Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder characterized by progressive muscular dystrophy and paralysis; most ALS patients die from respiratory failure within 3 to 5 years, and there is currently no effective treatment. Some studies have indicated sex differences in the incidence of ALS, and evidence suggests a neuroprotective role for estrogen.
Material/Methods
We used human Cu/Zn superoxide dismutase (hSOD1-G93A) transgenic mice to determine the effects of ovariotomy on the onset of disease and behavior; we also used Western blotting to measure the expression of aromatase and estrogen receptors, as well as the inflammatory cytokines and apoptosis markers, in the lumbar spinal cord to determine the mechanism of estrogen-mediated neuroprotection.
Results
Ovariectomy advanced the onset of disease, down-regulated aromatase and estrogen receptor alpha (ER-α) expression, and inhibited expression of the anti-inflammatory factors arginase-1 and the anti-apoptotic factor B-cell lymphoma-2 (Bcl-2) in the lumbar spinal cord of hSOD1-G93A transgenic mice.
Conclusions
Ovariectomy resulted in earlier disease onset and attenuated the anti-inflammatory and anti-apoptotic actions of estrogen in hSOD1-G93A transgenic mice. Therefore, estrogen may play an important role in protecting spinal cord motor neurons.
MeSH Keywords: Mice, Transgenic; Neurodegenerative Diseases; Ovariectomy
Background
Amyotrophic lateral sclerosis (ALS) is a destructive neurodegenerative disorder characterized by progressive muscular dystrophy and paralysis caused by the progressive and selective loss of motor neurons in the cerebral cortex, brain stem, and spinal cord; most ALS patients die of respiratory failure within 3 to 5 years. Unfortunately, ALS has no cure, and its exact pathogenesis remains unclear [1]. Approximately 2% of ALS cases are attributed to a mutation in the Cu/Zn superoxide dismutase (SOD1) gene [2], and transgenic mice carrying the human SOD-G93A (hSOD-G93A) mutation gene display similar clinical and histopathological features as ALS patients [3]; consequently, hSOD1-G93A transgenic mice are commonly used as an animal model to study ALS pathogenesis and treatment.
Some studies have identified potential sex differences in the incidence of ALS [4–6]. These sex differences prompted us to consider the role of estrogen in ALS. Indeed, increasing evidence supports a neuroprotective role for estrogen in a variety of neurodegenerative disease models, including ALS [7–10]. In the central nervous system (CNS), circulating estrogen derived from the ovaries, as well as local estrogen synthesized via aromatase, may exhibit neuroprotective effects, which include increasing the survival of motor neurons [9] and increasing neurotrophic factors [11,12]. Classically, neuroprotective female sex hormones activate 2 nuclear receptors: estrogen receptor alpha (ER-α) and estrogen receptor-beta (ER-β) [13,14]. In addition, G protein-coupled receptor 30 (GPR30), a novel membrane-bound G-protein-coupled receptor expressed in the CNS [15,16], has been found to show a high affinity for estrogen. Therefore, GPR30 may participate in the neuroprotective mechanism of estrogen [17,18].
Although numerous studies have investigated the effects of ovariectomy in hSOD1-G93A transgenic mice, there are only a few reports that describe the effects on the age of disease onset [7,8]. The literature also contains few reports describing the mechanisms underlying the effects of ovariectomy in this ALS animal model. Therefore, in the present study, we determined the effects of ovariotomy on the onset of disease and behavior in hSOD1-G93A transgenic mice; we also used Western blotting to measure the expression of aromatase and estrogen receptors, as well as the inflammatory cytokines and apoptosis markers, in the lumbar spinal cord to determine the mechanism of estrogen-mediated neuroprotection.
Material and Methods
Animal model
Female hSOD1-G93A transgenic mice, the offspring of a transgenic male and a B6/SJL F1 female, were bred in a temperature-controlled room with a 12: 12 h light/dark schedule. The mice received sterilized specific pathogen-free (SPF) rodent food and sterile water. All animal experiments were performed in accordance with the Laboratory Animal Management Guidelines established by the Ministry of Science and Technology of the People’s Republic of China, as well as the internationally recognized guidelines issued by the National Institutes of Health.
Identification of hSOD1-G93A transgenic mice
The ends of the tails from approximately 30-day-old offspring were cut and placed in sterile centrifuge tubes containing 150 μl of 50 mmol/l NaOH in a 95°C water bath for 30 min. Then, 12.5 μl of 1 mol/l Tris-HCl (pH 8.0) was added to each tube, followed by centrifugation for 2 min; the supernatant contained the genomic DNA. Next, polymerase chain reaction (PCR) assays were performed to identify hSOD1-G93A gene expression. Specific primers for the hSOD1-G93A gene were used in the PCR amplification (forward: 5′-CAT CAG CCC TAA TCC ATC TGA-3′; reverse: 5′-CGC GAC TAA CAA TCA AAG TGA-3′). The PCR amplification conditions were as follows: initial denaturation at 95°C for 3 min; 35 cycles of denaturation at 95°C for 45 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s; and a final extension at 72°C for 5 min. The PCR amplification products were separated on a 1.5% agarose gel at 80 V for 45 min, and the gel was photographed on a GBOX-HR fully automated gel imaging system. Mice with bands at 200–300 bp (236 bp) were identified as hSOD1-G93A-positive transgenic mice; mice without these bands were identified as non-hSOD1-G93A transgenic mice.
Experimental groups
We randomly divided 45-week-old hSOD1-G93A mice into 3 experimental groups (n ≥12): the ovariectomized group (OVX), the sham operation group (Sham), and the positive control group (NO-OVX). The negative control group (CON) included non-transgenic mice that were the same age as the experimental mice. These mice were used in behavioral studies.
Similarly, another 9 hSOD1-G93A mice of the same age were divided into 3 groups (OVX, Sham, and NO-OVX) of 3 mice per group. These and another 3 non-transgenic mice were used for Western blotting.
Ovariectomy and sham operation
At 5 weeks of age, the mice in the OVX group were anesthetized using 10% chloral hydrate, and a longitudinal incision was made 0.5 cm above where the upper border line of the 2 hind limbs connect with the back. The skin incision was pulled approximately 0.5 cm horizontally to the left or the right; then, we were able to visualize 2 soft, white, shiny fat masses next to the lower pole of the kidneys. The left and right ovaries of the mouse could be found in the respective fat masses, and they were bi-laterally ovariectomized. After the skin incision was sutured, the mice were placed in a heated chamber to recover.
Mice in the Sham group underwent a similar procedure as the OVX group, except the ovaries were left intact and only some adipose tissue near the ovaries was removed.
Determination of symptom onset of mice
Mice were assessed using Vercelli’s score of 1–5 as the reference standard [19]: a score of 4, which indicated that hind limb tremors appeared when the tail was suspended, was defined as the time of onset.
Behavior assessment
Motor function was evaluated by assessing changes in the weights and step lengths of the mice, as well as their performance on the rotarod test and hanging-wire test.
Changes in animal weight were measured twice a week from the eighth week and 3 times a week from the eleventh week to the end-point.
Mouse footprints were collected once a week from the ninth week. The hind feet of the mice were dyed with different colored ink. Mice were placed at one end of the groove and driven forward toward the other end. Footprints from 4 continuous runs of each mouse were collected and the average of the values of 3 separate distances was taken on each side. Then, the average of the 2 sides was recorded.
Rotarod tests were administered starting from the ninth week. The mice were trained for 5 days to adapt them to the experiment, and measurements were then conducted once per week. The rollers started at 1 revolution per minute (rpm) and accelerated to 15 rpm over 3 min. The time that the mouse continued to move on the roller was measured (counted in seconds), and the longest duration was recorded.
The hanging-wire test was used to assess the muscle strength of the mice 1 day after the rotarod test. The mice were suspended upside down from the cage cover, with the bottom of the cage covered with a soft cushion. Three trials were administered for each animal, and the longest latency until falling was analyzed.
Western blotting
The lumbar spinal cords of the mice were dissected at disease onset and frozen at −80°C. Total protein was then extracted using a protein extraction kit (Beijing Applygen Technologies Inc., P1250). Forty micrograms of protein from each sample was denatured and separated via 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Then, the proteins were transferred to PVDF membranes (Millipore, Billerica, MA, USA) at 100 V for 120 min. Afterwards, the membranes were blocked for 1 h with 5% non-fat milk and incubated overnight with primary antibodies at 4°C. The next day, the membranes were incubated for 1 h with fluorescence-conjugated secondary antibodies at room temperature. Then, the bands of interest on the membrane were detected using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA).
The following primary antibodies were used: anti-aromatase (1: 500, Abcam, ab18995), anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1: 5000; Bioworld, AP0063), anti-ER-α (1: 200; Abcam, ab75635), anti-ER-β (1: 500; Abcam, ab3576), anti-GPR30 (1: 250; Abcam, ab39742), anti-β-actin (1: 1000; Santa Cruz, SC-47778), anti-arginase-1 (1: 500, Santa Cruz, SC-18355), anti- transforming growth factor-beta1 (TGF-β1, 1: 500, Proteintech, 18978-1-AP), anti-tumor necrosis factor-alpha (TNF-α, 1: 500, Proteintech, 60291-1-lg), anti- B-cell lymphoma-2 (Bcl-2, 1: 500; Immunoway, YT0470), and anti- Bcl-2-associated X protein (Bax, 1: 500; Immunoway, YT0459).
Statistical analysis
Onset age was calculated using the Kaplan-Meier method. The behavioral assessments were analyzed by two-way analysis of variance (ANOVA), and other statistical analyses were performed using one-way ANOVA followed by least significant difference (LSD) tests with SPSS 21.0 statistical software. All values are expressed as the mean ± standard deviation (SD). Differences were considered statistically significant when P<0.05.
Results
Ovariectomy accelerates disease onset in hSOD1-G93A transgenic mice compared with NO-OVX mice
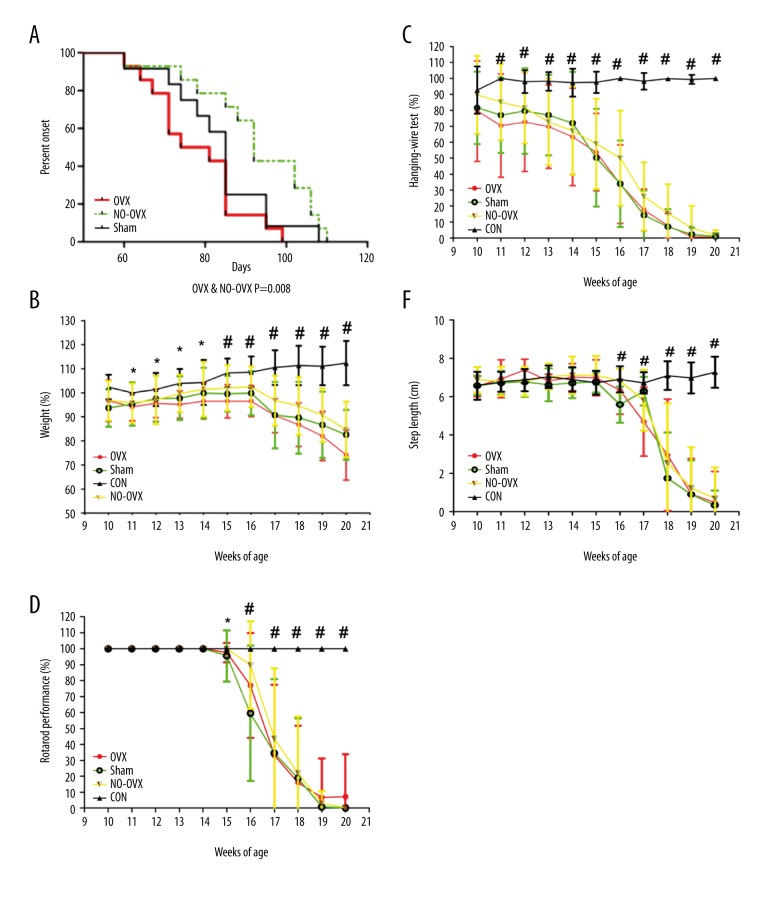
The onset time for the OVX group was 78.07±3.091 days, which was 14 days earlier than that for the NO-OVX group (92.50±3.907 days, P=0.008). The onset times were 83.50±3.598 days for the Sham group, which was not significantly different from either the NO-OVX group or the OVX group (Table 1, Figure 1A).
Table 1.
Age at disease onset in hSOD1-G93A transgenic mice.
| Group | OVX | Sham | NO-OVX |
|---|---|---|---|
| N | 14 | 12 | 14 |
| Onset (days ±SD) | 78.07±3.091** | 83.50±3.598 | 92.50±3.907 |
P<0.01 OVX vs. NO-OVX.
Figure 1.
Ovariectomy accelerates disease onset in hSOD1-G93A transgenic mice compared with NO-OVX mice (A). Ovariectomy increased weight loss and decreased rotarod test time but did not alter other motor functions in hSOD1-G93A mice. (B) Weights of hSOD1-G93A mice. (C) Motor function assessment using the hanging-wire test. (D) Motor function assessment using the rotarod test. (E) Motor function assessment using the step length. * P<0.05, OVX vs. CON. # P<0.05, transgenic groups vs. CON.
Behavior
Significant weight loss relative to the CON group was observed 4 weeks earlier in the OVX group than in the other positive groups (P<0.05). From the eleventh week to the fourteenth week, the weights of only the mice in the OVX group decreased significantly compared with those of mice in the CON group. However, at the fifteenth week, the weights of mice in all 3 positive groups (OVX, Sham, and NO-OVX groups) were significantly different compared with those of mice in the CON group (Figure 1B).
From the eleventh week to the twentieth week, the results of the hanging-wire test for the 3 positive groups were significantly different compared with those for the CON group (Figure 1C).
A significantly decreased rotarod test time relative to the CON group was observed 1 week earlier in the OVX group than in the other positive groups (P<0.05). On the fifteenth week, the rotarod test time of the OVX group differed significantly from that of the CON group. From the sixteenth to the twentieth week, the rotarod test times of all 3 positive groups were significantly different compared with that of the CON group (Figure 1D).
From the sixteenth week to the twentieth week, the step lengths of the 3 positive groups were significantly different compared with those of the CON group (Figure 1E).
Ovariectomy reduced aromatase expression in the lumbar spinal cord
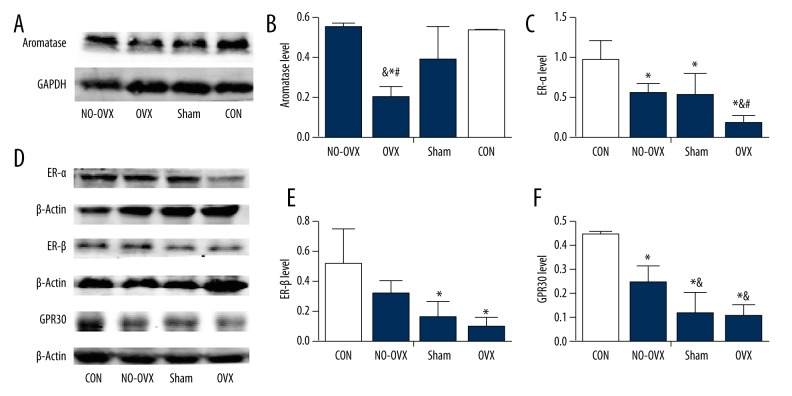
Western blotting revealed that ovariectomy reduced the expression of aromatase in the lumbar spinal cord and that the level of aromatase in the OVX group was the lowest among all the groups (F=8.853, P=0.006, Figure 2A, 2B).
Figure 2.
Aromatase and estrogen receptors levels in the lumbar spinal cord at the onset stage. Aromatase level (A, B). ER-α level (C, D). ER-β level (D, E). GPR30 level (D, F). * P<0.05 vs. CON. & P<0.05 vs. NO-OVX. # P<0.05 vs. Sham.
In the lumbar spinal cord, ovariectomy decreased ER-α expression but not ER-β expression, while both ovariectomy and the sham operation decreased GPR30 expression
There were obvious differences in the levels of ER-α in the 4 experimental groups (F=8.742, P=0.002; Figure 2C, 2D). ER-α levels in the OVX group were the lowest among all 4 groups (P<0.05); ER-α levels were lower in the NO-OVX group and the Sham group than in the CON group (P<0.05), but did not differ significantly from each other (P>0.05).
The largest difference of ER-β protein levels was between the OVX and CON groups (Figure 2D, 2E); however, no difference was detected between the OVX and NO-OVX groups (P>0.05).
Western blotting indicated that GPR30 protein expression in the OVX group was significantly lower than that in the CON and NO-OVX groups (P<0.05, Figure 2D, 2F), but we found no significant difference between the OVX and Sham groups (P>0.05).
Ovariectomy decreased the expression of the anti-inflammatory factors arginase-1
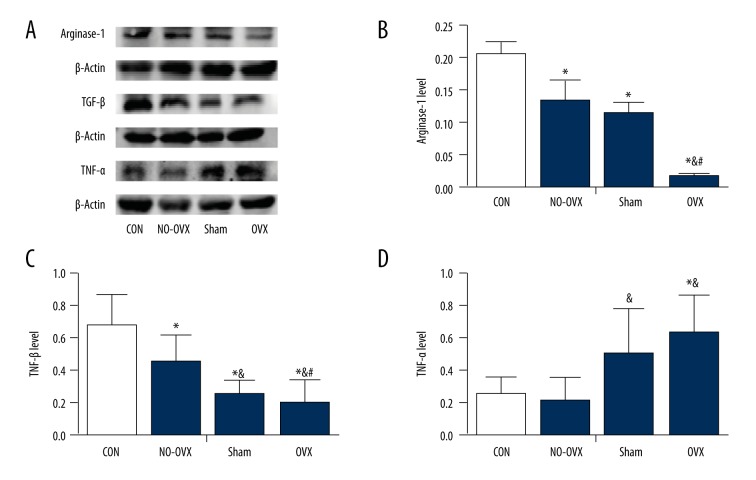
Western blotting revealed significant differences in arginase-1 levels among the 4 groups (F=36.141, P=0.000, Figure 3A, 3B), with arginase-1 levels in the OVX group being the lowest among all other groups (P<0.05). Arginase-1 levels in the NO-OVX and Sham groups were also lower than those in the CON group (P<0.05); however, arginase-1 levels were not significantly different between these 2 transgenic groups (P>0.05). TGF-β expression in all experiment groups was similar to that of arginase-1 (Figure 3A, 3C), although we found no significant changes between the OVX group and Sham group. TNF-α levels were significantly up-regulated in the OVX group (Figure 3A, 3D) compared with the CON and NO-OVX groups (P<0.05) but were not different compared with the Sham group (P>0.05).
Figure 3.
The effect of ovariectomy on the inflammatory factors in the lumbar spinal cord at the onset stage. The expression of arginase-1 (A, B). The expression of TGF-β (A, C). The expression of TNF-α (A, D). * P<0.05 vs. CON. & P<0.05 vs. NO-OVX. # P<0.05 vs. Sham.
Ovariectomy promoted apoptosis by down-regulating the expression of the anti-apoptotic protein Bcl-2
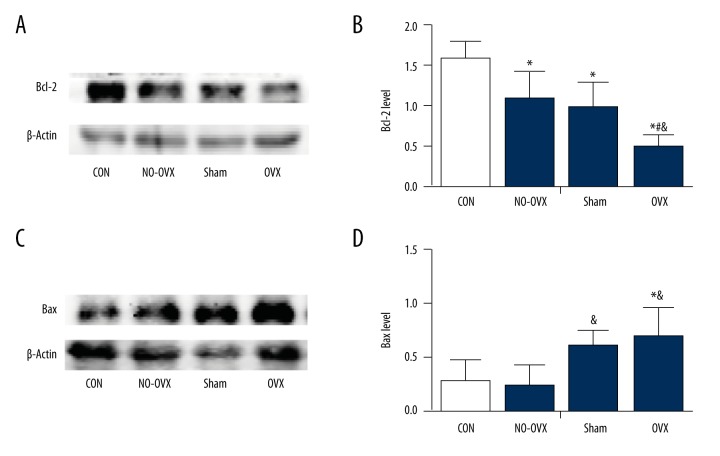
Bcl-2 expression was the lowest in the OVX group (F=9.799, P=0.002; P=0.000 vs. CON; P=0.011 vs. NO-OVX; P=0.032 vs. Sham, Figure 4A, 4B). Furthermore, Bcl-2 levels in the NO-OVX and Sham groups were lower than those in the CON group (P<0.05), but no significant differences were detected between the NO-OVX and Sham groups (P>0.05). In contrast, Bax expression was higher in the OVX group than that in the CON or NO-OVX group (P<0.05, Figure 4C, 4D).
Figure 4.
The effect of ovariectomy on the anti-apoptotic protein Bcl-2 (A, B) and the pro-apoptotic protein Bax expression (C, D). * P<0.05 vs. CON. & P<0.05 vs. NO-OVX. # P<0.05 vs. Sham.
Discussion
In the present study, significant weight loss and decreased rotarod test times relative to the CON group were observed 4 weeks earlier and 1 week earlier, respectively, in the OVX group compared with the other hSOD1-G93A groups. However, the hanging-wire test and the step length test showed no significant differences among the 3 positive groups. Therefore, weight changes and rotarod test results are more sensitive for evaluating the behavior of hSOD1-G93A transgenic mice when assessing the effect of ovariectomy.
As stated earlier, circulating estrogen and local estrogen both exert neuroprotective effects. In the present study, ovariectomy accelerated ALS onset in hSOD1-G93A transgenic mice; this earlier onset was accompanied by weight loss 4 weeks earlier and decreased rotarod test times 1 week earlier than observed in non-ovariectomized mice. Therefore, we inferred that the decrease in circulating estrogen levels after ovariectomy likely plays a neuroprotective role in this ALS animal model.
Aromatase catalyzes the rate-limiting enzyme in estrogen synthesis and is widely expressed in the nervous system; aromatase exerts neuroprotective effects through its role in producing estrogen [20,21]. The present study showed that aromatase expression was reduced in the spinal cords of mice after ovariectomy; consequently, we inferred that estrogen synthesis in the spinal cord was decreased and that the decrease in locally synthesized estrogen likely contributed to the earlier disease onset in the OVX group.
The neuroprotective effects of estrogen are well known to be mediated through the activation of estrogen receptors, including ERα, ERβ, and GPR30 [11,20]. Therefore, as the targets of estrogen, estrogen receptors levels in the lumbar spinal cord were assessed in the present study. Ovariectomy down-regulated ER-α expression but had no effect on that of ER-β, while ovariectomy and the sham operation both decreased GPR30 in the lumbar spinal cord at the onset stage. Furthermore, these results indicated that reduced estrogen levels resulted in decreased neuroprotection via reducing expression of ER-α, and probably GPR30. Studies demonstrated that the protective effects of 17β-estradiol were mediated through ER-α rather than ER-β [22,23], consistent with our findings. Furthermore, GPR30 might be another important component of estrogen-mediated neuroprotection [24,25], or possibly combined signaling through both ER-α and GPR30, but further study is needed to determine the exact mechanisms.
Previous studies have shown that inflammation, apoptosis, and oxidative stress participate in the pathogenesis of ALS [26–28] and that the activation of microglia is involved in the mechanism of this disease [29]. Moreover, previous studies have shown that estrogen exerts neuroprotection effects via its anti-inflammatory [30] and anti-apoptotic [31] functions. Our study agrees with these previous studies. Microglia is known to adopt 2 different phenotypes: the classically activated phenotype (M1) and the alternatively activated phenotype (M2). M1 microglia secrete pro-inflammatory cytokines, such as TNF-α, nitric oxide (NO), and interleukin-1 (IL-1) [32]. M2 microglia are likely involved in neuroprotection and repair following injury because they express the cytokines IL-4, IL-10, chitinase-like 3 (Ym1), TGF-β and arginase-1, which may exert anti-inflammatory effects and trigger the production of neurotrophic factors [30,33]. Our findings revealed that ovariectomy promoted inflammation by decreasing the expression of the anti-inflammatory cytokines arginase-1 and TGF-β, the latter also being inhibited by the sham operation, and by increasing the expression of the pro-inflammatory factor TNF-α. Furthermore, ovariectomy increased apoptosis by down-regulating the expression of the anti-apoptotic protein Bcl-2, while both ovariectomy and the sham operation up-regulated the expression of the pro-apoptotic protein Bax. These results show that ovariectomy decreased the anti-inflammatory and anti-apoptotic effects of estrogen and also demonstrate that estrogen can prevent inflammation and apoptosis.
Thus, we speculate that estrogen exerts its neuroprotective effects by inhibiting inflammation and apoptosis in hSOD1-G93A transgenic mice. The neuroprotective effects of estrogen were also confirmed by many previous animal experiments [7–10]. However, in contrast to our study, previous clinical studies did not demonstrate that estrogen replacement was effective in delaying the onset of ALS [34]. At present, the protective effect of estrogen on ALS is controversial, as well as on other neurodegenerative diseases, such as Alzheimer’s disease (AD) [35,36]. The reasons for these inconsistent results here are uncertain and may be related to the different duration of estrogen deprivation, the type of hormone deprivation, or different window for estrogenic intervention. Further analysis is necessary.
In our study, we used a Sham operation group as one of the control groups. Unexpectedly, although the onset time was 5 days later in the Sham group than in the OVX group, this difference was not significant. Moreover, GPR30, TGF-β, TNF-α, and Bax expression levels were not significantly different between the Sham and OVX groups. Mice in the Sham group underwent the sham operation, which may be considered a trauma. Prior studies have reported that trauma is likely associated with ALS risk [37,38]. Based on these results and our experimental results, we inferred that trauma might contribute to the onset of ALS in hSOD1-G93A transgenic mice. In our study, trauma may have triggered ALS through a mechanism partly similar to that of estrogen, but this hypothesis needs further study.
Conclusions
Ovariectomy accelerated the onset of ALS and down-regulated the expression of aromatase, ER-α, and GPR30 (the last of which was also inhibited by the sham operation) in the spinal cord of hSOD1-G93A transgenic mice. Moreover, ovariectomy promoted inflammation and apoptosis. Therefore, we speculate that the anti-inflammatory and anti-apoptotic effects of estrogen likely occur via its binding to ER-α and probably GPR30; these effects might be responsible for the neuroprotective properties of estrogen in hSOD1-G93A transgenic mice. In addition, trauma may contribute to the onset of ALS in hSOD1-G93A transgenic mice.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–55. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–75. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 4.Huisman MHB, de Jong SW, van Doormaal PTC, et al. Population based epidemiology of amyotrophic lateral sclerosis using capture-recapture methodology. J Neurol Neurosurg Psychiatry. 2011;82:1165–70. doi: 10.1136/jnnp.2011.244939. [DOI] [PubMed] [Google Scholar]
- 5.Manjaly ZR, Scott KM, Abhinav K, et al. The sex ratio in amyotrophic lateral sclerosis: A population based study. Amyotroph Lateral Scler. 2010;11:439–42. doi: 10.3109/17482961003610853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riar AK, Burstein SR, Palomo GM, et al. Sex specific activation of the ERα axis of the mitochondrial UPR (UPRmt) in the G93A-SOD1 mouse model of familial ALS. Hum Mol Genet. 2017;26:1318–27. doi: 10.1093/hmg/ddx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi CI, Lee YD, Gwag BJ, et al. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci. 2008;268:40–47. doi: 10.1016/j.jns.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Groeneveld GJ, Van Muiswinkel FL, Sturkenboom JM, et al. Ovariectomy and 17β-estradiol modulate disease progression of a mouse model of ALS. Brain Res. 2004;1021:128–31. doi: 10.1016/j.brainres.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Heitzer M, Kaiser S, Kanagaratnam M, et al. Administration of 17β-estradiol improves motoneuron survival and down-regulates inflammasome activation in male SOD1(G93A) ALS mice. Mol Neurobiol. 2017;54:8429–43. doi: 10.1007/s12035-016-0322-4. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Magranè J, Starkov AA, et al. The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis. Brain. 2012;135:2865–74. doi: 10.1093/brain/aws208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16:17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 12.Engler-Chiurazzi EB, Brown CM, Povroznik JM, et al. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Prog Neurobiol. 2017;157:188–211. doi: 10.1016/j.pneurobio.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morissette M, Le Saux M, D’Astous M, et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;20:1006–14. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson S, Mäkelä S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 15.Brailoiu E, Dun SL, Brailoiu GC, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 16.Funakoshi T, Yanai A, Shinoda K, et al. G protein-coupled receptor 30 is an estrogen receptor in the plasma membran. Biochem Biophys Res Commun. 2006;346:904–10. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 17.Abdelhamid R, Luo J, Vandevrede L, et al. Benzothiophene selective estrogen receptor modulators provide neuroprotection by a novel GPR30-dependent mechanism. ACS Chem Neurosci. 2011;2:256–68. doi: 10.1021/cn100106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu SB, Zhao MG. Neuroprotective effect of estrogen: Role of nonsynaptic NR2B-containing NMDA receptors. Brain Res Bull. 2013;93:27–31. doi: 10.1016/j.brainresbull.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Vercelli A, Mereuta OM, Garbossa D, et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31:395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Barakat R, Oakley O, Kim H, et al. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016;49:488–96. doi: 10.5483/BMBRep.2016.49.9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roselli CE, Liu M, Hurn PD. Brain aromatization: Classical roles and new perspectives. Semin Reprod Med. 2009;27:207–17. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polanczyk M, Zamora A, Subramanian S, et al. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003;163:1599–605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bains M, Cousins JC, Roberts JL. Neuroprotection by estrogen against MPP+-induced dopamine neuron death is mediated by ERα in primary cultures of mouse mesencephalon. Exp Neurol. 2007;204:767–76. doi: 10.1016/j.expneurol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu SB, Zhang N, Guo YY, et al. G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J Neurosci. 2012;32:4887–900. doi: 10.1523/JNEUROSCI.5828-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang H, Zhang QG, Yang LC, et al. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol Cell Endocrinol. 2014;387:52–58. doi: 10.1016/j.mce.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browne EC, Abbott BM. Recent progress towards an effective treatment of amyotrophic lateral sclerosis using the SOD1 mouse model in a preclinical setting. Eur J Med Chem. 2016;121:918–25. doi: 10.1016/j.ejmech.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 27.Hooten KG, Beers DR, Zhao W, et al. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12:364–75. doi: 10.1007/s13311-014-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mccombe PA, Henderson RD. The role of immune and inflammatory mechanisms in ALS. Curr Mol Med. 2011;11:246–54. doi: 10.2174/156652411795243450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata N, Kakita A, Takahashi H, et al. Activation of signal transducer and activator of transcription-3 in the spinal cord of sporadic amyotrophic lateral sclerosis patients. Neurodegener Dis. 2009;6:118–26. doi: 10.1159/000213762. [DOI] [PubMed] [Google Scholar]
- 30.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 31.Sales S, Ureshino RP, Pereira RT, et al. Effects of 17β-estradiol replacement on the apoptotic effects caused by ovariectomy in the rat hippocampus. Life Sci. 2010;86:832–38. doi: 10.1016/j.lfs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2012;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JH, Wei ZZ, Cao W, et al. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol Dis. 2016;96:248–60. doi: 10.1016/j.nbd.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudnicki SA. Estrogen replacement therapy in women with amyotrophic lateral sclerosis. J Neurol Sci. 1999;169:126–27. doi: 10.1016/s0022-510x(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 35.Engler-Chiurazzi EB, Brown CM, Povroznik JM, et al. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Prog Neurobiol. 2017;157:188–211. doi: 10.1016/j.pneurobio.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merlo S, Spampinato SF, Sortino MA. Estrogen and Alzheimer’s disease: Still an attractive topic despite disappointment from early clinical results. Eur J Pharmacol. 2017;817:51–58. doi: 10.1016/j.ejphar.2017.05.059. [DOI] [PubMed] [Google Scholar]
- 37.Feddermann-Demont N, Junge A, Weber KP, et al. Prevalence of potential sports-associated risk factors in Swiss amyotrophic lateral sclerosis patients. Brain Behav. 2017;7:1–10. doi: 10.1002/brb3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seals RM, Hansen J, Gredal O, et al. Physical trauma and amyotrophic lateral sclerosis: A population-based study using Danish National Registries. Am J Epidemiol. 2016;183:294–301. doi: 10.1093/aje/kwv169. [DOI] [PMC free article] [PubMed] [Google Scholar]