | 0.74 (0.07–7.87) | 0.80 | ||
| Preformed DSA | 0.75 (0.07–7.63) | 0.81 | ||
| De novo DSA | 76.49 (8.83–97.55) | 0.0001 | 25.42 (7.72–89.73) | <0.0001 |
| Duration of TPN (mos) | 1.01 (0.99–1.02) | 0.22 | ||
| Number of surgery | 1.21 (0.71–1.98) | 0.75 | ||
| Rejection number ≤ 360 days | 3.21 (1.05–5.73) | 0.03 | 1.93 (1.46–2.57) | 0.04 |
| Donor | ||||
| Age (years) | 0.99 (0.91–1.08) | 0.88 | ||
| Male | 0.66 (0.19–2.26) | 0.51 | ||
| Cold-ischemic times, minutes | 1.47 (0.81–2.66) | 0.20 | ||
| Positive CMV | 5.87 (1.41–24.42) | 0.02 | 2.72 (1.68–13.24) | 0.003 |
| Liver allograft | 0.04 (0.00–0.53) | 0.014 | 0.21 (0.04–0.79) | 0.03 |
PRA, panel reactive antibody; HLA, human leukocyte antigen; DSA, donor-specific antibody; TPN, total parenteral nutrition; CMV, cytomegalovirus.
Discussion
In this retrospective study, we compared the incidence of immune-mediated injury, causes of intestinal graft loss and patient outcomes between LETx and LITx to ascertain the immunoprotective role of a co-transplanted liver. Our results showed that: (i) although the trend of overall patient survival was lower in LITx than LETx, death-censored intestinal graft survival after LITx is superior to LETx; (ii) rejection is the leading cause of graft loss in LETx, while infection is the leading cause of graft loss in LITx; (iii) the frequency and severity of ACR and acute ABMR are reduced after LITx; (iv) the incidence of chronic rejection is lower after LITx. Our results indicate that LITx appears to render the graft more tolerable to rejection, but increases the risks of post-transplant infections. Our findings emphasize that LITx has characteristic immunologic and clinical features. Accordingly, ITx recipients should be managed mainly based on either liver-inclusive or liver-exclusive transplants. Different degree of immunosuppression between LITx and LETx may be used to better control rejection and prevent post-transplant serious infections.
In the current larger series of patients, we show decreased frequency of ACR and lower incidences of moderate to severe ACR after LITx, supporting the immunoprotective effect of liver allografts on simultaneously transplanted intestine. Selvaggi et al. reported 209 ITx recipients and found an increased incidence of overall rejection and severity in recipients of the isolated intestinal and combined liver-intestinal allograft compared to the multivisceral allograft with a liver or without a liver [25]. The discrepancy between two observations may be related to the differences in the patient population and in the time span to study the frequency of rejection. In their study, pediatric patients accounted for 61%, which may contribute less frequency and severity of acute rejection after transplantation of a larger amount of lymphoid tissue. The relative immatured immune system in pediatric patients may be more “‘immunologically educable”’ at a central or peripheral level than the matured immune system in adults [25,26]. In addition, our study focused on ACR within the first year after ITx to make our two study groups more comparable. Several potential mechanisms have been proposed to explain the reduced cellular immune response in transplant recipients. It has been noted that the liver allograft harbors donor-derived hematopoietic cells, which migrate out of the liver graft at the time of graft implantation to establish a state of chimerism [27]. Persistent chimerism can lead to clonal depletion of host alloreactive T cells, which has been speculated as one of the major factors that help promote long-term graft survival or tolerance induction after transplantation [28,29]. Recent data indicate that chimerism is associated with a lower incidence of rejection after intestinal and multivisceral transplantation [30].
Our earlier study examined the risk factors for acute ABMR and showed that the presence of a liver component was associated with a lower risk of developing acute ABMR after ITx [21]. In this observation, we further compared the recipients between LITx and LETx to confirm a protective effect of liver allografts against humoral rejection. There were no significant differences between LETx and LITx in terms of the proportions of a positive CDC-XM, pre-existing DSA and the levels of PRA, indicating that these two groups might face a similar immunologic challenge pre transplantation. Given that a positive CDC-XM was not contraindication to ITx, similar immunosuppression was used while B-cell-targeted therapies were not used, this observation can better explain the unique immunoprotective effect of liver allografts on ABMR. It is also worth noting that there were two cases (2.6%) of acute ABMR after LITx, implying that the liver is not always protected. Caution should be taken when transplanting highly sensitized patients. Several potential mechanisms for the immunoprotective effect of the liver have been hypothesized to play a role. It has been speculated that the transplanted liver may promote the clearance of alloantibodies via absorption by either surface-bound or soluble alloantigens. Recent evidence by Taner et al. further showed that the immunoprotective effect of the liver involves more than simply absorbing donor-specific antibodies, with a shift in the pattern of gene expression in kidneys away from proinflammatory toward preservation of tissue integrity [31].
During average follow-up of 44 months, chronic rejection occurred as high as 16.9% of the recipients. Our results showed that the presence of co-transplanted liver allografts significantly reduced the incidence of chronic rejection. Several donor and recipient factors have been reported to be associated with the development of chronic rejection [32,33]. Based on our findings, a post-transplant de novo DSA was closely related to the development of chronic rejection. Several pieces of evidence suggest that the major target of HLA antibodies appears to be the graft endothelium, which can be activated and injured via a complement-dependent or complement-independent pathway [34–36]. The mechanisms by which HLA antibodies lead to chronic rejection are not well understood, and whether the presence of antibodies is an initiating event or merely a response to tissue damage remains to be defined. We also identified a higher frequency of ACR episodes as an independent risk factor for chronic rejection. It has been speculated that repeated immune-mediated damage exhausts the recipient’s natural repair mechanism, leading to chronic rejection. Lower incidence of chronic rejection in LITx may be a secondary effect of the immunoprotection conferred by the donor liver on the intestinal allograft.
Our data show that a significant reduction in rejection did not improve patient survival and that infection accounts for the majority of patient deaths after LITx. In this study, infections during the early period appear to be related to the high morbidity of intestinal recipients at the time of transplantation and surgical complications. The type, timeline and site of infections were consistent with previous report [37–40]. In our previous report, we showed that overall infection-caused graft failure was around 11% and was the second most common cause of graft loss. Recent data by Silva et al. showed that infection-related graft loss was the major cause of mortality after ITx. But they did not exam specific contributions of the liver to infection [40]. Our findings indicate that the LITx recipient is more vulnerable to infections than the LETx when exposed to a similar degree of immunosuppression. The LITx candidates may have more co-morbidities that can result in multiple admission, prolonged lengths of stay and many surgical procedures. Most of the LITx recipients underwent splenectomy at the time of transplantation, which may decrease the risk of humoral and cellular-mediated rejection but may increase the risk of infection. The liver is roughly 10 times larger than the heart or kidney, and its large transplanted tissue mass has been hypothesized to dilute cytokines and alloreactive T lymphocytes, leading to exhaustion of the recipient immune response. The higher co-morbidities, an asplenic state, the use of relatively heavy immunosuppressive agents, a heavy burden of alloantigens, etc. might make recipients’ immune systems over-suppressed, as reflected by de novo malignancy, GVHD and increased incidences of severe infections. Our findings indicate that lower immunosuppression may need to be considered for the patients who undergo LITx to attenuate increased risk of infection. Finding a delicate balance between over- and under-immunosuppression remains as a major challenge for the optimal management of ITX recipients.
Our study is a retrospective analysis at a single center and has several important limitations. Although the size of the cohort is relatively large given the low case volumes of intestinal transplant procedures performed worldwide, it has the limitations of a small sample size. Due to its retrospective nature, the patients in the two groups were not matched and higher co-morbidities of LITx recipients might exist at the time of transplantation. As the assessment of HLA antibodies was not routine in our program, more detailed analyses of the DSA including C1q-binding capacity were not performed. These will need to be included in future studies, to better understand the role of the liver in reducing antibody-mediated immune injury.
In summary, intestinal allografts with a liver component appear to decrease the risk of rejection but increase the risk of infection. Lower immunosuppression may need to be considered to address the increased risk of infection associated with LITx.
Acknowledgements
The authors would like to thank the surgical team and the nursing staff at the Intestinal Rehabilitation and Transplant Center, University of Pittsburgh Medical Center, for their excellent patient care. We thank Mr Yinglun Wu for his help in correcting English. Ethics committee approval was obtained from the University of Pittsburgh Medical Center.
Conflict of interest statement: none declared.
References
- 1. Grant D, Abu-Elmagd K, Mazariegos G. et al. Intestinal transplant registry report: global activity and trends. Am J Transplant 2015;15:210–19. [DOI] [PubMed] [Google Scholar]
- 2. Sudan D. The current state of intestine transplantation: indications, techniques, outcomes and challenges. Am J Transplant 2014;14:1976–84. [DOI] [PubMed] [Google Scholar]
- 3. Reyes JD. Intestinal transplantation: an unexpected journey. Robert E. Gross Lecture. J Pediatr Surg 2014;49:13–18. [DOI] [PubMed] [Google Scholar]
- 4. Loo L, Vrakas G, Reddy S. et al. Intestinal transplantation: a review. Curr Opin Gastroenterol 2017;33:203–11. [DOI] [PubMed] [Google Scholar]
- 5. Fishman JA. Infection in organ transplantation. Am J Transplant 2017;17:856–79. [DOI] [PubMed] [Google Scholar]
- 6. Bharadwai S, Tandon P, Gohel TD. et al. Current status of intestinal and multivisceral transplantation. Gastroenterol Rep (Oxf) 2017;5:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meier D, Rumbo M, Gondolesi GE.. Current status of allograft tolerance in intestinal transplantation. Int Rev Immunol 2014;33:245–60. [DOI] [PubMed] [Google Scholar]
- 8. Ruiz P. Updates on acute and chronic rejection in small bowel and multivisceral allografts. Curr Opin Organ Transplant 2014;19:293–302. [DOI] [PubMed] [Google Scholar]
- 9. Starzl TE, Zinkernagel RM.. Transplantation tolerance from a historical perspective. Nat Rev Immunol 2001;1:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dresske B, Lin X, Huang DS. et al. Spontaneous tolerance: experience with the rat liver transplant model. Hum Immunol 2002;63:853–61. [DOI] [PubMed] [Google Scholar]
- 11. McCaughan GW, Bowen DG, Bertolino P.. Operational tolerance in liver transplantation: shall we predict or promote? Liver Transplant 2013;19:933–6. [DOI] [PubMed] [Google Scholar]
- 12. Waki K, Sugawara Y, Mizuta K. et al. Predicting operational tolerance in pediatric living-donor liver transplantation by absence of HLA antibodies. Transplantation 2013;95:177. [DOI] [PubMed] [Google Scholar]
- 13. Wong TW, Gandhi MJ, Daly RC. et al. Liver allograft provides immunoprotection for the cardiac allograft in combined heart-liver transplantation. Am J Transplant 2016;16:3522–31. [DOI] [PubMed] [Google Scholar]
- 14. Simpson N, Cho YW, Cicciarelli JC. et al. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS Database. Transplantation 2006;82:1298–303. [DOI] [PubMed] [Google Scholar]
- 15. Olausson M, Mjornstedt L, Norden G. et al. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant 2007;7:130–6. [DOI] [PubMed] [Google Scholar]
- 16. Cheng EY, Busuttil RW.. Liver transplantation for pediatric hepatoblastoma and hepatocellular carcinoma: out of the unknown. JAMA Surg 2015;150:1158–9. [DOI] [PubMed] [Google Scholar]
- 17. Abu-Elmagd K, Reyes J, Bond G. et al. Clinical intestinal transplantation: a decade of experience at a single center. Ann Sug 2001;234:404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abu-Elmagd KM, Costa G, Bond GJ. et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg 2009;250:567–81. [DOI] [PubMed] [Google Scholar]
- 19. Abu-Elmagd KM. The small bowel contained allografts: existing and proposed nomenclature. Am J Transplant 2011;11:184–5. [DOI] [PubMed] [Google Scholar]
- 20. Mazariegos GV, Steffick DE, Horslen S. et al. Intestine transplantation in the United States, 1999–2008. Am J Transplant 2010;10:1020–34. [DOI] [PubMed] [Google Scholar]
- 21. Wu GS, Cruz RJ Jr., Cai JC.. Acute antibody-mediated rejection after intestinal transplantation. World J Transplant 2016;6:719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu T, Abu-Elmagd K, Bond G. et al. A schema for histologic grading of small intestine allograft acute rejection. Transplantation 2003;75:1241. [DOI] [PubMed] [Google Scholar]
- 23. Wu GS. Updates on antibody-mediated rejection in intestinal transplantation. World J Transplant 2016;6:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee RG, Nakamura K, Tsamandas AC. et al. Pathology of human intestinal transplantation. Gastroenterology 1996;110:1820–34. [DOI] [PubMed] [Google Scholar]
- 25. Selvaggi G, Gaynor JJ, Moon J. et al. Analysis of acute cellular rejection episodes in recipients of primary intestinal transplantation: a single center, 11-year experience. Am J Transplant 2007;7:1249–57. [DOI] [PubMed] [Google Scholar]
- 26. Fan X, Ang A, Pollock-Barziv SM. et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med 2004;10:1227–33. [DOI] [PubMed] [Google Scholar]
- 27. Verdonk RC, Haagsma EB, Jongsma T. et al. A prospective analysis of the natural course of donor chimerism including the natural killer cell fraction after liver transplantation. Transplantation 2011;92:e22. [DOI] [PubMed] [Google Scholar]
- 28. Okumi M, Fishbein JM, Griesemer AD. et al. Role of persistence of antigen and indirect recognition in the maintenance of tolerance to renal allografts. Transplantation 2008;85:270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carney EF. Transplantation: induction of chimerism and immune tolerance using belatacept. Nat Rev Nephrol 2015;11:66. [DOI] [PubMed] [Google Scholar]
- 30. Zuber J, Rosen S, Shonts B. et al. Macrochimerism in intestinal transplantation: association with lower rejection rates and multivisceral transplants, without GVHD. Am J Transplant 2015;15:2691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taner T, Park WD, Stegall MD.. Unique molecular changes in kidney allografts after simultaneous liver-kidney compared with solitary kidney transplantation. Kidney Int 2017;91:1193–202. [DOI] [PubMed] [Google Scholar]
- 32. Swanson BJ, Talmon GA, Wisecarver JW. et al. Histologic analysis of chronic rejection in small bowel transplantation: mucosal and vascular alterations. Transplantation 2013;95:378–82. [DOI] [PubMed] [Google Scholar]
- 33. Parizhskaya M, Redondo C, Demetris A. et al. Chronic rejection of small bowel grafts: pediatric and adult study of risk factors and morphologic progression. Pediatr Dev Pathol 2003;6:240–50. [DOI] [PubMed] [Google Scholar]
- 34. Torres IB, Salcedo M, Moreso F. et al. Comparing transplant glomerulopathy in the absence of C4d deposition and donor-specific antibodies to chronic antibody-mediated rejection. Clin Transplant 2014;28:1148–54. [DOI] [PubMed] [Google Scholar]
- 35. Touzot M, Couvrat-Desvergnes G, Castagnet S. et al. Differential modulation of donor-specific antibodies after B-cell depleting therapies to cure chronic antibody mediated rejection. Transplantation 2015;99:63–8. [DOI] [PubMed] [Google Scholar]
- 36. Jolly EC, Key T, Rasheed H. et al. Preformed donor HLA-DP-specific antibodies mediate acute and chronic antibody-mediated rejection following renal transplantation. Am J Transplant 2012;12:2845–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Primeggia J, Matsumoto CS, Fishbein TM. et al. Infection among adult small bowel and multivisceral transplant recipients in the 30-day postoperative period. Transpl Infect Dis 2013;15:441–8. [DOI] [PubMed] [Google Scholar]
- 38. Oltean M, Herlenius G, Gabel M. et al. Infectious complications after multivisceral transplantation in adults. Transplant Proc 2006;38:2683–5. [DOI] [PubMed] [Google Scholar]
- 39. Guaraldi G, Cocchi S, Codeluppi M. et al. Outcome, incidence, and timing of infectious complications in small bowel and multivisceral organ transplantation patients. Transplantation 2005;80:1742–8. [DOI] [PubMed] [Google Scholar]
- 40. Silva JT, San-Juan R, Fernandez-Caamano B. et al. Infectious complications following small bowel transplantation. Am J Transplant 2016;16:951–9. [DOI] [PubMed] [Google Scholar]