Abstract
Background
Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) has become the preferred method to diagnose pancreatic masses due to its minimally invasive approach and diagnostic accuracy. Many studies have shown that rapid on-site evaluation (ROSE) improves diagnostic yield by 10–30%; however, more recent studies have demonstrated effective diagnostic accuracy rates without ROSE. Our study aims to examine whether the current standard of performing ROSE after each FNA pass adds diagnostic value during EUS-guided FNA of pancreatic masses.
Methods
We conducted a retrospective case series on patients who underwent EUS-guided FNA of pancreatic masses between February 2011 and October 2014. All cases were performed by one of three endoscopists at Emory University Hospital. Patient demographics, radiologic details of pancreatic masses and pathology reports of the biopsied pancreatic masses were examined.
Results
A total of 184 procedures performed in 171 patients were reviewed. The final pathology reports of the biopsied pancreatic masses showed 128 (70%) with confirmed malignancy. Only 64 (50%) of these 128 cases initially showed malignant cells during ROSE. Among these 64 cases, 23% required 5 or more FNA passes to first detect malignant cells.
Conclusions
The use of ROSE during EUS-guided FNA of pancreatic masses may increase the diagnostic yield, since malignant cells were often detected during later FNA passes that would otherwise be missed if tissue sampling stopped prematurely. In addition, sample preparation for ROSE may be suboptimal, since malignant cells were only detected in 50% of cases.
Keywords: endoscopic ultrasound, fine needle aspiration, rapid on-site evaluation, pancreatic mass
Introduction
Pancreatic cancer remains the eighth and ninth leading causes of death in men and women, respectively, in the USA [1]. Pancreatic ductal adenocarcinoma constitutes 85% of all pancreatic cancer, making it the most common type [2]. Many diagnostic modalities can be used to detect pancreatic cancer, including cross-sectional imaging such as computed tomography (CT) and magnetic resonance imaging (MRI). However, endoscopic ultrasound (EUS) has proven to be one of the most effective diagnostic methods for detecting pancreatic masses [3]. EUS has been shown to have diagnostic rates higher than 90%, with a sensitivity of 99% for masses less than 3 cm in size [4].
EUS-guided fine needle aspiration (FNA) has become the preferred method for diagnosing pancreatic cancer due to its minimally invasive approach and its effective diagnostic yield [5]. Rapid on-site evaluation (ROSE) by a cytopathologist of tissue samples obtained after each FNA pass to check for malignant cells in real time has been shown to improve diagnostic yield by 10–30% [6–10] and reduce the number of FNA passes required to make a diagnosis [11]. However, more recent studies have demonstrated effective diagnostic accuracy rates over 90% without ROSE [12,13]. Overall, data on the efficacy of ROSE are limited. Our study aims to examine how much diagnostic value the current standard of performing ROSE after each FNA pass adds during EUS-guided FNA of pancreatic masses.
Methods
This study was approved by the Institutional Review Board at the Emory University School of Medicine. We present a retrospective case series on consecutive patients who underwent EUS-guided FNA of pancreatic masses between February 2011 and October 2014. The software Endoworks (Olympus, Tokyo, Japan) was used to generate a list of all patients who underwent EUS of the pancreas in the aforementioned time frame. All patients who had EUS without FNA were excluded. All patients who underwent FNA of non-pancreatic masses and pancreatic cysts were also excluded. In addition, patients who had FNA of pancreatic masses without ROSE were excluded, which occurred when the cytopathologist was not available. Enrolled patients’ demographics, radiologic details of pancreatic masses and procedure details were retrospectively examined.
All procedures were performed by one of three endoscopists (Q.C., F.W. and K.W.) at Emory University Hospital. The curvilinear array echoendoscope (GF-UC140P, Olympus America, Center Valley, PA) was used in all cases. The procedures were performed under moderate sedation in the Endoscopy Unit. After detecting a lesion, its location, dimensions and echogenicity were noted. A 22-gauge needle (EUS N-1, Cook Medical, Winston Salem, NC) was then used to perform FNA. A stylet was used at the discretion of the endoscopist and continuous suction was applied during each FNA pass. During each procedure, the endoscopist continued tissue sampling of the pancreatic mass by FNA until malignant cells were seen on ROSE. If no malignant cells were detected in real time, the procedure was terminated after between six and eight FNA passes.
In all the examined cases, ROSE was performed by an on-site cytopathologist. After each FNA pass, the first drop of the sample was extracted onto a glass slide using an air-filled syringe. The rest of the sample was rinsed into 10% buffered formalin for cell block. The glass slides were air dried and stained with modified Giemsa for immediate on-site evaluation. A comprehensive pathology evaluation was later performed on the pooled tissue obtained during the entire procedure. Evaluation of the cell block and ROSE were performed by the same cytopathologist.
Cytopathology reports of ROSE performed during each case were reviewed and compared to the final pathology reports of the pooled cell block of biopsied pancreatic masses. We specifically examined the proportion of cases in which malignant cells were detected during ROSE among the cases with confirmed malignancy on final pathology reports. We also analysed on which FNA pass malignant cells were first detected during ROSE.
Results
A total of 475 patients underwent EUS of the pancreas between February 2011 and October 2014. Among these patients, 285 patients were excluded because no FNA was performed or because the patient underwent FNA of a pancreatic cyst or a pancreatic mass without ROSE. An additional 19 patients were excluded because they underwent FNA of non-pancreatic masses; generally, these were peripancreatic lymph nodes or liver lesions. Finally, a total of 184 procedures performed in 171 patients were included for further analysis (Figure 1). Of the cases reviewed, 85 (50%) were male, the average long-axis length of the pancreatic masses biopsied was 31 mm (range 8–70 mm) and the average procedure time was 50 minutes (range 21–85 minutes) (Table 1).
Figure 1.
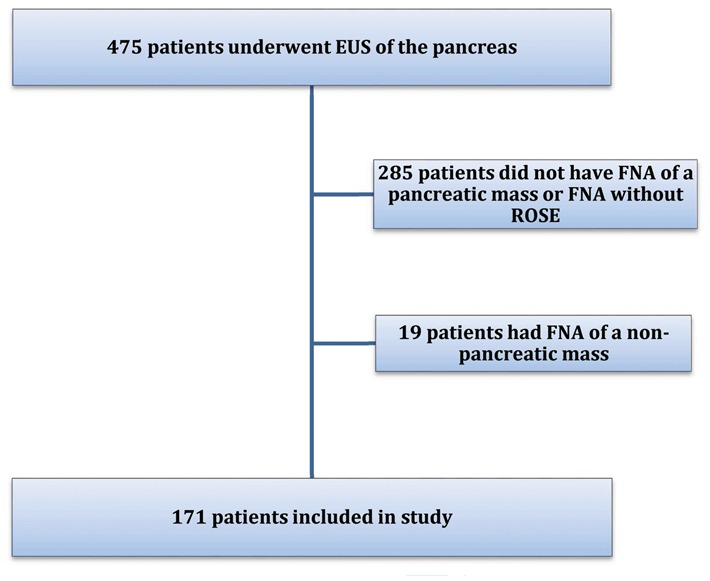
Summary of patients included in this study.
Table 1.
Characteristics of patients undergoing EUS-guided FNA of pancreatic masses
| Total procedures | 184 |
| Total patients | 171 |
| Male, n (%) | 85 (50%) |
| Average age, years | 64 |
| Average long-axis length of pancreatic massa, mm | 31 (range 8–70) |
| Average short axis length of pancreatic massb, mm | 26 (range 7–60) |
| Average procedure time, min | 50 (range 21–85) |
aLong-axis measurement not reported in five cases.
bShort-axis measurement not reported in 42 cases.
Of the 184 included procedures, final pathology reports of the biopsied pancreatic masses showed 128 (70%) with confirmed malignancy, 12 (6%) with atypical cells, 24 (13%) with no malignancy and 20 (11%) indeterminate cases. Only 64 (50%) of the 128 cases with confirmed malignancy initially showed malignant cells during ROSE. In the remaining cases, 17 (13%) showed atypical cells and 47 (37%) did not show malignant cells during ROSE (Figure 2). Of the 12 cases that showed atypical cells on cell bock, 8 were later confirmed to be malignant either through repeat EUS-guided FNA or through CT guided biopsy of the pancreatic mass. Of the 20 indeterminate cases on cell block, only 2 were later found to be malignant. Only one case showed malignant cells during ROSE that was later confirmed to be non-malignant on cell block evaluation from the same procedure.
Figure 2.
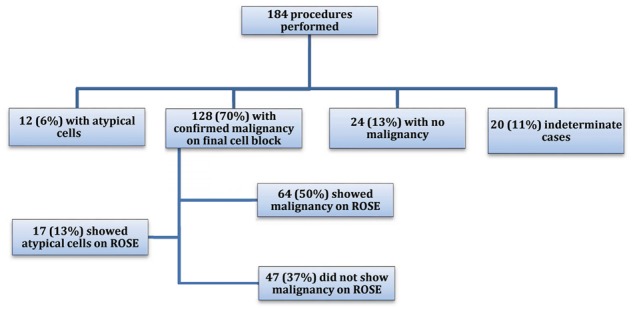
Summary of findings of during EUS-guided FNA of pancreatic masses, both on pathology reports and during ROSE.
Of the 184 total procedures examined, 3 or fewer FNA passes were performed in 65 cases (35%), with the remainder of cases undergoing 4 or more FNA passes. There was no notable difference in the size or location of the pancreatic mass between the group of patients that received three or fewer FNA passes and the group that underwent four or more FNA passes (both p > 0.05). On average, 3.5 FNA passes (range 1–6 passes) were required to confirm malignant cells during ROSE. Among the 64 cases where malignant cells were first detected during ROSE, only 11% showed malignant cells on the first FNA pass. The majority of malignant cells were first seen on ROSE during the second, third and fourth passes (66%), while 23% of cases first showed malignant cells during the fifth, sixth and seventh passes (Figure 3).
Figure 3.
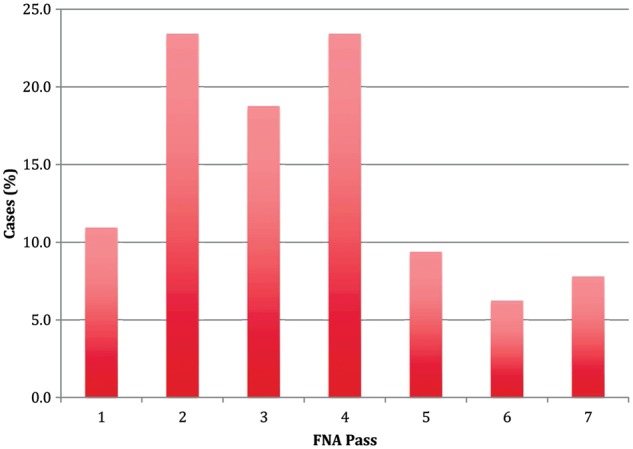
This figure indicates on which FNA pass attempt malignant cells were first seen during rapid on-site evaluation (n=64).
Discussion
Many studies have investigated the efficacy of ROSE during EUS-guided FNA of pancreatic masses with mixed results [6–13]. To our knowledge, this study is the first to specifically examine how often malignant cells are detected during ROSE on each FNA pass. Based on our results, we can conclude that ROSE on its own is not a reliable method to detect malignancy during EUS-guided FNA of pancreatic masses, since only about 50% of our cases with confirmed malignancy initially detected malignant cells during ROSE. The remaining 50% were diagnosed with malignancy during examination of the pooled cell block.
The reason for ROSE’s low efficacy remains unclear. One potential explanation could be that tissue samples are inadequately prepared for ROSE, leading to decreased detection of malignant cells. The current method involves performing ROSE on a smear of the first drop of sample from each FNA pass. Preparing tissue in this way may lead to a paucity of cells in the sample and a poor diagnostic yield. Perhaps if a smear of mixed tissue from each FNA pass were used for ROSE instead of the first drop alone, the diagnostic yield of ROSE would increase.
Despite the relatively low diagnostic yield of ROSE on its own, it does appear to add diagnostic value during EUS-guided FNA of pancreatic masses. About one-quarter of our cases first showed malignant cells during the fifth through seventh passes. If tissue sampling of pancreatic masses were to stop after five passes, a significant number of malignant diagnoses could be missed. However, during the cases where malignant cells were first detected after the fifth FNA pass, it is possible that adequate tissue was initially obtained during earlier passes and malignant cells were simply not detected during ROSE due to inadequate sample preparation, as discussed above. While it is difficult to interpret whether not detecting malignant cells during ROSE accurately predicts inadequate tissue sampling, ROSE may still increase the diagnostic yield by encouraging additional FNA passes during EUS-guided FNA of pancreatic masses.
Our study does have some limitations, primarily that the study was retrospective with a relatively small sample size. In addition, it is difficult to interpret whether the efficacy of ROSE on its own was low due to sample preparation or due to other unknown factors. It is also unclear whether ROSE truly increases the overall diagnostic yield of EUS-guided FNA of pancreatic masses by confirming whether an adequate tissue sample was obtained. It is possible that performing a higher number of FNA passes increases the diagnostic yield simply because more tissue is obtained for final pathology evaluation, regardless of whether ROSE is performed. Recent studies also suggest that there is no difference in diagnostic yield of malignancy during EUS-guided FNA of pancreatic masses with or without ROSE [13]. Further prospective studies are needed to investigate ideal sample preparation to maximize the diagnostic yield of ROSE, and also to determine the optimal number of FNA passes to perform during this procedure. For instance, a study could be designed where the samples from each FNA pass undergo ROSE and are also placed in separate cell blocks. The results of ROSE and cell block evaluation from each FNA pass could then be compared. However, this study design would be very cumbersome for the cytopathologist.
Of note, new technologies such as EUS-guided core biopsy appear to have comparable pathologic diagnostic yield to EUS-guided FNA with fewer passes required [14], possibly due to the greater tissue sample obtained with a larger-gauge needle. However, ROSE did significantly improve the cytologic diagnostic yield of EUS-guided core biopsy compared to when ROSE was not used, and this procedure was also associated with a higher adverse event rate then EUS-guided FNA [14]. However, ROSE may have more positive outcomes when EUS-guided FNA is performed by less experienced endoscopists. The value of ROSE appears to diminish with increased procedure proficiency, perhaps due to the development of an improved sampling technique after performing a large number of cases.
In summary, the use of ROSE during EUS-guided FNA of pancreatic masses does appear to increase the diagnostic yield by encouraging additional FNA passes when malignant cells are not initially detected. Current methods in sample preparation for ROSE may also need to change to optimize the detection of atypical cells during this procedure.
Conflict of interest statement: none declared.
References
- 1. Siegel R, Ma J, Zou Z. et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2. Ryan DP, Hong TS, Bardeesy N.. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039–49. [DOI] [PubMed] [Google Scholar]
- 3. Larino Noia J. Pancreatic tumors: recent developments. Gastroenterol Hepatol 2014;37 Suppl 3:98–106. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalo-Marin J, Vila JJ, Perez-Miranda M.. Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. World J Gastrointest Oncol 2014;6:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hewitt MJ, McPhail MJ, Possamai L. et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastroint Endosc 2012;75:319–331. [DOI] [PubMed] [Google Scholar]
- 6. Iglesias-Garcia J, Larino-Noia J, Abdulkader I. et al. Rapid on-site evaluation of endoscopic ultrasound guided fine needle aspiration diagnosis of pancreatic masses. World J Gastroenterol 2014;20:9451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hebert-Magee S, Bae S, Varadarajulu S. et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology 2013;24:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I. et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol 2011;106:1705–10. [DOI] [PubMed] [Google Scholar]
- 9. Klapman JB, Logrono R, Dye CE. et al. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol 2003;98:1289–94. [DOI] [PubMed] [Google Scholar]
- 10. Alsohaibani F, Girgis S, Sandha GS.. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound-guided needle aspiration biopsy? Can J Gastroenterol 2009;23:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt RL, Walker BS, Howard K. et al. Rapid on-site evaluation reduces needle passes in endoscopic ultrasound-guided fine-needle apiration for solid pancreatic lesions: a risk-benefit analysis. Dig Dis Sci 2013;58:3280–6. [DOI] [PubMed] [Google Scholar]
- 12. O’Connor K, Cheriyan DG, Li-Chang HH. et al. Gastrointestinal endoscopic ultrasound-guided fine-needle aspiration biopsy specimens: adequate diagnostic yield and accuracy can be achieved without on-site evaluation. Acta Cytol 2015;59:305–10. [DOI] [PubMed] [Google Scholar]
- 13. Wani S, Mullady D, Early DS. et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol 2015;110:1429–39. [DOI] [PubMed] [Google Scholar]
- 14. DiMaio CJ, Kolb JM, Benias PC. et al. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): results of a large North American multicenter study. Endosc Int Open 2016;4; E974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


