Abstract
Study Objectives:
The long-term effect of continuous positive airway pressure (CPAP) on health-related quality of life (HRQOL) in patients with high cardiovascular disease risk and obstructive sleep apnea (OSA) without severe sleepiness is uncertain. We aimed to determine the effect of CPAP treatment on HRQOL in individuals with moderate or severe OSA and cardiovascular disease (CVD) or multiple CVD risk factors without severe sleepiness.
Methods:
In this randomized, controlled, parallel group study, 169 participants were assigned to treatment with CPAP or the control group (conservative medical therapy [CMT] or CMT with sham CPAP). Analyses were based on an intention-to-treat approach. Linear mixed effect models were fitted to compare the changes in the Medical Outcomes Study Short Form-36 (SF-36) and in subjective sleepiness (Epworth Sleepiness Scale [ESS]) between groups from baseline to the average of 6- and 12-month measurements.
Results:
CPAP improved several domains of HRQOL including bodily pain (treatment effect 9.7 [95% confidence interval, CI 3.9 to 15.4]; p = .001), vitality (5.7 [95% CI 1.5 to 9.9]; p = .008), general health (8.2 [95% CI 3.7 to 12.7]; p < .001), physical functioning (5.5 [95% CI 1.1 to 10.0]; p = .016), and the physical health summary score (3.3 [95% CI 1.4 to 5.3]; p = .001). CPAP also resulted in less daytime sleepiness (mean change in ESS −1.0 point [95% CI −2.0 to −0.0]; p = .040).
Conclusions:
In patients with moderate–severe OSA at high risk of cardiovascular events and without severe sleepiness, CPAP improved daytime sleepiness and multiple domains of HRQOL over 6 to 12 months of follow-up, with the largest improvement observed for bodily pain.
Keywords: sleep apnea, CPAP, quality of life, clinical trial, sleepiness.
Statement of Significance
Obstructive sleep apnea (OSA) and cardiovascular disease (CVD) each impairs health-related quality of life (HRQOL). However, the effectiveness of CPAP in improving outcomes in patients without severe sleepiness is debated. We evaluated the effect of CPAP on long-term changes in HRQOL in high-risk patients with moderate to severe OSA and without severe sleepiness in one of the largest clinical trials to date. Despite only modest CPAP compliance and exclusion of participants with severe daytime sleepiness, CPAP improved several domains of HRQOL, including bodily pain and daytime sleepiness, compared to the control arm. Our findings may motivate clinicians and patients when considering treatment for OSA and may also provide data for policy makers to reexamine the current insurance criteria for CPAP use.
INTRODUCTION
Cardiovascular disease (CVD) remains the leading cause of death in the United States and is a significant cause of morbidity and health-care expenditures.1 Obstructive sleep apnea (OSA) affects an estimated 26% of the general population, and its prevalence among individuals with CVD is even higher.2–4 Increasing evidence suggests that OSA is an important modifiable risk factor for CVD, including stroke, coronary artery disease, heart failure, and atrial fibrillation.5–8 OSA is also associated with excessive daytime sleepiness, an increased risk of injuries from motor vehicle crashes and industrial injuries, and impaired health-related quality of life (HRQOL).9–14
Continuous positive airway pressure (CPAP) is currently the treatment of choice for OSA. CPAP has been shown to improve both subjective and objective measures of sleepiness.9 A number of cohort studies and randomized controlled trials have also evaluated the impact of CPAP on HRQOL in OSA patients although not all studies have shown an improvement in HRQOL with treatment.15–17 Most of these studies had relatively short study durations (3 months or less), and only a few studies have evaluated the impact of CPAP on HRQOL in patients with OSA and CVD.18–21 Mansfield et al. found that 3 months of CPAP therapy improved several domains of HRQOL in patients with heart failure.18 In comparison, a similar effect was not observed in a 6-week randomized controlled crossover study comparing autotitrating CPAP to sham CPAP in patients with stable symptomatic heart failure and OSA.19 More recently, McEvoy et al. showed improvements in HRQOL with CPAP use in patients with coronary or cerebrovascular disease.21 Given the limited and inconsistent data in patients with CVD and the short duration of follow-up in many of these studies, we aimed to investigate the impact of 6 to 12 months of CPAP therapy compared to control on key measures of HRQOL, using data from a randomized control trial.
The Best Apnea Interventions for Research (BestAIR) trial was designed as a planning study to assess key feasibility and optimal study design features in the context of a cardiovascular intervention trial in OSA.22,23 A secondary, prespecified objective was to compare the changes in HRQOL as assessed by the Medical Outcomes Study Short Form-36 questionnaire (SF-36) and subjective daytime sleepiness as measured by the Epworth Sleepiness Scale (ESS) between participants with cardiovascular comorbidity randomized to CPAP versus control. We hypothesized that in this group of high-risk patients without severe sleepiness CPAP treatment would significantly improve HRQOL and reduce sleepiness compared to the control group.
METHODS
The rationale and protocol for the BestAIR study has been previously reported.22,23 Detailed methods are reported in the Online Supplement.
Study Design
BestAIR was a randomized, parallel-group clinical trial with blinded assessment of outcomes. Participants were recruited from outpatient clinics from three medical centers in Boston, Massachusetts. The four treatment arms were: conservative medical therapy (CMT), CMT + sham CPAP, CMT + CPAP, and CMT + CPAP + motivational enhancement (ME). CMT consisted of education on sleep hygiene, healthy lifestyle, and nasal dilator strips for use during sleep. ME consisted of a behavioral intervention to improve CPAP adherence, as described previously.24 Owing to the longer-than-expected time to complete enrollment, patients randomized after January 2013 were only followed for 6 months.22,23 The study was approved by the Institutional Review Board at each participating center. All participants provided written informed consent.
Eligibility criteria were used to identify those at high risk of cardiovascular events as may be recruited in future large-scale trials. Eligible subjects had an apnea–hypopnea index (AHI) 4% ≥10 events/hour or AHI 3% ≥15 events/hour and were either aged 45 to 75 years with established CVD (coronary artery disease, ischemic stroke, or diabetes) or aged 55 to 75 years with 3 or more CVD risk factors (male, body mass index [BMI] ≥30 kg/m2, hypertension, dyslipidemia, or ≥10 pack-years of smoking). Major exclusion criteria were a cardiovascular event <4 months prior to enrollment, prior CPAP use, and excessive sleepiness (ESS score >14 or report falling asleep while driving within the past 2 years). Sleep studies to determine eligibility were completed either as part of routine care (in-laboratory polysomnography) or administered by the study investigators (Embletta Gold or X100, Embla, Ontario, Canada). Participants who had not undergone an in-lab CPAP titration used an auto-adjusting device for a minimum of 5 days to identify the pressure for ongoing fixed CPAP pressure.
Participants were asked to use a nasal CPAP mask open to atmosphere without a CPAP device and complete a diary during a 2-week run-in period. Those who reported wearing the mask for a majority of nights and were willing to continue in the study were randomized in a 1:1:1:1 ratio to one of the 4 study arms described earlier using sequence generated off-site with a block size of 4, based on 3 stratification factors: diagnostic type (full- or split-night titration), site, and CVD status (established or risk factors).
Outcomes
The key patient reported outcomes in the current analysis were HRQOL as assessed by the Medical Outcomes Study Short Form-36 questionnaire (SF-36) and subjective daytime sleepiness as measured by the ESS. Outcomes were measured at baseline, 6, and 12 months. The SF-36 is a generic HRQOL instrument that has been shown to have excellent reliability and validity in patients with OSA.25 The questionnaire measures 8 domains of health: physical functioning, role limitation due to physical problems, bodily pain, general health, vitality/energy, social functioning, role limitation due to emotional problems, and mental health. Scores from the 8 domains and the 2 derived summary scores (physical health and mental health summary scores) are standardized such that a mean score of 50 with an SD of 10 would reflect the mean score in the U.S. general population. The ESS is a self-administered 8-item questionnaire that is the most widely used index to measure subjective sleepiness in OSA. Scores range from 0 to 24, with a cutoff of >10 representing clinically significant sleepiness.26,27
Statistical Analysis
Analyses reported in this article compare data from the combined control arms (CMT and CMT + sham CPAP) with the combined CPAP arms (CMT + CPAP and CMT + CPAP + ME) as specified in the protocol, to provide greater power to detect any CPAP effect than pairwise comparisons among individual subarms. Between-group cross-sectional comparisons were made using Fisher’s exact tests for categorical data and 2-sample t-tests or Wilcoxon rank-sum tests for continuous data depending on whether or not substantial departure from normality in data distributions was observed. The primary analysis was based on an intent-to-treat approach. Mixed effects linear regression models were used to compare the longitudinal profiles between the combined CPAP and control groups. Observational time (0, 6, and 12 months) was modeled as a categorical variable. Stratification factors were included as covariates. To further explore the influence of adherence on study findings, we performed a sensitivity analysis comparing the differences in changes in outcomes from baseline to 6 months by adherence status among CPAP users (adherence defined using Medicare definition of ≥ 4 hours per night for 70% of days over 6 months).
Mixed effects linear regression analyses were performed using R version 3 or higher, with functions from the “lme4” package.28 All other analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC). Two-sided p values <.05 were considered statistically significant.
RESULTS
Study Population
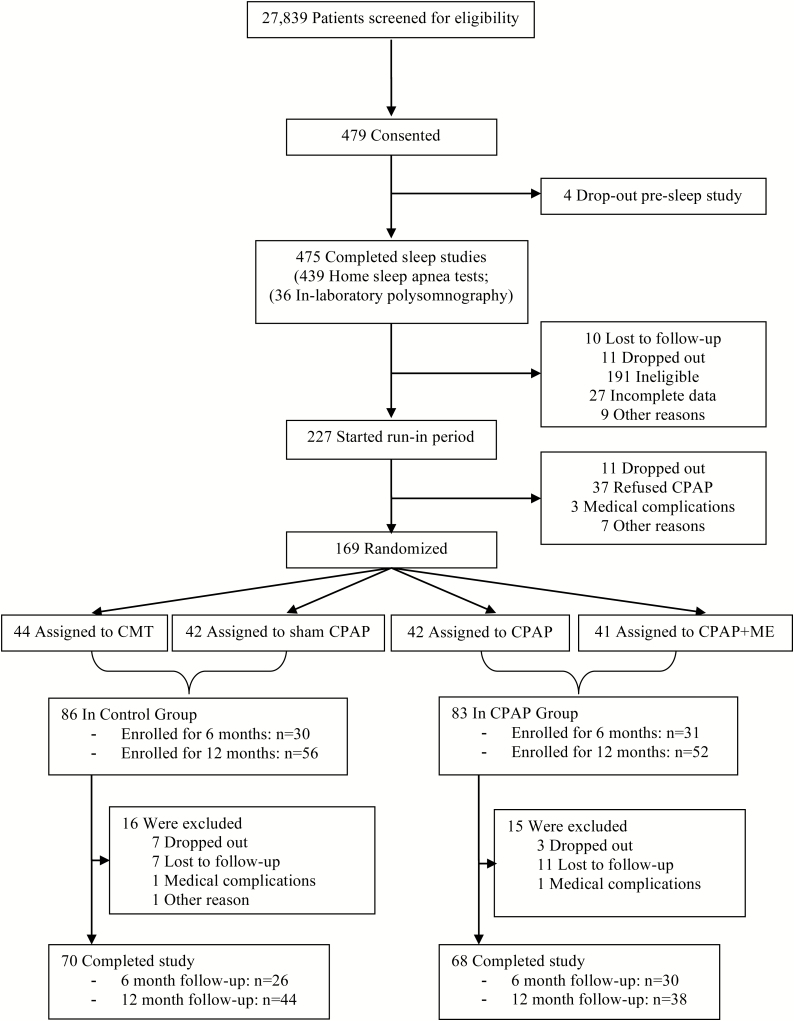
Screening began in February 2011, and randomization took place from April 2011 to August 2013, with the final follow-up visit completed in March 2014.22 A total of 475 patients completed screening sleep studies (in-laboratory polysomnography n = 36, home sleep apnea test n = 439), with 227 patients identified with moderate or severe OSA and completing the run-in phase (Figure 1). Of the 169 participants randomized, 108 were recruited before January 2013 and were followed for 12 months, and the remainder who were recruited later was followed for 6 months.
Figure 1.
Study flowchart.
Baseline characteristics of the participants were comparable for the CPAP and control groups (Table 1). The majority (85.2%) of the participants had hypertension, 34.3% had coronary artery disease, and 37.3% had diabetes. Mean (SD) age was 63.8 (7.3) years, and 65.1% were men. Mean BMI and AHI were 31.7 (5.9) kg/m2 and 29.2 (16.6) events/hr, respectively. At 6- and 12 months, the mean (SD) CPAP use was 3.82 (2.86) and 3.44 (2.99) hours per night, respectively.
Table 1.
Baseline characteristics of the study populationa.
| All patients, N = 169 | Control group, N = 86 | CPAP group, N = 83 | |
|---|---|---|---|
| Age (years) | 63.8 (7.3) | 63.7 (6.9) | 63.8 (7.8) |
| Male sex, n (%) | 110 (65.1) | 55 (64.0) | 55 (66.3) |
| Race/ethnicity, n (%)b | |||
| White | 151 (89.3) | 77 (89.5) | 74 (89.2) |
| Black | 11 (6.5) | 6 (7.0) | 5 (6.0) |
| Hispanic | 6 (3.6) | 2 (2.3) | 4 (4.8) |
| Other | 7 (4.1) | 3 (3.5) | 4 (4.8) |
| Education, n (%)b | |||
| <High school | 2 (1.2) | 1 (1.2) | 1 (1.2) |
| High school graduate | 57 (33.7) | 24 (27.9) | 33 (39.8) |
| Bachelors or higher | 110 (65.1) | 61 (70.9) | 49 (59.0) |
| Body mass index (kg/m2)c | 31.7 (5.9) | 32.3 (6.5) | 31.1 (5.2) |
| Neck circumference, cm | 41.5 (3.9) | 41.9 (3.9) | 41.1 (4.0) |
| Smoking history, n (%) | |||
| Current | 13 (7.7) | 5 (5.8) | 8 (9.6) |
| Former | 85 (50.3) | 40 (46.5) | 45 (54.2) |
| Never | 71 (42.0) | 41 (47.7) | 30 (36.2) |
| History of hypertension, n (%) | 144 (85.2) | 73 (84.9) | 71 (85.5) |
| History of CVD or diabetes, n (%) | |||
| Coronary artery disease | 58 (34.3) | 28 (32.6) | 30 (36.1) |
| Diabetes | 63 (37.3) | 35 (40.7) | 28 (33.7) |
| Stroke | 4 (2.4) | 2 (2.3) | 2 (2.4) |
| AHI, events/hr | |||
| Mean (SD) | 29.2 (16.6) | 32.0 (19.1) | 26.2 (12.9) |
| Median (IQR) | 23.9 (17.4–33.4) | 26.1 (18.2–37.4) | 22.7 (16.6–31.4) |
| Sleep time with SpO2 <90%, % | |||
| Mean (SD) | 9.2 (14.3) | 9.9 (15.2) | 8.5 (13.3) |
| Median (IQR) | 3.5 (0.9–11.0) | 3.7 (1.2–12.0) | 3.2 (0.6–10.8) |
Abbreviations: ACE, angiotensin-converting enzyme; AHI, apnea–hypopnea index; CVD, cardiovascular disease; IQR, interquartile range; CPAP, continuous positive airway pressure; SD, standard deviation; SpO2, oxygen saturation measured by pulse oximetry.
aData presented as mean (SD) unless otherwise specified.
bEducation, race and ethnic group were self-reported.
cThe body-mass index is the weight in kilograms divided by the square of the height in meters.
Outcomes
Baseline scores in all 8 domains of the SF-36 and the 2 component summary scores were similar between the CPAP and the control groups (Table 2). Overall, participants had above population average scores, with some scores higher than those reported in prior studies of OSA patients.18,29,30 No significant differences in SF-36 scores or ESS were observed within the 2 CPAP or within the 2 control subarms at baseline or at follow-up (results not shown). At the end of the study, CPAP improved several domains of the SF-36 compared to the control group. The greatest effect was observed for bodily pain, with an estimated treatment effect of 9.7 points (95% confidence interval [CI] 3.9 to 15.4; p = .001) comparing the CPAP to control group. Significant improvements were also seen for vitality (estimated treatment effect 5.7 points [95% CI 1.5 to 9.9]; p = .008), general health (8.2 points [95% CI 3.7 to 12.7]; p < .001), and physical functioning 5.5 points [95% CI 1.1 to 10.0]; p = .016). The physical health summary score improved by 3.3 points (95% CI 1.4 to 5.3; p = .001), but no significant improvement in the emotional or social role functioning, mental health subscale, or summary score was noted (all p > .05).
Table 2.
Patient-reported outcomes (Epworth Sleepiness Scale and SF-36).
| Control group | CPAP Group | Treatment Effect (95% CI) | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6-month follow-up | 12-month follow-up | Baseline | 6-month follow-up | 12-month follow-up | |||
| ESS | 8.5 (4.5) | 7.6 (4.2) | 7.7 (4.0) | 8.0 (4.5) | 6.2 (3.8) | 6.0 (4.0) | −1.0 (−2.0 to −0.0) | .040 |
| ESS >10, n (%) | 24 (27.9) | 16 (23.5) | 12 (27.9) | 20 (24.1) | 10 (14.7) | 5 (15.2) | – | – |
| SF-36 Scales | ||||||||
| Vitality | 56.0 (19.8) | 58.8 (21.6) | 60.9 (18.1) | 56.0 (20.0) | 63.5 (21.2) | 63.5 (18.8) | 5.7 (1.5 to 9.9) | .008 |
| General Health | 57.9 (20.1) | 57.5 (21.9) | 57.7 (21.2) | 56.5 (21.8) | 61.8 (21.8) | 61.5 (17.8) | 8.2 (3.7 to 12.7) | <.001 |
| Physical Functioning | 69.4 (25.5) | 67.6 (26.4) | 69.6 (23.4) | 73.2 (24.9) | 75.5 (23.1) | 76.4 (21.7) | 5.5 (1.1 to 10.0) | .016 |
| Bodily Pain | 64.7 (23.9) | 61.8 (25.7) | 56.3 (22.3) | 62.3 (25.0) | 65.7 (23.0) | 68.1 (22.8) | 9.7 (3.9 to 15.4) | .001 |
| Emotional Role Functioning | 79.8 (24.7) | 84.4 (21.6) | 84.8 (24.6) | 81.3 (24.6) | 84.8 (21.4) | 83.1 (23.8) | −0.4 (−6.5to 5.8) | .904 |
| Physical Role Functioning | 72.5 (26.1) | 70.2 (25.8) | 76.2 (23.5) | 73.8 (26.0) | 73.5 (27.2) | 73.1 (30.1) | 1.1 (−5.0 to 7.2) | .718 |
| Social Role Functioning | 80.9 (22.5) | 80.9 (22.7) | 86.3 (20.6) | 79.7 (27.0) | 81.5 (27.0) | 83.0 (25.6) | 1.0 (−3.9 to 5.9) | .682 |
| Mental Health | 75.7 (16.6) | 76.9 (16.2) | 80.9 (17.9) | 74.8 (19.8) | 77.7 (18.0) | 76.2 (14.8) | 1.2 (−2.6 to 5.0) | .541 |
| Physical Health Summary Score | 44.3 (9.6) | 42.7 (10.1) | 42.9 (8.7) | 45.2 (9.4) | 45.6 (9.7) | 46.2 (9.6) | 3.4 (1.4 to 5.3) | <.001 |
| Mental Health Summary Score | 50.3 (9.6) | 52.2 (9.7) | 54.0 (11.5) | 49.7 (12.0) | 52.2 (11.3) | 51.3 (9.7) | 0.2 (−2.1 to 2.4) | .869 |
Abbreviations: CI, confidence interval; ESS, Epworth Sleepiness Scale; SF-36, Medical Outcomes Study Short Form 36 Health Survey.
aData presented as mean (SD) unless otherwise specified. N = 86 subjects in the control group and N = 83 subjects in the CPAP group had one or more data points and were included in the analysis of ESS. N = 85 in the control group and N = 81 in the CPAP group had one or more data points and were included in the analysis of the various SF-36 scales.
The baseline ESS was similar between the CPAP and the control groups (Table 2). Despite the exclusion of participants with severe daytime sleepiness (mean ESS 8.3 [SD 4.5]), CPAP led to greater reduction in the ESS score (estimated treatment effect −1.0 point [95% CI −2.0 to −0.0]; p = .040 compared to the control group.
Over the first 6 months, the mean (SD) CPAP use was 3.8 (2.9) hours. Only 51.8% of participants in the treatment group used CPAP for an average of ≥4 hr/night over 6 months, and only 43.4% of participants were adherent by Medicare definition (≥ 4 hours per night for 70% of days). The degree of improvement in HRQOL did not correlate with CPAP compliance, but higher average nightly CPAP use was associated with more improvement in ESS scores (Pearson correlation r = −0.29, p = .018; Supplemental Table 1). Changes in HRQOL indices over 6 months in those who met or did not meet Medicare adherence criteria are shown in Supplemental Table 2. The magnitude of changes in ESS and HRQOL were not statistically different between individuals characterized by low or higher CPAP adherence; however, the power to detect differences was low.
DISCUSSION
The present study is one of the largest long-term randomized controlled trials to examine the effect of CPAP on HRQOL in patients with moderate or severe OSA at high risk of CVD. In this group of participants without severe sleepiness and with generally above-average HRQOL at baseline, CPAP led to significant 6- to 12-month improvements in several domains of HRQOL, with the greatest improvement seen in bodily pain. CPAP also led to a modest but significant improvement in subjective daytime sleepiness. These improvements were seen despite an average CPAP use of less than 4 hours per night.
Patient-reported outcomes such as symptoms and HRQOL are increasingly recognized as important components of disease management to improve patient well-being and are recommended to be included as secondary end points in clinical trials.30 CVD is known to impair HRQOL.31 Sleep apnea, even if mild, has also been associated with impaired HRQOL.10,11,29,32 Thus, patients with both OSA and CVD may be at particular risk of poorer HRQOL. CPAP pneumatically stents open the airway, thereby abolishing apneas and hypopneas and reduces sleep apnea-related arousals and sleep fragmentation. Several previous studies have demonstrated that CPAP improves HRQOL in symptomatic patients with OSA.29,33 However, the impact of CPAP on these outcomes in nonsleepy patients has been debated. In particular, Barbé et al. found that 6 weeks of treatment with CPAP compared to sham CPAP in nonsleepy patients with severe OSA did not improve HRQOL as measured by the SF-36 and the Functional Outcomes of Sleep Quality questionnaires.34 In contrast, in our study of patients without severe sleepiness (mean ESS 8.3), significant improvements in several domains of SF-36 were seen. The difference between our study and Barbé et al. may be explained by the longer duration of treatment in our study (6- to 12 months vs. 6 weeks) and the inclusion of patients with significant cardiovascular comorbidity in our study.
Prior studies in patients with heart disease have not consistently demonstrated a benefit of CPAP on HRQOL.17–21 In a study of 55 patients with chronic heart failure, Mansfield and colleagues showed that 3 months of CPAP therapy significantly improved the physical role, vitality, social functioning, and mental health domains of the SF-36, compared to control.18 In a separate study, Smith et al did not show an improvement in HRQOL with 6 weeks of autotitrating CPAP compared to sham CPAP in patients with stable symptomatic heart failure and OSA.19 Similar to the Mansfield study, we showed that CPAP improved vitality. However, no significant improvement in physical role, social functioning, and mental health were noted in our study. One explanation may be that our participants did not have heart failure and tended to have high scores in the physical role, social functioning, and mental health domains at baseline (>70). More recently, improvements in HRQOL with CPAP use were also reported in an international trial of patients with moderate-to-severe OSA and coronary or cerebrovascular disease.21 However, a direct comparison of our study results to that of the SAVE trial is not possible, given the latter did not report a detailed analysis of the HRQOL measures.
Unlike previous studies, which did not show significant improvements in pain domains of HRQOL with CPAP use,18,35-37 we observed the greatest improvement in the bodily pain scale of the SF-36 (mean treatment effect 9.7, 95% CI 3.9 to 15.4, p = .001). Chronic pain has become the most common reason for outpatient medical visits, and its treatment has led to a dramatic rise in habitual narcotic use.38 Studies show that patients with chronic pain have diminished HRQOL and are more likely to develop psychological disorders, cognitive impairment, and sexual dysfunction.39 Chronic pain syndromes also have significant cost implications, both in terms of increased health-care costs and lost productivity with increased absenteeism from work and reduced performance while at work.40 There is emerging data indicating the close association between sleep quality, duration, and pain, with sleep and pain sharing common neurological pathways.41 New data suggest that sleep apnea may be associated with chronic pain syndromes.42 Our results point to the possibility that improvement in sleep apnea may improve pain and suggest that sleep apnea may potentially be an important therapeutic target in the management of chronic pain syndromes.
Subjective sleepiness is absent in many individuals with significant sleep disordered breathing.43 In particular, many patients with CVD and sleep apnea do not report excessive daytime sleepiness.20,44 Therefore, our findings of improved HRQOL with CPAP use in a group of participants with ESS scores in the normal to mildly elevated range have important clinical implications. These findings suggest that treatment benefits occur even in the absence of a high ESS score. Furthermore, an expectation of improved HRQOL may motivate patients to undergo testing and treatment for sleep apnea. Given that symptom relief with CPAP treatment has been consistently shown among the strongest predictors of CPAP compliance,45,46 improved HRQOL may promote better CPAP adherence. The larger changes in HRQOL measures compared to change in the ESS also suggest the potential utility in tracking change in HRQOL with treatment.
The 6- to 12-month treatment duration in our study is longer than most previous randomized controlled trials, which were generally 3 months or shorter in duration. Our results indicate that improvements in HRQOL seen with CPAP use are sustained in this patient population, even in a group of patients with an average duration of use of less than 4 hours per night. Long-term CPAP coverage is currently limited by the Center for Medicare and Medicaid Services to those who demonstrate adherence and subjective benefit during an initial 90-day trial period. Adherence is defined as CPAP use ≥4 hours per night for 70% of days within a consecutive 30-day period. Of note, although change in sleepiness was associated with level of CPAP adherence, we observed no significant associations with average hours of CPAP use and change in the HRQOL measures. Research by Weaver et al. suggest that there are different dose–response associations between CPAP use and different outcomes.47 Additional research is needed to further investigate the nature of the dose–response relationship for CPAP and relevant outcomes. Given the importance of HRQOL to patients, these data suggest that more liberal CPAP coverage criteria should be considered.
Our study had several limitations. A potential limitation was the use of a generic HRQOL instrument. While the use of disease-specific instruments such as the Functional Outcomes of Sleep Questionnaire and the Sleep Apnea Quality of Life Index may detect more subtle effects of CPAP on HRQOL, the use of SF-36 allows for cross-study and cross-population comparisons.10 The SF-36 is also one of the most frequently used HRQOL instrument in sleep research.10 Although we did not have 12-month follow-up data for all of our study participants, the use of mixed effects linear regression models allowed use of all data points at 6- and 12-months. In addition, the participants who were followed to 12 months were those randomized before a certain date and not those who chose to remain in the study long term; thus, we do not believe that the 12-month data are biased toward those more motivated to use the therapy. Finally, despite intensive efforts to promote CPAP adherence, average use was only modest, and 56.6% of subjects did not meet the Medicare adherence criteria for coverage over 6 months. It is possible larger effects may have been observed with greater CPAP use, although we did not observe a correlation between improvements in HRQOL and CPAP compliance. Nonetheless, these findings suggest that even modest use may result in clinically significant improvements in HRQOL, and may motivate reexamination of current insurance criteria for CPAP use.
In conclusion, CPAP improved multiple domains of HRQOL in relatively asymptomatic patients with moderate to severe OSA at high risk of CVD. Our findings are likely important for patients and clinicians to help inform therapeutic choices and may also have important implications for policy makers and reimbursement decisions.
FUNDING
This study was supported by the National Institutes of Health (NIH) and the National Heart, Lung, and Blood Institute (NHLBI) 1U34HL105277 and a supplemental grant from the ResMed Foundation. Equipment was donated by ResMed Inc. and Philips Respironics.
INSTITUTION AT WHICH THE WORK WAS PERFORMED
Study subjects were recruited from the Brigham and Women’s Hospital, Beth Israel Deaconess Medical Center, and Joslin Diabetes Center, Boston, MA.
CONTRIBUTORS
Conception and design: RW, EL, SQ, JW, MM, SR; analysis and interpretation: all authors; drafting the manuscript: YZ, RW, KG, JW, MM, SR. SR takes full responsibility for the work as a whole including the study design, access to data, and the decision to submit and publish the manuscript. All authors approved this manuscript in its final form.
CLINICAL TRIAL REGISTRATION
URL: http://www.clinicaltrials.gov; Unique identifier: NCT 01261390.
DISCLOSURE STATEMENT
None disclosed.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all study participants as well as research staff including Hannah Buettner, Beatriz Oropeza, Erin Reese, Tricia Tiu, and Christina Zenobi. The study is also grateful to members of the Data Safety and Monitoring Board (Drs. Mark Espeland, Mike Sharma, Richard Bootzin, Mark Dyken, Ileana Piña), the external Medical Monitor, Dr. Sergio Waxman, the local Medical Monitor, Dr. Sanjay Patel, and Dr. Dennis Drotar for his expertise in adherence fidelity monitoring.
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015; 131(4): e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013; 177(9): 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012; 126(12): 1495–1510. [DOI] [PubMed] [Google Scholar]
- 4. Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008; 52(8): 686–717. [DOI] [PubMed] [Google Scholar]
- 5. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010; 122(4): 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005; 353(19): 2034–2041. [DOI] [PubMed] [Google Scholar]
- 7. Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004; 110(4): 364–367. [DOI] [PubMed] [Google Scholar]
- 8. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005; 365(9464): 1046–1053. [DOI] [PubMed] [Google Scholar]
- 9. Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003; 163(5): 565–571. [DOI] [PubMed] [Google Scholar]
- 10. Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2001; 2(6): 477–491. [DOI] [PubMed] [Google Scholar]
- 11. Finn L, Young T, Palta M, Fryback DG. Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998; 21(7): 701–706. [PubMed] [Google Scholar]
- 12. Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001; 24(1): 96–105. [DOI] [PubMed] [Google Scholar]
- 13. Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999; 340(11): 847–851. [DOI] [PubMed] [Google Scholar]
- 14. Lindberg E, Carter N, Gislason T, Janson C. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001; 164(11): 2031–2035. [DOI] [PubMed] [Google Scholar]
- 15. Jing J, Huang T, Cui W, Shen H. Effect on quality of life of continuous positive airway pressure in patients with obstructive sleep apnea syndrome: a meta-analysis. Lung. 2008; 186(3): 131–144. [DOI] [PubMed] [Google Scholar]
- 16. Giles TL, Lasserson TJ, Smith B, White J, Wright JJ, Cates CJ. Continuous positive airways pressure for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006; Issue 3 Art. No.; CD001106 DOI: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 17. Batool-Anwar S, Goodwin JL, Kushida CA, Walsh JA, Simon RD, Nichols DA, Quan SF. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA). J Sleep Res 2016; 25(6):731–738. doi: 10.1111/jsr.12430. [Epub ahead of print] PubMed PMID: 27242272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004; 169(3): 361–366. [DOI] [PubMed] [Google Scholar]
- 19. Smith LA, Vennelle M, Gardner RS, et al. Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J. 2007; 28(10): 1221–1227. [DOI] [PubMed] [Google Scholar]
- 20. Parra O, Sánchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011; 37(5): 1128–1136. [DOI] [PubMed] [Google Scholar]
- 21. McEvoy RD, Antic NA, Heeley E, et al. ; SAVE Investigators and Coordinators. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016; 375(10): 919–931. [DOI] [PubMed] [Google Scholar]
- 22. Gleason K, Shin D, Rueschman M, et al. Challenges in recruitment to a randomized controlled study of cardiovascular disease reduction in sleep apnea: an analysis of alternative strategies. Sleep. 2014; 37(12): 2035–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yaggi HK, Mittleman MA, Bravata DM, et al. Reducing cardiovascular risk through treatment of obstructive sleep apnea: 2 methodological approaches. Am Heart J. 2016; 172: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bakker JP, Wang R, Weng J, et al. Motivational Enhancement for Increasing Adherence to CPAP: A Randomized Controlled Trial. Chest. 2016; 150(2): 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997; 6(3): 199–204. [DOI] [PubMed] [Google Scholar]
- 26. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991; 14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 27. Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993; 103(1): 30–36. [DOI] [PubMed] [Google Scholar]
- 28. Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48. [Google Scholar]
- 29. Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care Med. 1999; 159(2): 461–467. [DOI] [PubMed] [Google Scholar]
- 30. Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD; CONSORT PRO Group Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013; 309(8): 814–822. [DOI] [PubMed] [Google Scholar]
- 31. Li C, Ford ES, Mokdad AH, Balluz LS, Brown DW, Giles WH. Clustering of cardiovascular disease risk factors and health-related quality of life among US adults. Value Health. 2008; 11(4): 689–699. [DOI] [PubMed] [Google Scholar]
- 32. Gall R, Isaac L, Kryger M. Quality of life in mild obstructive sleep apnea. Sleep. 1993; 16(8 Suppl): S59–S61. [DOI] [PubMed] [Google Scholar]
- 33. Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999; 353(9170): 2100–2105. [DOI] [PubMed] [Google Scholar]
- 34. Barbé F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001; 134(11): 1015–1023. [DOI] [PubMed] [Google Scholar]
- 35. Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999; 353(9170): 2100–2105. [DOI] [PubMed] [Google Scholar]
- 36. Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001; 164(4): 608–613. [DOI] [PubMed] [Google Scholar]
- 37. Shaw JE, Punjabi NM, Naughton MT, et al. The Effect of Treatment of Obstructive Sleep Apnea on Glycemic Control in Type 2 Diabetes. Am J Respir Crit Care Med. 2016; 194(4): 486–492. [DOI] [PubMed] [Google Scholar]
- 38. Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2006. National Health Statistics Reports, No 8. Hyattsville, MD: National Center for Health Statistics; 2008 [PubMed] [Google Scholar]
- 39. Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 2011; 12(7): 996–1004. [DOI] [PubMed] [Google Scholar]
- 40. Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003; 290(18): 2443–2454. [DOI] [PubMed] [Google Scholar]
- 41. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013; 14(12): 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanders AE, Essick GK, Beck JD, et al. Periodontitis and Sleep Disordered Breathing in the Hispanic Community Health Study/Study of Latinos. Sleep. 2015; 38(8): 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005; 28(4): 472–477. [DOI] [PubMed] [Google Scholar]
- 44. Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006; 166(16): 1716–1722. [DOI] [PubMed] [Google Scholar]
- 45. Catcheside PG. Predictors of continuous positive airway pressure adherence. F1000 Med Rep 2010; 2: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008; 5(2): 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007; 30(6): 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.