Abstract
Study Objective:
To compare cognitive behavioral therapy for insomnia (CBT-I) + antidepressant medication (AD) against treatments that target solely depression or solely insomnia.
Design:
A blinded, randomized split-plot experimental study.
Setting:
Two urban academic clinical centers.
Participants:
107 participants (68% female, mean age 42 ± 11) with major depressive disorder and insomnia.
Interventions:
Randomization was to one of three groups: antidepressant (AD; escitalopram) + CBT-I (4 sessions), CBT-I + placebo pill, or AD + 4-session sleep hygiene control (SH).
Measurements and Results:
Subjective sleep was assessed via 2 weeks of daily sleep diaries (use of medication was covaried in all analyses); although there were no statistically significant group differences detected, all groups improved from baseline to posttreatment on subjective sleep efficiency (SE) and total wake time (TWT) and the effect sizes were large. Objective sleep was assessed via overnight polysomnographic monitoring at baseline and posttreatment; analyses revealed both CBT groups improved on TWT (p = .03), but the AD + SH group worsened. There was no statistically significant effect for PSG SE (p = .07). There was a between groups medium effect observed for the AD + SH and CBT + placebo group differences on diary TWT and both PSG variables. All groups improved significantly from baseline to posttreatment on the Hamilton Rating Scale for Depression (HAMD-17); the groups did not differ.
Conclusions:
Although all groups self-reported sleeping better after treatment, only the CBT-I groups improved on objective sleep, and AD + SH’s sleep worsened. This suggests that we should be treating sleep in those with depression with an effective insomnia treatment and relying on self-report obscures sleep worsening effects. All groups improved on depression, even a group with absolutely no depression-focused treatment component (CBT-I + placebo). The depression effect in CBT-I only group has been reported in other studies, suggesting that we should further investigate the antidepressant properties of CBT-I.
Keywords: insomnia, depression, CBT-I.
INTRODUCTION
Major depressive disorder (MDD) is a condition with markedly reduced quality of life, increased health-care utilization and suicide risk, and impaired social/occupational functioning.1–3 Antidepressant medications (for review see4) and psychological interventions (for review see5) have well-established efficacy for treating MDD and restoring normal functioning in many with this condition. However, those MDD patients who present with clinically significant insomnia complaints comprise a particularly challenging group to treat. For many such patients, insomnia represents a long-standing and problematic condition that can: (1) predate the onset of MDD,6,7 (2) increase the risk of suicide,8,9 (3) show a suboptimal response to traditional depression treatment,10,11 (4) remain after successful depression treatment,12,13 and (5) increase risk for MDD relapse (e.g., Nierenberg et al14). Perhaps because the insomnia of MDD patients traditionally has been viewed as an MDD symptom rather than a comorbid disorder, the sleep-specific treatment needs of MDD patients have been largely ignored until recently. Addressing this gap in the literature is important when the prevalence of MDD and sleep complaints are considered; 90% of those in clinical settings presenting with MDD complain of sleep problems,15,16 and MDD plus insomnia patients actually outnumber insomnia-only patients in sleep clinics by almost 2:1.17–19 Considering the persistence and potential long-term significance that insomnia may have for MDD patients, well-designed studies to test the benefits of insomnia-targeted therapies for those who present comorbid MDD and insomnia are urgently needed.
Of the available treatments, Cognitive Behavior Therapy for Insomnia (CBT-I) represents an attractive option for comorbid MDD/insomnia patients. CBT-I is currently considered a safe, efficacious, and durable therapy for the treatment of insomnia disorder (ID).20 Studies suggest that the psychological factors targeted by CBT-I presumed to perpetuate the sleep problems among ID sufferers generally are also common in insomnia patients with comorbid depression.12,21 In addition, CBT-I has been shown to successfully reduce these sleep-disruptive factors and to improve the sleep of patients with insomnia and comorbid mental disorders, even in those receiving pharmacotherapy for depression.22 One study evaluating CBT-I for those with residual MDD and refractory insomnia after adequate pharmacotherapy for depression found that both sleep and depression improved significantly more among the CBT-I group than those treated as usual with only pharmacotherapy.23 Furthermore, research suggests that significant improvements in sleep are associated with mood improvements and even remission of the depressive episode.24–26
Taken together, these studies provide convincing early evidence that CBT-I is effective for improvement in sleep and may also lead to additional improvement in depression symptoms. Unfortunately, the studies conducted to date lacked both proper control groups and objective (polysomnography [PSG]) verification of sleep changes. An exploration of PSG-measured sleep is important because we know that antidepressants can cause objective sleep problems (for review see27). The purpose of this randomized controlled trial was to compare a therapy that combines CBT-I with antidepressant medication (CBT-I + AD) against treatments that target solely depressive symptoms (AD + sleep hygiene control [SH]) or insomnia symptoms (CBT-I + placebo drug [PD]) for improving sleep and depression symptoms in patients with comorbid insomnia and MDD. It was hypothesized that (1) the combined CBT-I + AD therapy would produce significantly greater pre-to-post therapy improvements on subjective and objective sleep continuity measures than the two monotherapy conditions; (2) the combined CBT-I + AD therapy would produce significantly greater pre- to posttherapy improvements in clinician-assessed depression (Hamilton Rating Scale for Depression [HAMD-17]); and (3) posttreatment sleep would predict posttreatment depression symptoms.
METHODS
Design
This trial employed a randomized split-plot experimental design with three between-group cells (CBT-I + AD, CBT-I + PD, and AD + SH) and four within-group cells (baseline, mid-treatment, posttreatment, and 6-month follow-up). The behavioral therapies (i.e., CBT-I and SH) were delivered in a single-blind manner, whereas the medication components of treatment (i.e., AD and PD) were delivered in double-blind fashion. Sample size was determined using a priori analyses. Power estimates were calculated with Proc Power in SAS using the one-way analysis of variance (ANOVA) option as an approximation for the statistical model. Previous depression literature indicated an expected 20% dropout rate before the posttreatment assessment and another 25% dropout prior to follow-up assessment. To accommodate a 45% dropout rate by the follow-up time point, the recruitment goal was 67 subjects per group (Total N = 201) which would allow sufficient subjects (37 per group) to maintain >.80 power to detect the expected group differences.
Participants
Men and women aged 18–64 years old meeting diagnostic criteria for both MDD and ID were recruited via clinics and media advertisements. The advertisements invited participation in a study for depression and insomnia and did not reveal study hypotheses. Recruitment took place at two study sites: Duke University in North Carolina from 2008 to 2009 and Ryerson University in Toronto, Canada, from 2009 to 2014. Study protocols were approved by both Duke University and Ryerson University ethics review boards, and all participants provided written consent for participation.
Participants were included if they (1) were in good health as determined by medical and psychiatric history and physical examination; (2) had an insomnia complaint of at least 1-month duration that met Research Diagnostic Criteria for an Insomnia Disorder; (3) had a score ≥15 on the Insomnia Severity Index; (4) showed a mean sleep diary total wake time (TWT) ≥60 min per night and a mean sleep diary sleep efficiency (SE = [Total Sleep Time / Time in Bed] × 100%) <85% during a 1-week screening period; (5) met criteria for a Major Depressive Episode (without psychotic features) as verified by the mood module of the SCID; and (6) had a score of ≥15 on the 17-item HAMD17. Participants were included if they were currently taking antidepressant medication, as long as they and their treating physician were agreeable to discontinuing the antidepressant medication throughout the trial.
Excluded from the study were those who (1) needed immediate psychiatric (e.g., imminently suicidal patients) or medical care (e.g., patients with acute cardiac symptoms) or have attempted suicide in the past 6 months; (2) had a sleep-disruptive comorbid medical condition (e.g., moderate to severe rheumatoid arthritis); (3) had a positive urine pregnancy test (to prevent possible adverse effects with the study drug escitalopram and a fetus); (4) were not cognitively intact (a score <27 on the Mini-Mental Status Examination); (5) met criteria for another psychiatric disorder on the basis of an SCID interview that could account for or worsen the insomnia; (6) met criteria for sleep apnea, restless legs syndrome, or Circadian Rhythm Sleep Disorder on the basis of the Duke Structured Interview of Sleep Disorders and/or an apnea–hypopnea index ≥15 or periodic limb movement-related arousal index ≥15 per hour of sleep during a screening laboratory polysomnogram; (7) had a history of alcohol, narcotic, benzodiazepine, or other substance abuse or dependence in the 6 months prior to screening or have a positive urine drug or alcohol test on the night of the screening PSG; (8) had any medical conditions that would preclude them from taking the study drug (e.g., a disorder characterized by altered metabolism, seizure disorder, severe renal impairment) or used any drugs known or suspected to affect hepatic or renal clearance of escitalopram or drugs that could interact with escitalopram; and (9) were hypnotic dependent (i.e., they reported that they were unwilling or unable to abstain from prescription medications for sleep during the 8-week treatment phase of the study).
Treatments
The therapists were novice graduate students naive to sleep treatments. They were master’s-level students with at least 1 year of practicum training in other CBTs. They were trained by the principal investigator (PI)/author of the standardized treatment protocols (CBT-I and SH) published elsewhere.28 New therapists began by observing at least two therapy sessions by a doctoral-level student, and they proceeded to taking the lead in cotherapy with a doctoral-level student in the room. Once the doctoral-level student and the supervising PI/first author agreed the student was competent in the therapy, the therapist was approved for therapy alone but under the weekly supervision of the PI (i.e., a behavioral medicine specializing in CBT for comorbid insomnia). There was no formal competence evaluation beyond observation and supervision. Participants were randomized to one of three groups; all received 8 weeks of active treatment delivered in four biweekly sessions: (1) CBT-I + AD; (2) CBT-I + PD; (3) AD + SH (sleep hygiene control). Double-blinding procedures were accomplished through a compounding pharmacy that was responsible for receiving participant group information from one study administrator who was not blinded to treatment group. The primary study coordinator, PI, study physician, research assistant trained in the HAMD17 assessments, and participants were all blind to group status. The inert placebo substance used was Avicel, a microcrystalline cellulose.
Escitalopram was selected as the study medication because it is safe, well-tolerated, and efficacious for treating depression.29 It allowed for the use of a single and standard daily medication dosage (10 mg) in this trial, thus eliminating subject variability in dose and in the time to reach the final dose. As with all SSRIs, insomnia may occur as a side effect, but this occurs for only 7% of patients taking 10 mg of escitalopram, a rate only slightly higher than the insomnia rate reported for placebo (4%). In fact, patients with depression and an insomnia complaint report greater subjective sleep improvement with escitalopram than with placebo,30 and escitalopram has extremely low rates of adverse drug effects that lead to discontinuing medication and/or initiating a new or additional therapy, so it seemed a good choice. Escitalopram is currently one of the most frequently prescribed medications, perhaps due to its rating as one of the most cost-effective SSRIs.29,31,32 Adherence to the placebo and escitalopram medications was verified at each physician appointment with pill counts.
CBT-I + AD
Participants in this treatment group received CBT-I and the antidepressant, escitalopram. As part of the CBT-I treatment, patients were first presented a standardized animated Powerpoint presentation containing sleep education and information relating to CBT for insomnia based on materials from Edinger and Carney.28 This education is designed to address unhelpful beliefs about sleep by providing corrective information about sleep (e.g., age-appropriate sleep norms, the effects of aging on sleep, influence of circadian rhythms, and the effects of sleep deprivation). During the remainder of the session, patients were provided a regimen instructing them to (a) establish a standard wake time; (b) get out of bed whenever awake for >20 minutes; (c) avoid reading, watching television, eating, worrying, or other sleep-incompatible behaviors in the bed or bedroom; (d) refrain from daytime napping; and (e) limit their total time in bed (TIB) to mean total sleep time (from baseline sleep diary) + 30 min (to allow for normal sleep onset latency and brief awakenings). The remaining three treatment sessions were used to reinforce instructions provided in the first session and to make needed adjustments in TIB prescriptions. The TIB was increased by 15-min increments when the SE was >85%, and there was a report of daytime sleepiness. TIB was decreased by 15-min increments each week the subject showed an average SE <80%. Further, in sessions 2–4, remaining sleep-interfering beliefs were targeted by designing/conducting behavioral experiments (e.g., testing the belief that, “I have limited resources to cope with daytime symptoms,” or that, “I need to try to sleep”). There were four 1-hr CBT-I biweekly sessions (treatment phase is thus 8 weeks). A biweekly four-session schedule was selected because it has been shown empirically to be the optimal dose/schedule.61
Participants assigned to this condition also received a 10-mg daily dose of the medication, escitalopram, and they were maintained on this dose throughout the trial as there are no data supporting greater efficacy at a higher dose than 10 mg. The study physician met with each participant in this condition weekly to provide medication instructions and to assess and monitor adverse events; an MD research assistant blinded to conditions and trained in HAMD17 administration completed the HAMD17 ratings. During the first visit, the physician gave the participant an 8-week supply of escitalopram and instructed them to take one dose of this medication (i.e., a single 10-mg pill) each morning throughout the trial. Beginning at the second visit and continuing throughout treatment, the study physician conducted a thorough assessment of adverse events, and any events reported were documented and discussed with the participant. Those who “responded” (i.e., had a > 50% decrease on HAMD17 and a > 50% decrease in ISI from baseline) did not meet current criteria for MDD, evidenced no significant adverse medication effects, and had HAMD17 and ISI scores <15 continued on their medication into the 6-month follow-up period or until they met removal criteria.
CBT-I + PD medication
Participants assigned to the CBT-I + PD treatment group received the same CBT-I treatment described earlier but were given the inactive placebo in place of the escitalopram. The placebo medication condition was used to control for the effects of “pill taking” that are operative in the CBT-I + AD and AD + SH groups. Participants received the same instructions as the escitalopram (e.g., a single pill is to be taken each morning) and underwent the same adverse event assessment and weekly HAMD17 as the CBT-I + AD group.
AD + SH
Participants assigned to the AD + SH group received the same medication (10 mg escitalopram), and weekly physician-directed adverse event assessments and research assistant-administered HAMD17 as the AD + CBT-I condition. However, they received generic sleep hygiene instructions instead of an active cognitive behavioral sleep treatment (e.g., CBT-I). Preliminary studies support the perceived credibility of SH as an insomnia treatment. Also, SH recommendations have good “face validity,” as they are often used in clinical practice. Nonetheless, sleep hygiene recommendations are ineffective for treating insomnia when used in the absence of other insomnia therapies.33,34 In the current trial, each participant assigned to this condition received the SH intervention during 4 biweekly 1-hr individual therapy sessions with a study therapist. Thus, both groups had the same amount of therapist contact. During the first SH session, the therapist reviewed the patient’s sleep diaries without suggestions for altering the observed sleep schedule. SH consisted of descriptions of sleep stages, normal sleep architecture, and sleep cycles presented via an animated Powerpoint presentation. After presenting this educational information, the therapist discussed a series of recommendations from the manual entitled “Overcoming Insomnia,”28 including eliminating caffeine and alcohol, avoiding exercise and other physical activity just prior to bed, having a light snack before bed, and keeping the bedroom quiet, dark, and at a comfortable temperature. These recommendations were applied to the participant’s own circumstances in detail, such as planning a particular sleep-inducing snack (e.g., milk, peanut butter, and crackers) or not scheduling exercise right before bed. In addition to receiving these verbal instructions, SH patients were given a take-home pamphlet reiterating the sleep hygiene instructions. During subsequent SH sessions, the therapist reviewed the logs and the sleep hygiene recommendations and addressed treatment adherence problems.
Screening Measures
Duke Structured Interview for Sleep Disorders
The Duke Structured Interview for Sleep Disorders (DSISD) is an instrument designed to assist in ascertaining Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) and International Classification of Sleep Disorders, Second Edition (ICSD-2)35 sleep disorder diagnoses. The DSISD has acceptable reliability (κ values ranging from .71 to .86 across DSM & ICSD categories) and discriminant validity.36
Folstein Mini-Mental Status Exam
The Folstein Mini-Mental Status Exam (MMSE)37 was used to identify and exclude individuals who have cognitive deficits that make them unable to give informed consent or fully participate in an interactive treatment process. It contains items that assess orientation, attention, memory skills, mathematical abilities, and language skills. Following standard administration and scoring procedures, all individuals who obtained a total MMSE score <27 were excluded.
Structured Clinical Interviews for DSM-IV Axis I Disorders
The Structured Clinical Interviews for DSM-IV Axis I Disorders (SCID)38 was used to identify study candidates who met DSM-IV-TR inclusion criteria for MDD as part of the screening process and to identify patients who met criteria for a major psychiatric illness other than MDD (e.g., particular Anxiety Disorders, Bipolar Disorder, Schizophrenia, current substance or alcohol dependence, etc.) that would obviate study participation. The SCID methodology has a strong legacy attesting to its reliability and validity for ascertaining psychiatric diagnoses.
Outcome Measures
PSG
All study participants underwent three nights (one screening night and two baseline nights following the medication washout phase) of PSG monitoring prior to treatment and an additional two consecutive nights of PSG immediately after treatment. PSG data were collected using CompumedicsTM recording devices at Duke and SandmanTM devices at the Sleep and Alertness Clinic (SAC). These devices are small 32-channel digital PSG recorders that allow sampling rates up to 512 Hz and accommodate a wide range of recording montages. Software accompanying these devices allows for conventional scoring of records.
All overnight PSG recordings were conducted in the laboratory. The initial PSG was conducted solely to identify and exclude those with significant sleep-disordered breathing or periodic limb movements. The monitoring montage for this initial Screening PSG consisted of the following channels: two EEG (C3-M2 and Oz-Cz), one chin EMG, two EOG (left eye-M1 and right eye-M2), one airflow (nasal–oral thermistor), two respiratory effort (thoracic and abdominal impedance), pulse oximetry, two anterior tibialis EMG (right and left legs), and one for body position monitoring. The remaining for PSGs included only EEG, EOG, and submental EMG leads since these recordings were used only to derive sleep–wake measures used in treatment outcome analyses. Electrode attachments for all PSGs was performed in the sleep laboratory by trained technicians. Usual bed and rising times were ascertained for each participant via interview and 1 week of confirmatory sleep diary monitoring, and these “usual times” were used on the PSG recording nights. All PSGs acquired were scored by experienced sleep technologists using standard scoring criteria for sleep stage assignments,39 the identification of apneas/hpopneas,40 and periodic limb movement-related arousals.41,42 To ensure reliable PSG scoring, a randomly selected 10% of the PSGs conducted each month were rescored by ADK at Duke, and a second technologist at SAC and scoring results were compared. When such comparisons revealed <85% agreement for epoch-by-epoch comparisons of sleep stage scoring and/or <80% agreement for event scoring, the technologists resolved their scoring differences. When preparing for sleep monitoring, study participants were instructed to refrain from caffeine intake after 12:00 PM on the days their PSG studies were scheduled. They were also instructed to abstain from alcohol ingestion and illicit drug use on these days. To assess their compliance with these instructions, they underwent breath-alcohol testing and provided a urine sample for drug screening prior to each PSG.
Interactive Voice Response System Electronic Sleep Diary
Subjective sleep estimates were obtained using a touchtone telephone interactive program that automates the collection of subjective sleep data. The program, constructed by the PI using Voiceguide software (© Katalina Technologies, Inc.), presents questions about each night’s sleep (i.e., bedtime, sleep onset latency, number and length of nocturnal awakenings, time of final awaking, and rising time). The Interactive Voice Response System program includes queries about caffeine (food/beverages), sleep medication, and alcohol use. The wording of the items was based on the now-published Consensus Sleep Diary-Morning Version.43 At the end of the entries for 1 day, the program automatically records a time stamp to verify the time and date that data were entered. There are moderate correlations between diary and PSG measures of TWT (r = .73) and SE (r = .66).87 Those with hearing impairments were permitted to use paper diaries. Paper diary data were manually double entered into the diary database.
Sleep Parameters
Primary outcomes were the 2-week means of TWT and SE percentage (SE% = [Total Sleep Time / Time in Bed] × 100%) taken from sleep diaries. Secondary sleep outcome measures were TWT and SE% (mean values for two nights at baseline and post-treatment time points), taken from PSG. SE and TWT are widely used indices of sleep improvement in insomnia research because they are sensitive to all possible scenarios of sleep disruption (i.e., sleep onset, sleep maintenance problems, or both).
Insomnia Severity Index (ISI)44 is a 7-item scale that assesses the self-reported severity of DSM-IV-defined insomnia symptoms. The ISI has demonstrated good internal consistency and test–retest reliability.45
Mood Measures
Clinician ratings of depression were obtained by a physician research assistant using the 17-item version of the HAMD17,46 which assesses the severity of 17 depression symptom items over the past week. This measure has evidence of good reliability (interrater reliability coefficient = .8447; item–total correlations range from .45 to .78).48
Quality-of-Life Measure
The Medical Outcomes Study SF-36 (MOS SF-36)49: The SF-36 was used to assess changes in perceived health-related quality of life and served as a secondary outcome measure. This 36-item self-report instrument is a reliable and widely used questionnaire49 designed to assess respondents’ quality-of-life perceptions across a variety of functional domains, such as global Physical Composite (PC) and Mental Composite (MC) scores that respectively summarize global functioning in the physical and mental domains.
Medication Quantification Scale
The Medication Quantification Scale (MQS)50 provides a quantitative index for most types of common medications (prescription and nonprescription) consumed based on the known effects of their long-term use. It provides a means of quantifying different types of medications using a common scale. It was originally developed for pain patient trials and has sound validity and reliability.51 The MQS scores are derived on the basis of weights assigned to each type of medication and the actual dosage levels used. For example, a prn nonsteroidal anti-inflammatory medication is assigned a predetermined detrimental weight which is multiplied by dosage weights and then summed across days monitored. This provides a total score that can be entered as a covariate.
Measures of Adverse Events
The study physician conducted assessments of adverse events at each patient visit using the Adverse Events Form (AEF). Full definitions for adverse events and specifications for determining severity and the relation of adverse events to the study medications are contained in the AEF. These definitions are adapted from those used in a number of recent controlled trials.32,52
Therapy Evaluation Questionnaire
To assess acceptance of the two behavioral treatments (CBT-I and SH) and expectations for success, all study patients completed the Therapy Evaluation Questionnaire (TEQ),53 both after their first behavioral treatment session and again at the end of the study’s 8-week treatment phase. The TEQ consists of five questions (rated on a 7-point scale) assessing respondents’ perceived logic of and confidence in their assigned treatment condition, willingness to recommend it to a friend, and so on. The TEQ also includes 2 items assessing the quality of the therapeutic relationship. The TEQ has high internal consistency (Cronbach’s α = .79)54 and good face validity for assessing treatment credibility and therapist competence/warmth.
Treatment Component Checklist
To assess treatment fidelity and therapist adherence to specific elements of the two behavioral treatments administered, a Treatment Component Checklist (TCC) was used. This instrument consists of 20 items that describe a therapist verbal instruction or action that should occur either in the CBT-I or SH interventions. Ten of the items pertain specifically to CBT-I, whereas the other 10 specifically describe the SH therapy. An independent rater certified in behavioral sleep medicine (MDL) reviewed and rated the digital recordings of CBT-I and SH therapy sessions using the TCC. A therapy “fidelity” index for each treatment session was computed for each treatment session. A fidelity index = 1.00 suggests that the session included items solely from the treatment intended and was not “contaminated” by items from the alternate treatment.
Procedures
Study candidates telephoned the project coordinator in response to study advertising (i.e., advertisements and brochures in clinics). The project coordinator assessed their interest and eligibility using a brief telephone information and inclusion screen. Those found eligible were then scheduled for an in-lab screening interview at either the Ryerson Sleep and Depression Laboratory or the Duke Insomnia and Sleep Research Program. Participants attended this interview to provide study information, to obtain informed consent, and to complete the DSISD, HAMD17, SCID, SCID-II, MMSE, and ISI. Those who continued to meet inclusion criteria then completed (a) a standard medical evaluation that included a physical examination and laboratory studies (i.e., chem.-7, abc with differential, thyroid panel, urinalysis and urine drug screen, and a serum pregnancy test if applicable), (b) one night of PSG monitoring, and (c) 1 week of sleep log monitoring. Patients who continued to meet selection criteria and were also taking an antidepressant medication began the antidepressant medication washout phase under the direction of the study physician. The length of the washout phase varied depending on the half-life, dose, and duration of the antidepressant medication they were discontinuing. Participants were seen by the study physician weekly during this phase to assess safety. After the washout phase, participants saw the study physician for a safety assessment and began pretreatment (baseline) monitoring, including (a) maintaining IVR sleep diaries to describe each night’s sleep for 2 weeks; (b) two consecutive nights of PSG monitoring, and (c) outcome measures (ISI, SF-36). Those who continued to meet all study selection criteria were randomized to treatment groups following the baseline period. Randomization was accomplished by a computer-generated randomization paradigm.
As a result of random assignment, each study participant received one of three interventions during an 8-week treatment period. Treatment with CBT-I or SH components were provided in 4 weekly individual visits followed by 2 visits scheduled at Week 6 and Week 8, by one of two graduate therapists supervised by a BSM expert (i.e., the first author). All treatment sessions were conducted at the Duke Insomnia and Sleep Research Program clinic during Year 1 and then, for the remainder of the study, sessions were conducted at Ryerson’s Sleep and Depression Laboratory. The study physician provided patients with medication instructions and medication at the SAC. All participants were asked to maintain nightly IVR sleep diaries and completed treatment adherence ratings throughout the 8-week treatment phase of the project. Immediately after the 8-week treatment phase, study patients underwent a posttreatment assessment that included completion of HAMD17 and SCID mood module, all outcome questionnaires, two nights of Lab-PSG monitoring, and 2 weeks of sleep diary monitoring. All of these assessments except for PSG monitoring were repeated at the 6-month follow-up.
There was not a true follow-up period associated with this study because of stringent safety protocols. Once participants completed the posttreatment assessment, they continued into the study’s follow-up phase only if their pre- to posttreatment decline in both their HAMD17 and ISI scores were in the recovered range (minimum <15 at posttreatment for both); they did not meet current criteria for MDD on the mood module of the SCID; and they failed to meet Clinical Worsening criteria (clinical worsening is defined as: 1. an increase in HAMD17 item #10 or BDI-II items #2 or #9, 2. a 20 % increase in total HAMD17 score, or 3. a moderate AE, that is, the symptom results in some restriction of regular activities). Although only entering this subset into the follow-up phase limited the ability to examine this data in a meaningful way, it was done to prioritize safety given that some participants received placebos. Those included in the follow-up phase were maintained on their assigned medication and were asked to attend monthly assessments, conducted primarily for safety purposes, with the Study Physician at the SAC over the 6-month follow-up period. The MQS was completed during every week of the trial and at 6-month follow-up to track the use of nonstudy medications during the trial. At the end of the 6-month follow-up period, participants completed the ISI, SF-36, HAMD17, and 2 weeks of IVR sleep diary monitoring.
Interim analyses were conducted on the ISI and HAMD17 as a safety precaution. During this time, all investigators remained blinded to treatment group. Results from these interim analyses including the aggregate analysis of proportion of depression responders and insomnia responders was presented at the Association for Behavioral and Cognitive Therapies annual conference in Nashville, TN (2013). The trial was concluded as of November 30, 2013, at the end of the funding period.
RESULTS
Analyses
The primary analysis of these variables was conducted using imputed data and under intention to treat. The missing data on these variables were imputed using multiple imputation techniques. Secondary outcomes of HAMD17, BDI, and ISI variables were analyzed using complete case analysis. Comparisons across the three randomized groups were conducted using either general linear models (GLM) or a nonparametric Wilcoxon-signed rank test. For each variable, if the model was significant, pairwise comparisons between groups were conducted using the Tukey test for adjusted multiple comparisons.
Baseline Characteristics
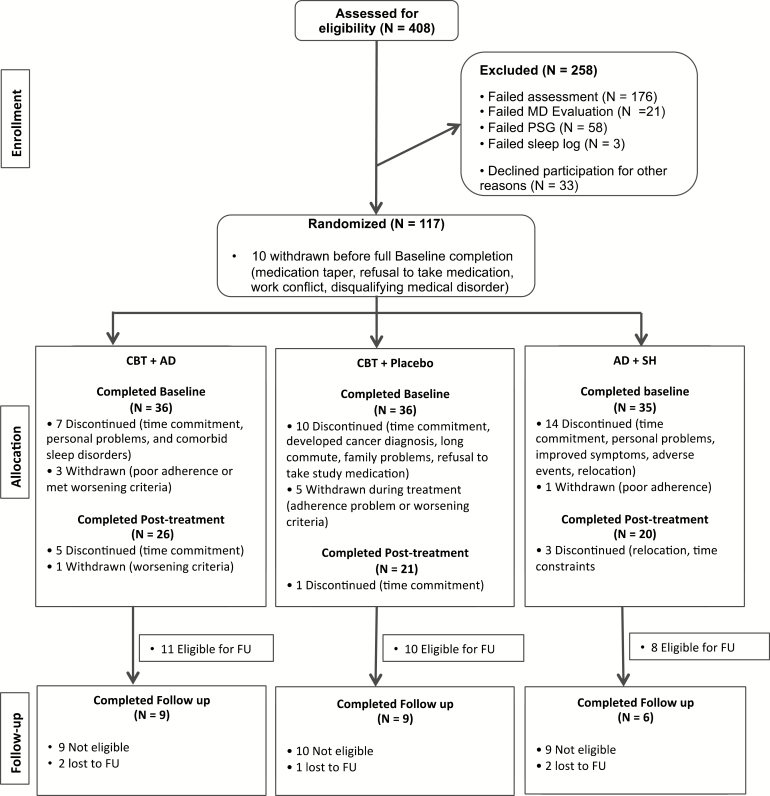
Participant flow from enrollment until the completion of follow-up for sleep diary variables is depicted in Figure 1. Main analyses were conducted on posttreatment data. Demographic and baseline characteristics of the sample are depicted in Table 1. There were no statistically significant differences between groups on age or gender; thus, these variables were not included as covariates in the analyses. Chi-square analyses revealed that there were no baseline group differences on AHI (p = .41) or PLM arousal index (p = .79).
Figure 1.
CONSORT Participant Flow.
Table 1.
Baseline characteristics.
| Variable | Total Sample, Mean (SD) (N = 107) | CBT + AD, Mean (SD) (N = 36) | CBT + Placebo, Mean (SD) (N = 36) | AD + Hygiene Mean (SD), (N = 35) |
|---|---|---|---|---|
| Age, years | 42.28 (11.39) | 44.97 (9.38) | 41.78 (12.89) | 40.11 (11.41) |
| Female | 68% | 55.56% | 72.22% | 62.86% |
| Hispanic | 10.4% | 2.9% | 16.7% | 8.6% |
| Caucasian non-Hispanic | 60.4% | 76.5% | 55.6% | 51.4% |
| Asian | 10.4% | 2.9% | 13.9% | 14.3% |
| African American | 10.4% | 2.9% | 16.7% | 11.4% |
| Other | 16.0% | 17.6% | 8.3% | 22.9% |
| PLM-I | 1.7 (2.8) | 1.3 (2.9) | 1.1 (2.0) | |
| AHI | 0.74 (1.56) | 0.67 (1.1) | 1.1 (1.02) |
Abbreviations: PLM-I, Periodic limb movement index; AHI, apnea–hypopnea index.
n = 24 for CBT + AD PLM-I and AHI; n = 25 for CBT + Placebo PLM-I and AHI; n = 28 for AD + Hygiene PLM-I and AHI.
Sleep
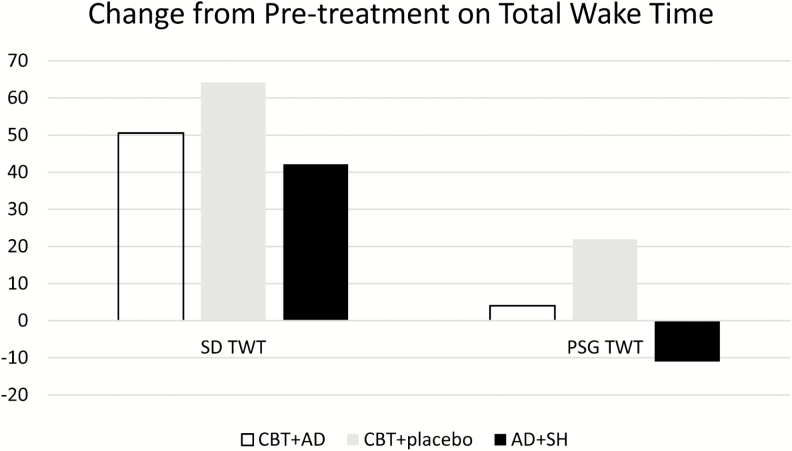
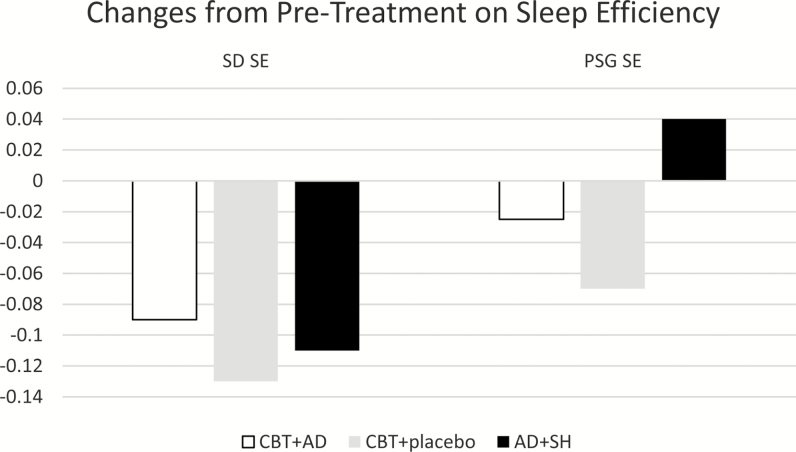
We verified that there were no statistically significant baseline differences and then analyzed baseline to posttreatment differences using an ANOVA (GLM) with pairwise comparisons, adjusting for medication use (covarying the Medication Quantification Score). Each of the groups improved from baseline to posttreatment (p < .05), and there were no group differences on mean Sleep Diary (SD) TWT [F = 7.97, p = .9] or on mean SD SE [F = 1.18, p = .7]. The between-group effect sizes for CBT+AD versus CBT+Placebo and CBT+AD versus AD+SH group for SD TWT were small77 (d = .3 and d = .2, respectively); the effect for CBT+Placebo and AD+ SH was medium (d = .5). The between-group effect sizes for CBT+AD versus CBT+Placebo, CBT+AD versus AD+SH, and CBT+Placebo versus AD+ SH for SD SE were small: d’s = .4, .2, and .3, respectively. Table 2 includes mean group sleep diary and PSG data as well as within-groups associated effect sizes.
Table 2.
Sleep and depression means (SD) and associated within-groups effect sizes (Cohen’s d on unadjusted data).
| Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CBT + AD, n = 36 | CBT + Placebo, n = 36 | AD + SH, n = 35 | |||||||
| Baseline | Post-tx | d | Baseline | Post-tx | d | Baseline | Post-tx | d | |
| SD TWTa | 130.54 (49.34) | 80.00 (34.76) | 1.20 | 147.35 (43.03) | 83.12 (29.58) | 1.77 | 142.46 (56.66) | 100.34 (27.84) | 0.96 |
| SD SE | 0.74 (0.10) | 0.83 (0.08) | −1.01 | 0.69 (0.09) | 0.82 (0.07) | −1.64 | 0.69 (0.11) | 0.80 (0.06) | −1.26 |
| PSG TWT | 63.62 (48.81) | 48.29 (40.53) | 0.35 | 65.99 (55.73) | 57.83 (53.49) | 0.15 | 52.01 (40.68) | 59.68 (55.58) | −0.16 |
| PSG SE | 0.74 (.19) | 0.78 (.17) | −0.23 | 0.78 (0.16) | 0.80 (0.15) | −0.12 | 0.79 (0.12) | 0.73 (0.14) | 0.43 |
| ISI | 26.21 (8.61) | 13.23 (9.90) | 1.42 | 29.53 (9.74) | 14.63 (10.43) | 1.50 | 30.80 (9.81) | 15.30 (9.03) | 1.67 |
| HAMD-17 | 24.13 (6.55) | 13.03 (8.72) | 1.46 | 25.52 (6.23) | 14.67 (9.58) | 1.36 | 24.88 (6.58) | 11.24 (6.40) | 2.13 |
Sample sizes for PSG data: CBT + AD: n = 33; CBT + placebo: n = 35; AD + SH: n = 36.
a indicates that sample size for CBT + Placebo: n = 35.
On objective (i.e., PSG) sleep, there were statistically significant group differences on PSG TWT (F = 4.65, p = .03); follow-up analyses revealed that CBT + PD improved but AD + SH worsened, and this difference was statistically significant (p = .02); both CBT groups improved and did not differ from each other (p = .4; see Figure 2). There were no significant group differences for increases in PSG SE (F = 3.3, p = .07; see Figure 3). The between-group effect sizes for CBT+AD versus CBT+PD and CBT+AD versus AD+SH group for PSG TWT were small: d = .36 and d = .32, respectively. The effect size for the between-group difference for CBT+PD versus AD+ SH was medium (d = .67). Although the trend was the same for efficiency as it was for PSG TWT, that is, CBT + PD improved but AD + SH worsened, this difference was not quite statistically significant (p = .059). The CBT groups did not differ from each other on follow-up analyses (p = .3). Likewise, the between-group effect sizes for CBT+AD versus CBT+PD and CBT+AD versus AD+SH group, for PSG SE were small (d = .29, d = .19, respectively), whereas the effect size for the between-group difference for CBT+PD versus AD+ SH was medium (d = .68).
Figure 2.
Polysomnography (PSG) change in total wake time.
Figure 3.
Polysomnography (PSG) change in sleep efficiency.
With respect to a global, subjective retrospective impression of their insomnia symptoms, all groups showed a statistically significant improvement from baseline to posttreatment (p < .05), but there were no group differences (p = .9). The between-group effect sizes for CBT+AD versus CBT+PD on the ISI was medium (d = .56), and the effects were small for CBT+AD versus AD+SH and CBT+PD versus AD+ SH (d’s = .29 and .25, respectively).
Mood
Depression (i.e., HAMD17 total scores) changes were tested to ensure there were no statistically significant baseline differences and then baseline to posttreatment were analyzed using an ANOVA (GLM) with pairwise comparisons, adjusting for medication use (covarying the Medication Quantification Score). All groups improved from baseline to posttreatment (p < .05), but there were no group differences on the HAMD17 (F = 37.86, p = 1.0). The HAMD17 between-group effect size for CBT+AD versus CBT+PD was d = .02 and was small for CBT+AD versus AD+SH and CBT+PD versus AD+SH (both d’s = .32). The results remained the same when the sleep items of the HAMD17 were removed (F = 43.04, p = 1.0). HAMD17 baseline and posttreatment group mean scores and associated effect sizes are depicted in Table 2.
Relation Between Depression and Insomnia
We conducted a logistic regression analysis predicting posttreatment depression remission status (remitted = HAMD17 < 8 and no MDD on SCID and nonremitted = HAMD17 ≥ 8 and /or meeting MDD criteria on the SCID). Table 3 provides a summary of the results. We entered baseline levels of sleep disturbance and insomnia and neither was a statistically significant predictor of depression treatment outcome. We also entered posttreatment sleep (ISI continuous score), and this was a significant predictor of depression treatment outcome (ß = −.403; p = .001).
Table 3.
Logistic regression analysis predicting posttreatment depression remission status.
| Predictor | ß | SE (ß) | Wald’s χ2 | df | p | eß (odds ratio) |
|---|---|---|---|---|---|---|
| Baseline ISI | .109 | .133 | .669 | 1 | .413 | 1.115 |
| Baseline HAMD | −.072 | .087 | .691 | 1 | .406 | .931 |
| Post-tx ISI | −.403 a | .125 | 10.392 | 1 | .001 | .668 |
a indicates significance at p < .05.
Because there are sleep items in the HAMD17, we conducted a multiple regression analysis predicting posttreatment depression score with sleep items removed (see Table 4). We entered baseline levels of depression (HAMD17 with sleep items removed) and insomnia (ISI) and neither was a statistically significant predictor of HAMD17 score at posttreatment (p = .07). When posttreatment ISI continuous score was entered into the model, it was significantly associated with depression treatment outcome (ß = .392; p = .002).
Table 4.
Multiple regression predicting posttreatment HAMD17 with sleep items removed.
| Predictor | ß | SE (ß) | F change | p | Adjusted R2 |
|---|---|---|---|---|---|
| Step One | |||||
| Baseline ISI | .063 | .15 | 2.8 | .07 | .06 |
| Baseline HAMD no sleep items | .294 | .23 | |||
| Step Two | |||||
| Post-tx ISI | .392a | .2 | 10.2 | .002 | .2 |
a indicates significance at p < .05.
To understand the clinical significance of the treatments, we tested whether there were any differences in those attaining self-reported good sleeper status at posttreatment (i.e., ISI < 8); the chi-square was not statistically significant (χ2 (2) = 2.3, p = .31). Insomnia remission (ISI < 8) was identical for both CBT groups (22% each) and lower, albeit not statistically, for the AD+SH group (only 6% meeting the good sleeper cut-score). Similarly, there was no group difference on depression remission (i.e., HAMD posttreatment score <8) with χ2 (2) = .034, p = .98; CBT+AD = 39%, CBT+placebo = 39% and AD+SH = 41% with a score lower than 8.
Quality of Life
We evaluated whether there were perceived group improvements from baseline to posttreatment on general health perception or physical functioning (PF) on the SF-36 using an ANOVA (GLM) with pairwise comparisons, adjusting for medication use (covarying the Medication Quantification Score). There were no group differences on GH (p = .99) or PF (p = .99).
Treatment Credibility
Treatment credibility was assessed at pre- and posttreatment via a repeated measures multivariate ANOVA. On the omnibus test, there was a main effect for time (p = .02) but no group × time interaction (p = .98). An examination of the follow-up ANOVAs revealed that only the items: “confident in recommending the treatment to a friend” and “confident that treatment will be successful for self” changed over time (both at p < .01). In both cases, ratings increased at posttreatment (i.e., they became more confident that it was successful for self and that they would recommend it to a friend). In other words, at baseline, the mean for recommending it to a friend was 5.62 (SD = 1.1), and it increased at posttreatment to M = 6.13 (SD = 1.1); the maximum score possible is 7, meaning that you are very likely to recommend it to a friend. Similarly, at baseline the mean for believing it would be successful was 5.13 (SD = 1.2), and this rating significantly increased to M = 5.72 (SD = 1.4); the maximum score possible is 7, meaning that they were confident that the treatment would be successful. At posttreatment they provided ratings of the therapist on a 7-point Likert-type scale. The 2 items were “How warm and caring did you find your therapist” and “How confident are you in your therapist’s skills?” A Likelihood ratio chi-square tested whether groups differed on these 2 items. The chi-square values for both were not statistically significant, χ2 (6) = 10.24, p = .12 and χ2 (6) = 10.24, p = .12, respectively]. Most of the sample (69% rated the therapist as very warm and 68% rated the therapist as very competent) gave the highest rating possible (i.e., 7).
Treatment Fidelity Check
Therapists were graduate students at Ryerson University supervised by a licensed psychologist with a specialty in behavioral sleep medicine (CEC). This psychologist is also the PI of the study and author of the treatment manual Overcoming Insomnia.28 Therapists completed a Therapy Component Checklist (TCC) at the end of each session, indicating which elements of the protocol were discussed in session. Therapists were randomly assigned to patients using a random number generator in Microsoft Excel. When a graduate student no longer worked on the trial, a new therapist was substituted into the randomization table for the former therapist. Seven of the reviewed Session 1 tapes were the SH group. The “first session” for SH consisted of a review of sleep logs and sleep complaints only and scores should be close to 0; that is, almost no elements of CBT should be heard on the tape. The mean score was 0.14 (SD = .38) for Session 1 of SH. The remaining reviewed tapes (N = 26) were in the CBT group; their score should be close to the maximum score of 15, meaning that most of the elements of Session 1 should be heard on the tape. The mean score for Session 1 of CBT was 13.25 (SD = 1.0). Digital audio files for the first session were sent to an independent rater for review. The independent rater (MDL) is a licensed psychologist certified in behavioral sleep medicine. The rater completed the same TCC, and we tested whether the independent rater’s TCC scores differed from the therapists’. The independent rater also evaluated (yes/no) whether the SH group received any CBT-specific interventions—there were no such violations. The resulting κ value revealed a very high rate of agreement (.9). Thus, by both PI supervision and oversight and independent rater treatment components, treatment fidelity was very high in this study.
DISCUSSION
Results from this trial indicate that although three combinations of depression and insomnia treatments were helpful in producing subjective improvements for insomnia and depression, a depression-only approach objectively worsened sleep. That is, on objective (PSG) indices, there was improvement in mean total time awake for the CBT-I groups, but the AD + SH group actually worsened on this index. The same trend was shown on objective SE. Subsequently, we explore the possible reasons for this difference in subjective versus objective sleep outcomes as well as discuss the depression response with an insomnia-specific treatment.
With respect to subjective insomnia indices, all groups did reasonably well, with improved scores on both ISI and sleep diary indices from baseline to posttreatment. One possible explanation may relate to sleep hygiene being a poor control sleep therapy condition. This therapy controlled for nonspecific effects, as the material was presented by the same therapists for the same amount of time as CBT-I groups. There is some support for sleep hygiene as a treatment for insomnia (e.g.,55,56); however, when considering the evidence collectively, this therapy fails to meet standards of evidence as an effective monotherapy33 (see also57). This group also was taking antidepressant medication, so the positive subjective effects may be related to the antidepressant itself; that is, in other studies, some depressed patients taking SSRIs report subjective sleep improvements.58,59 It is unclear whether this effect is due to the perception that the depression is improving with the medication and thus sleep also is rated more favorably. Previous research has shown that subjective assessment of insomnia using sleep items on depression inventories is associated with modest improvement in some58,59 and worsening in others.60 The lack of a follow-up period precludes us from knowing whether the subjective benefit would have any durability.
Another possible explanation for the lack of separation for the sleep hygiene and CBT-I groups on subjective insomnia improvement may relate to the dose of CBT-I in this trial. Participants in this study were those with both insomnia and MDD. Although 4 sessions are optimal in those with insomnia alone,61 it is possible that individuals with both insomnia and depression would benefit from a longer treatment period, as some studies62 report somewhat diminished treatment effect sizes in those with comorbidities. However, a meta-analysis including studies of differing treatment duration found similarly large effects, such as observed in this trial, in those with comorbid insomnias.63 Similarly, it is possible that the therapy was not potent enough because it was delivered by novices (i.e., graduate students). Although fidelity to the protocol was measured formally, there was no formal competence evaluation beyond the observation of the students and weekly supervision of these sleep-therapy naive novice therapists. Additionally, there was some inequality with the AD condition, in that a licensed, medical prescriber with decades more clinical experience was interacting with participants, in comparison to the inexperienced therapists for the cognitive behavioral components. Nonetheless, participant ratings of the therapist/therapy, fidelity assessments, and close supervision by a CBT-I expert (first author) support the idea that the cognitive behavioral components were delivered in a competent manner. In fact, it is promising for widespread dissemination that, similar to other studies similar to other studies,64–67 this brief insomnia treatment is effectively delivered to complex patients by novice nonsleep specialist providers. Although there was no separation on the mean subjective appraisal of sleep, the rate of those in the AD+SH group becoming good sleepers by the end of the treatment was only 6% as compared to over 20% in the CBT groups. Moreover, the differences in the between group effect sizes for subjective TWT and PSG indices were small for the comparison of the CBT groups and the CBT+AD and AD+SH group, but the effect size for the comparison of the CBT+Placebo and AD + SH group was medium. This consistent pattern suggests the possibility that there may be sleep differences for the two control group conditions (CBT-I + PD and AD+SH) that may have emerged if sufficiently powered.
Although there was no statistical separation on the mean subjective appraisal of sleep, there were statistically meaningful group differences in objective sleep; that is, in the group without the antidepressant (CBT-I + PD), there was improvement in the time spent awake via PSG, but in the antidepressant plus control therapy, there was a worsening in the amount of time spent awake. This finding is perhaps not surprising, as there are studies using objective polysomnographic indices finding that a proportion of SSRI-treated patients experience a disturbance of their sleep,68 and these objective sleep problems can last as long as 30 weeks after SSRI discontinuation.68–71 Thus, there were subjective improvements reported by many of those on antidepressants but an objective worsening if the antidepressant was paired with sleep hygiene (i.e., CBT-I was not used). Given the widespread use and belief that sleep hygiene is effective,57 this finding suggests antidepressants should not be augmented with sleep hygiene if improved objective sleep is desired. Although subjective sleep is the most typical target in insomnia treatment, growing research suggests that objective indices such as total sleep time may relate to adverse health outcomes such as cognitive impairment, hypertension, and diabetes.72–74 Thus, evidence of objective sleep worsening in antidepressant therapy is cause for concern.
There were no differences in depression whether someone received a depression treatment or not; that is, all three groups showed improvements with large effect sizes on depression. The between-group effects were small which has been found in other studies examining CBT-I.78 These results are also consistent with previous studies suggesting CBT-I is associated with improvements in depression symptoms as well as increased rates of depressive remission.75 In another study CBT-I was shown to potentiate the antidepressant effects of the medication,24 but this did not occur in this study. Although posttreatment sleep predicted posttreatment depression status, there was no evidence that the combined depression and insomnia treatment fared any better than the CBT-I alone group or the AD + SH group. It is unclear why we did not see this effect. The Manber et al. study was a small study and the only comparison study to date, so it is possible that these findings were anomalous. It is also possible that this study was statistically underpowered, since our final sample size was less than the a priori power analysis suggested was necessary.
The CBT-I + PD was no different than the active antidepressant medication treatment groups on depression symptom severity, and their sleep was objectively and subjectively improved (the effect sizes for this group in comparison to the AD+SH control were medium in size for diary and PSG TWT as well as PSG SE). Given that this group did well with respect to sleep, and just as well with respect to depression, it may be tempting to see this insomnia-only treatment choice as viable because it would be shorter than a lengthy antidepressant course; however, this interpretation should be considered in light of the following study design features. First, these participants were taking a placebo pill so we do not know what would happen if CBT-I was delivered in isolation. Second, this study is limited in that the sample included only those who met criteria for both insomnia disorder and MDD, and thus, these results, at best, can generalize only to those who have both disorders. There are some with MDD who do not have insomnia disorder and some who experience hypersomnia; thus, these results may not generalize to the range of people with depression. Additionally, as mentioned earlier, the analyses were underpowered according to the a priori power analysis, so it is possible that the antidepressant treatment groups could have produced superior depression treatment responses, but the insufficient sample size precluded adequate power analyses. The CBT-I plus placebo was not significantly different from the other CBT group on any index. Finally, safety precautions in the trial precluded a rigorous assessment of follow-up effects of all of the treatments, so we do not know the long-term effects of any of these treatments. This is a limitation that was necessary due to safety, but this design issue is significant because it is unknown as to whether the treatments would have had durable effects. It would be important to find a safe way to explore durability in future research.
The aforementioned caveats withstanding, there may be reasons why CBT-I seems to help with MDD. Although this trial did not contain an investigation of treatment mechanisms, CBT-I targets the time spent in bed in a 24-hr period via sleep restriction and stimulus control, which promotes more time out of bed and increased activation. Future studies should consider increased behavioral activation as a possible mechanism of change in CBT-I for depression. In addition, more time out of bed also generally means increased light exposure, which may be another possible mechanism. Excess rapid eye movement (REM) sleep has been shown to have a depressant effect,76 and since CBT-I tends to limit excessive time in bed in the morning hours, this may prevent excess REM sleep. Future studies should focus on the mechanisms of sleep improvement on mood.
In sum, CBT-I was a brief but effective treatment for insomnia in those with MDD, both on subjective and on objective measures of sleep, even when delivered by novice therapists, and with benefits on depression that were indistinguishable from antidepressant medication. Although the combination of an antidepressant medication with sleep hygiene produced a positive, subjective appraisal of sleep, the combination of antidepressant medication and sleep hygiene was associated with an objective increase in the time spent awake in bed. Thus, CBT-I appears to be an important component of depression management for those with both MDD and insomnia disorder, and future research should explore the antidepressant mechanisms of CBT-I.
FUNDING
National Institute of Mental Health R01 MH76856-02
CLINICAL TRIAL REGISTRATION NUMBER
Clintrial.gov id NCT0062079.
CITATION
Carney, C. E., Edinger, J. D, Kuchibhatla, M., Lachowski, A. M., Bogouslavsky, O., Krystal, A. D., & Shapiro, C.M. Cognitive behavioral insomnia therapy for those with insomnia and depression: A randomized controlled trial. SLEEP 2015.
ACKNOWLEDGMENTS
We wish to acknowledge the contributions of our Independent Assessor Dr J. Gojer and Independent Therapy Rater, Dr Meg Lineberger. We thank Dr Shen and the staff and technicians at the Sleep and Alertness Clinic. We also thank the student therapists who delivered the treatments and Dr R.M. Bagby who served as the Data Safety Officer. Lastly, we acknowledge the study patients who gave of their time for this study.
REFERENCES
- 1. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013; 10(11): e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malik S, Kanwar A, Sim LA, et al. The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: a systematic review and meta-analysis. Syst Rev. 2014; 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015; 76(2): 155–162. [DOI] [PubMed] [Google Scholar]
- 4. Turner EH, Rosenthal R. Efficacy of antidepressants. BMJ. 2008; 336(7643): 516–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beck AT, Dozois DJ. Cognitive therapy: current status and future directions. Annu Rev Med. 2011; 62: 397–409. [DOI] [PubMed] [Google Scholar]
- 6. Baglioni C, Spiegelhalder K, Nissen C, Riemann D. Clinical implications of the causal relationship between insomnia and depression: how individually tailored treatment of sleeping difficulties could prevent the onset of depression. EPMA J. 2011; 2(3): 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997; 42(2-3): 209–212. [DOI] [PubMed] [Google Scholar]
- 8. McCall WV, Black CG. The link between suicide and insomnia: theoretical mechanisms. Curr Psychiatry Rep. 2013; 15(9): 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woznica AA, Carney CE, Kuo JR, Moss TG. The insomnia and suicide link: toward an enhanced understanding of this relationship. Sleep Med Rev. 2015; 22: 37–46. [DOI] [PubMed] [Google Scholar]
- 10. Buysse DJ, Kupfer DJ, Cherry C, Stapf D, Frank E. Effects of prior fluoxetine treatment on EEG sleep in women with recurrent depression. Neuropsychopharmacology. 1999; 21(2): 258–267. [DOI] [PubMed] [Google Scholar]
- 11. Thase ME, Simons AD, Reynolds CF., 3rd Abnormal electroencephalographic sleep profiles in major depression: association with response to cognitive behavior therapy. Arch Gen Psychiatry. 1996; 53(2): 99–108. [DOI] [PubMed] [Google Scholar]
- 12. Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007; 68(2): 254–260. [DOI] [PubMed] [Google Scholar]
- 13. Carney CE, Harris AL, Friedman J, Segal ZV. Residual sleep beliefs and sleep disturbance following Cognitive Behavioral Therapy for major depression. Depress Anxiety. 2011; 28(6): 464–470. [DOI] [PubMed] [Google Scholar]
- 14. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010; 40(1): 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thase ME. Summary: defining remission in patients treated with antidepressants. J Clin Psychiatry. 1999; 60Suppl 22: 35–36. [PubMed] [Google Scholar]
- 16. Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005; 66(10): 1254–1269. [DOI] [PubMed] [Google Scholar]
- 17. Buysse DJ, Reynolds CF, 3rd, Kupfer DJ, et al. Clinical diagnoses in 216 insomnia patients using the International Classification of Sleep Disorders (ICSD), DSM-IV and ICD-10 categories: a report from the APA/NIMH DSM-IV Field Trial. Sleep. 1994; 17(7): 630–637. [DOI] [PubMed] [Google Scholar]
- 18. Coleman RM, Roffwarg HP, Kennedy SJ, et al. Sleep-wake disorders based on a polysomnographic diagnosis. A national cooperative study. JAMA. 1982; 247(7): 997–1003. [PubMed] [Google Scholar]
- 19. Jacobs EA, Reynolds CF, 3rd, Kupfer DJ, Lovin PA, Ehrenpreis AB. The role of polysomnography in the differential diagnosis of chronic insomnia. Am J Psychiatry. 1988; 145(3): 346–349. [DOI] [PubMed] [Google Scholar]
- 20. Vitiello MV. Cognitive-behavioural therapy for insomnia: effective, long-lasting and safe. Evid Based Ment Health. 2016; 19(1): e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kohn L, Espie CA. Sensitivity and specificity of measures of the insomnia experience: a comparative study of psychophysiologic insomnia, insomnia associated with mental disorder and good sleepers. Sleep. 2005; 28(1): 104–112. [DOI] [PubMed] [Google Scholar]
- 22. Manber R, Edinger J, San Pedro M, Kuo T. Combining escitalopram oxalate (ESCIT) and individual cognitive behavioral therapy for insomnia (CBTI) to improve depression outcome. In: Sleep. Vol 30 American Academy of Sleep Medicine; 2007:A232–A232. [Google Scholar]
- 23. Watanabe N, Furukawa TA, Shimodera S, et al. Brief behavioral therapy for refractory insomnia in residual depression: an assessor-blind, randomized controlled trial. J Clin Psychiatry. 2011; 72(12): 1651–1658. [DOI] [PubMed] [Google Scholar]
- 24. Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008; 31(4): 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor DJ, Lichstein KL, Weinstock J, Sanford S, Temple JR. A pilot study of cognitive-behavioral therapy of insomnia in people with mild depression. Behav Ther. 2007; 38(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 26. Lancee J, van den Bout J, van Straten A, Spoormaker VI. Baseline depression levels do not affect efficacy of cognitive-behavioral self-help treatment for insomnia. Depress Anxiety. 2013; 30(2): 149–156. [DOI] [PubMed] [Google Scholar]
- 27. Jindal RD, Thase ME. Treatment of insomnia associated with clinical depression. Sleep Med Rev. 2004; 8(1): 19–30. [DOI] [PubMed] [Google Scholar]
- 28. Edinger JD, Carney CE. Overcoming Insomnia: A Cognitive-Behavioral Therapy Approach, Therapist Guide. Oxford University Press; 2014. [Google Scholar]
- 29. Einarson TR. Evidence based review of escitalopram in treating major depressive disorder in primary care. Int Clin Psychopharmacol. 2004; 19(5): 305–310. [DOI] [PubMed] [Google Scholar]
- 30. Lader M, Andersen HF, Baekdal T. The effect of escitalopram on sleep problems in depressed patients. Hum Psychopharmacol. 2005; 20(5): 349–354. [DOI] [PubMed] [Google Scholar]
- 31. Hemels ME, Kasper S, Walter E, Einarson TR. Cost-effectiveness analysis of escitalopram: a new SSRI in the first-line treatment of major depressive disorder in Austria. Curr Med Res Opin. 2004; 20(6): 869–878. [DOI] [PubMed] [Google Scholar]
- 32. Sullivan PW, Valuck R, Saseen J, MacFall HM. A comparison of the direct costs and cost effectiveness of serotonin reuptake inhibitors and associated adverse drug reactions. CNS Drugs. 2004; 18(13): 911–932. [DOI] [PubMed] [Google Scholar]
- 33. Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999; 281(11): 991–999. [DOI] [PubMed] [Google Scholar]
- 34. Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006; 7(2): 123–130. [DOI] [PubMed] [Google Scholar]
- 35. American Sleep Disorders Association. The international classification of sleep disorders: diagnostic and coding manual. 1990. [Google Scholar]
- 36. Edinger JD, Wyatt JK, Olsen MK, et al. Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening. SLEEP 2009;32:265–274. [Google Scholar]
- 37. Folstein MF, Folstein SE, McHugh PR. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 38. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition (SCID-I/P).New York Psychiatric Institute: New York Biometrics Research; 2002. [Google Scholar]
- 39. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Arch Gen Psychiatry 1968;20. [DOI] [PubMed] [Google Scholar]
- 40. Phillipson EA, Remmers JE. American Thoracic Society Consensus Conference on Indications and Standards for Cardiopulmonary Sleep Studies. Am Rev Resp Dis 1989;139:559–568. [DOI] [PubMed] [Google Scholar]
- 41. ASDA Task Force. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 42. Coleman RM. Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome. In: Sleeping and Waking Disorders: Indications and Techniques.; 1982:265–295. [Google Scholar]
- 43. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012; 35(2): 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford; 1993. [Google Scholar]
- 45. Blais FC, Gendron L, Mimeault V, Morin CM. [Evaluation of insomnia: validity of 3 questionnaires]. Encephale. 1997; 23(6): 447–453. [PubMed] [Google Scholar]
- 46. HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hedlund JL, Vieweg BW. The Hamilton Rating Scale for Depressoin: a comprehensive review. J Oper Psychiatry 1979;10(2):149–165. [Google Scholar]
- 48. Schwab JJ, Bialow MR, Holzer CE. A comparison of two rating scales for depression. J Clin Psychol. 1967; 23(1): 94–96. [DOI] [PubMed] [Google Scholar]
- 49. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30(6): 473–483. [PubMed] [Google Scholar]
- 50. Masters Steedman S, Middaugh SJ, Kee WG, Carson DS, Harden RN, Miller MC. Chronic-pain medications: equivalence levels and method of quantifying usage. Clin J Pain. 1992; 8(3): 204–214. [PubMed] [Google Scholar]
- 51. Stormo KJ, Kee WG, Steedham S, Middaugh S. Medication quantification scores and evaluation of patient pharmacology profiles. Curr Rev Pain 1998;2:171–174. [Google Scholar]
- 52. Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003; 26(7): 793–799. [DOI] [PubMed] [Google Scholar]
- 53. Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry 1972;3:257–260. [Google Scholar]
- 54. Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive Behavioral Therapy for Treatment of Chronic Primary Insomnia. JAMA J Am Med Assoc 2001;285:1856–1864. [DOI] [PubMed] [Google Scholar]
- 55. Chesson AL, Jr, Anderson WM, Littner M, et al. Practice parameters for the nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 1999; 22(8): 1128–1133. [DOI] [PubMed] [Google Scholar]
- 56. Schoicket SL, Bertelson AD, Lacks P. Is sleep hygiene a sufficient treatment for sleep-maintenance insomnia? Behav Ther 1988;19:183–190. [Google Scholar]
- 57. Moss TG, Lachowski AM, Carney CE. What all treatment providers should know about sleep hygiene recommendations. Behav Ther 2013;36:76–83. [Google Scholar]
- 58. Asnis GM, Chakraburtty A, DuBoff EA, et al. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry. 1999; 60(10): 668–676. [DOI] [PubMed] [Google Scholar]
- 59. Fava M, Asnis GM, Shrivastava RK, et al. Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011; 72(7): 914–928. [DOI] [PubMed] [Google Scholar]
- 60. Zajecka J, Amsterdam JD, Quitkin FM, et al. Changes in adverse events reported by patients during 6 months of fluoxetine therapy. J Clin Psychiatry. 1999; 60(6): 389–394. [DOI] [PubMed] [Google Scholar]
- 61. Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep. 2007; 30(2): 203–212. [DOI] [PubMed] [Google Scholar]
- 62. Edinger JD, Olsen MK, Stechuchak KM, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009; 32(4): 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive Behavioral Therapy for Insomnia Comorbid With Psychiatric and Medical Conditions: A Meta-analysis. JAMA Intern Med. 2015; 175(9): 1461–1472. [DOI] [PubMed] [Google Scholar]
- 64. Baillargeon L, Demers M, Ladouceur R. Stimulus-control: nonpharmacologic treatment for insomnia. Can Fam Physician. 1998; 44: 73–79. [PMC free article] [PubMed] [Google Scholar]
- 65. Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007; 30(5): 574–584. [DOI] [PubMed] [Google Scholar]
- 66. McCrae CS, Ross A, Stripling A, Dautovich ND. Eszopiclone for late-life insomnia. Clin Interv Aging. 2007; 2(3): 313–326. [PMC free article] [PubMed] [Google Scholar]
- 67. Richardson GS, Roth T. Future directions in the management of insomnia. J Clin Psychiatry. 2001; 62Suppl 10: 39–45. [PubMed] [Google Scholar]
- 68. Armitage R, Yonkers K, Cole D, Rush AJ. A multicenter, double-blind comparison of the effects of nefazodone and fluoxetine on sleep architecture and quality of sleep in depressed outpatients. J Clin Psychopharmacol. 1997; 17(3): 161–168. [DOI] [PubMed] [Google Scholar]
- 69. Keck PE, Jr, Hudson JI, Dorsey CM, Campbell PI. Effect of fluoxetine on sleep. Biol Psychiatry. 1991; 29(6): 618–619. [DOI] [PubMed] [Google Scholar]
- 70. Minot R, Luthringer R, Macher JP. Effect of moclobemide on the psychophysiology of sleep/wake cycles: a neuroelectrophysiological study of depressed patients administered with moclobemide. Int Clin Psychopharmacol. 1993; 7(3-4): 181–189. [DOI] [PubMed] [Google Scholar]
- 71. Trivedi MH, Rush AJ, Armitage R, et al. Effects of fluoxetine on the polysomnogram in outpatients with major depression. Neuropsychopharmacology. 1999; 20(5): 447–459. [DOI] [PubMed] [Google Scholar]
- 72. Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012; 60(4): 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010; 33(9): 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009; 32(11): 1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Morawetz D. Insomnia and depression: which comes first. Sleep Res Online 2003;5(2):77–81. [Google Scholar]
- 76. Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev. 2013; 17(5): 377–390. [DOI] [PubMed] [Google Scholar]
- 77. Cohen J. Statistical power analysis for the behavioural sciences. Hillside. NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 78. Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry. 2014; 26(2): 205–213. [DOI] [PubMed] [Google Scholar]