Abstract
background
A 2007 national report identified North Carolina’s Edgecombe County as having among the highest breast cancer incidence and mortality rates nationally, motivating the initiation of a task force and other local efforts to address the problem. The goal of this study is to examine county breast cancer characteristics before and after the report, including whether geographic variation may mask racial disparities in this majority African American community.
method
With guidance from community partners, breast cancer cases from 2000 to 2012 in Edgecombe, Nash, and Orange Counties (N = 2,641) were obtained from the North Carolina Central Cancer Registry. Bivariate and trend analyses of tumor and treatment characteristics were examined by county and race.
results
Women in Edgecombe and Nash Counties were diagnosed with more advanced stage, higher grade tumors. African Americans in Edgecombe and Nash Counties were diagnosed with advanced disease more often than African Americans in Orange County. Average time-to-treatment was well within guideline recommendations. Incidence and mortality rates appear to have declined, with variation in measures of racial differences over time.
limitations
Changes in coding standards across the observation period required reliance on coarse measures that may partially mute useful findings.
conclusions
Racial disparities remain a concern in North Carolina; however, they appear to be less profound than in the 2007 national report. The portentous statistics in the report represent an all-time high, after which some, but not all, measures reflect positive change amidst ongoing local efforts to improve breast cancer knowledge and care.
Breast cancer is the most commonly diagnosed cancer in women and the second most common cause of cancer deaths, with 246,660 new cases and 40,450 deaths estimated for 2016 in the United States [1]. From 2008 to 2012, 43,740 women were diagnosed in North Carolina and 6,357 died from breast cancer [2, 3].
Many factors influence breast cancer outcomes, including stage at diagnosis, tumor grade (aggressiveness), and hormone receptor status [4–6]. Tumor characteristics determine treatment options, which vary in effectiveness [7]. African American (AA) women are at disproportionate risk for poor outcomes for several reasons. Among them, AA women are more likely than Caucasian American (CA) women to be diagnosed with higher stage (more advanced) disease, higher grade tumors, and hormone receptor negative tumors, which have fewer treatment options [8–11]. Differing cultural norms and insufficient monetary or local health care resources decrease the likelihood of having a regular source of health care or being able to adhere to cancer screening guidelines, resulting in diagnostic and treatment delays [12–14]. These factors are particularly relevant for Edgecombe County, North Carolina, where 58% of residents are AA, median income is much lower than other areas of the state, and health care resources are limited as denoted by its designation as a Medically Underserved Area [15, 16].
In 2007, Susan G. Komen for the Cure, the nation’s most well-known breast cancer advocacy group, listed North Carolina’s Edgecombe County as having the 16th-worst breast cancer mortality rate in the nation, with 38% of women with breast cancer dying from the disease compared to 26% nationally [17]. The report sent shockwaves through the North Carolina cancer community and sparked several efforts to understand and address the factors responsible for these poor outcomes. Funded by the National Cancer Institute, the Carolina Community Network (CCN) is a regional cancer network program that aims to reduce cancer disparities among AAs in 13 North Carolina counties, including Edgecombe County. In response to the Edgecombe community’s concern about the Komen report’s findings, CCN leveraged supplemental funding to examine the elevated rates together with a community advisory board (CAB).
The goal of this examination is to provide a contemporary understanding of breast cancer incidence and mortality rates following the release of the Komen report, including geographic differences in factors associated with breast cancer mortality and whether racial disparities may be masked by these geographic differences. We describe population breast cancer characteristics before and after the Komen report, and illustrate local community efforts (see Table 1) intended to address the disproportionate burden of breast cancer experienced in Edgecombe County.
TABLE 1.
Timeline: Susan G. Komen for the Cure Breast Cancer Report and Edgecombe County Community Response
| Founded 1969 | Opportunities Industrialization Center, Inc. (OIC) in Rocky Mount, North Carolina provides comprehensive employment, training, business, and health services to strengthen the communities they serve. OIC has a mobile health unit, community education center, and a federally qualified health center. |
| Founded 1974 | Rural Health Group, Inc. (RHG) in northeastern North Carolina (Halifax and Northampton counties) is a nonprofit, federally qualified community health center providing primary care, dental care, and other health-related services for the underserved. The RHG Community-based Outreach Program trained and coordinated breast cancer lay health advisors. |
| Founded 1999 | Crossworks, Inc. is a faith-based nonprofit that provides health and nutrition education to fight chronic disease in Nash, Edgecombe, and the surrounding area. |
| 2004 | Creation of the UNC Center for Community Research, a community-based research center located in the Area L Area Health Education Center (AHEC), which targeted several counties. The goal was to reduce health disparities through community-academic partnerships. Crossworks, RHG, and OIC were influential community leaders and research partners already addressing health concerns in the community. |
| 2005 | National Cancer Institute Center to Reduce Cancer Health Disparities awarded infrastructure funds to UNC-Chapel Hill, establishing the Carolina Community Network (CCN). CCN used the infrastructure of the UNC Center for Community Research to target and address breast, colorectal, and prostate cancers in several counties. |
| 2006 | Initiation of CCN Community Grants Program. Crossworks and RHG received community grants from the CCN to address cancer disparities in Edgecombe county. |
| 2007 | Susan G. Komen for the Cure released report |
| September 2007 | Breast Cancer Summit, Heritage Hospital, Tarboro, North Carolina (Now Vidant Edgecombe Hospital) |
| November 2007 | Edgecombe County Breast Cancer Taskforce (ECBCT) created and first official meeting held |
| 2007 | Crossworks initiated Breaking Free Breast Cancer Lay Health Advisor Program. Received funding from the Susan G. Komen Foundation and North Carolina Office of Minority Health and Health Disparities Health and Wellness Program. |
| 2008 | ECBCT obtained $135,000 funding from Susan G. Komen for the Cure |
| May 2008 | ECBCT hired Project Manager |
| 2008–2009 | Community Health Assessment for breast cancer with East Carolina University; recommendations include lay health advisor and patient navigation programs |
| August 2009 | Patient navigator hired at Heritage Hospital |
| 2008–2010 | UNC-Chapel Hill provided Breast Cancer Screening Program train-the-trainer to 6 ECBCT trainers |
| 2009–2010 | Extensive Mammography Campaign |
| 2010 | Obtained $68,000 funding from Susan G. Komen for the Cure |
Methods
Community Advisors
The use of CABs promotes understanding of local organizations and improves the relevance, integrity, and accountability of research efforts and their prospective positive impact in the community. CABs have been shown to be an effective approach to addressing disparities in health outcomes [18]. CAB members included 3 CCN community partners representing the local community, local health care providers, community-based public health organizations, and community support and development organizations. One member pioneered the formation of the volunteer Edgecombe County Breast Cancer Task Force that included community members, health care providers, and local organizations in response to the Komen report.
The CCN and CAB collaboratively identified the study questions and comparator counties. CCN team members independently performed all data management and analysis, including development of results and their primary interpretation. Meanwhile, CAB members articulated the community-based efforts and offered additional interpretation of results and discussion of implications for their community.
Comparison Counties
Two counties, Nash and Orange, were selected as comparators to Edgecombe County because they are proximal CCN area counties with similarities to Edgecombe. However, there were key differences between the counties. For example, Nash County is adjacent to Edgecombe but differs in race group composition (Nash: 55.9% CA, 37.2% AA; Edgecombe: 38.8% CA, 57.4% AA), and Orange County is more affluent with substantial local health care resources, including 2 academic medical centers with National Cancer Institute-designated cancer centers [19]. It was perceived that if racial inequities exist, they may be better seen in comparing Edgecombe to Nash; if resource and access issues exist, they may be seen in comparing Orange.
North Carolina Central Cancer Registry (NCCCR) data were obtained for 2,803 women living in Edgecombe, Nash, or Orange County and diagnosed with breast cancer between years 2000 and 2012. The sample was restricted to AA and CA women because limited numbers from other minority populations precluded their examination while adhering to the data use agreement. To minimize bias, we examined analytic cases—those that were diagnosed and received most treatment at a North Carolina facility (N = 2,641; 94.2% of all cases)—and excluded those diagnosed at autopsy or treated primarily out of state.
Breast Cancer Characteristics
The study examined variables associated with breast cancer incidence and outcomes, such as age at diagnosis, race, tumor characteristics (including stage at diagnosis), and first course of therapy [4, 5]. Measures, including stage at diagnosis, tumor grade, and hormone receptor status, were selected because they are associated with differences in survival and may inform future research or interventions [6]. Summary stage at diagnosis was used because it was the most complete and consistent measure of tumor stage across the 13-year study period. Tumor receptor status includes estrogen-receptor (ER) and progesterone-receptor (PR) status; human epidermal growth factor receptor (HER2) status was not consistently reported during the study period and is not included. These tumor characteristics are primary determinants of relevant treatment options [7], which were analyzed as informed by consensus guidelines. For early-stage breast cancer, consensus guidelines recommend either breast conserving surgery (BCS) plus radiation therapy (RT) or mastectomy, which have equivalent survival, though BCS/RT is often preferred because it is less disfiguring and has lower morbidity [20]. Women who have BCS with no RT were examined because they have reduced survival compared to women who have BCS [21]. For more advanced disease, chemotherapy improves survival [22]; however, women with hormone receptor negative disease (“Triple-negative”) have fewer treatment options, contributing to comparatively worse survival. We examined number of days from diagnosis to treatment initiation since delays of 3 or more months are associated with worse survival [23].
County-level and state-level incidence rates and mortality rates are presented as reported by the NCCCR to promote consistency with official, previously-reported statistics [2, 3, 17]. Five-year rates reflect the sum of 5 years of incidence or mortality and are used to smooth out spurious fluctuation, are per 100,000 population, and are age-adjusted to the year 2000 US Census.
Statistical Analysis
Subsets of time periods were examined to explore trends or changes over time as well as how the population experience may have differed before and after the Komen report. Chi-square tests examined distributional differences in categorical data and excluded individuals with missing/unknown data. Two-sided t-tests were used for continuous variables. Analyses of small sample sizes were confirmed using Fisher’s exact test. All P-values are unadjusted and two-tailed. Because treatment schemas differ by stage at diagnosis, treatment data were analyzed by stage at diagnosis. Analysis examined receipt of mastectomy or BCS plus RT as markers for guideline-concordant care in the context of localized disease and, separately, regional disease. Limited sample size precluded examination of distant disease [24]. Time between diagnosis and treatment initiation was examined for all groups.
Results
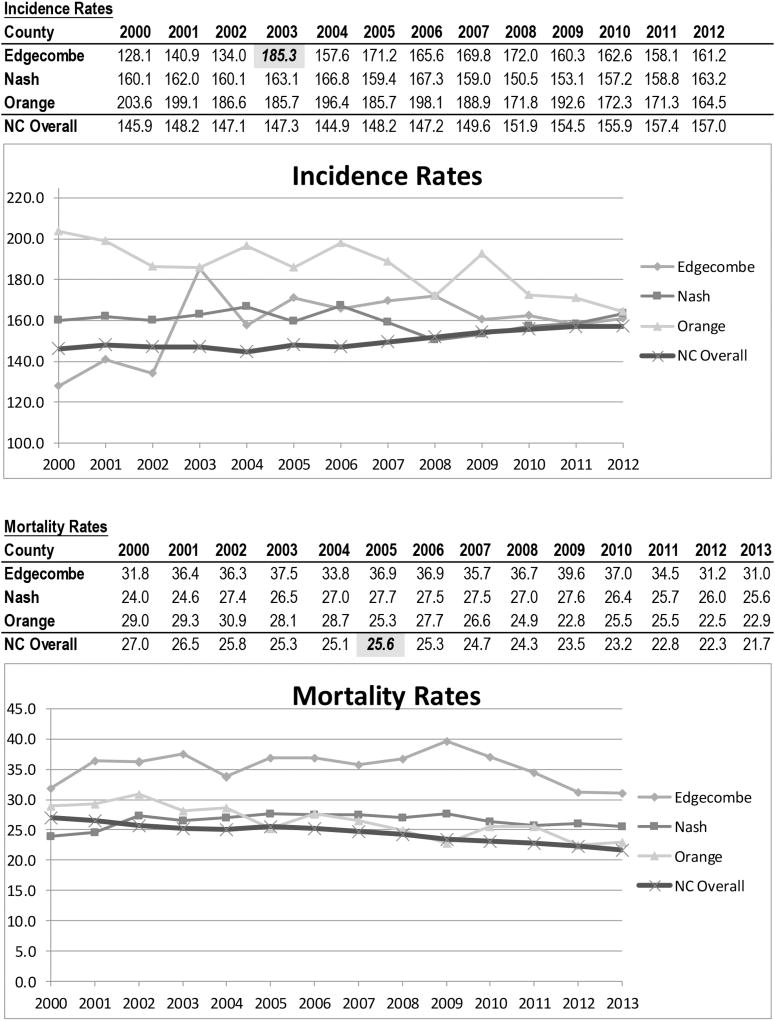
Edgecombe County’s 2003 breast cancer incidence rate of 185.3/100,000 was featured in the 2007 Komen report [17], which drew from NCCCR reports [25], but appears to be a peak, before and after which incidence rates were notably lower (see Figure 1). North Carolina’s 2005 breast cancer mortality rate of 25.6/100,000 was also featured, after which it appears to be declining. Since that time, Edgecombe County has experienced a 16% decline, though with some volatility, while the other counties have experienced apparently less substantial changes.
figure 1. Age-Adjusted 5-Year Incidence and Mortality Rates* for Breast Cancer, All Races and Ages.
*Rates are per 100,000 population and age adjusted to the year 2000 US Census. Rates reflect 5 years of incidence or mortality (eg, 2012 incidence rates reflect the total incidence for years 2008–2012).
Note. Highlighted incidence and mortality statistics are those presented in the 2007 Komen Report.
Source. North Carolina Central Cancer Registry.
Overall demographic and tumor characteristics are presented by county in Table 2 and relate to geographic differences in mortality. Women in Edgecombe and Nash Counties were more likely in 2007–2012 to be diagnosed with more advanced stage of disease compared to women in Orange County (P = .001, P = .013, respectively). Throughout the study observation period, they were also more likely than women in Orange County to be diagnosed with higher grade disease, though statistical significance varied. They were consistently much more likely to be diagnosed with hormone receptor negative disease compared to women in Orange County (P <0.001).
TABLE 2.
Demographic and Tumor Characteristics of the Study Population Analytics Cases, by County
| 2000–2006 | 2007–2012 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| P-value | P-value | |||||||||||||
| Total | Edge combe |
Nash | Orange | E vs O | E vs N | N vs O |
Total | Edge combe |
Nash | Orange | E vs O | E vs N | N vs O |
|
|
|
|
|||||||||||||
| Number of cases Race [N, (%)] | 1,379 | 300 | 505 | 574 | 1,262 | 255 | 428 | 579 | ||||||
| African American | 395 (28.6) | 158 (52.7) | 158 (31.3) | 79 (13.8) | <.001 | <.001 | <.001 | 361 (28.6) | 140 (54.9) | 148 (34.6) | 73 (12.6) | <.001 | <.001 | <.001 |
| Caucasian American | 984 (71.4) | 142 (47.3) | 347 (68.7) | 495 (86.2) | 901 (71.4) | 115 (45.1) | 280 (65.4) | 506 (87.4) | ||||||
| Mean Age at diagnosis, (SD) | ||||||||||||||
| 60.5 (14.0) | 60.6 (13.9) | 60.4 (13.8) | 60.6 (14.2) | .974 | .801 | .796 | 62.3 (13.4) | 63.1 (13.6) | 62.2 (13.4) | 62.1 (13.3) | .301 | .397 | .875 | |
| Cancer Summary Stage, Overall [n, (%)] | ||||||||||||||
| Localized | 870 (63.1) | 190 (63.3) | 325 (64.4) | 355 (61.9) | .745 | .930 | .616 | 800 (63.4) | 140 (54.9) | 265 (61.9) | 395 (68.2) | .001 | .236 | .013 |
| Regional | 430 (31.2) | 92 (30.7) | 151 (29.9) | 187 (32.6) | 397 (31.5) | 95 (37.3) | 135 (31.5) | 167 (28.8) | ||||||
| Distant | 62 (4.5) | 10–20 (~5) | 20–30 (~5) | 20–30 (~4) | 53 (4.2) | 10–20 (~6) | 20–30 (~6) | 10–20 (~3) | ||||||
| Unknown | 17 (1.2) | <11 (~1) | <11 (~1) | <11 (~1) | 12 (1.0) | <11 (~2) | <11 (~1) | <11 (~1) | ||||||
| Tumor Grade [N, (%)] | ||||||||||||||
| Grade l–ll | 657 (47.6) | 126 (42.0) | 215 (42.6) | 316 (55.1) | .003 | .342 | .020 | 690 (54.7) | 136 (53.3) | 178 (41.6) | 376 (64.9) | .057 | .197 | <.001 |
| Grade lll–IV | 513 (37.2) | 127 (42.3) | 186 (36.8) | 200 (34.8) | 387 (30.7) | 83 (32.6) | 137 (32.0) | 167 (28.8) | ||||||
| Other/Unknown | 209 (15.2) | 47 (15.7) | 104 (20.6) | 58 (10.1) | 185 (14.7) | 36 (14.1) | 113 (26.4) | 36 (6.2) | ||||||
| ER/PR Status [N, (%)] | ||||||||||||||
| ER+/PR+ | 735 (53.3) | 130 (43.3) | 250 (49.5) | 355 (61.9) | <.001 | .188 | <.001 | 777 (61.6) | 130 (51.0) | 240 (56.1) | 407 (70.3) | <.001 | .433 | <.001 |
| ER−/PR− | 291 (21.1) | 83 (27.7) | 133 (26.3) | 75 (13.1) | 276 (21.9) | 72 (28.2) | 109 (25.5) | 95 (16.4) | ||||||
| Other/Unknown | 353 (25.6) | 87 (29.0) | 122 (24.2) | 144 (25.1) | 209 (16.6) | 53 (20.8) | 79 (18.5) | 77 (13.3) | ||||||
Note. E vs N, Edgecombe County vs Nash County; E vs O, Edgecombe County vs Orange County; N vs O, Nash County vs Orange County; SD, standard deviation.
+ Two-sided T-test for continuous variables; Chi-square test for categorical variables, which exclude “unknown” category.
In some instances (eg, <11, (~5)) actual values are suppressed due to small sample size, per data use agreement requirements.
Demographic and tumor characteristics are further broken down by race in Table 3 and relate to whether geographic differences may mask racial disparities. The racial distribution of new breast cancer cases roughly paralleled overall population characteristics in each county, with AAs representing 54.9% of incident cases in Edgecombe County, 34.6% of cases in Nash County, and 12.6% of cases in Orange County in 2007–2012. CA women tended to be older at diagnosis compared to AA women, though statistical significance varied by county and time period. AA women tended to be diagnosed with more advanced disease compared to CA women, particularly in Edgecombe and Nash Counties, though in 2007–2012 this was only statistically significant in Nash County (P = .018). In addition, AA women were consistently more likely than CA women to be diagnosed with higher grade disease. AA women were more likely to be hormone receptor negative than CAs in all counties and years, though the strength of this difference may be lessening over time.
TABLE 3.
Demographic and Tumor Characteristics of the Study Population Analytics Cases, by County
| 2000–2006 | 2007–2012 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||||||||
| Edgecombe | Nash | Orange | AA, E vs O |
AA, E vs N |
AA, N vs O |
Edgecombe | Nash | Orange | AA, E vs O |
AA, E vs N |
AA, N vs O |
|||||||||||||
|
|
|
|||||||||||||||||||||||
| AA | CA | P | AA | CA | P | AA | CA | P | P-value | AA | CA | P | AA | CA | P | AA | CA | P | P-value | |||||
|
|
|
|||||||||||||||||||||||
| Number of cases | 158 | 142 | 158 | 347 | 79 | 495 | 140 | 115 | 148 | 280 | 73 | 506 | ||||||||||||
| Mean Age at diagnosis, (SD) | 59.8 (13.6) | 61.5 (14.3) | .312 | 57.2 (14.0) | 61.8(13.4) | <.001 | 58.0 (16.4) | 61.0 (13.7) | .076 | .720 | .797 | .387 | 61.2 (13.7) | 65.4 (13.2) | .015 | 58.8 (13.7) | 64.0 (12.9) | <.001 | 62.3 (13.8) | 62.0 (13.2) | .847 | .014 | .345 | .042 |
| Cancer Stage [N, (%)] | ||||||||||||||||||||||||
| Localized | 95 (60.1) | 95 (66.9) | .494 | 94 (59.5) | 231 (66.6) | .304 | 43 (54.4) | 312 (63.0) | .163 | .709 | .950 | .662 | 71 (50.7) | 69 (60.0) | .198 | 79 (53.4) | 186 (66.4) | .018 | 51 (69.9) | 344 (68.0) | .863 | .019 | .811 | .039 |
| Regional | 52 (32.9) | 40 (28.2) | 54 (34.2) | 97 (28.0) | 28 (35.4) | 159 (32.1) | 58 (41.4) | 37 (32.2) | 56 (37.8) | 79 (28.2) | 19 (26.0) | 148 (29.3) | ||||||||||||
| Distant | <11 (~6) | <11 (~4) | <11 (~5) | 10–20 (~5) | <11 (~8) | 10–20 (~4) | <11 (~7) | <11 (~4) | 10–20 (~8) | 10–20 (~4) | <11 (~3) | 10–20 ('3) | ||||||||||||
| Unknown | <11 (~1) | <11 (~1) | <11 (~1) | <11 (~1) | <11 (~3) | <11 (~1) | <11 (~1) | <11 (~3) | <11 (~1) | <11 (~1) | <11 (~1) | <11 (~1) | ||||||||||||
| Tumor Grade [N, (%)] | ||||||||||||||||||||||||
| Grade 1–II | 55 (34.8) | 71.(50.0) | .004 | 51 (32.3) | 164 (47.3) | .007 | 32 (40.5) | 284 (57.4) | .006 | .455 | .068 | .019 | 65 (46.4) | 71 (61.7) | .049 | 41 (27.7) | 137 (48.9) | <.001 | 39 (53.4) | 337 (66.6) | .002 | .596 | .002 | .001 |
| Grade III–IV | 81 (51.3) | 46 (32.4) | 69 (43.7) | 117 (33.7) | 40 (50.6) | 160 (32.3) | 53 (37.9) | 30 (26.1) | 67 (45.3) | 70 (25.0) | 23 (31.5) | 144 (28.5) | ||||||||||||
| Unknown | 22 (13.9) | 25 (17.6) | 38 (24.1) | 66 (19.0) | 7 (8.9) | 51 (10.3) | 22 (15.7) | 14 (12.2) | 40 (27.0) | 73 (26.1) | 11 (15.1) | 25 (4.9) | ||||||||||||
| ER/PR Status [N, (%)] | ||||||||||||||||||||||||
| ER+/PR+ | 52 (32.9) | 78 (54.9) | <.001 | 69 (43.7) | 181 (52.2) | .026 | 41 (51.9) | 314 (63.4) | .038 | .013 | .091 | .134 | 65 (46.4) | 65 (56.5) | .060 | 66 (44.6) | 174 (62.1) | <.001 | 45 (61.6) | 362 (71.5) | .113 | .108 | .878 | .057 |
| ER−/PR− | 57 (36.1) | 26 (18.3) | 54 (34.2) | 79 (22.8) | 17 (21.5) | 58 (11.7) | 48 (34.3) | 24 (20.9) | 55 (37.2) | 54 (19.3) | 10–20 ('25) | 77 (15.2) | ||||||||||||
| Other / Unknown | 49 (31.0) | 38 (26.8) | 35 (22.2) | 87 (25.1) | 21 (26.6) | 123 (24.9) | 27 (19.3) | 26 (22.6) | 27 (18.2) | 52 (18.6) | <11 (~15) | 67 (13.2) | ||||||||||||
Note. AA, African Americans; CA, Caucasian Americans; E vs N, Edgecombe County vs Nash County; E vs O, Edgecombe County vs Orange County; N vs O, Nash County vs Orange County; SD, standard deviation.
+ Two-sided T-test for continuous variables; Chi-square test for categorical variables, which exclude “unknown” category.
In some instances (eg, <11, (~5)) actual values are suppressed due to small sample size, per data use agreement requirements.
Treatment characteristics are examined in Table 4. Among those with localized disease, AAs experienced greater time from diagnosis to treatment initiation in 2000–2006 in Nash (P = .007) and Orange Counties (P = .081), though this difference of only several days was well within consensus guidelines and no longer statistically significant in 2007–2012. Treatment patterns were generally similar. Among women receiving BCS, AA BCS patients in Edgecombe County were less likely than AA BCS patients in Nash County to receive RT in 2000–2006 (67% receiving RT vs 80%, P = .082). The proportion of early-stage Edgecombe County AA BCS patients receiving RT appeared to improve in the 2007–2012 time period (85% vs 67% previously, trend P = .090) and was no longer significantly different from other counties. AA BCS patients in Orange County were less likely than AA BCS patients in Nash County to receive RT in the more recent time period (73% with RT vs 90%, P = .039).
TABLE 4.
Initial Treatment Characteristics by Race and County
| 2000—2006 | 2007–2012 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||||||||
| Edgecombe | Nash | Orange | AA, E vs O |
AA, E vs N |
AA, N vs O |
Edgecombe | Nash | Orange | AA, E vs O |
AA, E vs N |
AA, N vs O |
|||||||||||||
|
|
|
|||||||||||||||||||||||
| AA | CA | p | AA | CA | p | AA | CA | p | p-value | AA | CA | p | AA | CA | p | AA | CA | p | p-value | |||||
|
|
|
|||||||||||||||||||||||
| Number of cases | 158 | 142 | 158 | 347 | 79 | 495 | 140 | 115 | 148 | 280 | 73 | 506 | ||||||||||||
| Patients with Localized Disease | ||||||||||||||||||||||||
| Diagnosis to 1st Treatment, Days (SD) | 23.0 (3.5) | 17.4 (2.3) | .186 | 24.6 (3.3) | 14.8 (24.6) | .007 | 25.1 (3.1) | 19.8 (1.0) | .081 | .704 | .738 | .920 | 21.3 (2.2) | 21.5 (2.3) | .945 | 22.9 (2.8) | 20.5 (1.7) | .444 | 26.6 (2.4) | 23.5 (1.0) | 240 | .116 | .649 | .359 |
| Surgery [n, (%)] | ||||||||||||||||||||||||
| Yes | 95 (100.0) | 94 (~98) | .101 | 94 (100.0) | 231 (100.0) | .180 | 42 (~98) | 305 (~98) | .339 | .105 | .313 | .101 | 69 (~97) | 66 (~96) | .740 | 78 (~99) | 184 (~99) | .609 | 48 (~94) | 335 (~97) | 280 | .701 | .623 | .959 |
| No/Unknown | 0 (0.0) | <11 (~2) | 0 (0.0) | 0 (0.0) | <11 (~2) | <11 (~2) | <11 (~3) | <11 (~4) | <11 (~1) | <11 (~1) | <11 (~6) | <11 (~6) | ||||||||||||
| Type of Surgery [N, (%)] | ||||||||||||||||||||||||
| BCS | 71 (74.7) | 68 (72.3) | .897 | 68 (72.3) | 163 (70.6) | .955 | 24 (57.1) | 198 (64.9) | .454 | .087 | .800 | .133 | 50 (72.4) | 44 (66.7) | .438 | 55 (70.5) | 125 (67.9) | .529 | 30 (62.5) | 207 (61.8) | 924 | .117 | .701 | .176 |
| Mastectomy | 24 (25.3) | 26 (27.7) | 26 (27.7) | 68 (29.4) | 18 (42.9) | 107 (35.1) | 19 (27.5) | 22 (33.3) | 23 (29.5) | 59 (32.1) | 18 (37.5) | 128 (38.2) | ||||||||||||
| BCS+/−Radiation Therapy [N, (%)] | ||||||||||||||||||||||||
| BCS plus RT | 47 (67.1) | 54 (79.4) | .104 | 53 (80.3) | 132 (82.5) | .697 | 17 (~71) | 140 (72.5) | .860 | .738 | .082 | .339 | 43 (~85) | 34 (~75) | .273 | 48 (~90) | 105 (85.4) | .348 | 22 (~73) | 159 (79.9) | 410 | .160 | .470 | .039 |
| BCS no RT | 23 (32.9) | 14 (20.6) | 13 (19.7) | 28 (17.5) | <11 (~30) | 53 (27.5) | <11 (~15) | <11 (~25) | <11 (~10) | 18 (14.6) | <11 (~27) | 40 (20.1) | ||||||||||||
| Patients with Regional Disease | ||||||||||||||||||||||||
| Diagnosis to 1st Treatment, Days (SD) | 23.5 (27.1) | 21.1 (23.1) | .654 | 19.9 (20.6) | 15.8 (19.0) | .224 | 25.5 (324) | 19.9 (19.3) | .205 | .764 | .438 | .336 | 25.5 (24.2) | 19.9 (19.1) | .238 | 19.2 (19.6) | 19.7 (17.4) | .879 | 32.4 (45.5) | 21.8 (16.4) | 041 | .388 | .136 | .076 |
| Surgery [N, (%)] | ||||||||||||||||||||||||
| Yes | 51 (98.1) | 40 (100.0) | .378 | 50 (92.6) | 92 (94.9) | .895 | 25 (89.3) | 145 (91.2) | .746 | .085 | .317 | .409 | 56 (~97) | 35 (~95) | .643 | 49 (~88) | 78 (~98) | .006 | 17 (~90) | 136 (91.9) | .642 | .228 | .073 | .819 |
| No / Unclear | <11 (~2) | 0 (0.0) | <11 (~7) | <11 (~5) | <11 (~10) | 14(8.8) | <11(~3) | <11 (~5) | <11 (~12) | <11 (~2) | <11 (~10) | 12 (8.1) | ||||||||||||
| Type of Surgery [N, (%)] | ||||||||||||||||||||||||
| BCS | 23 (44.2) | 14 (35.0) | .330 | 29 (56.9) | 38 (39.6) | .047 | <11 (~25) | 64 (43.5) | .059 | .075 | .195 | .005 | 34 (59.7) | 14 (37.8) | .049 | 30 (57.7) | 32 (40.5) | .057 | <11 (~30) | 53 (38.4) | .444 | .075 | .957 | .074 |
| Mastectomy | 29 (55.8) | 26 (65.0) | 22 (43.1) | 58 (60.4) | 19 (~75) | 83 (56.5) | 23 (40.4) | 23 (62.2) | 22 (42.3) | 47 (59.5) | 12 (~70) | 85 (61.6) | ||||||||||||
| Surgery +/− Radiation [N, (%)] | ||||||||||||||||||||||||
| Surgery plus RT | 23 (44.2) | 19 (47.5) | .755 | 27 (50.0) | 41 (42.3) | .360 | 12 (42.9) | 71 (44.7) | .860 | .906 | .552 | .539 | 41 (70.7) | 21 (56.8) | .164 | 37 (66.1) | 43 (54.4) | .175 | <11 (~54) | 90 (60.8) | .493 | .149 | .596 | .295 |
| Surgery no/unclear RT | 29 (55.8) | 21 (52.5) | 27 (50.0) | 56 (57.7) | 16 (57.1) | 88 (55.4) | 17 (29.3) | 16 (43.2) | 19 (33.9) | 36 (45.6) | <11 (~46) | 58 (39.2) | ||||||||||||
| Chemotherapy | ||||||||||||||||||||||||
| Yes | 38 (73.1) | 25 (62.5) | .232 | 40 (74.1) | 63 (65.0) | .377 | 18 (~64) | 119 (74.8) | .374 | .142 | .864 | .181 | 46 (79.3) | 22 (59.5) | .025 | 47 (~84) | 52 (65.8) | .025 | 16 (~84) | 110 (74.3) | .728 | .837 | .478 | .758 |
| No / Unknown | 14 (26.9) | 15 (37.5) | 14 (25.9) | 34 (35.1) | <11 (~36) | 40 (25.2) | 12 (20.7) | 15 (40.5) | <11 ('16) | 27 (34.2) | <11 ('16) | 38 (25.7) | ||||||||||||
| Patients with Distant Disease | ||||||||||||||||||||||||
| Analysis not feasible due to small sample size | ||||||||||||||||||||||||
Note. AA, African Americans; BCS, Breast Conserving Surgery; CA, Caucasian Americans; E vs N, Edgecombe County vs Nash County; E vs O, Edgecombe County vs Orange County; N vs O, Nash County vs Orange County; RT, radiation therapy; SD, standard deviation.
+ Two-sided T-test for continuous variables; Chi-square test for categorical variables, which exclude “unknown” category.
In some instances (eg, <11, (~5)) actual values are suppressed due to small sample size, per data use agreement requirements.
Among those with regional disease, racial differences in time from diagnosis to treatment were modest and only statistically significant in Orange County in 2007–2012 (P = .041). AA women in Edgecombe and Nash Counties appeared to be more likely than CA women to have chemotherapy in the most recent time period (P = .025 for each). Small sample size precluded substantive analysis of those with distant disease.
Discussion
Cancer health disparities remain a concern in North Carolina in general, and in Edgecombe and Nash Counties specifically; however, these findings suggest that geographic and racial disparities in breast cancer in Edgecombe County are not as profound as suggested by the 2007 Komen Report. Notably, the 2003 incidence rate of 185.3/100,000 presented in the Komen Report appears to be an all-time high for Edgecombe County. Since then, Edgecombe appears to have followed a trend more comparable to that seen in other counties, which generally reflects recent trends seen nationally [1]. The Komen Report spurred several efforts to increase community awareness, coordinate regional breast cancer resources, and build upon community-based efforts already underway. The declining mortality rate may in-part reflect that these efforts have begun to bear fruit.
Among community efforts (see Table 1), a lay health advisor program—initiated in the early 2000s based in local beauty shops and more recently enhanced through grant funding [26]—has sought to overcome cultural barriers by providing information on breast cancer in general and increasing people’s awareness of breast cancer screening specifically, including by referring people to the health department and elsewhere for screening. Throughout the 2000s, several other efforts were funded by agencies like the North Carolina Office of Minority Health and Health Disparities, the Susan G. Komen Foundation, and even the CCN, and the community was introduced to new levels of detail regarding breast cancer, including the existence of different disease subtypes and AA women’s differential susceptibility to more aggressive subtypes like hormone receptor negative disease. Of course, both the Komen Report itself and the activities of these community groups may have fueled local health care organizations’ more global discussions of affiliation or collaboration with major medical centers, which may in turn have contributed to accelerated adoption of treatment innovations or quality of care protocols; however, while there may be some association between these efforts and changes in outcomes, such a causal association cannot be firmly attributed. This said, the task force that formed following the Komen report continues to utilize cancer data to review community needs, available resources, and service access issues, and coordinate activities to address them. As an example, Edgecombe County’s Vidant Health, a member of the task force, has been addressing some of the identified gaps by leveraging the successes of the lay health advisor program and integrating previously-trained advisors into the health care system as cancer health navigators. However, the sustainability of such services is unclear, as the external grant funding that helped initiate the development of these many efforts has drawn to a close.
While some measures suggest there has been positive progress, other measures do not, suggesting there has not been a comprehensive or systematic shift in the profile of Edgecombe County’s breast cancer burden. Accordingly, the details regarding the specific cause, strength, and permanence of the changes in incidence and mortality remain unclear. For example, a traditional marker for a successful cancer screening program is when disease is increasingly detected at an earlier, more treatable stage with associated longer survival. This analysis reveals that women in both Edgecombe and Nash counties remain more likely to be diagnosed with advanced disease compared to women in Orange County. While there is no statistically significant trend, Orange County appears to be consistently improving and diverging from the other 2 counties. There appears to be a positive shift in other tumor characteristics though, as tumors have tended to be lower grade and more likely to be hormone receptor positive over time. This less aggressive, more treatable disease may be playing a role with the improvement in mortality. Taken together, this shift in population tumor characteristics may actually partly reflect a demographic aberration that warrants further attention, as the population of these 2 counties is both shrinking and aging [16]. This may partly explain the slight trend toward less aggressive (grade) disease among residents, since breast cancer among older women is often a less aggressive phenotype [27].
Several measures of treatment characteristics are consistent across time, suggesting changes in access to treatment may not necessarily be the primary drivers of the apparent improvement in mortality. For example, while there were some geographic and racial differences in the time between diagnosis and treatment initiation, the differences were not substantial and all groups were well within consensus recommendations. This said, there appears to have been a notable change in quality of care vis-à-vis improvement in the guideline-concordant use of RT following surgery for those diagnosed with either local or regional disease. This was apparent in Edgecombe County overall and especially among AA women. For example, among AA women with early-stage disease, the proportion receiving BCS/RT increased from 67% in 2000–2006 to approximately 85% in 2007–2012. This change was similar among AA women with regional disease among whom BCS/RT increased from approximately 44% to approximately 71%. Nash and Orange Counties saw a less substantial improvement in this measure for these populations. This finding is particularly important for understanding changes in quality of care in Edgecombe County both overall and in the context of racial disparities, since a much greater proportion of the population is AA in Edgecombe (54%) and Nash (33%) compared to Orange (13%). An increase in RT use after surgery is expected to lead to an improvement in long-term overall survival for patients with localized and regional breast cancer [28–30], and the impact of these positive changes may only be beginning to be seen in the decline in mortality.
Among policy implications, programs to spur economic development and job (re)training in Edgecombe County may help. Economic development can translate to more jobs/employment, which typically is associated with better insurance coverage and local health resources, which in turn can be associated with better access to cancer prevention/early detection and treatment. It may also help stabilize the apparent out-migration of younger residents, the full implications of which are unclear. Additional targeted funding from government or grant sources could help support some of the programs presented in Table 1, while additional research works to identify root causes of breast cancer incidence and mortality and effective interventions to resolve them.
Limitations
State tumor registry data are widely perceived as well-suited for characterizing patient and tumor characteristics at diagnosis and hospital-based treatment. Data on surgery are considered to be of highest quality, followed closely by radiation therapy, and more distantly by chemotherapy [24], which is almost exclusively delivered in the outpatient setting. Among study limitations, these factors and the limited sample size for patients with advanced disease inhibited the characterization of treatment for the chemotherapy population and require thoughtful interpretation. Notably, since chemotherapy data may be better reported for hospital-associated outpatient clinics compared with free-standing physician practices, apparent geographic variation in chemotherapy use may in part reflect infrastructure and reporting differences and not quality of care differences.
The American Joint Committee on Cancer (AJCC) TNM (tumor, node, and metastases) Staging System provides the contemporary criteria for disease characterization and treatment determination based on tumor characteristics and extent of disease. The more-precise TNM staging was not the standard throughout the study period, and completeness was limited and varied by county. The measure of summary stage (ie, local, regional, distant stage at diagnosis) was more complete and consistent; however, its coarseness—relative to more current staging systems with their more precise stage categories—may mute important yet subtle changes in outcomes, such as shifts in stage at diagnosis that may result from improved screening rates associated with nascent community screening programs. As they mature, the more fine-grained measures may yield more nuanced understanding and inform future efforts to improve treatment and outcomes.
For several reasons, including the impracticality of power calculations given the data source/sample as well as the broad goals of the manuscript, we present the unadjusted P-values; only measures with highly significant P-values should be viewed most confidently.
Conclusion
These findings document the beginning of positive changes in outcomes amidst ongoing community efforts to address the disproportionate burden of breast cancer in Edgecombe County. These efforts originated through local community and academic partnerships and were supported by grants from foundations, the North Carolina Office of Minority Health and Health Disparities, and research programs such as the CCN [26]. Despite improvement among some measures in recent years, the apparent progress may be short-lived given the variation in findings among other measures and the declines in funding for community-based health promotion and prevention. Going forward, additional targeted funding may support these community-identified needs for a more comprehensive knowledge of breast health and improvements in care that can yield lasting betterment in breast cancer outcomes. Future research should examine these issues and programs more specifically, with an eye toward identifying the root causes of the disproportionate breast cancer burden that are amenable to scientifically testable interventions. It is our hope that this study will inform such research and interventions.
Acknowledgments
The Carolina Community Network is supported by the National Cancer Institute’s Center to Reduce Cancer Health Disparities through its Community Networks Program (U01 CA 114629) and Community Networks Program Centers (U54 CA 153602). We are grateful to Soundarya Radhakrishnan and the North Carolina Central Cancer Registry for their kind assistance with regard to data access and interpretation. We acknowledge with appreciation the efforts and contribution of Reuben Blackwell with Opportunities Industrialization Center, Inc. (OIC) in Rocky Mount, North Carolina.
Footnotes
Potential conflicts of interest. All authors have no relevant conflicts of interest.
Contributor Information
Anissa I. Vines, Department of Epidemiology, Gillings School of Global Health; fellow, UNC Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
William R. Carpenter, Department of Health Policy and Management, Gillings School of Global Health; member, UNC Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Ronald C. Chen, Department of Radiation Oncology, UNC School of Medicine; member, UNC Lineberger Comprehensive Cancer Center; senior fellow, UNC Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Michele W. Cherry, Grants and Special Projects, Vidant Edgecombe Hospital, Tarboro, North Carolina.
Debra G. Long, Crossworks LLC, Rocky Mount, North Carolina.
Keith D. Amos, UNC Lineberger Comprehensive Cancer Center; assistant professor, Department of Surgery, UNC School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Paul A. Godley, Diversity and Inclusion; Rush S. Dickson distinguished professor, Division of Hematology/Oncology, UNC School of Medicine; member, UNC Lineberger Comprehensive Cancer Center; senior fellow, UNC Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
References
- 1.American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Accessed August 9, 2017]. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2016/cancer-facts-and-figures-2016.pdf. [Google Scholar]
- 2.North Carolina Central Cancer Registry. 2008–2012 North Carolina Cancer Incidence Rates by Race and Ethnicity per 100,000 Population Age-Adjusted to the 2000 Census. Raleigh, NC: North Carolina Central Cancer Registry; 2016. [Accessed August 9, 2017]. http://www.schs.state.nc.us/schs/CCR/incidence/2012/5yearRatesbyRaceEth_v3.pdf. [Google Scholar]
- 3.North Carolina Central Cancer Registry. 2008–2012 North Carolina Cancer Mortality Rates by Race and Ethnicity per 100,000 Population Age-Adjusted to the 2000 Census. Raleigh, NC: North Carolina Central Cancer Registry; 2014. [Accessed August 9, 2017]. http://www.schs.state.nc.us/schs/CCR/mort2012re.pdf. [Google Scholar]
- 4.Kushi LH, Kwan ML, Lee MM, Ambrosone CB. Lifestyle factors and survival in women with breast cancer. J Nutr. 2007;137(1 Suppl):236S–242S. doi: 10.1093/jn/137.1.236S. [DOI] [PubMed] [Google Scholar]
- 5.Masood S. Prognostic/predictive factors in breast cancer. Clin Lab Med. 2005;25(4):809–825. doi: 10.1016/j.cll.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45(Suppl 1):27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 7.Polacek GN, Ramos MC, Ferrer RL. Breast cancer disparities and decision-making among U.S. women. Patient Educ and Couns. 2007;65(2):158–165. doi: 10.1016/j.pec.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–48. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 9.Dunn BK, Agurs-Collins T, Browne D, Lubet R, Johnson KA. Health disparities in breast cancer: biology meets socioeconomic status. Breast Cancer Res Treat. 2010;121(2):281–292. doi: 10.1007/s10549-010-0827-x. [DOI] [PubMed] [Google Scholar]
- 10.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11(7):601–607. [PubMed] [Google Scholar]
- 11.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sassi F, Luft HS, Guadagnoli E. Reducing racial/ethnic disparities in female breast cancer: screening rates and stage at diagnosis. Am J Public Health. 2006;96(12):2165–2172. doi: 10.2105/AJPH.2005.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97(6):1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 14.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 15.Health Resources and Services Administration. HRSA website; [Accessed August 9, 2017]. Medically Under-served Areas and Populations. https://bhw.hrsa.gov/shortage-designation/muap. Updated October 2016. [Google Scholar]
- 16.US Census Bureau. Quickfacts: Edgecombe County. North Carolina: US Census Bureau website; [Accessed August 9, 2017]. http://www.census.gov/quickfacts/table/POP060210/37065. [Google Scholar]
- 17.Susan G Komen for the Cure. Breast Cancer Mortality Report: Closing the Gaps in Eight Communities. Susan G Komen for the Cure; 2007. [Accessed October 30, 2017]. http://www.komennyc.org/pdfs/ClosingTheGap-Mortality-Report-Narratives.pdf. [Google Scholar]
- 18.Newman SD, Andrews JO, Magwood GS, Jenkins C, Cox MJ, Williamson DC. Community advisory boards in community-based participatory research: a synthesis of best processes. Prev Chronic Dis. [PMC free article] [PubMed] [Google Scholar]
- 19.US Census Bureau. Census Data Quick Facts. US Census Bureau website; [Accessed August 9, 2017]. https://www.census.gov/quickfacts/table/PST045216/00. [Google Scholar]
- 20.NIH Consensus Conference. Treatment of early breast cancer. JAMA. 1991;265(3):391–395. [PubMed] [Google Scholar]
- 21.Truong PT, Bernstein V, Lesperance M, Speers CH, Olivotto IA. Radiotherapy omission after breast-conserving surgery is associated with reduced breast cancer-specific survival in elderly women with breast cancer. Am J Surg. 2006;191(6):749–755. doi: 10.1016/j.amjsurg.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Briest S, Stearns V. Chemotherapeutic strategies for advanced breast cancer. Oncology (Williston Park) 2007;21(11):1325–1335. [PubMed] [Google Scholar]
- 23.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 24.Malin JL, Kahn KL, Adams J, Kwan L, Laouri M, Ganz PA. Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst. 2002;94(11):835–844. doi: 10.1093/jnci/94.11.835. [DOI] [PubMed] [Google Scholar]
- 25.North Carolina Central Cancer Registry. Statistics and reports. North Carolina: Health Statistics website; [Accessed August 9, 2017]. http://www.schs.state.nc.us/data/cancer.cfm. Updated April 3, 2017. [Google Scholar]
- 26.Teal R, Moore AA, Long DG, Vines AI, Leeman J. A community-academic partnership to plan and implement an evidence-based lay health advisor program for promoting breast cancer screening. J Health Care Poor Underserved. 2012;23(2 Suppl):109–120. doi: 10.1353/hpu.2012.0076. [DOI] [PubMed] [Google Scholar]
- 27.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36(3):237–49. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 29.Early Breast Cancer Trialists’ Collaborative Group. Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Early Breast Cancer Trialists’ Collaborative Group. McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]