Abstract
Klebsiella pneumoniae is an increasingly recognized cause of a unique invasive syndrome manifesting as pyogenic liver abscess and hematogenous extrahepatic dissemination to a variety of sites, including the lung. Originally described only in Asia, this entity has now been reported across continents and ethnicities. Intrathoracic complications of invasive Klebsiella pneumoniae liver abscess syndrome (IKPLAS) have been characterized sporadically but have not been the subject of an all-encompassing investigation. Review of the English-language literature yields no reports of the acute respiratory distress syndrome as a consequence of IKPLAS. Herein we report what, to our knowledge, is the first such description.
Key words: ARDS, liver abscess, Klebsiella pneumoniae
Introduction
Klebsiella pneumoniae is an increasingly recognized cause of a unique invasive syndrome consisting of pyogenic liver abscess and hematogenous extrahepatic dissemination to a variety of sites, including the lung. Originally described in Taiwan, this entity has now been reported across continents and ethnicities.1-5 Intrathoracic complications of invasive Klebsiella pneumoniae liver abscess syndrome (IKPLAS) have been characterized sporadically but have not been the subject of a systematic investigation. The frequency with which they are encountered varies broadly among series.6-10 Review of the English-language literature yields no reports of the acute respiratory distress syndrome (ARDS) as a consequence of IKPLAS. Herein we describe what, to our knowledge, is the first such occurrence.
Case Report
A 38-year-old woman originally from Mexico with a past medical history of type 2 diabetes mellitus (DM) treated with a sulfonylurea presented to a New York City hospital with abdominal pain. She also reported polyuria and polydipsia as well as nausea and vomiting. Her initial vital signs were as follows: blood pressure 93/53 mmHg, pulse 119 beats/min, respiratory rate 28 breaths/min, oxygen saturation 99% while receiving oxygen at 2L/min via nasal cannula, and a temperature of 97.8F. Physical examination revealed Kussmaul breathing, normal cardiopulmonary auscultation, and a soft, non-tender abdomen. She was fully alert and oriented. Initial laboratory evaluation revealed a blood pH of 6.98 with a serum bicarbonate of 5mmol/L (reference range 23-32). The serum anion gap was 22 with a normal serum lactate. The serum glucose level was 506mg/dL, and the urinalysis was positive for ketones. Urine pregnancy testing was negative. Her initial portable chest radiograph (CXR) showed subtle prominence of the interstitial markings and multiple ill-defined nodular opacities (Figure 1A). In the Emergency Department (ED), the patient received 4 liters of intravenous crystalloid, and an insulin infusion was started. Blood cultures were collected, and she was admitted to the intensive care unit (ICU) for the management of diabetic ketoacidosis.
Figure 1.
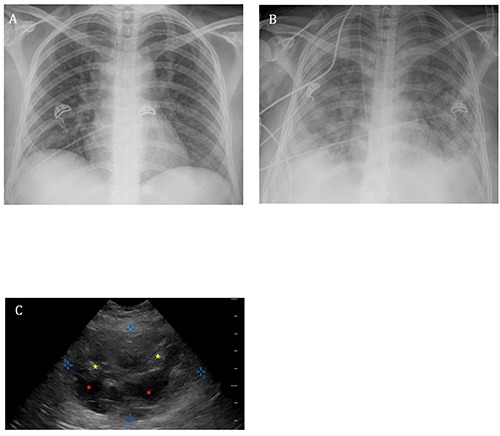
A) The patient’s portable chest radiograph on admission showing subtle prominence of the interstitial markings and multiple ill-defined nodular opacities. B) Portable chest radiograph taken following endotracheal intubation demonstrating interval development of diffuse bilateral interstitial and alveolar opacities as well as diffuse granular opacity and possible right pleural effusion. C) Ultrasonographic image of the liver obtained at the bedside showing a large hepatic mass-like lesion demarcated by the blue calipers with both cystic (red stars) and solid (yellow stars) components.
In the ICU, the patient became progressively more hypoxemic and dyspneic despite escalation to a non-rebreather mask. Her temperature rose to as high as 104F, and her level of consciousness declined to obtundation. Diffuse crackles were now heard on lung auscultation. She was endotracheally intubated for hypoxemic respiratory failure and placed on volume assistcontrol ventilation. Repeat CXR showed diffuse bilateral opacities (Figure 1B). While receiving a fraction of inspired oxygen (FiO2) of 70%, the patient’s PaO2 was measured at 59 mmHg for a PaO2/FiO2 (P/F) ratio of 84. Lung-protective ventilation for the diagnosis of ARDS was initiated by reducing the tidal volume to 350ml and increasing the positive end-expiratory pressure (PEEP) to 7.5 cmH2O. Vancomycin and piperacillin/tazobactam were started empirically. Soon thereafter, blood cultures drawn in the ED were reported as growing gram negative bacilli, so piperacillin/tazobactam was switched to imipenem. The patient’s hemodynamics deteriorated rapidly to the point of requiring high-dose norepinephrine infusion along with vasopressin to maintain a mean arterial pressure >65 mmHg. By ICU day #3, her FiO2 requirement had risen to 100%, and the PEEP had been increased to 15 cmH2O. Together with inhaled nitric oxide therapy, these settings were sufficient to maintain an oxygen saturation of >90%. At that time, the gram negative bacilli growing in blood culture were identified as pan-sensitive Klebsiella pneumoniae. Antibiotic coverage was narrowed to ceftriaxone. Abdominal ultrasonography performed in search of the source of her bacteremia revealed a complex avascular hepatic mass-like lesion with both cystic and solid components measuring 7.7x5.1x8.0 cm (Figure 1C). Based on the suspicion of liver abscess, interventional radiology was called for bedside placement of a drainage catheter. During the procedure, the patient developed pulseless electrical activity (PEA) with return of spontaneous circulation following 5 minutes of cardiopulmonary resuscitation. The insertion was aborted and therefore abscess specimens could not be sent for culture. Thereafter, PEA recurred multiple times, so, after consultation with the patient’s relatives, resuscitative efforts were ultimately aborted, and the patient expired. Her family granted permission for an autopsy. The autopsy confirmed the presence of a liver abscess with extensive necrosis. Lung sections demonstrated diffuse abscess formation completely obliterating alveolar architecture over a large territory (Figure 2A). Direct examination of the tissue revealed no organisms. In addition, many of the preserved alveoli contained hyaline membranes, the histological hallmark of diffuse alveolar damage (Figure 2B). The post-mortem diagnosis was thus invasive KLA syndrome leading to diffuse lung abscess formation complicated by ARDS.
Figure 2.
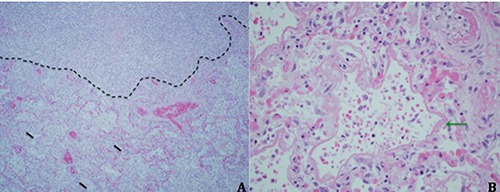
A) Section of lung tissue obtained at autopsy demonstrating destruction of lung parenchyma with an abscess (above dotted black line) adjacent to an area with preserved alveolar structures and intra-alveolar neutrophils (black arrows) (hematoxylin & eosin, original magnification x 200). B, Close-up of alveoli lined with fibrinous, eosinophilic material (green arrow) consistent with hyaline membranes (hematoxylin & eosin, original magnification x 400).
Discussion
Historically, pyogenic liver abscess has represented a localized form of infection caused most commonly by Escherichia coli and afflicting patients with underlying hepatobiliary pathology.11 Its normally localized nature is perhaps the reason why the literature contains no reports of ARDS as a direct sequela of liver abscess. The landscape of this disease has changed over the last several decades, beginning in the 1980s with reports from Taiwan documenting patients with liver abscess caused by K. pneumoniae and complicated by bacteremia and dissemination, specifically endophthalmitis. 1,12 Subsequently, a number of other Asian countries - a region in which fecal Klebsiella carriage rates are remarkably high - reported encounters with the socalled IKPLAS and its extrahepatic manifestations, including hematogenous lung seeding.13,14 Early on, DM was recognized to be a significant risk factor for this emerging infection. It was also noted that the majority of IKPLAS cases were attributable to the hypervirulent capsular subtypes of K. pneumoniae known as K1 and K2, which are especially prevalent in Asian countries. 7,12,15 These strains are known for their resistance to phagocytic clearance by macrophages, particularly if they also exhibit the characteristic mucoviscous phenotype conferred by the rmpA gene and identified by positivity of the so-called string test (Figure 3).16-18 The impairment in phagocytic function typical of the diabetic milieu has been shown to further promote the evasiveness of K1/K2 serotypes and thereby their propensity for entry into the bloodstream and distant spread.19 In recent decades, experience with IKPLAS has been published by investigators from Europe, the Americas, and Australia describing cases in both Asian immigrants as well as in the native population.2-5 Of note, a 2008 series from our institution found a comparable incidence of IKPLAS between Asian- Americans and Hispanic-Americans such as the patient in this report (12 or 60% vs 8 or 40%, respectively).2 Clinically, fever is a near-universal initial sign, but presentations are typically non-specific, and development of metastatic infection frequently provides a very delayed clue. Right upper quadrant tenderness may be absent. Klebsiella bacteremia is detected in about 50% of cases and is often what prompts a search for liver abscess.9 CT and ultrasound are the preferred imaging modalities for that purpose.
Figure 3.
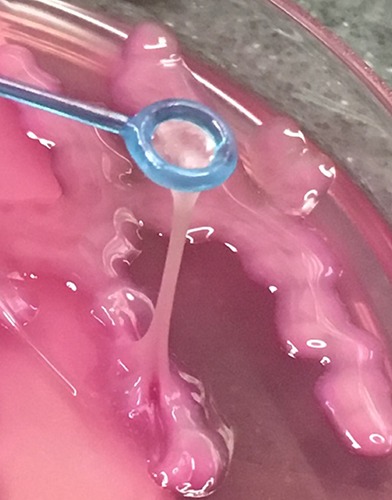
Illustration of a positive string test in a sample from another patient exhibited by the hypermucoviscous phenotype of K. pneumoniae.
The literature on the scope of intrathoracic manifestations of disseminated KLA is still in evolution; there are no Englishlanguage publications comprehensively analyzing chest imaging in this disease. Data on such manifestations are currently limited to case reports and series, studies of KLA in general that report lung findings, as well as descriptions of septic pulmonary emboli (SPE) caused by this infection.6-10,20,21 Some groups, though not all, have found the lung to be the most common site of extrahepatic seeding, ranging from 16.3% to 43.4% of cases.7,9,10 Among the specific types of intrathoracic complications, septic embolization diagnosed radiologically is the best described. Consolidations, mass-like lesions, interstitial opacities, and pleural effusions, including empyema, have also been reported.8,10,22 Review of our institutional experience indicates that pleural effusions may actually be the most frequent radiological finding in the chest of invasive KLA patients (40%), followed by consolidations at 20% and masses/ nodules at 7.5% (unpublished data). These numbers echo those previously published by a Taiwanese group.8 No reports known to us have heretofore linked IKPLAS to the development of ARDS.
As is the case with other distant sites of infection that can complicate KLA, we posit that our patient incurred a heavy and progressive burden of hematogenous lung seeding by K. pneumoniae, leading to what pathologically appeared to be diffuse lung abscess formation. This mechanism of pulmonary involvement is analogous to septic embolization and is to be distinguished from the typical endobronchial acquisition of conventional pneumonia caused by Klebsiella spp. The septic embolic insult to the lung was so severe that it resulted in the development of catastrophic lung injury in the form of ARDS.
Conclusions
In summary, IKPLAS is increasingly being recognized across the globe as a potentially morbid infection that carries a mortality rate of 4-11%, but one that can be effectively treated with prompt drainage and appropriate antibiotics.1,23 It is unique among causes of liver abscess in its propensity for hematogenous dissemination to other organs, including the lung. The extent of pulmonary involvement can be sufficient to cause respiratory failure in otherwise normal hosts. Based on our report, clinicians should add IKPLAS to the list of extrapulmonary infections capable of seeding the lungs and resulting in ARDS.
References
- 1.Fazili T, Sharngoe C, Endy T, et al. Klebsiella pneumoniae liver abscess: an emerging disease. Am J Med Sci 2016; 351:297-304. [DOI] [PubMed] [Google Scholar]
- 2.Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008;6:228-33. [DOI] [PubMed] [Google Scholar]
- 3.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004;39:1654-9. [DOI] [PubMed] [Google Scholar]
- 4.Moore R, O’Shea D, Geoghegan T, et al. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013;41:681-6. [DOI] [PubMed] [Google Scholar]
- 5.Dulku G, Tibballs J. Cryptogenic invasive Klebsiella pneumoniae liver abscess syndrome (CIKPLA) in Western Australia? Australas Med J 2014;7:436-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Z, Gong Z, Zheng J, et al. Computed tomography features of septic pulmonary embolism caused by Klebsiella pneumoniae liver abscess associated with extrapulmonary metastatic infection. J Comput Assist Tomogr 2016;40:364-9. [DOI] [PubMed] [Google Scholar]
- 7.Chung DR, Lee SS, Lee HR, et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect 2007;54:578-83. [DOI] [PubMed] [Google Scholar]
- 8.Yang PW, Lin HD, Wang LM. Pyogenic liver abscess associated with septic pulmonary embolism. J Chin Med Assoc 2008;71:442-7. [DOI] [PubMed] [Google Scholar]
- 9.Alsaif HS, Venkatesh SK, Chan DS, Archuleta S. CT appearance of pyogenic liver abscesses caused by Klebsiella pneumoniae. Radiology 2011;260:129-38. [DOI] [PubMed] [Google Scholar]
- 10.Shin SU, Park CM, Lee Y, et al. Clinical and radiological features of invasive Klebsiella pneumoniae liver abscess syndrome. Acta Radiologica 2013;54:557-63. [DOI] [PubMed] [Google Scholar]
- 11.Mavilia MG, Molina M, Wu GY. The evolving nature of hepatic abscess: a review. J Clin Transl Hepatol 2016;4:158-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang CT, Lai SY, Yi WC, et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 2007;45:284-93. [DOI] [PubMed] [Google Scholar]
- 13.Siu LK, Yeh KM, Lin JC, et al. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012;12:881-7. [DOI] [PubMed] [Google Scholar]
- 14.Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh KM, Kurup A, Siu LK, Koh YL, Fung CP, Lin JC, et al. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol 2007;45: 466-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YC, Lu MC, Tang HL, et al. Assessment of hypermucoviscosity as a virulence factor in experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscositynegative strain. BMC Microbiol 2011;11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu VL, Hansen DS, Ko WC, et al. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis 2007;13:986-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis 2006;42:1351-8. [DOI] [PubMed] [Google Scholar]
- 19.Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006;91:3084-7. [DOI] [PubMed] [Google Scholar]
- 20.Chou DW, Wu SL, Chung KM, Han SC. Septic pulmonary embolism caused by a Klebsiella pneumoniae liver abscess: clinical characteristics, imaging findings, and clinical courses. Clinics (Sao Paulo) 2015;70:400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagiya H, Kuroe Y, Nojima H, et al. Emphysematous liver abscesses complicated by septic pulmonary emboli in patients with diabetes: two cases. Intern Med 2013;52:141-5. [DOI] [PubMed] [Google Scholar]
- 22.Sano A, Tsuchiya T. Right pleural empyema secondary to liver abscess due to Klebsiella pneumoniae. PLEURA 2015; 1:1-3. [Google Scholar]
- 23.Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect 2004; 37:17-84. [PubMed] [Google Scholar]


