Abstract
Lassa virus (LASV) is the most prevalent rodent-borne arenavirus circulated in West Africa. With population at risk from Senegal to Nigeria, LASV causes Lassa fever and is responsible for thousands of deaths annually. High genetic diversity of LASV is one of the challenges for vaccine R&D. We developed multivalent virus-like particle vectors (VLPVs) derived from the human Venezuelan equine encephalitis TC-83 IND vaccine (VEEV) as the next generation of alphavirus-based bicistronic RNA replicon particles. The genes encoding VEEV structural proteins were replaced with LASV glycoproteins (GPC) from distantly related clades I and IV with individual 26S promoters. Bicistronic RNA replicons encoding wild-type LASV GPC (GPCwt) and C-terminally deleted, non-cleavable modified glycoprotein (ΔGPfib), were encapsidated into VLPV particles using VEEV capsid and glycoproteins provided in trans. In transduced cells, VLPVs induced simultaneous expression of LASV GPCwt and ΔGPfib from 26S alphavirus promoters. LASV ΔGPfib was predominantly expressed as trimers, accumulated in the endoplasmic reticulum, induced ER stress and apoptosis promoting antigen cross-priming. VLPV vaccines were immunogenic and protective in mice and upregulated CD11c+/CD8+ dendritic cells playing the major role in cross-presentation. Notably, VLPV vaccination resulted in induction of cross-reactive multifunctional T cell responses after stimulation of immune splenocytes with peptide cocktails derived from LASV from clades I-IV. Multivalent RNA replicon-based LASV vaccines can be applicable for first responders, international travelers visiting endemic areas, military and lab personnel.
Keywords: Multivalent Lassa virus vaccine, T cells cross-reactivity, protection, Alphavirus replicons
1. Introduction
Lassa virus (LASV) is the most prevalent African arenavirus [1] with the highest impact in public health. The virus causes Lassa fever (LF) which is endemic to a large geographical area of sub-Saharan Africa [2–4]. It is quite likely that population at risk includes countries from Senegal to Nigeria and can be as high as 200 million people [5]. With the exception of Dengue fever, the estimated global burden of LF is the highest among viral hemorrhagic fevers [6]. In 2016, WHO identified LASV as a top priority emerging pathogen and recommended accelerated vaccine R&D [7].
LASV has a bisegmented negative strand RNA genome [8,9]. The L segment encodes the L protein (viral polymerase) and the Z (matrix) protein. The S segment encodes the glycoprotein precursor (GPC) and nucleoprotein (NP). Upon cleavage by cellular signal peptidase in the lumen of the endoplasmic reticulum (ER), the GPC is extensively N-glycosylated and further processed into the peripheral GP1 (attached factor) and transmembrane GP2 responsible for fusion [10]. Both glycoproteins are required and sufficient for induction of protective immunity in non-human primates, NHPs [11,12].
The genetic variation of LASV is the highest among mammalian arenaviruses [13,14]. Diversity of LASV likely contributes to under-estimating its prevalence [15]. Overall, strain variation was found to be as high as 27% and 15% at the nucleotide and amino acid levels, respectively. Phylogenetically, LASV strains comprise of at least four lineages (clades) [13]. Clades I-III have been found in Nigeria. LASV strains circulated in Guinea, Liberia, and Sierra Leone are included in the largest lineage IV. Genetic LASV diversity poses a great challenge for vaccine development.
Epidemiological observations in West Africa suggest that in immunocompetent individuals, a single attenuated natural LASV infection results in lengthy protection against fatal LF. Re-infection with heterologous LASV strains provides boosting protective immunity [11,16]. In line with these observations, a single injection of a MOP/LAS reassortant (clone ML29), effectively protected animals against LF caused by homologous or distantly-related LASV [17]. The ML29 is a promising live-attenuated vaccine candidate against LF [18].
Alphavirus replicon technology provides a reasonable compromise, in terms of safety and immunogenicity, between “killed” and “live” vaccines. Alphavirus replicon particles are single-cycle, replication-defective, virus-like-particles vehicles (vectors) (VLPVs). They are not able to spread beyond the initially infected cells, but can deliver and transduce the gene(s) of interest in target cells (e.g., dendritic cells, DCs). Numerous alphavirus replicon-based vaccine candidates are in pre-clinical and clinical development [19].
We have prepared VLPVs from the human Venezuelan equine encephalitis vaccine (VEEV) TC-83 (U.S. FDA IND No. 142) [20] as bicistronic RNA replicons [21]. The multivalent feature of this system is beneficial for optimization of LASV vaccine formulation. In this study, we designed two bicistronic RNA replicons. Each replicon expressed two LASV GPCs (from clades I and IV) under control of two 26S promoters simultaneously and efficiently. In addition, RNA replicons expressing truncated and metabolically stable (uncleaved) LASV GPs promoting cross-priming CD8+ T cell responses were also designed and encapsidated in VLPVs. Humoral and cross-reactive T cell responses as well as protective efficacy of these bivalent VLPVs were tested in mice.
2. Materials and methods
2.1. Design of bivalent VLPVs
LASV GPC genes from clades I and IV, LASV/NIG/LP (AF181853), and LASV/Josiah (AY628203), respectively, were assembled from synthetic oligonucleotides and/or from PCR products. The strategy for creation of monovalent VLPVs expressing truncated, metabolically stable LASV GP fused with fibritin (ΔGPfib) [21] was previously described. Bicistronic vectors were generated as depicted in Fig. S1. For production of VLPVs, optimized RNA formulations containing 1 μg of RNA (LASV GP vector, E1&E2-helper, and C-helper) were transfected into CHO-K1 cells and purified from culture medium as described [22,23]. High yielding monovalent and bivalent VLPVs (Fig. S1) were selected for further studies.
2.2. Identity and potency assays
Identity and potency of VLPVs were assessed by immunofluorescence (IFA) and in western blot (WB) [21–23]. For IFA staining, LASV GP-specific monoclonal antibodies [22] and LASV GP1- and GP2-specific [24] rabbit polyclonal antibodies were used. Rabbit anti-phage T4 fibritin, affinity-purified IgG was produced by Covance (Denver, PA). For WB analysis, VLPV-infected cells were lysed in buffer (40 mM Tris-HCl pH 7.2, 300 Mm NaCl, 10 mM EDTA, 2% NP40 and 20% glycerol) supplemented with protease inhibitors. The lysates (100 μg/well) were run in 5–14% SDS-PAGE gel, proteins were electroblotted to PVDF membranes, incubated with either anti-fibritin or anti-LASV GP2 antibodies and treated with second antibodies (HRP-conjugated rabbit IgG or HRP-conjugated mouse IgG). Titration of VLPVs was performed in IFA. VLPV stocks with titers 1 × 107–1 × 108 fluorescent focus units (FFU/ml) were used in this study.
2.3. Co-staining and apoptosis protocols
Vero E6 cells were infected with LASV VLPVs at multiplicity of infection (MOI) of 1. On day 2 (D2), cells were fixed with 4% paraformaldehyde, permeabilized, stained with antibodies against LASV GPC and/or against fibritin, calnexin (clone 1D4), syntaxin-6 (LifeSpan BioSciences) and analyzed with a Zeiss AX10 microscope. LASV GP expression was also measured by flow cytometry (FACScalibur, BD Bioscience, San Diego, CA) using LASV GP2 or fibritin antibody and Texas Red-conjugated anti-rabbit IgG. Apoptosis was measured by TUNEL assay using the ApopTag Red Apoptosis Detection Kit.
2.4. Immunization and challenge studies in mice
LASV VLPV vaccine immunogenicity was evaluated in a CBA/J-ML29 model [25]. In brief, CBA/J mice (Harlan® Labs) were subcutaneously (SC) immunized with 1 × 107 FFU of VLPV1 or VLPV2 (Fig. S1) or with blended formulation, VLPV3 (VLPV1&LPV2). Control groups include mock- and ML29-vaccinated mice (single shot, 1 × 103 PFU, SC). All groups were challenged on D21 and monitored during 21 days post-challenge. Mice were euthanized at 7-day interval during 42 days. Blood and spleen samples were collected for evaluation of LASV-specific immunity.
2.5. LASV-specific adaptive immune responses
LASV antibodies were measured in IgG ELISA and in plaque reduction neutralization test, PRNT [25], to detect neutralizing antibodies (nAbs). LASV ΔGP2 ectodomain expressed in insect cells was used as antigen in IgG ELISA (Fig. S3). Validation of this antigen with plasma samples collected from LASV-infected individuals [26] or from infected NHPs [18] documented LASV antigenic identity. PRNT was performed using a constant dose of virus and serial dilutions of plasma and calculated as the highest serum dilution resulting in 50% plaque reduction. For T cell activation markers, splenocytes from vaccinated mice were stained with antibodies for CD3e-PerCP-cy5.5, CD8a-APC, MHC I-PE, CD80-FITC, and CD86-PE markers (eBioscience). For detection of the mouse CD8+ DC subsets with cross-presentation capabilities, splenocytes were stained for CD3e-PerCP-cy5.5, CD8a-APC, CD11b-APC, and CD11c-Alexa Fluor 488. IFN-γ/IL-2 ELISPOT was performed according to manufacturer’s protocol (Cellular Technology Ltd., Cleveland, OH). Splenocytes were stimulated with LASV GPC peptide cocktails (Mimotopes Ltd, Melbourne, Australia). Each cocktail contained 69 overlapping 20-mer peptides derived from LASV phylogenetic lineages I–IV [13] (Table S1). After stimulation, splenocytes secreted IFN-γ and/or IL-2 antibodies were developed (with Red and Blue Developer Solutions) and counted using C.T.L. Ltd. Immunospot® S6 Universal analyzer and Immunospot® software 5.3.
2.6. Statistical analysis
Results are reported as means ± SEM (n = 4–5). ANOVA with Bonferroni’s post hoc test (for parametric data) or Mann-Whitney Rank Sum test (for nonparametric data) was used for the determination of statistical significance among treatment groups, as appropriate. Statistical analysis (mean, SD, T-test) and graphics were performed using the OriginLab 2016 package (OriginLab Corporation, Northampton, MA).
3. Results
3.1. Bivalent VLPVs simultaneously express wild type and metabolically stable LASV glycoproteins
The TC-83 genetic backbone was used to design bicistronic VLPVs expressing LASV GPCwt (from clades I or IV) and ΔLJGPfib (I or IV, Fig. S1). The LASV ΔGPfib-transduced cells were positively stained with LASV GP1- and GP2-specific antibodies indicating that genetic modification of LASV GPC did not affect antigenic identity of ΔLGPfib. Co-staining experiments (Fig. 1A) revealed that LASV GPCwt was predominantly co-localized with a Golgi marker; while LASV ΔLJGPfib was expressed as a cell-associated protein and costained with ER. Approximately 10% of the ΔLGPfib was recovered from culture medium (not shown).
Fig. 1.
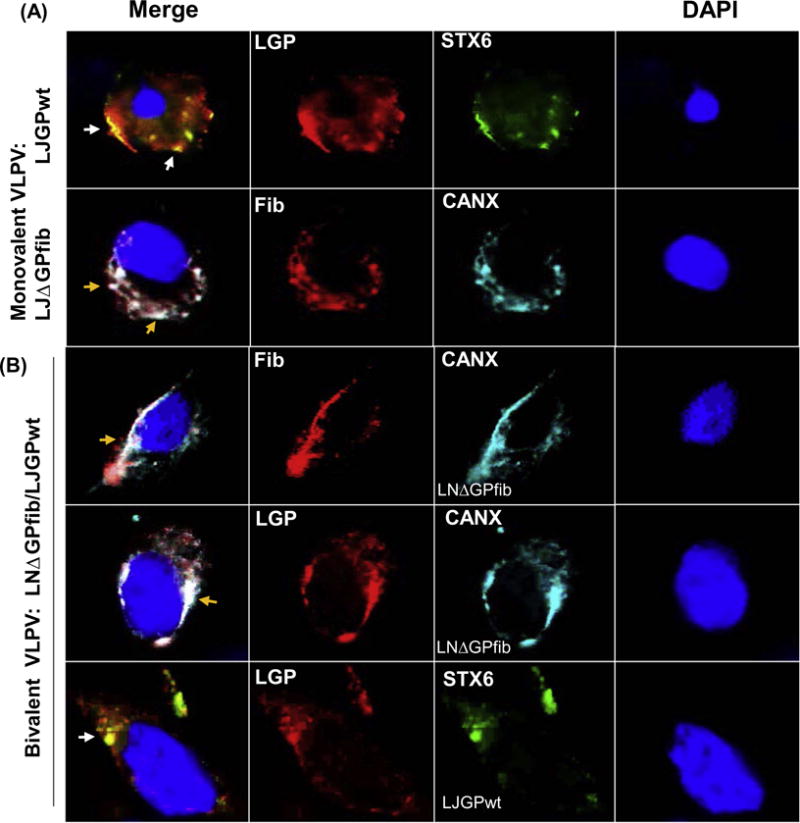
Co-staining of wild type and genetically modified LASV GPs with Golgi and ER markers. (A), infection with monovalent VLPVs. Vero E6 cells were exposed to monovalent VLPV LJGPwt or LDGPfib and stained with anti-GP LASV antibody, or with antibody against T4fib (red), Golgi (Syntaxin 6, green), or ER (Calnexin, blue). White and yellow arrows indicate colocalization of LJGPwt and Golgi; and LJΔGPfib and ER, respectively. (B), expression of both antigens, LNΔGPfib and LJGPwt, in cells transduced with bivalent VLPV1 was detected in both compartments, in ER and in Golgi.
Based on the stability and yields, two bivalent VLPVs were selected for further studies (Fig. S1): VLPV1 expressing ΔGPfib from LASV LP/NIG (clade I) and GPCwt from JOS/SL (IV); and VLPV2 expressing GPCwt from LP/NIG and ΔGPfib from JOS/SL. In VLPV-transduced cells, ΔGPfib was stained either with virus-specific or with fibritin-specific antibodies and localized in perinuclear ER compartment as assessed by co-staining with CANX (Fig. 1B). Meanwhile, in the same cells, the LASV GPCwt cleavage products (GP1 and GP2) were mostly co-stained on the surface of cells with Golgi marker, STX6.
Cells infected/transduced with bivalent VLPVs expressed almost equal amounts of LASV GPCwt and ΔGPfib (Fig. 2A). In these cells, LASV GP was detected as a ~250 kDa-trimer (Fig. 2B, right panel) or as GP2 glycoprotein (32 kD), a product of GPCwt cleavage (Fig. 2B). Un-cleaved, monomeric LNΔGPfib or LJΔGPfib was also detected at low amount. LASV GP1 is poorly detected in WB due to antigenicity damage during SDS-PAGE and blotting.
Fig. 2.
LASV ΔGPfib migrated as trimers in VLPV-exposed cells and induced ER stress. (A), VLPV1 were exposed to Vero or RAW-264.7 cells (MOI = 3) and stained 24 h after infection with anti-GP LASV antibody or anti-fib antibody. Positively stained cells were assessed by flow cytometry. (B), expression of LASV GP. Vero cells exposed to monovalent VLPV (MOI = 1 or 5) were analyzed by WB using anti-GP2 polyclonal antibody. Left panel, expression of LASV GPwt. Right panel, expression of LASV ΔGPfib after exposure cells with VLPV1. Arrows indicate positions of GP trimers, un-cleaved GPC, and GP2. (C), (D) LASV ΔGPfib retained in ER induce ER stress and apoptosis, as assessed in WB with antibodies against PERK (markers of unfolded protein responses) and CHOP, a marker of ER-induced apoptosis. pPERK, phosphorylated PERK. In panel (C), cells were exposed with bivalent VLPV at 0 (mock), 1 and 5 of MOI. In panel (D), cells were exposed with ML29 expressing LJGPwt, monovalent VPLV expressing LNΔGPfib and with bivalent VLPV, LJGPwt/LNΔGPfib (MOI = 5). At 24 h after infection, cell lysates were analyzed by WB. Relative density of the bands was quantified and normalized to GAPDH.
3.2. Bivalent VLPVs trigger ER stress and apoptosis
Accumulation of metabolically stable LASV ΔGPfib glycoproteins in ER compartment can overload folding capacities of ER resulting in activation of the unfolded protein response (UPR) and apoptosis [27,28]. Indeed, as seen in Fig. 2C and D bivalent LASV VLPVs induced PERK (PRK-like ER kinase), one of the three master UPR regulators. Notably, phosphorylated form of PERK was strongly upregulated in VLPV-infected cells in comparison with mock- and ML29-infected cells expressing LAS/Jos GPCwt (Fig. 2D).
The UPR failure to properly fold proteins in the stressed ER can induce apoptosis. CHOP (C/EBP homologous protein), a member of the C/EBP transcription factor family [29], is one of the genes induced during ER-stressed apoptosis. The expression of CHOP was upregulated in cells exposed to VLPVs expressing ΔGPfib (Fig. 2C and D). TUNNEL method confirmed this observation (Fig. S2). On day 3, 12.5% VLPV-exposed cells were positively stained on apoptotic markers, while only 2% mock-infected cells were positively stained by Annexin-V during 3 days of incubation. In addition, ~8–10% of cells bivalent VLPV-exposed cells were positively co-stained with lysosomal markers (not shown), suggesting that LASV antigens were also subjected to proteolytic degradation.
3.3. LASV VLPV vaccines protects mice against fatal challenge
In CBA/J mice, a model developed to evaluate LASV vaccine candidates at early stage of pre-clinical development [25,30], all mock-immunized mice experienced weight loss after challenge and met euthanasia criteria on D9 (Fig. 3). In contrast, all animals receiving experimental VLPV and control (ML29) vaccines were fully protected against lethal challenge. There were no statistically significant differences in body weight between vaccinated/challenged groups.
Fig. 3.
Vaccination-challenge experiments. Mice in experimental “VLPV groups” were primed with 5 ×106 FFU of VLPV1, VLPV2, or VLPV3 (blended formulation, VLPV1&VLPV2) and boosted 14days later with the same regimens. On Day 21, all mice were challenged with 1 × 103 PFU/dose of ML29 (intracranially), as previously described [25]. Mice immunized with a single dose of ML29 (1 × 103 PFU, SC) were used as a vaccine control group; mock-vaccinated animals were included in a challenge control group. (A), survival rate, %. (B), weight.
3.4. LASV-specific humoral responses
By the day of the challenge, barely detectable antibody responses were detected in vaccinated mice with relatively higher responses in VLPV2 group (Fig. S3). After challenge, humoral IgG responses markedly increased in all VLPV-vaccinated mice with the highest titers in VLPV1 group. Vaccination with blended VLPV3 did not result in higher titers. ML29 control group demonstrated lower titers. In line with previous observations [18,25], nAbs were not detectable in vaccinated mice.
3.5. VLPV vaccines induce cross-reactive T cell-mediated responses
LASV specific T cell responses were assessed in IL-2/IFN-γ ELI-SPOT using antigen stimulation with peptide cocktails derived from LASV GPC from clade I-IV [13,14]. On D7 after VLPV vaccination, LASV-specific T cells (counted as spot-forming cells, SFC) were barely detected. Immunization and challenge up-regulated SFC and on D42, ≥1,000/106 IL-2 SFC were detected in splenocytes (Fig. 4A). IFN-γ SFC demonstrated similar kinetics but numbers were 3–4–fold lower. The strongest T cell responses were observed in VLPV1-immunized group after stimulation cells with GPC cocktail I and IV (Fig. 4A and B). We did not observe statistically significant differences in T cell responses after stimulation with GPC cocktails derived from LASV lineage II and III (not shown).
Fig. 4.
Cross-reactive T cell responses in VLPV-infected mice. (A)-(C), vaccination with VLPV1, VLPV2 and VLPV3 (blended formulation, VLPV1&VLPV2), respectively. VLPV1 antigen formulation is LNΔGPfib/LJGPwt; VLPV2 is LJΔGPfib/LNGPwt, where LJ and LN, LASV-Josiah and LASV-Nigeria from distantly-related phylogenetic groups IV and I, respectively. GPwt and ΔGpfib, wild-type and genetically modified LASV GP, respectively (see Fig. 1). (D), vaccination with ML29. Splenocytes from vaccinated and challenged mice were collected at indicated time points, stimulated with GP peptide cocktails I (LASV/LP/NIG) or IV (LASV/JOS/SL) and cells secreting individual cytokines, IL-2 (panel A), IFN-γ (panel B), or both, IFN-γ/IL-2 (panels C and D), were counted as described in Methods.
Boost immunization strongly increased numbers of double-stained, IL-2/IFN-γ-secreted T cells (dsSFC), while the effect of challenge on dsSFC was less prominent. In the control vaccine group, strong LASV-specific T cell responses were detected after a single injection of ML29 in line with previous observations [18,25]. ML29-vaccinated mice generated higher number of dsSFC. However, the number of these double-stained T cells declined at the end of week 2. Still, on D42, number of poly-functional T cells in control mice was comparable with VLPV1 group.
3.6. Vaccination with LASV VLPV induces co-stimulatory markers and upregulates CD8α+ dendritic cells (DCs) promoting antigen cross presentation
In addition to cytokine secretion, expression of co-stimulatory molecules provides additional and indispensable signals for development of effective adaptive CD8+ T cell responses. As seen in Fig. 5, vaccination with VLPVs induced expression of CD80, CD86 and MHC-I molecules. Boost immunization strongly upregulated expression of the co-stimulatory molecules. One of the aims of this study was to design VLPV-based LASV vaccines with enhanced immunogenicity and cross-reactivity. To this end, metabolically stable trimers, LJΔGPfib or LNΔGPfib, were expressed using advanced alphavirus replicons. As expected, LASV ΔGPfib induced ER stress and apoptosis. Based on this and previous observations, we assumed that the releasing metabolically stable LASV ΔGPfib antigens from apoptotic cells would be picked up by antigen-presenting cells and then presented to CD8+ T cells.
Fig. 5.
T cells activation markers in VLPV-immunized and challenged mice. T cells activation markers, MHC I, CD80, CD86, were detected by flow cytometry among splenocytes collected from immunized mice at indicated time points. (A), MHC I+; (B), CD80+; (C), CD86++. Asterisks (* and **) indicate statistically significant different differences in frequency when compared with mock vaccine (p < .05 and p < .01), respectively.
Flow cytometry analysis (Fig. 6) documented that VLPV immunization upregulated population of CD11c+/CD8+ DCs specialized in capturing dying cells and cross-presenting of antigens on MHC class. Population of cross-presenting DCs was elevated in all VLPV vaccination groups as early as D7 and increasing during an observation period. Stimulation of CD8+ DCs constitutively equipped with mechanisms for antigen cross-presentation seems to be important strategy to enhance efficacy of vaccines based on T cell-mediated protection.
Fig. 6.
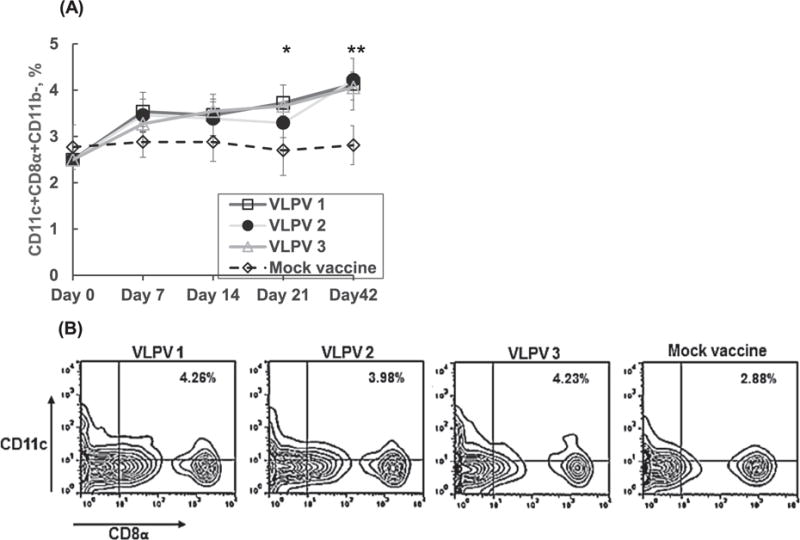
VLPV vaccination upregulates population of CD8α+CD11c+ dendritic cells with cross-presenting capabilities. (A), detection of CD8α+CD11c+ at different time points after prime immunization. (B), the panel is based on gating CD8+ CD11c+ cells at the end of the experiment, day 42 after prime immunization. Asterisks * and **, p < .05 and p < .001, respectively, between VLPV1 and mock vaccine.
4. Discussion
Molecular dating suggests that LASV originated in Nigeria approximately a thousand years ago [14]. During last several centuries the virus moved into neighboring countries, Sierra Leone, Liberia, and Guinea. Phylogenetically, LASV isolates from these countries were placed into clade IV. The prototypic JOS/SL/76/H is the most extensively studied LASV isolate. The information on Nigerian strains belong to clades I-III is limited. Two most advanced LF vaccine candidates, MOP/LAS reassortant (clone ML29) [18] and rVSVΔG/LASV-GPC [31], were designed using LASV/JOS/SL. In spite of high efficacy, these replication-competent vaccines have potential safety concerns.
Alphavirus self-replicating RNA molecules encoding antigens of interest demonstrated promising safety and immunogenicity features. Several alphavirus replicon vaccines are currently in clinical development [19]. We used advanced bicistronic VEEV TC83-based replicons to design multivalent LASV vaccines simultaneously expressing GPCs from both distantly related clades I and IV of LASV. The ability of alphavirus replicon VLPVs to express several genes from the same pathogen (e.g., LASV) or from two different pathogens (e.g., LASV and EBOV) was already documented [22].
Alphavirus VLPVs target professional antigen-presenting cells and promote CD8+ T cells responses [32], a feature which is especially attractive for LASV vaccines [33]. We designed bivalent VLPVs expressing metabolically stable LASV GPs, ΔLGPfib (from clade I or IV) to enhance T cell responses through a cross-priming pathway. For cross-priming CD8+ T cell responses, vaccines have to express metabolically stable antigens [34,35]. It has been documented, that stable antigens improved vaccine immunogenicity compared with antigens subjected to accelerated proteosomal degradation [36].
In VLPV-infected cells, ΔLGPfib was expressed as a trimer with molecular mass of approximately 220–250 kDa (Fig. 3B). Staining with a panel of LASV CPC-specific antibodies confirmed antigenic identity of LASV ΔLGPfib. The similar strategy (Fig. S1) was used to design non-cleavable variant of LCMV GP [37]. The GP trimers, from LCMV [37] and LASV (Fig. 2), accumulated in ER compartment. We documented that LASV ΔLGPfib affected ER homeostasis and induced ER-stressed apoptosis (Fig. 2C, S2). Metabolically stable LCMV GP induced a cross-primed CD8+ CTL 10,000-fold better than those induced with wild-type GP [37]. In line with these results, we previously demonstrated [21] that monovalent VLPV expressing ΔLGPfib induced stronger T cell responses than those induced by VLPV expressing LASV GPCwt which is not cross-presented naturally. Immunization with bivalent VLPVs expressing both antigens, ΔLGPfib and LGPwt, resulted in induction of CD11c+/CD8+ dendritic cells (Fig. 6) playing the major role in cross-presentation of antigens on MHC class I and induction of CD8+ T cell responses.
Immunogenicity of LASV VLPV vaccines was assessed in a CBA/J-ML29 mouse model [25]. In this model and in NHPs [38], protection is associated with T cell responses rather than with antibody production. In contrast to monovalent LASV VLPVs [25], bivalent VLPVs expressing GPCwt and ΔLGPfib resulted in higher immunogenicity as assessed by IgG ELISA (Fig. S3). LASV VLPV vaccines did not induce detectable levels of nAbs. During natural LASV infection, protection and recovery are not associated with nAbs which appeared too late during the infection (rev. by [38]). However, recent success in generation of recombinant nAbs from B cells of LASV-exposed individuals provides opportunity to use these mAbs for post-exposure treatment [39]. The possibility that non-nAbs may also play a role in protection cannot be excluded.
While antigen formulation in VLPV1-3 vaccines had no effect on protection, bivalent VLVPs induced stronger LASV-specific IgG responses in comparison with a ML29 control. Notably, bivalent VLPV1 vaccine formulation (LNΔGPfib/LJGPwt) induced stronger T cell responses than VLPV2 vaccine (LNGPwt/LJΔGPfib) or combination of both vectors, VLPV1&VLPV2, (Fig. S1, 7). With similar levels of expression of both antigens, LGPwt and ΔLGPfib, higher immunogenicity (in terms of induction of T cells) of VLPV1 formulation remains to be additionally investigated. Stimulation of splenocytes of VLPV1-immunized mice with peptide cocktails derived from opposite LASV clades, I and IV, resulted in similar responses (including stimulation with peptides from clades II and III, not shown). Interestingly, control ML29 vaccine induced similar poly-functional T cell responses after stimulation with both cocktails from clade I and IV. While kinetics and scale of ML29-induced T cell responses were different in comparison VLPV1-immunized mice (Fig. 4D), both experimental vaccines induced comparable cross-reactive responses. It should be mentioned, that these vaccine candidates, VLPVs and ML29, share GP2 epitopes responsible for cross-reactivity [40]. In case of ML29, NP protein seems to additionally contribute to cross-protective T cell responses [41].
In conclusion, advanced TC83-based alphavirus bivalent VLPV vaccines expressing a combination of LASV GPCwt and ΔLGPfib from strains belonging to clades I and IV induce cross-reactive T cells responses comparable (or better) with those induced by live attenuated ML29 experimental vaccine. Certainly, a mouse challenge model used in this study has limitations. LASV is a rodentborne virus and LASV infection is treated differently by immune system of rodents (mice, guinea pigs) and NHPs. Currently, there is no challenge model for LASV from clade I. LASV/LP does not induce fatal LF-like disease in strain 13 guinea pigs, the most sensitive model for LASV/Jos, and there is no information on LASV/LP in NHPs. At these circumstances, a mouse model is the only currently available model to assess LASV/LP-specific T cell responses and cross-protective efficacy of experimental vaccines. Ongoing vaccination-challenge experiments with bivalent LASV VLPV vaccines in NHPs will provide additional valuable information on cross-protective efficacy. With more favorable safety profile, replication-incompetent experimental VLPV vaccines described in this study are well positioned for “organized” groups at risk (first responders, personnel of local hospitals in endemic areas, international travelers visiting endemic areas, military personnel, and staff of BSL4 labs working with LASV) where prime-boost vaccination is feasible.
Supplementary Material
Acknowledgments
These studies were supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01 AI093450 (to I.S. Lukashevich and P. Pushko). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Gretchen Holz and Nicole Warner for assistance with animal studies.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2017.12.046.
Footnotes
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Radoshitzky SR, Bào Y, Buchmeier MJ, Charrel RN, Clawson AN, Clegg CS, et al. Past, present, and future of arenavirus taxonomy. Arch Virol. 2015;160:1851–74. doi: 10.1007/s00705-015-2418-y. [DOI] [PubMed] [Google Scholar]
- 2.Fichet-Calvet E, Rogers DJ. Risk maps of lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3(3) doi: 10.1371/journal.pntd.0000388. doi: e38810.1371/journal.pntd.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson AT, Moses LM, Bausch DG. Mapping transmission risk of Lassa fever in West Africa: the importance of quality control, sampling bias, and error weighting. PLoS One. 2014;9(8):e100711. doi: 10.1371/journal.pone.0100711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sogoba N, Feldmann H, Safronetz D. Lassa fever in West Africa: evidence for an expanded region of endemicity. Zoonoses Public Health. 2012;59:43–7. doi: 10.1111/j.1863-2378.2012.01469.x. Epub. [DOI] [PubMed] [Google Scholar]
- 5.Richmond JK, Deborah JB. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003;327(7426):1271–5. doi: 10.1136/bmj.327.7426.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falzarano D, Feldmann H. Vaccines for viral hemorrhagic fevers - progress and shortcomings. Curr Opin Virol. 2013;3:1–9. doi: 10.1016/j.coviro.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. 2017 Annual review of diseases prioritized under the Research and Development Blueprint. Geneva, Switzerland: < http://wwwwhoint/csr/research-and-development/documents/2017-Prioritization-Long-Reportpdf?ua=1.2017> (24-25 January 2017) [Google Scholar]
- 8.Lukashevich IS, Stelmakh TA, Golubev VP, Stchesljonok EP, Shkolina TV. Ribonucleic acids of Machupo and Lassa viruses. Arch Virol. 1984;79:189–203. doi: 10.1007/BF01310811. [DOI] [PubMed] [Google Scholar]
- 9.Lukashevich IS, Salvato MS. Lassa virus genome. Curr Genom. 2006;7:351–79. [Google Scholar]
- 10.Burri DJ, da Palma JR, Kunz S, Pasquato A. Envelope glycoprotein of arenaviruses. Viruses. 2012;4:2162–81. doi: 10.3390/v4102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher-Hoch SP, McCormick JB. Lassa fever vaccine: a review. Expert Rev Vaccines. 2004;3:103. doi: 10.1586/14760584.3.2.189. https://doi.org/10.1586/14760584.3.2.189. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Dalebout TJ, Bredenbeek PJ, Carrion R, Jr, Brasky K, Patterson J, et al. Yellow fever 17D-vectored vaccines expressing Lassa virus GP1 and GP2 glycoproteins provide protection against fatal disease in guinea pigs. Vaccine. 2011;29(6):1248–57. doi: 10.1016/j.vaccine.2010.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen M, Rollin P, Ksiazek T, Hustad H, Bausch D, Demby A, et al. Genetic diversity among Lassa virus strains. J Virol. 2000;74(15):6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen Kristian G, Shapiro BJ, Matranga Christian B, Sealfon R, Lin Aaron E, Moses Lina M, et al. Clinical sequencing uncovers origins and evolution of lassa virus. Cell. 2015;162(4):738–50. doi: 10.1016/j.cell.2015.07.020. https://doi.org/10.1016/j.cell.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emmerich P, Gunter S, Schmitz H. Strain-specific antibody response to Lassa virus in the local population of West AFrica. J Clin Virol. 2008;42(1):40–4. doi: 10.1016/j.jcv.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 17.Carrion R, Jr, Patterson JL, Johnson C, Gonzales M, Moreira CR, Ticer A, et al. A ML29 reassortant virus protects guinea pigs against a distantly related Nigerian strain of Lassa virus and can provide sterilizing immunity. Vaccine. 2007;25(20):4093–102. doi: 10.1016/j.vaccine.2007.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukashevich IS, Patterson J, Carrion R, Moshkoff D, Ticer A, Zapata J, et al. A live attenuated vaccine for lassa fever made by reassortment of lassa and mopeia viruses. J Virol. 2005;79(22):13934–42. doi: 10.1128/JVI.79.22.13934-13942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pushko P, Tretyakova I. Aphavirus replicon vectors for prophylactic applications and cancer intervention. In: Lukashevich IS, Shirwan H, editors. Novel technologies for vaccine development. Wienn: Springer; 2014. pp. 61–85. [Google Scholar]
- 20.Pittman PR, Makuch RS, Mangiafico JA, Cannon TL, Gibbs PH, Peters CJ. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine. 1996;14(4):337–43. doi: 10.1016/0264-410x(95)00168-z. [DOI] [PubMed] [Google Scholar]
- 21.Carrion R, Bredenbeek P, Jiang X, Tretyakova I, Pushko P, Lukashevich IS. Vaccine platforms to control arenaviral hemorrhagic fevers. J Vaccines Vaccination. 2012;3(7):1000160. doi: 10.4172/2157-7560.1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001;75(23):11677–85. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;2(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 24.Bredenbeek PJ, Molenkamp R, Spaan WJM, Deubel V, Marianneau P, Salvato MS, et al. A recombinant Yellow Fever 17D vaccine expressing Lassa virus glycoproteins. Virology. 2006;345(2):299–304. doi: 10.1016/j.virol.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goicochea MA, Zapata JC, Bryant J, Davis H, Salvato MS, Lukashevich IS. Evaluation of Lassa virus vaccine immunogenicity in a CBA/J-ML29 mouse model. Vaccine. 2012;30(8):1445–52. doi: 10.1016/j.vaccine.2011.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukashevich IS, Clegg JC, Sidibe K. Lassa virus activity in Guinea: distribution of human antiviral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen. J Med Virol. 1993;40(3):210–7. doi: 10.1002/jmv.1890400308. [DOI] [PubMed] [Google Scholar]
- 27.Hegde RS, Ploegh HL. Quality and quantity control at the endoplasmic reticulum. Curr Opin Cell Biol. 2010;22:437–46. doi: 10.1016/j.ceb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ron D, Habener JF. CHOP, a novel developmentally regu-lated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–53. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 30.Lukashevich IS. The search for animal models for Lassa fever vaccine development. Expert Rev Vaccines. 2013;12(1):71–86. doi: 10.1586/erv.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geisbert T, Jones S, Fritz E, Shurtleff A, Geisbert J, Liebscher R, et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005;2:e183. doi: 10.1371/journal.pmed.0020183. https://doi.org/10.1371/journal.pmed.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson JM, Whitmore AC, Staats HF, Johnston RE. Alphavirus replicon particles acting as adjuvants promote CD8+ T cell responses to co-delivered antigen. Vaccine. 2008;26(33):4267–75. doi: 10.1016/j.vaccine.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukashevich IS. Advanced vaccine candidates for lassa fever. Viruses. 2012;4(11):2514–57. doi: 10.3390/v4112514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norbury C, Basta S, Donohue KB, Tscharke DC, Princiotta MF, Berglund P, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–21. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 35.Huckriede A, Bungener L, Holtrop M, de Vries J, Waarts B-L, Daemen T, et al. Induction of cytotoxic T lymphocyte activity by immunization with recombinant Semliki Forest virus: indications for cross-priming. Vaccine. 2004;22(9–10):1104–13. doi: 10.1016/j.vaccine.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Schliehe C, Bitzer A, van den Broek M, Groettrup M. Stable antigen is most effective for eliciting CD8+ T-cell responses after DNA vaccination and infection with recombinant vaccinia virus in vivo. J Virol. 2012;86(18):9782–93. doi: 10.1128/JVI.00694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freigang S, Eschli B, Harris N, Geuking M, Quirin K, Schrempf S, et al. A lymphocytic choriomeningitis virus glycoprotein variant that is retained in the endoplasmic reticulum efficiently cross-primes CD8+ T cell responses. Proc Natl Acad Sci USA. 2007;104(33):13426–31. doi: 10.1073/pnas.0704423104. https://doi.org/10.1073/pnas.0704423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescott JB, Marzi A, Safronetz D, Robertson SJ, Feldmann H, Best SM. Immunobiology of Ebola and Lassa virus infections. Nat Rev Immunol. 2017;17(3):195–207. doi: 10.1038/nri.2016.138. https://doi.org/10.1038/nri.2016.138. [DOI] [PubMed] [Google Scholar]
- 39.Mire CE, Cross RW, Geisbert JB, Borisevich V, Agans KN, Deer DJ, et al. Human-monoclonal-antibody therapy protects nonhuman primates against advanced Lassa fever. Nat Med. 2017;23(10):1146–9. doi: 10.1038/nm.4396. https://doi.org/10.1038/nm.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meulen J, Badusche M, Satoguina J, Strecker T, Lenz O, Loeliger C, et al. Old and New World arenaviruses share a highly conserved epitope in the fusion domain of the glycoprotein 2, which is recognized by Lassa virus-specific human CD4+ T-cell clones. Virology. 2004;321(1):134–43. doi: 10.1016/j.virol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 41.ter Meulen J, Badusche M, Kuhnt K, Doetze A, Satoguina J, Marti T, et al. Characterization of human CD4+ T-cell clones recognizing conserved and variable epitopes of the Lassa virus nucleoprotein. J Virol. 2000;74(5):2186–92. doi: 10.1128/jvi.74.5.2186-2192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


