Abstract
Hyperleptinemia, associated with obesity, is related with immune dysfunction and carcinogenesis. Natural Killer (NK) cells, a major component of the innate immune system are mediators of anti-tumor immunity and the most actively migrating cells among leukocytes. Actin rearrangement, promoted by cofilin plays a central role in cellular migration. Leptin affects the phosphorylation- dependent activity of cofilin and thus actin remodeling. We used human NK-92 cells to explore the in vitro effects of leptin on co-localization of cofilin and F-actin and on morphological changes in NK cells. NK- 92 cells were incubated with different leptin concentrations (10 and 100 ng/mL) for 30 min and 24 h and immunocytochemically stained. Results demonstrate a dose- and time-dependent influence of leptin on cellular morphology. Utilizing confocal microscopy, we observed that the co-localization of cofilin-1 and F-actin was slightly influenced by leptin. In summary, the present study demonstrates an impact of a physiological leptin stimulation on the filopodia length, and a time-dependent effect on the co-localization of cofilin and F-actin in NK- 92 cells.
Key words: Actin, leptin, adipokine, NK cells, cell movement, migration, obesity, immunity
Introduction
Obesity is a medical condition characterized by excessive body fat with a body mass index (BMI) exceeding 30 kg/m2 and is one of the most serious and escalating public health problems affecting all age and socioeconomic groups in developed as well as developing countries. In 2014, the World Health Organization reported that over 1.9 billion adults (39%) were overweight and more than 600 million adults (13%) were obese.1 Both conditions are associated with an increased production of metabolic hormones coupled with a chronic low-grade state of inflammation that is linked to various disease entities, such as type II diabetes and cardiovascular disease.2 Furthermore, several analyses have shown an association between high BMI and an increased incidence of certain types of cancer, like kidney, liver and colorectal cancer.3-4 Adipose tissue not only plays an important role as an energy storage site, but also modulates immune cells through the release of adipokines, such as leptin, resistin, adiponectin, as well as cytokines such as interleukin (IL) -6 and tumor necrosis factor (TNF)-α.5 Leptin is the most widely studied adipocyte-derived hormone whose production and circulating levels depend on the amount of adipose tissue.6 Leptin, the product of obese (ob) gene, is a 16-kDa non-glycosylated protein, mainly secreted by adipocytes.7,8 Structurally, leptin is a member of the helical cytokine family, including IL-6, IL-11, IL-12, and granulocytemacrophage colony-stimulating factor.9,10 Furthermore, leptin also affects actin microfilaments interfering with cofilin-severing function.11 In the last years it became evident that leptin also modulates both the adaptive and the innate immune system.12,13 Natural Killer (NK) cells, with their capability of lysing certain infected or tumor cells are a major component of the innate immune system. On the one hand, NK cells mediate their anti-tumor response by direct cellular induction of target cell lysis via exocytosis of granzymes and perforin. On the other hand, NK cells activate the adaptive immune system by secreting different cytokines, like interferon- (IFN-) γ and TNF-α, in order to minimize tumor progression and metastases.14 NK-92 is a wellestablished human cell line for investigating NK cell physiology since they exhibit functional and phenotypical characteristics of primary NK cells.15 Cell movement or motility is a highly dynamic phenomenon that is essential for a variety of biological processes such as cancer metastasis and immune responses.16 The most active migrating cells among leukocytes are NK cells.17 Cell movement begins with restructuring of the actin cytoskeleton and can mostly be divided into three stages.18 First, the cell orients and reorganizes the actin network at its leading edge. Secondly, it adheres to the substrate at the leading edge and de-adheres at the cell body and rear of the cell. Finally, contractile forces pull the cell forward.19 Protrusive structures at the leading edge of motile cells are highly dynamic and contain dense arrays of actin filaments. The simplest protrusive structures are filopodia, thin cylinders that can extend tens of microns from the main cortex. 20 Filopodia contain a tight bundle of long actin filaments oriented in the direction of protrusion21 and they are used for the exploration of the extracellular matrix. Protrusion and building of filopodia are based on actin filament turnover. The turnover is the continuous polymerization and de-polymerization of actin filaments. Cofilin-1 (19kDa, non-muscle cofilin) is a small ubiquitous actin binding protein, which binds to F-actin and plays an essential role in enhancing actin-filament dynamics and accelerating the depolymerization of actin filaments.22 The activities of cofilin are reversibly regulated by phosphorylation and de-phosphorylation at Ser3, with the phosphorylated form being inactive.23 One of the major signaling pathways regulating the remodeling of the actin cytoskeleton is the small G protein Ras homolog gene family (Rho)/Rho-associated coiled-coil-forming protein kinase (ROCK) pathway.24 Activated Rho/ROCK relays intracellular signals through phosphorylating the actindepolymerizing factor cofilin, which leads to the loss of its ability to disassemble Factin. 25 Interestingly, leptin has been shown to induce the Rho/ROCK pathway and to affect phosphorylation of cofilin.26
The aim of the present study is therefore to elucidate the dose- and time-dependent impact of leptin stimulation on the cellular morphology of NK-92 cells and on the colocalization of cofilin and F-actin.
Materials and Methods
Cell culture
The well-established human NK cell line NK-92 was cultivated in RPMI 1640 medium (Biochrom AG, Berlin, Germany) supplemented with 10% fetal bovine serum (FBS, Biochrom AG), 1% penicillin, 1% streptomycin, 1% sodium-pyruvate, 1% Lglutamine (Biochrom AG), and 200 U/mL human recombinant interleukin- (IL-) 2 (Novartis AG, Basel, Switzerland).
Stimulation with leptin and immunofluorescence staining
In three independent experiments, human NK-92 cells (5x105) were incubated in 1 mL RPMI-Medium 1640 with 20% FBS and 200 U/mL IL-2 on 13 mm cover slips coated with 20 μg/mL human Fibronectin (Culturex® Human Fibronectin, PathClear®, Amsbio, Abington, UK) and stimulated with 10 ng/mL (physiological concentration) and 100 ng/mL (pathophysiological concentration) recombinant human leptin (R&D Systems, Minneapolis, MN, USA) for 30 min and 24 h. Thereafter, cells were fixed and permeabilized with 4% PSA (Formalin solution, 10%, Sigma-Aldrich, St. Louis, MO, USA), incubated with the primary monoclonal anti-Cofilin-1 antibody (1:50; D3F9, Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature and stained with Phalloidin - TRITC (1:1,000; Sigma-Aldrich, St. Louise, MO, USA) for 1 h at room temperature, donkey anti-rabbit Alexa 488 (1:310; Dianova GmbH, Hamburg, Germany) for 1 h at room temperature and DAPI (1:50,000; Sigma-Aldrich) for 5 min at room temperature. Slides were mounted with ProLong ® Gold (Thermo Fisher Scientific, Waltham, MA, USA).
Confocal laser scanning microscopy and data acquisition
Images were acquired with a Leica - TCS SP5 X confocal microscope equipped with a white light laser and hybrid detectors using a 63x oil immersion objective (NA 1.40) and standardized settings. From each cover slip 5-6 images were collected. The images were detected and analyzed with Fiji/ImageJ 2.0.0-rc-59/1.51j.
Cell morphology
In order to investigate the influence of leptin, filopodia length and NK cell circumference were measured in 44-45 cells per group (control 30 min, control 24 h, 10 ng/mL leptin 24 h, 100 ng/mL leptin 30 min and 100 ng/mL leptin 24 h). Due to a mounting problem in the group stimulated with 10 ng/mL leptin for 30 min only 35 cells could be analyzed in this group. The filopodia length was measured beginning from the proper end of the cell membrane. Per group 600-1200 filopodia lengths were measured. To ensure statistical correctness, all measured lengths of filopodia in each group were randomized by software for n = 600.
Co-localization analysis
For the examination of the co-localization of cofilin and F-actin the Mander’s colocalization coefficients were evaluated in regions of interest (ROI) with Coloc 2 in ImageJ. Coloc 2 is a Fiji /ImageJ plugin for co-localization analysis. For each experimental group, 28-30 cells were measured and Costes regression was performed. The ROIs were determined with ROI plugin. The nucleus area was subtracted. To avoid a manipulation of the co-localization coefficients no background subtractions were performed. For the analysis with Coloc2 an auto-threshold was set. Co-occurrence can be quantified by expressing the number of co-occurring pixels as a fraction of the total number or by using the Mander’s co-localization coefficients (MCC) M1 (fractional co-localization coefficient for F-actin to cofilin) and M2 (fractional co-localization coefficient for cofilin to F-actin) which, separately for each fluorophore, record the fraction of the total fluorescence that cooccurs. These coefficients are proportional to the amount of fluorescence of the colocalizing proteins like F-actin and cofilin, relatively to the total fluorescence in that region of interest. The measuring points range from 0 to 1, whereas 0 means no and 1 means 100% overlap.27
Statistical analysis
Data analysis was performed with the one-way ANOVA with the Tukey multiple comparison test for post-hoc analysis using Prism 7.0 (GraphPad. Software, Inc., La Jolla, CA, USA). P<0.05 was considered as statistical significant and marked with a star. Data from three independent experiments are depicted as means + SEM.
Results
Short-term leptin treatment alters length and number of filopodia but not cell circumferences
The incubation with 10 ng/mL leptin for 30 min resulted in a significantly shorter length of filopodia compared to control cells (Figure 1A), while the incubation for 24 h revealed similar results in all groups (Figure 1B). The treatment with 100 ng/mL leptin had no effect on the length of filopodia. The highest maximal length of filopodia was found in the group of cells stimulated with 10 ng/mL leptin for 24 h (15% higher compared to cells stimulated with 10 ng/mL for 30 min) (Table 1). Furthermore, numbers of filopodia per cell after stimulation with 10 ng/mL leptin for 24 h were slightly enhanced compared to the numbers after the treatment with 10 ng/mL for 30 min (Figure 2 A,B).
Figure 1.
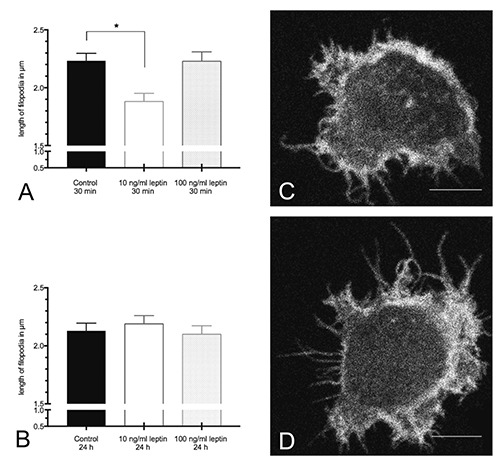
Length of filopodia (A,B). A) 30 min leptin stimulation; *P=0.0013 as compared to control. B) 24 h leptin stimulation; data are expressed as mean + SEM from three independent experiments (n=600 filopodia per group). C,D) Representative immunocytochemistry sections of NK cells (gray scaled) showing the differences in the length of filopodia between 10 ng/mL leptin for 30 min (C) and 10 ng/mL leptin for 24 h (D). C,D) scale bars: 6 μm.
Table 1.
Minimal and maximal length of filopodia (in μm) for each group of all measured lengths.
| Control 30 min | 10 ng/mL leptin 30 min | 100 ng/mL leptin 30 min | Control 24 h | 10 ng/mL leptin 24 h | 100 ng/mL leptin 24 h | |
|---|---|---|---|---|---|---|
| Minimum | 0.3 | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 |
| Maximum | 11.8 | 14.7 | 14.0 | 11.3 | 17.3 | 13.5 |
Figure 2.
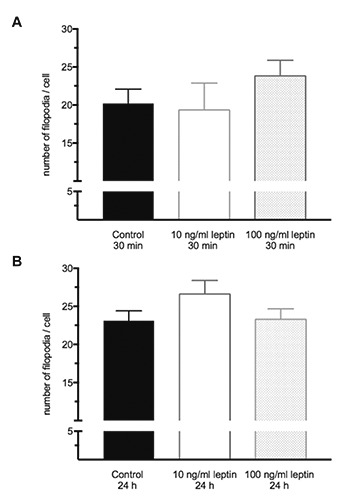
Number of filopodia per cell. A) 30 min leptin stimulation; B) 24 h leptin stimulation. Data are expressed as mean + SEM from three independent experiments (n=9 per group).
In the present study, no effect of a leptin stimulation on the NK cell circumference could be shown (Figure 3 A,B).
Figure 3.
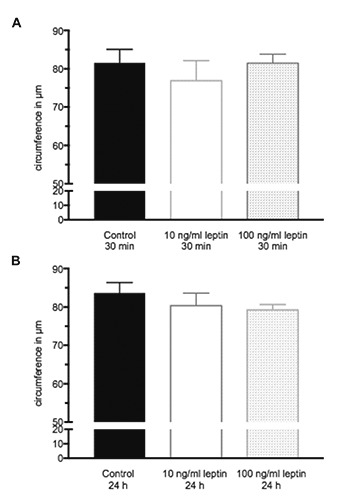
Circumference of NK-92 cells. A) 30 min leptin stimulation; B) 24 h leptin stimulation. Data are expressed as mean + SEM from three independent experiments (n=70-90 per group).
Influence of leptin on the co-localization of F-actin and cofilin in NK- 92 cells
Table 2 shows the fractional co-localization coefficients for F-actin (red fluorescence; tM1) and cofilin (green fluorescence; tM2) with auto-threshold used by Coloc 2. The overlap of F-actin to cofilin (represented by tM1) after short-term (30 min) leptin stimulation ranged from 53% to 58% between all groups. The overlap decreased by approximately 10% after long-term (24 h) stimulation and ranges from 45% to 49% between all groups. A slightly higher, but not significant overlap of cofilin to F-actin (represented by tM2) could be observed after the treatment with 10 ng/mL leptin for 30 min compared to the control cells. Interestingly, the incubation with 10 ng/mL leptin for 24 h showed a lower overlap of cofilin to F-actin as compared to the corresponding control. Concerning the intracellular localizations, Figures 4 and 5 show a ubiquitous occurrence of cofilin in the cells and a co-localization with F-actin at the membrane. The significantly shorter filopodia length after shortterm stimulation (10 ng/mL for 30 min) can be seen in Figure 4.
Table 2.
Mander’s co-localization coefficients, at 30 min and 24 h.
| Mander’s co-localization coefficients | Control 30 min | 10 ng/mL leptin 30 min | 100 ng/mL leptin 30 min | Control 24 h | 10 ng/mL leptin 24 h | 100 ng/mL leptin 24 h |
|---|---|---|---|---|---|---|
| tM1 | 0.572 (57%) | 0.577 (58%) | 0.529 (53%) | 0.452 (45%) | 0.456 (46%) | 0.487 (49%) |
| ± 0.049 (5%) | ± 0.042 (4%) | ± 0.046 (5%) | ± 0.050 (5%) | ± 0,040 (4%) | ± 0.044 (4%) | |
| tM2 | 0.497 (50%) | 0.561 (56%) | 0.542 (54%) | 0.536 (54%) | 0.480 (48%) | 0.511 (51%) |
| ± 0.030 (3%) | ± 0.020 (2%) | ± 0.022 (2%) | ± 0.028 (3%) | ± 0.024 (2%) | ± 0.020 (2%) |
tM1, fractional co-localization coefficient for F-actin; tM2, fractional co-localization coefficient for cofilin; mean ± SEM; n=28-30 per group.
Figure 4.

Representative images showing a ubiquitous occurrence of cofilin and a co-localization with F-actin at the membrane in NK- 92 cells. Each panel shows an enlarged view of one NK-92 cell. Green channel shows cofilin, red channel shows actin. Rightmost images show merged image. Original images 2048x2048; scale bars: 10 μm.
Discussion
Leptin, can be considered as a link between the immune system and cancer.28,29 Several studies have shown a positive correlation between high leptin levels, an increased cancer incidence and altered immune cell functions.29-31 Obesity is associated with significantly increased leptin levels in the blood.32 NK cells are a subgroup of lymphocytes playing an essential role in cell-based immune defense against virus infected and transformed cells.33 To date, only sparse information on the influence of leptin regarding the migratory performance, actin turnover rate and phosphorylation of cofilin is available. Mattioli et al. could show that physiological leptin concentrations time-dependently enhanced the amount of F-actin in immature human dendritic cells.11 In addition, O’Malley et al. could demonstrate an effect of leptin on filopodia outgrowth in neurons via the activation of MAPK (ERK) signaling pathway. 34 For the first time, the present study investigated the influence of leptin on filopodia and the extent of morphological changes in NK cells. To explore the doseand time-dependent impact of leptin on parameters of NK cell motility, an in vitro experiment with NK-92 cells was performed and the length and numbers of filopodia per cell and the circumference of the cells were investigated. Filopodia are known as the simplest protrusion tool during cell movement, containing high amounts of actin filaments.20 Several former studies demonstrated that filopodia influence cell migration.35,36 Here we report on a dose- and time-dependent influence of leptin on the filopodia length. The lengths of filopodia were significantly decreased in cells after physiological leptin stimulation with 10 ng/mL for 30 min compared to cells of all other groups. This result may indicate an altered migratory behavior of these NK- 92 cells. Xue et al. showed filopodia alterations during cell migration cycle in B16F1 mouse melanoma cells.37 It could be shown that during the protrusion phase filopodia were initiated, elongated and remained within the lamellopodium. During the retraction phase the projected filopodia were persistently growing, while the lamellipodium edge was retracted towards the filopodia base. Furthermore, the number of stationary filopodia increased and redecreased while the cell was moving.37 In contrary to the stimulation with physiological leptin concentrations the treatment with higher leptin levels did not affect the filopodia length. Furthermore, the amount of filopodia per cell was almost constant in all investigated groups, with a slight increase in cells after a long-term stimulation with physiological leptin dosages. It has to be taken into consideration that in the present study solely two time points could be investigated. In view of the relatively short sequences of cell migration cycles and concomitant alterations in filopodia length within the time-frame of a few minutes, future studies should investigate timedependent dynamics of NK cell migration patterns induced by a leptin stimulation via live cell imaging. The influence of leptin on filopodia and consequently on the movement of NK cells is important. NK cells play an important role in cellular immune defense. An impairment of NK cells movement results in a restricted immune defense against tumor cells. This study shows for the first time, that physiological concentrations of the adipokine leptin could increase the motility of NK cells and thus possibly support immune defense in different tissues. The stimulation with pathophysiologically high levels of leptin showed no influence on the filopodia length, number of filopodia per cell and the cell circumferences. However, several former studies have demonstrated that high concentrations of leptin impair NK cell cytotoxicity.38-40 Possibly, pathophysiologically high concentrations of leptin affect NK cells less on a morphological and more on a cytotoxic level. In a rodent lung metastasis model Spielmann et al. could demonstrate significantly increased lung metastasis in dietinduced obese rats accompanied with decreased numbers of NK cells in the lung tissue, reduced NK cell-tumor cell contacts and reduced expression of the activating NK cell receptor NKG2D.41
The comparison of the circumference of the NK cells indicated no influence of leptin. Somersalo et al. showed morphological alterations of human NK cells during migration on fibronectin-coated filters. NK cells migrating through untreated filters exerted mostly round shapes compared to prominent spread cells which migrated on fibronectin-coated filters.42 The results of the present study refer to the circumference and not to the morphological shape. Thus, it is conceivable that the cell shape is altered without a quantifiable effect on the cell circumference. Morphological parameters of cells are one aspect of the complex process of cell motility. Intracellular signaling pathways need to be analyzed and linked with morphological changes in NK cells. To explore the impact of leptin on the co-localization of cofilin and F-actin in NK-92 cells, regions of interest (ROI) were defined and the overlap of the fluorescence for both proteins was quantified by using Mander’s co-localization coefficient. The results indicated a time-dependent effect of the colocalization of cofilin to F-actin. The overlap of cofilin to F-actin was influenced by the treatment with 10 ng/mL leptin. The relevance of cofilin as an essential protein for the actin dynamics during cell motility has been well investigated.43,44 The proximity of cofilin to F-actin is required for a cell movement. Furthermore, a higher overlap could suggest an increased interaction between cofilin and F-actin, and consecutively an increased migratory behavior. We assume that the short-term treatment with leptin in a physiological concentration stimulates the overlap of cofilin and F-actin in NK-92 cells and facilitates cell migration. This result further supports the positive effect of leptin on NK cell morphology in a healthy microenvironment and the lack of this stimulatory impulse in a pathophysiological (obese) microenvironment. One possible underlying molecular mechanism for the impact of leptin on the co-localization of cofilin and F-actin could be an activation of the RhoA/ROCK/LIMK/cofilin-2 cascade as shown by Li et al. in nucleus pulposus cells.26
Nevertheless, a close co-localization does not prove a biochemical interaction. Further studies with NK cells are required to investigate the contribution of leptin to the interaction of cofilin and actin and intracellular pathways during NK cell migratory processes. Furthermore, Ngo et al. showed with high-speed atomic force microscopy technology that cofilin-bound actin filaments were merely 2 nm thicker compared to the corresponding actin filaments without bounded cofilin.45 The assessment of colocalization in the present study is susceptible to the resolution limits of a confocal microscope. Furthermore, analyzes of 2D images of 3D cells depend on intersecting planes. Overlapping structures from other levels cannot be ruled out. In summary, the present study demonstrates an impact of physiological leptin stimulation on the filopodia length and a time-dependent effect on the co-localization of cofilin and F-actin in NK-92 cells.
Figure 5.

Enlarged view of an NK-92 cell with detailed view of the cell membrane and co-localized (merge, yellow) cofilin (green) and F-actin (red); scale bar: 6 μm.
Acknowledgments
The authors gratefully acknowledge the technical assistance of S. Möschter, M. Helwig, as well as the support of image acquisition by Dr. N. Bley and D. Misiak from the Core Facility Imaging of the Medical Faculty of the Martin Luther University Halle-Wittenberg. The funding of the article processing fee by the publication fund of the Martin Luther University Halle- Wittenberg is gratefully acknowledged.
References
- 1.WHO. Obesity and Overweight. WHO fact sheet N 311. World Health Organisation, Geneva, Switzerland; 2015. [Google Scholar]
- 2.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92:347-55. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker- Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579-91. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772-83. [DOI] [PubMed] [Google Scholar]
- 6.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995;1:1155-61. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;1:425-32. [DOI] [PubMed] [Google Scholar]
- 8.Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci USA 1995;18:6957-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, et al. Crystal structure of the obese protein leptin-E100. Nature 1997;8:206-9. [DOI] [PubMed] [Google Scholar]
- 10.Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett 1995;2:13-8. [DOI] [PubMed] [Google Scholar]
- 11.Mattioli B, Straface E, Matarrese P, Quaranta MG, Giordani L, Malorni W, et al. Leptin as an immunological adjuvant: enhanced migratory and CD8+ T cell stimulatory capacity of human dendritic cells exposed to leptin. FASEB J 2016;6:2012-22. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol 2000;199: 15-24. [DOI] [PubMed] [Google Scholar]
- 13.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol 2004;4:371-9. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CA, Vivier E, Colonna M. Strategies of natural killer cell recognition and signaling. Curr Top Microbiol Immunol 2006;298:1-21. [DOI] [PubMed] [Google Scholar]
- 15.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994;4:652-8. [PubMed] [Google Scholar]
- 16.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. Garland Science; 2002. [Google Scholar]
- 17.Lang K, Drell TL, Niggemann B, Zanker KS, Entschladen F. Neurotransmitters regulate the migration and cytotoxicity in natural killer cells. Immunol Lett 2003;90:165-72. [DOI] [PubMed] [Google Scholar]
- 18.Abercrombie M. The Croonian lecture, 1978. The crawling movement of metazoan cells. Proc R Soc B 1980;207:129-47. [Google Scholar]
- 19.Ananthakrishnan R, Ehrlicher A. The forces behind cell movement. Rev Int J Biol Sci 2007;3:303-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Rev Cell Press 1996;84:371-9. [DOI] [PubMed] [Google Scholar]
- 21.Small JV. The actin cytoskeleton. Electron Microsc Rev 1988;1:155-74. [DOI] [PubMed] [Google Scholar]
- 22.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 1999;15:185-230. [DOI] [PubMed] [Google Scholar]
- 23.Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem 1995;270:17582-7. [DOI] [PubMed] [Google Scholar]
- 24.Zeidan A, Javadov S, Karmazyn M. Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc Res 2006;72:101-11. [DOI] [PubMed] [Google Scholar]
- 25.Thirone AC, Speight P, Zulys M, Rotstein OD, Szaszi K, Pedersen SF, et al. Hyperosmotic stress induces Rho/Rho kinase/LIM kinase-mediated cofilin phosphorylation in tubular cells: Key role in the osmotically triggered Factin response. Am J Physiol Cell Physiol 2009;296:C463-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Liang J, Wu WK, Yu X, Yu J, Weng X, et al. Leptin activates RhoA/ROCK pathway to induce cytoskeleton remodeling in nucleus pulposus cells. Int J Mol Sci 2014;15:1176-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dual-color confocal images. J Microsc 1993;169:375-82. [DOI] [PubMed] [Google Scholar]
- 28.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, et al. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev 2010;29:641-53. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Riejos P, Najib S, Santos- Alvarez J, Martin-Romero C, Perez- Perez A, Gonzalez-Yanes C, et al. Role of leptin in the activation of immune cells. Mediators Inflamm 2010;2010: 568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 2007;14: 189-206. [DOI] [PubMed] [Google Scholar]
- 31.Jarde T, Perrier S, Vasson MP, Caldefie- Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer 2011;47:33-43. [DOI] [PubMed] [Google Scholar]
- 32.Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One 2012;7:e33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008;9:503-10. [DOI] [PubMed] [Google Scholar]
- 34.O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neuroscience 2007;35:559-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuxworth RI, Weber I, Wessels D, Addicks GC, Soll DR, Gerisch G, et al. A role for myosin VII in dynamic cell adhesion. Curr Biol 2001;11:318-29. [DOI] [PubMed] [Google Scholar]
- 36.Guillaume J, Hellyeh H, Johanna I. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr Opin Cell Biol 2015;36:23-31. [DOI] [PubMed] [Google Scholar]
- 37.Xue F, Janzen D.M, Knecht DA. Contribution of filopodia to cell migration: a mechanical link between protrusion and contraction. Int J Cell Biology 2010;ID 507821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bähr I, Goritz V, Doberstein H, Hiller GG, Rosenstock P, Jahn J, et al. Dietinduced obesity is associated with an impaired NK cell function and an increased colon cancer incidence. J Nutr Metab 2017;2017:4297025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrann CD, Laue T, Hübner L, Kuhlmann S, Jacobs R, Goudeva L, et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am J Physiol Endocrinol Metab 2012;302:E108-16. [DOI] [PubMed] [Google Scholar]
- 40.Lamas B, Goncalves-Mendes N, Nachat-Kappesetal R. Leptin modulates dose-dependently the metabolic and cytolytic activities of NK-92 cells. J Cell Physiol 2013;228:1202-9. [DOI] [PubMed] [Google Scholar]
- 41.Spielmann J, Hanke J, Knauf D, Ben- Eliyahu S, Jacobs R, Stangl GI, et al. Significantly enhanced lung metastasis and reduced organ NK cell functions in diet- induced obese rats. BMC Obesity 2017;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somersalo K, Saksela E. Fibronectin facilitates the migration of human natural killer cells. Eur J Immunol 1991;21:35-42. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh M Song X Mouneimne G Sidani M David S. Lawrence DSet al. Cofilin promotes actin polymerization and defines the direction of cell motility. Science 2004;304:743-6. [DOI] [PubMed] [Google Scholar]
- 44.DesMarais V, Ghosh M, Eddy R, Condeelis J. Cofilin takes the lead. J Cell Sci 2005;18:19-26. [DOI] [PubMed] [Google Scholar]
- 45.Ngo KX, Kodera N, Katayama E, Ando T, Uyeda T. Cofilin-induced unidirectional cooperative conformational changes in actin filaments revealed by high-speed atomic force microscopy. eLife 2015;4:e04806. [DOI] [PMC free article] [PubMed] [Google Scholar]


