Abstract
Objectives
To examine cardiac biomarkers over time in youth-onset type 2 diabetes, and relate serum concentrations to cardiovascular disease risk factors, and left ventricular structure and function.
Study design
TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) was a multicenter randomized trial of 3 treatments including 521 participants with type 2 diabetes, aged 10–17 years, and with 2–6 years of follow-up. Participants were 36% male, obese, and ethnically diverse. Annual serum concentrations of brain natriuretic peptide, troponin, tumor necrosis factor (TNF)-α, receptors 1 and 2 were related to blood pressure, body mass index, hemoglobin A1c, and left ventricular ejection fraction, diastolic function, relative wall thickness, and mass.
Results
Elevated concentrations of brain natriuretic peptide (≥100 pg/mL), TNF-α (≥5.6 pg/mL) and troponin (≥0.01 ng/mL), were present in 17.8%, 18.3%, and 34.2% of the cohort, respectively, at baseline, and in 15.4%, 17.1%, and 31.1% at the end of the study, with wide variability over time, without persistence in individuals or clear relationship to glycemia or cardiovascular structure/function. TNF receptors concentrations were increased at baseline and not significantly different from end-of-study concentrations. Adverse echocardiographic measures were more likely in the highest TNF receptor tertile (all P < .05): higher left ventricular mass (39.3 ± 9.0 g/m2.7), left atrial internal dimension (3.7 ± 0.4 cm) and E/Em ratio, a measure of diastolic dysfunction (6.2 ± 1.9). After adjustment for body mass index, these relationships were no longer significant.
Conclusions
Elevated serum concentrations of cardiac biomarkers were common in youth with type 2 diabetes, but their clinical significance is unclear and will require further long-term study.
Youth with type 2 diabetes (T2D) are at high lifetime risk for cardiovascular disease (CVD).1 The Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study, a randomized clinical trial of 3 glycemic control treatments in newly diagnosed youth with T2D, reported that CVD risk worsened over 2–6 years.2–4 Adverse profiles of inflammatory markers including high-sensitivity C-reactive protein, interleukin-6, plasminogen activator inhibitor-1, and homocysteine were described, and these did not improve.4 Cross-sectional echocardiographic studies of adolescents with T2D demonstrate high concentrations of left ventricular (LV) mass, high measures of relative wall thickness to cavity dimensions, high left atrial (LA) size, and possible diastolic dysfunction.5–7 In adults with T2D, measurement of cardiovascular and inflammatory biomarkers adds to CVD risk stratification.8,9
Cardiac biomarkers, including brain natriuretic peptide (BNP), and high-sensitivity troponin (troponin), have been associated with adverse cardiac structure or function changes in adults with T2D.10 Tumor necrosis factor alpha (TNF-α), an inflammatory marker, is also associated with the presence of T2D. A study of Asian Indians with youth-onset T2D showed this relationship in obese and nonobese patients. Laboratory studies have implicated TNF in regulation of myocardial cell function.11 Two large cohort studies of T2D suggest TNF receptor 1 (R1) and TNF receptor 2 (R2) predict future kidney disease, CVD, and total mortality.12–14
There are limited longitudinal data on cardiac biomarkers in children and adolescents, including at-risk populations, such as those with youth-onset T2D. The purpose of this report is to determine the (1) natural history of BNP, troponin, TNF-α, TNF-R1, and TNF-R2 over time in youth with T2D, (2) relationship of these cardiac biomarkers to CVD risk factors and hemoglobin A1c (HbA1c), and (3) relationships of these biomarkers to echocardiographic measures of LV structure and function. The finding of worsening biomarker profiles over time or a relationship to cardiac structure and function would suggest subclinical diabetic cardiomyopathy in this young population.
Methods
The TODAY study was a multicenter, randomized clinical trial (ClinicalTrials.gov: NCT00081328) of 3 treatments for T2D in youth: metformin alone, metformin and intensive lifestyle, and metformin and rosiglitazone.2 Eligibility included age 10–17 years, T2D duration <2 years, body mass index (BMI) ≥85th percentile, negative pancreatic autoantibodies, fasting C-peptide >0.6 ng/mL, and an adult caregiver willing to support study participation. Subjects were excluded for refractory hypertension or creatinine clearance <70 mL/min. The primary objective was to compare treatment arms on time with treatment failure (HbA1c ≥8% [≥64 mmol/mol] for 6 months or sustained metabolic decompensation requiring insulin). One-half of the cohort reached the primary endpoint and results demonstrated that adding rosiglitazone to metformin was associated with more durable glycemic control.2
The protocol was approved by an External Evaluation Committee convened by the National Institute of Diabetes and Digestive and Kidney Disease and by the Institutional Review Boards for the Protection of Human Subjects of each participating institution. All participants provided informed consent and minor children confirmed assent according to local guidelines. A Data and Safety Monitoring Board convened by the National Institute of Diabetes and Digestive and Kidney Disease reviewed progress, safety, and interim analyses throughout the study.
Assessments were obtained at months 0, 12, 24, 36, 48, and 60, as previously described.5 These included measurements of height, weight, blood pressure (BP) and laboratory testing. Hypertension was defined as BP ≥130/80 mm Hg or ≥95th percentile for age, sex, and height2,3 and was treated with an angiotensin-converting enzyme inhibitor. Additional medications were added as needed.
HbA1c and cardiac biomarkers measurements were performed at the Northwest Lipid Research Laboratory, University of Washington, Seattle, Washington. Blood samples were collected yearly in EDTA tubes, centrifuged, frozen immediately upon sample processing and stored in 24/7 monitored −80°C freezers. Analyses were performed immediately after samples were thawed. Serum BNP concentrations were measured by ELISA (Raybio Tech Inc, Norcross, Georgia), with intra-assay and interassay coefficient of variations of 10% and 12%, respectively. Troponin, TNF-α, TNF-R1, and TNF-R2 assays were performed using a Multiplex protein arrays system using magnetic beads (R&D systems, Minneapolis, Minneapolis, and EMD Millipore Inc, Gibbstown, NJ). Intra-assay and interassay CVs were 6.5% and 11% for troponin respectively, 7.2% and 12.5% for TNF-α, 3.0% and 3.3% for TNF-R1, and 2.3% and 2.8% for TNF-R2.
Two-dimensional guided echocardiograms were performed on participants during the last year of the study according to American Society of Echocardiography standards by certified technicians as previously described.5,15 Images were transferred to a central reading laboratory where MMode measurements of LV wall thicknesses in diastole, LV dimensions in systole and diastole, and dimension were performed; tissue Doppler imaging was used to measure diastolic function. The tricuspid annular plane systolic excursion was measured to assess right ventricular function. Study quality was graded and only studies of fair or better quality were included. Quality control procedures showed a coefficient of variation for repeat measurement of all parameters of <10%.
Statistical Analyses
Statistical analyses of the biomarker data were performed on TODAY participants (n = 521) with at least 2 annual assessments including baseline. TODAY participants completed an average of 4 annual examinations (SD, 1; min-max, 2–6). Abnormal risk categories, based on current consensus, were applied to 3 of the 5 cardiac biomarkers (BNP, ≥100 pg/mL16; troponin, ≥0.01 pg/mL17; TNF-α, ≥5.6 pg/mL18). A cutoff of ≥ 0.04 pg/mL19 for troponin was also examined.
Participants were grouped into 3 categories for BNP, troponin, and TNF-α: (A) always normal (values less than the cutoff at all annual visits), (B) intermittent (at least 1 visit with a value ≥l to the cutoff), and C) always high (values greater than or equal to the cutoff at all annual visits). TNF-R1 and TNF-R2 were normally distributed and analyzed as continuous variables or by tertiles. Generalized linear models were used to assess the relationships between treatment group, race-ethnicity, T2D parameters, risk factors, or echocardiography parameters with the cardiac biomarkers concentrations at (a) baseline, (b) end of the study, and (c) grouped into categories (always abnormal, intermittent, always normal). Models evaluating longitudinal data accounted for the multiple observations per participant as appropriate. Analyses included all data available up to as many as 6 annual visit time points (range, 24–60 months). Sensitivity analyses were conducted on those with a minimum of 4 visits (n = 340) to ensure reported results were not biased by those with fewer visits. Echocardiography variables not normally distributed were log-transformed. All models were adjusted for time in the study. Models for BNP were further adjusted for sex and antihypertensive medications use. Treatment group and failure to maintain glycemic control were not included as covariates in the final models because no univariate associations were identified with the biomarkers and their adjustment in the models did not impact the relationships between the biomarkers and the CVD risk factors and echocardiography measures. Analyses were performed in SAS (version 9.4 for Windows; SAS Institute, Cary, North Carolina), and considered exploratory and hypothesis generating. P < .05 was used to determine statistical significance.
Results
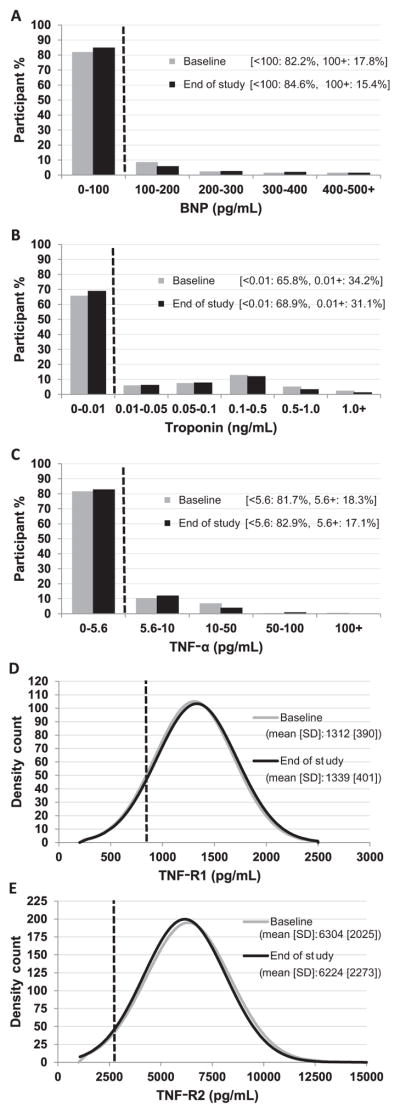
Participants were 36% male, obese (BMI 35 kg/m2 at baseline and 37 kg/m2 at the end of the study), and ethnically diverse: 34% African American, 43% Hispanic, and 20% white (Table I). By the end of the study, the median duration of T2D was 4.1 years with about one-half achieving the primary end-point (failure to maintain glycemic control). Hypertension requiring antihypertensive medications increased from baseline (4.4% vs 27.8%). Average LV mass and LA size were increased (Table I).5 Baseline and end-of-study values for the 5 cardiac biomarkers are presented in the Figure.
Table I.
TODAY participant characteristics and demographics at baseline and end-of-study visit (n = 521)*
| Baseline Visit | End-of-study visit | |
|---|---|---|
| T2D duration (y) | 0.7 (0.5) | 4.0 (1.4) |
| Age (y) | 13.9 (2.0) | 17.3 (2.3) |
| Male (%) | 36.1% | |
| Race-ethnicity (%) | ||
| Non-Hispanic black | 33.6% | |
| Hispanic | 43.0% | |
| Non-Hispanic white | 19.8% | |
| Other | 3.6% | |
| Treatment group (%) | ||
| Metformin | 34.0% | |
| Metformin + rosiglitazone | 31.6% | |
| Metformin + lifestyle | 34.4% | |
| Failed to maintain glycemic control (%) | 47.7% | |
| HbA1c (%) | 6.0 (0.7) | 7.7 (2.5) |
| Mean HbA1c during study (%) | 6.9 (1.6) | |
| BMI (kg/m2) | 34.6 (7.8) | 36.5 (8.4) |
| Mean BMI during study (kg/m2) | 35.7 (8.0) | |
| Systolic BP (mm Hg) | 112.7 (11.2) | 115.1 (11.4) |
| Mean systolic BP during study (mm Hg) | 114.3 (9.4) | |
| Diastolic BP (mm Hg) | 66.4 (8.3) | 69.3 (8.9) |
| Mean diastolic BP during study (mm Hg) | 68.1 (6.7) | |
| Antihypertensive medication use (%) | 4.4% | 27.8% |
| LV mass (g) | 154.0 (45.9) | |
| LV mass/height2.7 (g/m2.7) | 37.6 (9.2) | |
| LV relative wall thickness | 0.34 (0.06) | |
| LV fractional shortening (%) | 38.3 (5.1) | |
| LA internal dimension (cm) | 3.59 (0.46) | |
| LA internal dimension/height (cm/m) | 2.14 (0.26) | |
| TAPSE (cm) | 2.16 (0.36) | |
| Doppler diastology | ||
| LV E (cm/s) | 92.2 (18.5) | |
| LV Em (cm/s) | 16.9 (4.6) | |
| E/Em ratio | 5.80 (1.82) | |
TAPSE, tricuspid annular plane systolic excursion.
Mean (SD) or percent are shown; based on a sample of n = 521 TODAY participants with at least 2 annual assessments collected including a baseline visit; mean (SD) duration in the study was 3.4 (1.2) years at the end-of-study visit.
Figure.
Baseline and end-of-study distributions of measures of A, BNP, B, troponin, C, TNF-α, D, TNF-R1, and E, TNF-R2. A reference line (dashed line) and the percent of participants above a pre-specified abnormal cutoff is illustrated in the graph for BNP (cutoff, 100 pg/mL), troponin (cutoff, 0.01 ng/mL), and TNF-α (5.6 pg/mL). TNF-R1 and TNF-R2 are normally distributed and therefore the mean (SD) is given; the reference line (dashed line) represents the upper normal range reported in the literature (TNF-R1: 821 pg/mL, and TNF-R2: 2622 pg/mL).
Elevated serum BNP concentrations (≥100 pg/mL) were found in 17.5% at baseline (Figure, A). The BNP distribution was positively skewed, with no overall change in the prevalence of abnormal BNP values (ie, ≥100 pg/mL) over the course of the study. Overall, 128 (24.6%) of participants had elevations of BNP; 57 (44.5%) had abnormal values on every assessment and 48 (37.5%) had abnormal values on ≥ 50% of assessments.
To determine if BNP was associated with treatment group, race-ethnicity, T2D duration, HbA1c, loss of glycemic control, sex, BMI, antihypertensive medication use, BP, or echocardiography parameters, the cohort was divided into 3 groups: always normal (n = 393), always high (n = 57), and intermittent (n = 71). For LV diastolic function, there was a trend toward a higher E (P = .0210) and E/Em ratio (P = .0223) in those with consistently high BNP level (mean [SD], always high 97.5 [20.5] and 6.3 [2.2] vs normal 92.3 [17.9] and 5.9 [1.8] cm/s, respectively); these individuals were also more likely to be male. No relationships were found between baseline BNP or BNP group and any echocardiographic parameters (Table II; available at www.jpeds.com). At the end of the study, those with elevated BNP (ie, ≥100 pg/mL) had higher LV E values compared with those with normal BNP concentrations (mean [SD], 96.1 [20.4] vs 91.7 [18.0] cm/s respectively; P = .046), after accounting for sex and antihypertensive medications use differences. BNP level was unrelated to treatment group, ethnicity, HbA1c, primary outcome, diabetes duration, BMI, or CVD risk factors.
Table II.
TODAY participant (n = 521) end-of-study and overall* characteristics, T2D parameters, and echocardiography parameters by BNP group†
| (A) | (B) | (C) | ||
|---|---|---|---|---|
| BNPs (pg/mL) | Always normal (<100) (n = 393) | Intermittent (n = 71) | Always high (≥100) (n = 57) | P value |
| T2D duration (y) | 4.0 (1.4) | 4.5 (1.3) | 4.0 (1.4) | .0159 B vs A,C |
| Male (%) | 34.6% | 32.4% | 52.6% | .0179 C vs A,B |
| Race-ethnicity (%) | ||||
| Non-Hispanic black | 33.9% | 32.8% | 37.5% | NS |
| Hispanic | 45.7% | 41.8% | 46.4% | |
| Non-Hispanic white | 20.5% | 25.4% | 16.1% | |
| Treatment group (%) | ||||
| Metformin | 36.1% | 23.9% | 31.6% | NS |
| Metformin + rosiglitazone | 30.0% | 38.0% | 35.1% | |
| Metformin + lifestyle | 33.8% | 38.0% | 33.3% | |
| Failed to maintain glycemic control (%) | 47.1% | 49.3% | 45.6% | NS |
| End-of-study HbA1c (%) | 7.7 (2.4) | 7.5 (2.4) | 7.9 (2.8) | NS |
| Mean HbA1c during study (%) | 6.9 (1.6) | 6.8 (1.3) | 7.1 (1.8) | NS |
| End-of-study BMI (kg/m2) | 36.3 (8.3) | 36.6 (8.7) | 37.2 (8.4) | NS |
| Mean BMI during study (kg/m2) | 35.4 (7.9) | 35.7 (7.8) | 36.4 (8.0) | NS |
| End-of-study systolic BP (mm Hg) | 115.2 (11.4) | 113.8 (12.4) | 116.1 (11.5) | NS |
| Mean systolic BP during study (mm Hg) | 114.4 (9.5) | 113.0 (8.9) | 115.2 (9.5) | NS |
| End-of-study diastolic BP (mm Hg) | 69.4 (9.0) | 68.5 (8.5) | 69.3 (9.4) | NS |
| Mean diastolic BP during study (mm Hg) | 68.2 (6.7) | 68.0 (6.8) | 67.6 (6.9) | NS |
| End-of-study ACE inhibitor/angiotensin receptor blocker use (%) | 30.0% | 21.1% | 21.0% | NS |
| LV mass (g) | 151.9 (43.6) | 151.7 (40.7) | 166.2 (56.9) | NS |
| LV mass/height2.7 (g/m2.7) | 37.2 (8.9) | 37.6 (8.4) | 40.0 (9.9) | NS |
| LV relative wall thickness | 0.34 (0.06) | 0.33 (0.06) | 0.35 (0.07) | NS |
| LV fractional shortening (%) | 38.4 (5.0) | 38.0 (5.4) | 38.0 (5.5) | NS |
| LA internal dimension (cm) | 3.57 (0.45) | 3.62 (0.43) | 3.66 (0.46) | NS |
| LA internal dimension/height (cm/m) | 2.13 (0.26) | 2.17 (0.26) | 2.18 (0.25) | NS |
| TAPSE (cm) | 2.16 (0.35) | 2.13 (0.35) | 2.20 (0.41) | NS |
| Doppler diastology | ||||
| LV E (cm/s) | 92.3 (17.9) | 89.3 (19.2) | 97.5 (20.5) | .0210 A,B vs C |
| LV Em (cm/s) | 16.8 (4.6) | 17.4 (4.3) | 16.3 (4.2) | NS |
| E/Em ratio | 5.9 (1.8) | 5.4 (1.5) | 6.3 (2.2) | .0223 B vs C |
ACE, angiotensin-converting enzyme.
Mean during the study.
All models adjusted for time in study (ie, similar to duration of diabetes), antihypertensive medications use, and sex. All echocardiography LV and Doppler diastology parameters are log transformed before testing due to skewed distributions. Pairwise comparisons are given for the significant associations found (P < .05).
The distribution of troponin was positively skewed, with most values close to zero (Figure, B). The prevalence of abnormal troponin values (≥0.01 pg/mL) was not statistically different at the end-of-study visit (31.1%) compared with baseline (34.2%; P = NS), with tremendous variability from visit to visit. Only 43.0% of the cohort (n = 224) had a normal value (<0.01 pg/mL) at every annual visit; 19.8% (n = 103) had an abnormal value at 1 visit only, 15.4% (n = 80) at 2 visits, 11.1% (n = 58) at 3 visits, and 10.8% (n = 56) at all visits.
When the cohort was divided into 3 groups—always normal (<0.01 pg/mL; n = 224), always high (n = 56), and intermittent (n = 241)—no relationship was found for any of the parameters or characteristics, except for BMI (Table III; available at www.jpeds.com). The mean [SD] was higher for BMI (37.9 [8.7] kg/m2) in those with troponin concentrations that alternated between high and low compared with those with normal (35.2 [8.1] kg/m2) or elevated (34.7 [6.7] kg/m2) elevated troponin values. No relationship was found with baseline troponin and any of the parameters. At the end of the study, those with elevated troponin had slightly higher LV relative wall thickness vs normal (mean [SD], 035 [0.06] vs 0.33 [0.06]) and lower tricuspid annular plane systolic excursion (2.10 [SD 0.35] vs normal (mean [SD], 2.10 [0.35] vs 2.18 [0.36] cm), both adverse differences. No other differences related to presence of elevated troponin were found by treatment group, race-ethnicity, diabetes parameters, risk factors, or other echocardiography parameters. When analyses were performed with a 0.04 pg/mL cutoff, similar results were found.
Table III.
TODAY participant (n = 521) end-of-study and overall* characteristics, T2D parameters and echocardiography parameters by troponin group†
| (A) | (B) | (C) | ||
|---|---|---|---|---|
| Troponins (ng/mL) | Always normal (<0.01) (n = 224) | Intermittent (n = 241) | Always high (≥0.01) (n = 56) | P value |
| T2D duration (y) | 4.0 (1.4) | 4.2 (1.3) | 3.6 (1.4) | .0057 A,B vs C |
| Male (%) | 34.4% | 37.8% | 37.5% | NS |
| Race-ethnicity (%) | ||||
| Non-Hispanic black | 39.8% | 31.2% | 24.1% | NS |
| Hispanic | 41.2% | 47.4% | 51.8% | |
| Non-Hispanic white | 19.0% | 21.4% | 24.1% | |
| Treatment group (%) | ||||
| Metformin | 33.5% | 36.1% | 26.8% | NS |
| Metformin + rosiglitazone | 29.9% | 34.0% | 28.6% | |
| Metformin + lifestyle | 36.6% | 29.9% | 44.6% | |
| Failed to maintain glycemic control (%) | 46.4% | 49.4% | 41.1% | NS |
| End-of-study HbA1c (%) | 7.6 (2.5) | 7.8 (2.5) | 7.5 (2.3) | NS |
| Mean HbA1c during study (%) | 6.9 (1.6) | 7.0 (1.6) | 6.7 (1.4) | NS |
| End-of-study BMI (kg/m2) | 35.2 (8.1) | 37.9 (8.7) | 34.7 (6.7) | .0013 B vs A,C |
| Mean BMI during study (kg/m2) | 34.4 (7.6) | 37.0 (8.2) | 33.9 (6.5) | .0007 B vs A,C |
| End-of-study systolic BP (mm Hg) | 115.1 (11.7) | 115.2 (11.0) | 114.8 (12.8) | NS |
| Mean systolic BP during study (mm Hg) | 114.2 (9.4) | 114.8 (9.4) | 112.8 (10.1) | NS |
| End-of-study diastolic BP (mm Hg) | 69.5 (8.7) | 69.1 (8.9) | 69.0 (10.2) | NS |
| Mean diastolic BP during study (mm Hg) | 68.3 (6.4) | 68.1 (6.8) | 67.6 (8.0) | NS |
| LV mass (g) | 150.2 (46.1) | 155.8 (44.2) | 155.5 (42.9) | NS |
| LV mass/height2.7 (g/m2.7) | 36.7 (8.9) | 38.1 (9.0) | 38.4 (8.8) | NS |
| LV relative wall thickness | 0.33 (0.06) | 0.34 (0.06) | 0.35 (0.07) | NS |
| LV fractional shortening (%) | 38.7 (5.5) | 38.0 (4.9) | 38.2 (4.6) | NS |
| LA internal dimension (cm) | 3.55 (0.44) | 3.63 (0.46) | 3.54 (0.48) | NS |
| LA internal dimension/height (cm/m) | 2.11 (0.25) | 2.16 (0.27) | 2.12 (0.25) | NS |
| TAPSE (cm) | 2.19 (0.35) | 2.14 (0.37) | 2.08 (0.31) | NS |
| Doppler diastology | ||||
| LV E (cm/s) | 92.7 (18.7) | 92.8 (18.3) | 89.2 (18.1) | NS |
| LV Em (cm/s) | 16.8 (4.6) | 17.0 (4.5) | 16.1 (4.0) | NS |
| E/Em ratio | 5.9 (1.8) | 5.8 (1.7) | 5.9 (2.0) | NS |
Mean during the study.
All models adjusted for time in study (ie , similar to duration of diabetes). All echocardiography LV and Doppler diastology parameters are log transformed before testing due to skewed distributions. Pairwise comparisons are given for the significant associations found (P < .05).
The distribution of TNF-α was positively skewed, with elevated serum concentrations (≥5.6 pg/mL) in 18.3% of participants at baseline (Figure, C). There was no change in the prevalence of abnormal TNF-α values over the course of the study. Values were normal at all visits in 67.6% of participants, with 11.9% having a single elevation, and 8.1% being elevated at all examinations. When divided into 3 groups (always normal, intermittent, and always high), those with at least 1 elevation during the study (n = 127) had higher LV mass index than those always normal (mean [SD], 161.2 g [46.2] vs 150.2 g [44.9]; P = .048). In analyses restricted to baseline TNF-α, no significant associations were identified. At the end of the study, those with an elevated TNF-α value had a trend toward higher HbA1c compared with those with normal TNF-α (mean [SD], 7.2% [1.8] vs 6.9% [1.5]; P = .052), and BP. No other differences related to presence of elevated TNF-α (treatment group, race-ethnicity, HbA1c, risk factors, or other echocardiography parameter; Table IV [available at www.jpeds.com]).
Table IV.
TODAY participant (n = 521) end-of-study and overall* characteristics, T2D parameters, and echocardiography parameters by TNF-α group†
| (A) | (B) | (C) | ||
|---|---|---|---|---|
| TNF-αs (pg/mL) | Always normal (<5.6) (n = 352) | Intermittent (n = 127) | Always high (≥5.6) (n = 42) | P value |
| T2D duration (y) | 4.1 (1.4) | 4.0 (1.3) | 3.9 (1.4) | NS |
| Male (%) | 34.7% | 38.6% | 42.9% | NS |
| Race-ethnicity (%) | ||||
| Non-Hispanic black | 33.9% | 30.9% | 45.2% | NS |
| Hispanic | 44.5% | 49.6% | 38.1% | |
| Non-Hispanic white | 21.5% | 19.5% | 16.7% | |
| Treatment group (%) | ||||
| Metformin | 33.8% | 34.6% | 33.3% | NS |
| Metformin + rosiglitazone | 30.7% | 32.3% | 38.1% | |
| Metformin + lifestyle | 35.5% | 33.1% | 28.6% | |
| Failed to maintain glycemic control (%) | 46.6% | 48.8% | 47.6% | NS |
| End-of-study HbA1c (%) | 7.7 (2.5) | 7.7 (2.6) | 7.7 (2.2) | NS |
| Mean HbA1c during study (%) | 6.9 (1.5) | 6.9 (1.7) | 7.1 (1.6) | NS |
| End-of-study BMI (kg/m2) | 35.9 (8.7) | 37.4 (8.0) | 37.2 (6.4) | NS |
| Mean BMI during study (kg/m2) | 35.1 (8.0) | 36.4 (7.8) | 36.6 (6.5) | NS |
| End-of-study systolic BP (mm Hg) | 114.5 (11.1) | 116.3 (13.0) | 116.1 (10.0) | NS |
| Mean systolic BP during study (mm Hg) | 113.6 (9.3) | 115.9 (10.1) | 115.0 (8.0) | NS |
| End-of-study diastolic BP (mm Hg) | 69.0 (8.8) | 70.0 (9.4) | 69.3 (8.9) | NS |
| Mean diastolic BP during study (mm Hg) | 67.8 (6.7) | 68.9 (7.1) | 68.5 (5.5) | NS |
| LV mass (g) | 150.2 (44.9) | 161.2 (46.2) | 156.8 (38.6) | .0476 A vs B |
| LV mass/height2.7 (g/m2.7) | 36.9 (9.2) | 38.9 (8.5) | 38.7 (8.2) | NS |
| LV relative wall thickness | 0.34 (0.06) | 0.34 (0.06) | 0.34 (0.06) | NS |
| LV fractional shortening (%) | 38.5 (5.2) | 38.1 (5.2) | 37.5 (4.0) | NS |
| LA internal dimension (cm) | 3.57 (0.46) | 3.66 (0.46) | 3.54 (0.36) | NS |
| LA internal dimension/height (cm/m) | 2.13 (0.26) | 2.17 (0.26) | 2.12 (0.24) | NS |
| TAPSE (cm) | 2.15 (0.36) | 2.20 (0.38) | 2.07 (0.29) | NS |
| Doppler diastology | ||||
| LV E (cm/s) | 92.4 (18.3) | 92.7 (18.8) | 91.7 (19.7) | NS |
| LV Em (cm/s) | 16.8 (4.6) | 16.6 (4.2) | 17.1 (4.5) | NS |
| E/Em ratio | 5.8 (1.9) | 5.9 (1.7) | 5.6 (1.6) | NS |
Mean during the study.
All models adjusted for time in study (ie, similar to duration of diabetes). All echocardiography LV and Doppler diastology parameters are log transformed before testing due to skewed distributions. Pairwise comparisons are given for the significant associations found (P < .05).
TNF-R1 and TNF-R2 serum concentrations were normally distributed across the cohort (Figure, D and E). Mean values were significantly higher than reported in nondiabetic cohorts.18 Visit-to-visit correlation for TNF-R1 and TNF-R2 were strong (correlation coefficient, 0.70–0.86). Longitudinal repeated measures analyses indicated no change over time in receptor concentrations. For both TNF-R1 and TNF-R2, there was a graded relationship with participants in the highest tertiles having greater BMI and BMI-associated risk factors: systolic BP, LV mass, LV relative wall thickness, and LA diameter (Table V and Table VI; available at www.jpeds.com). There was also a relationship to LV Em and E/Em ratio tissue Doppler imaging (Table V). Because TNF-R1 and TNF-R2 were strongly related to BMI, relevant comparisons were adjusted for BMI. All significance disappeared after adjustment for BMI, except for the relationship between end-of-study TNF-R1 and TNF-R2 with LV Em (P = .01 and P = .007, respectively). No differences related to treatment group, race-ethnicity, diabetes duration, risk factors, or echocardiographic parameters were found. Similar results were obtained when analyses were repeated based on TNF-R1 and TNF-R2 tertiles during the study (data not shown).
Table V.
TODAY participant (n = 521) end-of-study and overall* characteristics, T2D parameters, and echocardiography parameters by end-of-study TNF-R1 tertile†
| (A) | (B) | (C) | ||
|---|---|---|---|---|
| End-of-study TNF-R1s (pg/mL) | Tertile 1 (<1155) | Tertile 2 (1155–1463) | Tertile 3 (1464+) | P value |
| T2D duration (y) | 4.0 (1.4) | 3.9 (1.4) | 4.2 (1.2) | NS |
| Male (%) | 30.6% | 40.7% | 37.6% | NS |
| Race-ethnicity (%) | ||||
| Non-Hispanic black | 39.6% | 32.9% | 29.1% | NS |
| Hispanic | 45.6% | 44.3% | 46.1% | |
| Non-Hispanic white | 14.8% | 22.7% | 24.8% | |
| Treatment group (%) | ||||
| Metformin | 34.7% | 34.9% | 32.9% | NS |
| Metformin + rosiglitazone | 31.8% | 31.4% | 31.2% | |
| Metformin + lifestyle | 33.5% | 33.7% | 35.8% | |
| Failed to maintain glycemic control (%) | 52.6% | 43.0% | 45.7% | NS |
| End-of-study HbA1c (%) | 8.0 (2.8) | 7.4 (2.2) | 7.6 (2.4) | NS |
| Mean HbA1c during study (%) | 7.1 (1.7) | 6.8 (1.4) | 6.9 (1.6) | NS |
| End-of-study BMI (kg/m2) | 33.5 (6.2) | 36.3 (8.2) | 39.4 (9.3) | P < .0001 A vs B,C, B vs C |
| Mean BMI during study (kg/m2) | 33.0 (6.1) | 35.5 (7.9) | 38.1 (8.6) | P < .0001 A vs B,C, B vs C |
| End-of-study systolic BP (mm Hg) | 113.7 (10.7) | 115.0 (11.8) | 116.6 (11.9) | NS |
| Mean systolic BP during study (mm Hg) | 113.1 (9.1) | 113.6 (9.3) | 116.2 (9.6) | .007 C vs A, B |
| End-of-study diastolic BP (mm Hg) | 69.1 (9.0) | 69.3 (9.0) | 69.4 (8.8) | NS |
| Mean diastolic BP during study (mm Hg) | 68.0 (7.0) | 67.6 (6.8) | 68.7 (6.4) | NS |
| LV mass (g) | 141.6 (42.6) | 157.9 (44.3) | 161.1 (45.5) | P < .0001 A vs B,C |
| LV mass/height2.7 (g/m2.7) | 35.0 (8.1) | 38.5 (9.1) | 39.3 (9.0) | P < .0001 A vs B,C |
| LV relative wall thickness | 0.33 (0.06) | 0.33 (0.06) | 0.35 (0.06) | NS |
| LV fractional shortening (%) | 38.4 (5.3) | 38.3 (5.5) | 38.3 (4.7) | NS |
| LA internal dimension (cm) | 3.51 (0.44) | 3.60 (0.48) | 3.66 (0.42) | .02 A vs C |
| LA internal dimension/height (cm/m) | 2.10 (0.26) | 2.14 (0.26) | 2.18 (0.24) | NS |
| TAPSE (cm) | 2.13 (0.34) | 2.18 (0.36) | 2.17 (0.37) | NS |
| Doppler diastology | ||||
| LV E (cm/s) | 92.1 (19.1) | 93.3 (18.2) | 91.9 (18.3) | NS |
| LV Em (cm/s) | 17.5 (4.5) | 17.3 (4.4) | 15.8 (4.3) | .0003 C vs A, B |
| E/Em ratio | 5.6 (1.8) | 5.7 (1.6) | 6.2 (1.9) | .004 C vs A, B |
Mean during the study.
All models adjusted for time in study (ie, similar to duration of diabetes). All echocardiography LV and Doppler diastology parameters are log transformed before testing due to skewed distributions. Pairwise comparisons are given for the significant associations found (P < .05). All significant relationships disappear after adjustment for BMI in the models, except for LV Em (adjusted P = .01).
Table VI.
TODAY participant (n = 521) end-of-study and overall* characteristics, T2D parameters, and echocardiography parameters by end-of-study TNF-R2 tertile†
| (A) | (B) | (C) | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| End-of-study TNF-R2s (pg/mL) | (<5180) | (5180–6870) | (6871+) | P value |
| T2D duration (y) | 3.9 (1.4) | 4.0 (1.4) | 4.1 (1.3) | NS |
| Male (%) | 35.8% | 35.8% | 37.2% | NS |
| Race-ethnicity (%) | ||||
| Non-Hispanic black | 32.7% | 37.1% | 31.9% | NS |
| Hispanic | 47.0% | 42.5% | 46.4% | |
| Non-Hispanic white | 20.2% | 20.4% | 21.7% | |
| Treatment group (%) | ||||
| Metformin | 30.6% | 35.3% | 36.6% | NS |
| Metformin + rosiglitazone | 31.2% | 32.9% | 30.2% | |
| Metformin + lifestyle | 38.2% | 31.8% | 33.1% | |
| Failed to maintain glycemic control (%) | 44.5% | 44.5% | 52.3% | NS |
| End-of-study HbA1c (%) | 7.6 (2.5) | 7.5 (2.4) | 8.0 (2.5) | NS |
| Mean HbA1c during study (%) | 6.8 (1.5) | 6.9 (1.5) | 7.1 (1.6) | NS |
| End-of-study BMI (kg/m2) | 32.5 (5.8) | 37.1 (7.6) | 39.5 (9.7) | P < .0001 A vs B,C, B vs C |
| Mean BMI during study (kg/m2) | 32.0 (5.8) | 36.4 (7.3) | 38.3 (8.9) | P < .0001 A vs B,C, B vs C |
| End-of-study systolic BP (mm Hg) | 113.3 (11.3) | 114.5 (11.2) | 117.6 (11.6) | .0022 C vs A, B |
| Mean systolic BP during study (mm Hg) | 112.2 (10.0) | 114.3 (9.0) | 116.3 (8.9) | .0005 A vs B,C |
| End-of-study diastolic BP (mm Hg) | 68.7 (9.2) | 68.7 (8.8) | 70.4 (8.6) | NS |
| Mean diastolic BP during study (mm Hg) | 68.5 (7.2) | 68.0 (6.6) | 68.9 (6.3) | NS |
| LV mass (g) | 143.1 (39.2) | 155.8 (49.3) | 162.2 (43.6) | .0007 A vs B,C |
| LV mass/height2.7 (g/m2.7) | 35.5 (8.0) | 38.2 (9.3) | 39.2 (9.1) | .0008 A vs B,C |
| LV relative wall thickness | 0.33 (0.06) | 0.34 (0.06) | 0.34 (0.06) | NS |
| LV fractional shortening (%) | 38.4 (5.1) | 38.0 (5.1) | 38.6 (5.2) | NS |
| LA internal dimension (cm) | 3.50 (0.46) | 3.57 (0.46) | 3.71 (0.40) | .0001 C vs A, B |
| LA internal dimension/height (cm/m) | 2.09 (0.27) | 2.13 (0.25) | 2.20 (0.24) | .0014 C vs A, B |
| TAPSE (cm) | 2.13 (0.36) | 2.16 (0.37) | 2.18 (0.35) | NS |
| Doppler diastology | ||||
| LV E (cm/s) | 89.9 (18.4) | 94.7 (18.5) | 92.6 (18.4) | NS |
| LV Em (cm/s) | 17.5 (4.8) | 17.3 (4.2) | 15.6 (4.2) | .0001 C vs A, B |
| E/Em ratio | 5.5 (1.7) | 5.8 (1.8) | 6.3 (1.9) | .0001 C vs A, B |
Mean during the study.
All models adjusted for time in study (ie, similar to duration of diabetes). All echocardiography LV and Doppler diastology parameters are log transformed before testing due to skewed distributions. Pairwise comparisons are given for the significant associations found (P < .05). All significant relationships disappear after adjustment for BMI in the model, except for LV Em (adjusted P = .0069).
Discussion
In this analysis of longitudinal trends in cardiac biomarkers in youth with T2D, there are novel and clinically relevant observations. Importantly, no adverse trends in biomarker concentrations during 2–6 years of treatment were observed, suggesting an absence of subclinical changes in cardiac function in the early course of adolescent T2D. For biomarkers that are not normally distributed (BNP, troponin, and TNF-α), we observed high visit-to-visit variability, especially for troponin, with a relatively high frequency of values outside the normal range at any individual visit. In a few individuals, BNP and TNF-α remained consistently high. These data suggest that, although abnormalities in these biomarkers are prevalent in adolescents with T2D, they should not be considered pathologic without repeat measurement and clinical correlation. TNF-R1 and TNF-R2 values were associated with systolic BP and several cardiac structure and function parameters, but these relationships seemed to be driven by BMI. TNF-α and BNP concentrations were not related to BMI, although there was a trend for those with variable troponin concentrations to have a higher BMI.
Nonetheless, the frequency of values outside reported ranges, particularly for BNP, troponin, and TNF-α receptor, remains concerning. In older adults, cardiovascular and in-flammatory biomarkers are related to CVD outcomes over a relatively short follow-up interval.8 Although adolescents with T2D have a significant lifetime risk for CVD, they may have a lower short-term risk for adverse outcomes than adults. These biomarkers also have been associated with other adverse outcomes. For example, higher TNF-α concentrations have been associated with BP disorders of pregnancy and lower newborn birth weight.20–24 Long-term follow-up of this cohort to determine the clinical implications for these findings is necessary.
There were few significant associations between the biomarkers, CVD risk factors, and echocardiographic parameters. There were no relationships to treatment group, race-ethnicity, diabetes duration, or risk factors (other than BMI for TNF-R1 and TNF-R2). TNF-α elevation was related to worse glycemic control, but the other markers did not show this association. With regard to echocardiographic parameters, persistently elevated troponin was related to higher LV relative wall thickness and lower tricuspid annular plane systolic excursion. TNF-α was related to higher LV mass index. However, given the large number of comparisons and .05 < P < .01 among the relationships, these findings should be considered hypothesis generating rather than confirmed positive associations. BNP was unrelated to any echocardiographic parameters.
Collectively, these data do not support a role for the routine measurement of these biomarkers during the care of adolescents with T2D. They may actually be misleading, for example, in the evaluation of chest pain. Most studies of biomarkers in children and adolescents report single measures and only cross-sectional correlations. A strength of this analysis is that these longitudinal measurements combined with other TODAY study findings allowed for evaluation of chronic exposure and relationships to baseline parameters.
BNP is most commonly measured for heart failure assessment.25,26 In this study, BNP elevations were not associated with measures of cardiac function. In healthy adults, BNP is not a marker for future heart failure or adverse events.27 In adults with T2D and/or hypertension, BNP predicts future events in older, but not younger, patients.26,28,29 There are many studies suggesting a link between BNP concentrations and future diabetes vascular complications; however, there are limited pediatric data.26 Because atrial and ventricular stretch may vary depending on diabetes control, blood volume, and other factors impacting cardiac chamber size, the variation of individual values may occur and explain the results presented. Long-term follow-up of adolescents with T2D will be needed to determine the prognostic value of BNP measurement.
Measurement of troponin is typically used to diagnose myocardial infarction in emergency or urgent care settings.17,30 Elevated troponin has been associated with myocardial injury, including myopericarditis.31 The value of troponin as a long-term predictor of myocardial outcomes is uncertain. There is no prior information on troponin in youth with T2D. The finding that more than one-half the participants in the TODAY study had an elevated value at least once suggests that subclinical and mild myocardial injury occurs in T2D. Elevated values in asymptomatic individuals should not be considered pathologic. Their value in symptomatic individuals in the TODAY age range is unknown, but our data suggest that there might be high false-positive rates.
TNF-α is a proinflammatory adipocytokine.32 Elevated concentrations may increase insulin resistance by downregulating genes required for insulin action and directly affecting insulin transduction to impair glucose metabolism and stimulate lipolysis.33–35 There is evidence that increased TNF-α concentrations may contribute directly to myocardial insulin resistance.36 In this study, TNF-α concentrations did not substantially change over 2–6 years of follow-up, suggesting that the inflammatory status as monitored by this biomarker was stable. TNF-α concentrations are not modified by bariatric surgery.37 This is in contrast with findings with regard to high-sensitivity C-reactive protein, interleukin-6, and plasminogen activator inhibitor-1 levels, which increased during TODAY follow-up.4 By the end of the study, TNF-α concentrations showed an association with LV mass, BP, and HbA1c.
TNF-R1 and TNF-R2 concentrations add to the assessment of TNF-α systemic activation, particularly when TNF-α is degraded rapidly. These biomarkers were initially associated with progression of renal impairment in T2D, but, more recently, have been associated with CVD and all-cause mortality.12,13 In this study, these variables were strongly associated with several CVD risk factors and echocardiographic traits; however, after adjustment for BMI, these relationships were completely attenuated. In the absence of an obese nondiabetic control group, we cannot be certain if this adverse distribution in the TODAY cohort is due to obesity alone or is related to diabetes.
The collection of biomarker data was not a primary aim of the TODAY study. A change in laboratory values related to prolonged storage or stability of specimens could have occurred. All specimens were analyzed simultaneously, without freeze–thaw cycles, minimizing assay variation risk. End-of-study laboratory data could have been collected up to 3 months from the time of the echocardiogram. There were a variable number of measurements of each biomarker (range, 2–6) across the cohort creating missing data, but sensitivity analyses did not suggest that this created a bias. There was no lean or obese non-diabetic control group and markers of adequate day-to-day hydration were not available. Epicardial fat as an independent measure of influence of fat on outcomes was not measured.
The role of cardiac biomarkers in the assessment and management of youth with T2D is uncertain. Long-term follow-up of this cohort is required to understand the prognostic value of these markers, particularly BNP, in identifying those at risk for future ventricular dysfunction, heart failure, or other CVD complications. Unlike conventional risk factors, such as lipids and BP, these cardiac biomarker concentrations did not worsen during follow-up of the TODAY cohort.3,4 Owing to the high variability of troponin, BNP, and TNF-α measurements, providers should exert caution in interpretation of isolated values, particularly in the absence of clinical symptoms or a CVD diagnosis.
Acknowledgments
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc; Pfizer; Sanofi Aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service Institution Review Boards or their members.
Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
Funded by the National Institute of Diabetes and Digestive and Kidney Disease and National Institutes of Health (U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, U01-DK61254, and T32-DK063687), the National Center for Research Resources General Clinical Research Centers Program (M01-RR00036 [Washington University School of Medicine], M01-RR00043-45 [Children’s Hospital Los Angeles], M01-RR00069 [University of Colorado Denver], M01-RR00084 [Children’s Hospital of Pittsburgh], M01-RR01066 [Massachusetts General Hospital], M01-RR00125 [Yale University], and M01-RR14467 [University of Oklahoma Health Sciences Center], and by the NCRR Clinical and Translational Science Awards (UL1-RR024134 [Children’s Hospital of Philadelphia], UL1-RR024139 [Yale University], UL1-RR024153 [Children’s Hospital of Pittsburgh], UL1-RR024989 [Case Western Reserve University], UL1-RR024992 [Washington University in St Louis], UL1-RR025758 [Massachusetts General Hospital], and UL1-RR025780 [University of Colorado Denver]). R.S.W. has participated in multicenter clinical trials sponsored by Medtronic Minimed Inc, Diasome Pharmaceuticals Inc, Calibra Medical Inc, and Mylan GmbH Inc. L.L.L is a consultant for Eli Lilly.
Glossary
- BMI
Body mass index
- BNP
brain natriuretic peptide*
- BP
blood pressure*
- CVD
cardiovascular disease*
- HbA1c
Hemoglobin A1c
- LA
left atrial*
- LV
left ventricular*
- T2D
Type 2 diabetes*
- TNF-R1
tumor necrosis receptor 1*
- TNF-R2
tumor necrosis receptor 2*
- TNF-α
tumor necrosis factor alpha*
- TODAY
Treatment Options for type 2 Diabetes in Adolescents and Youth*
- Troponin
high-sensitivity troponin*
Appendix
Listing of the TODAY Study Group
The following individuals and institutions constitute the TODAY Study Group (*principal investigator or director):
CLINICAL CENTERS Baylor College of Medicine: S. McKay*, M. Haymond*, B. Anderson, C. Bush, S. Gunn, H. Holden, S.M. Jones, G. Jeha, S. McGirk, S. Thamotharan Case Western Reserve University: L. Cuttler* (deceased), E.Abrams, T. Casey, W. Dahms (deceased), C. Ievers-Landis, B. Kaminski, M. Koontz, S. MacLeish, P. McGuigan, S. Narasimhan Children’s Hospital LosAngeles: M.Geffner*,V.Barraza, N.Chang, B. Conrad, D. Dreimane, S. Estrada, L. Fisher, E. Fleury-Milfort, S. Hernandez, B. Hollen, F. Kaufman, E. Law,V. Mansilla, D. Miller, C. Muñoz, R. Ortiz, A. Ward, K. Wexler, Y.K. Xu, P. Yasuda Children’s Hospital of Philadelphia: L. Levitt Katz*, R. Berkowitz, S. Boyd, B. Johnson, J. Kaplan, C. Keating, C. Lassiter, T. Lipman, G. McGinley, H. McKnight, B. Schwartzman, S. Willi Children’s Hospital of Pittsburgh: S. Arslanian*, F. Bacha, S. Foster, B. Galvin, T. Hannon, A. Kriska, I. Libman, M. Marcus, K. Porter, T. Songer, E. Venditti Columbia University Medical Center: R. Goland*, D. Gallagher, P. Kringas, N.Leibel, D.Ng, M.Ovalles, D.Seidman Joslin Diabetes Center: L. Laffel*, A. Goebel-Fabbri, M. Hall, L. Higgins, J. Keady, M. Malloy, K. Milaszewski, L. Rasbach Massachusetts General Hospital: D.M. Nathan*, A. Angelescu, L. Bissett, C. Ciccarelli, L. Delahanty, V. Goldman, O. Hardy, M. Larkin, L. Levitsky, R. McEachern, D.Norman, D.Nwosu, S.Park-Bennett, D.Richards, N. Sherry, B. Steiner Saint Louis University: S. Tollefsen*, S. Carnes, D. Dempsher, D. Flomo, T. Whelan, B. Wolff State University of NewYork Upstate Medical University: R.Weinstock*, D. Bowerman, S. Bristol, J. Bulger, J. Hartsig, R. Izquierdo, J. Kearns, R. Saletsky, P. Trief University of Colorado Denver: P. Zeitler* (Steering Committee Chair),N.Abramson, A.Bradhurst, N. Celona-Jacobs, J. Higgins, M. Kelsey, G. Klingensmith, K. Nadeau, T. Witten University of Oklahoma Health Sciences Center: K. Copeland* (Steering CommitteeVice-Chair), E. Boss, R. Brown, J. Chadwick, L. Chalmers, S. Chernausek, A. Hebensperger, C. Macha, R. Newgent, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, S. Sternlof University of Texas Health Science Center at San Antonio: J. Lynch*, N.Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, C. Ibarra, E. Morales, S. Rivera, G. Rupert, A. Wauters Washington University in St Louis: N. White*, A. Arbeláez, D. Flomo, J. Jones, T. Jones, M. Sadler, M. Tanner, A. Timpson, R. Welch Yale University: S. Caprio*, M. Grey, C. Guandalini, S. Lavietes, P. Rose, A. Syme, W. Tamborlane
COORDINATING CENTER George Washington University Biostatistics Center: K. Hirst*, S. Edelstein, P. Feit, N. Grover, C. Long, L. Pyle
PROJECT OFFICE National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder*
CENTRAL UNITS Central Blood Laboratory (Northwest Lipid Research Laboratories, University of Washington): S.M. Marcovina*, J. Harting DEXA Reading Center (University of California at San Francisco): J. Shepherd*, B. Fan, L. Marquez, M. Sherman, J. Wang Diet Assessment Center (University of South Carolina): M. Nichols*, E. Mayer-Davis, Y. Liu Echocardiogram Reading Center (Johns Hopkins University): J. Lima*, S Gidding, J. Puccella, E. Ricketts Fundus Photography Reading Center (University of Wisconsin): R. Danis*, A. Domalpally, A. Goulding, S. Neill, P. Vargo Lifestyle Program Core (Washington University): D. Wilfley*, D. Aldrich-Rasche, K. Franklin, C. Massmann, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren
OTHER Hospital for Sick Children, Toronto: M. Palmert Medstar Research Institute, Washington DC: R. Ratner Texas Tech University Health Sciences Center: D. Dremaine University of Florida: J. Silverstein
Footnotes
The other authors declare no conflicts of interest.
Trial registration ClinicalTrials.gov NCT00081328.
References
- 1.Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130:1532–58. doi: 10.1161/CIR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 2.TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–56. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1735–41. doi: 10.2337/dc12-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.TODAY Study Group. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1758–64. doi: 10.2337/dc12-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levitt Katz L, Gidding SS, Bacha F, Hirst K, McKay S, Pyle L, et al. Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatr Diabetes. 2015;16:39–47. doi: 10.1111/pedi.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah AS, Khoury PR, Dolan LM, Ippisch HM, Urbina EM, Daniels SR, et al. The effects of obesity and type 2 diabetes mellitus on cardiac structure and function in adolescents and young adults. Diabetologia. 2011;54:722–30. doi: 10.1007/s00125-010-1974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjornstad P, Truong U, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, et al. Cardiopulmonary dysfunction and adiponectin in adolescents with type 2 diabetes. J Am Heart Assoc. 2016;5:e002804. doi: 10.1161/JAHA.115.002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scirica BM, Bhatt DL, Braunwald E, Raz I, Cavender MA, Im K, et al. Prognostic implications of biomarker assessments in patients with type 2 diabetes at high cardiovascular risk: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:989–98. doi: 10.1001/jamacardio.2016.3030. [DOI] [PubMed] [Google Scholar]
- 9.Qiao YC, Shen J, He L, Hong XZ, Tian F, Pan YH, et al. Changes of regulatory T cells and of proinflammatory and immunosuppressive cytokines in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. 2016;2016:3694957. doi: 10.1155/2016/3694957. Epub 2016 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tehrani DM, Wong ND. Integrating biomarkers and imaging for cardiovascular disease risk assessment in diabetes. Curr Cardiol Rep. 2016;18:105. doi: 10.1007/s11886-016-0789-7. [DOI] [PubMed] [Google Scholar]
- 11.Vasudevan NT, Mohan ML, Gupta MK, Martelli EE, Hussain AK, Qin Y, et al. Gβγ-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor α-induced cardiac β-adrenergic receptor dysfunction. Circulation. 2013;128:377–87. doi: 10.1161/CIRCULATIONAHA.113.003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saulnier PJ, Gand E, Ragot S, Ducrocq G, Halimi JM, Hulin-Delmotte C, et al. SURDIAGENE Study Group. Association of serum concentration of TNFR1 with all-cause mortality in patients with type 2 diabetes and chronic kidney disease: follow-up of the SURDIAGENE Cohort. Diabetes Care. 2014;37:1425–31. doi: 10.2337/dc13-2580. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson AC, Östgren CJ, Nystrom FH, Länne T, Jennersjö P, Larsson A, et al. Association of soluble tumor necrosis factor receptors 1 and 2 with nephropathy, cardiovascular events, and total mortality in type 2 diabetes. Cardiovasc Diabetol. 2016;29:15–40. doi: 10.1186/s12933-016-0359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valgimigli M, van Mieghem CA, Ong AT, Aoki J, Granillo GA, McFad-den EP, et al. Short- and long-term clinical outcome after drug-eluting stent implantation for the percutaneous treatment of left main coronary artery disease: insights from the Rapamycin-Eluting and Taxus Stent Evaluated At Rotterdam Cardiology Hospital registries (RESEARCH and T-SEARCH) Circulation. 2005;111:1383–9. doi: 10.1161/01.CIR.0000158486.20865.8B. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Berry C, Kingsmore D, Gibson S, Hole D, Morton JJ, Byrne D, et al. Predictive value of plasma brain natriuretic peptide for cardiac outcome after vascular surgery. Heart. 2006;92:401–2. doi: 10.1136/hrt.2005.060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apple FS, Parvin CA, Buechler KF, Christenson RH, Wu AHB, Jaffe AS. Validation of the 99th percentile cutoff independent of assay imprecision (CV) for cardiac troponin monitoring for ruling out myocardial infarction. Clin Chem. 2005;51:2198–200. doi: 10.1373/clinchem.2005.052886. [DOI] [PubMed] [Google Scholar]
- 18.Putko BN, Wang Z, Lo J, Anderson T, Becher H, Dyck JR, et al. Circulating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PLoS ONE. 2014;9:e99495. doi: 10.1371/journal.pone.0099495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R., Jr National Academy of Clinical Biochemistry Standards of Laboratory Practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem. 1999;45:1104–21. [PubMed] [Google Scholar]
- 20.McDade TW, Borja JB, Largado F, Adair LS, Kuzawa CW. Adiposity and chronic inflammation in young women predict inflammation during normal pregnancy in the Philippines. J Nutr. 2016;146:353–7. doi: 10.3945/jn.115.224279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kac G, Mendes RH, Farias DR, Eshriqui I, Rebelo F, Benaim C, et al. Hepatic, renal and inflammatory biomarkers are positively associated with blood pressure changes in healthy pregnant women: a prospective cohort. Medicine (Baltimore) 2015;94:e683. doi: 10.1097/MD.0000000000000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzawa CW, Tallman PS, Adair LS, Lee N, McDade TW. Inflammatory profiles in the non-pregnant state predict offspring birth weight at Cebu: evidence for inter-generational effects of low grade inflammation. Ann Hum Biol. 2012;39:267–74. doi: 10.3109/03014460.2012.692810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siwetz M, Blaschitz A, El-Heliebi A, Hiden U, Desoye G, Huppertz B, et al. TNF-α alters the inflammatory secretion profile of human first trimester placenta. Lab Invest. 2016;96:428–38. doi: 10.1038/labinvest.2015.159. [DOI] [PubMed] [Google Scholar]
- 24.Lau SY1, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol. 2013;70:412–27. doi: 10.1111/aji.12138. [DOI] [PubMed] [Google Scholar]
- 25.Francis GS, Felker GM, Tang WH. A test in context: critical evaluation of natriuretic peptide testing in heart failure. J Am Coll Cardiol. 2016;67:330–7. doi: 10.1016/j.jacc.2015.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruden G, Landi A, Bruno G. Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research. Diabetes Care. 2014;37:2899–908. doi: 10.2337/dc14-0669. [DOI] [PubMed] [Google Scholar]
- 27.McKie PM, Cataliotti A, Lahr BD, Martin FL, Redfield MM, Bailey KR, et al. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55:2140–7. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballo P, Betti I, Barchielli A, Balzi D, Castelli G, De Luca L, et al. Prognostic role of N-terminal pro-brain natriuretic peptide in asymptomatic hypertensive and diabetic patients in primary care: impact of age and gender: results from the PROBE-HF study. Clin Res Cardiol. 2016;105:421–31. doi: 10.1007/s00392-015-0937-x. [DOI] [PubMed] [Google Scholar]
- 29.Kroon MH, van den Hurk K, Alssema M, Kamp O, Stehouwer CD, Henry RM, et al. Prospective associations of B-type natriuretic peptide with markers of left ventricular function in individuals with and without type 2 diabetes: an 8-year follow-up of the Hoorn Study. Diabetes Care. 2012;35:2510–4. doi: 10.2337/dc11-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brush JE, Jr, Kaul S, Krumholz HM. Troponin testing for clinicians. J Am Coll Cardiol. 2016;68:2365–75. doi: 10.1016/j.jacc.2016.08.066. [DOI] [PubMed] [Google Scholar]
- 31.Anaya P, Moliterno DJ. The evolving role of cardiac troponin in the evaluation of cardiac disorders. Curr Cardiol Rep. 2013;15:420. doi: 10.1007/s11886-013-0420-0. [DOI] [PubMed] [Google Scholar]
- 32.Swaroop JJ, Rajarajeswari D, Naidu JN. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2012;135:127–30. doi: 10.4103/0971-5916.93435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moller DE. Potential role of TNF-α in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–7. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 34.Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, et al. Effects of TNF-α neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2007;293:E102–9. doi: 10.1152/ajpendo.00089.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tangvarasittichai S, Pongthaisong S, Tangvarasittichai O. Tumor necrosis factor-A, interleukin-6, C-reactive protein levels and insulin resistance associated with type 2 diabetes in abdominal obesity women. Indian J Clin Biochem. 2016;31:68–74. doi: 10.1007/s12291-015-0514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu F, Zhao K, Li J, Xu J, Zhang Y, Liu C, et al. Direct evidence that myocardial insulin resistance following myocardial ischemia contributes to post-ischemic heart failure. Sci Rep. 2015;5:17927. doi: 10.1038/srep17927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, Inge TH. Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int J Obes (Lond) 2016;40:275–80. doi: 10.1038/ijo.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]