Abstract
Study Objectives
To determine whether sleep apnea, defined by polysomnography, accelerates kidney function decline in generally healthy adults not selected for sleep apnea or kidney disease.
Methods
We performed a retrospective cohort study in 855 participants from the Wisconsin Sleep Cohort Study, a large 20-year population-based study of sleep apnea, who had at least one polysomnogram and serial measurements of serum creatinine over time. Sleep apnea was defined as an apnea–hypopnea index ≥ 15 or positive airway pressure (PAP) use at baseline. We compared the slope of estimated glomerular filtration rate (eGFR) change and odds of rapid eGFR decline (>2.2 mL/minute/1.73 m2/year) for those with and without sleep apnea.
Results
The mean follow-up was 13.9 ± 3.4 years. The cohort was 50.4 ± 7.6 years, 55% male, and 97% white. The mean eGFR was 89.3 ± 13.8 mL/minute/1.73 m2 and 11% had sleep apnea. Overall, the mean eGFR change was −0.88 ± 1.12 mL/minute/1.73 m2/year. Compared with those without sleep apnea, participants with sleep apnea had a 0.2 mL/minute/1.73 m2/year slower eGFR decline though this was not statistically significant (95% CI [−0.06–0.45], p = .134). When we excluded those on PAP therapy (n = 17), eGFR decline was even slower among those with sleep apnea (0.36 mL/minute/1.73 m2/year slower, 95% CI [0.08–063], p = .012). Those with sleep apnea had lower odds of rapid eGFR decline but this was not statistically significant, even after excluding PAP users.
Conclusion
Among healthy middle-aged adults, the presence of sleep apnea at baseline did not accelerate kidney function decline compared with those without sleep apnea over time.
Keywords: sleep apnea, kidney function, estimated glomerular filtration rate
Statement of Significance
Utilizing data from a landmark, 20-year, population-based study of sleep outcomes in middle-aged adults, we determined the impact of sleep apnea on kidney function. Other studies have shown that sleep apnea accelerates kidney function decline but no study has examined this association in healthy adults, unselected for sleep disorders or kidney disease. Interestingly, our results suggest that, contrary to published literature, sleep apnea does not accelerate the decline of kidney function. As such, we provide another important perspective on the relationship between sleep apnea and kidney function. Taken together with existing literature, our findings suggest that the impact of sleep apnea on kidney function may be complex and vary by sleep apnea phenotype and the presence of pre-existing comorbidities.
INTRODUCTION
Sleep apnea (SA) is common among Americans, affecting one in four men and one in nine women in the United States.1 Repeated episodes of hypoxia, hypercapnia, and arousal during sleep, in severe cases over 30 times per hour of sleep, are hypothesized to contribute to excess risk of numerous negative health outcomes related to SA including hypertension, stroke, cardiovascular disease, and death.2,3 Through similar mechanisms, SA may impair kidney function.2
Chronic kidney disease (CKD) is also common in the United States, affecting more than 1 in 10 Americans.4 CKD, even at modest reductions in renal function, confers excess risk of adverse health outcomes.5 To date, beyond optimization of diabetes mellitus (DM) and hypertension (HTN) control, few modifiable risk factors for CKD are known. Should SA promote kidney injury, treatment of SA may represent a novel way to prevent development or progression of kidney disease and thus related negative outcomes.
Indeed, SA is more common among those with CKD, and those with SA are more likely to have CKD.6,7 However, literature to date relating SA to kidney injury has longitudinally been limited to the following: (1) retrospective studies performed in clinic populations selected for SA or CKD, (2) unselected populations in which diagnosis codes were used to identify SA, and (3) a single prospective study of diabetic patients with short follow-up.8–11 The results of these studies suggest that SA, defined by sleep study or a diagnosis code, is associated with greater odds of rapid decline in kidney function. To date, however, no study has examined the impact of SA diagnosed by polysomnography (PSG) on kidney function decline longitudinally in a population-based sample with extended follow-up.
We performed a longitudinal study of 855 participants enrolled in the Wisconsin Sleep Cohort Study (WSCS) who had at least one PSG study and serial measurements of kidney function over time to determine the association of SA with kidney function trajectory.
METHODS
Study Cohort
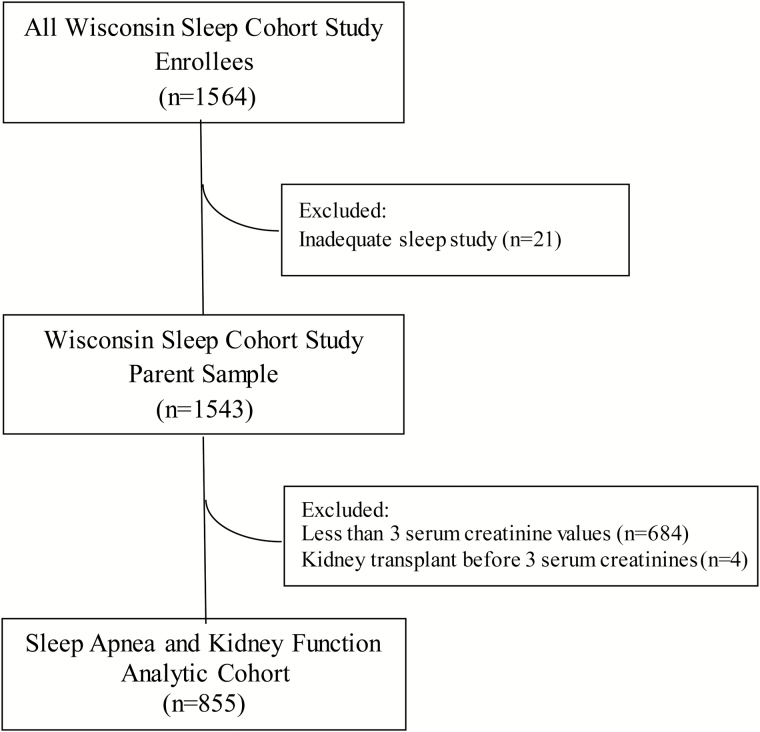
Protocols and written informed consent documents for this study were approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board. The Sleep Apnea and Kidney Function analytic cohort was developed as outlined in Figure 1.
Figure 1.
Genesis of the sleep apnea and kidney function analytic cohort.
The parent cohort for this study, the WSCS, is a prospective, population-based cohort study established in 1988 to study the natural history of SA in middle-aged men and women. The detailed recruitment methodology have been published previously.12,13 Briefly, 1564 men and women aged 30–60 years underwent baseline in-center PSG beginning in 1988. Of these, 1543 completed a successful baseline PSG with usable data. All participants were invited back to undergo repeated PSG approximately every 4 years through 2015. Beginning in 1993, serum creatinine levels were measured at each study visit, and 1366 participants had at least one visit with both PSG and a measurement of serum creatinine level. For the present study, we studied the subset of the WSCS enrollees (Sleep Apnea and Kidney Function Analytic Cohort, n = 855) who underwent at least one PSG coincident with a serum creatinine measurement and who had at least two subsequent serum creatinine measurements over the remaining follow-up time (Figure 1). Thus, timing of measurements were as follows: “baseline” serum creatinine was the first serum creatinine performed coincident with a PSG; thereafter, each of these participants had at least two subsequent serum creatinine measurements on average 4 years apart (because study visits occurred 4 years apart). Follow-up was truncated at the last serum creatinine measurement available for a given participant.
PSG Protocol and Definition of Sleep Variables
Participants underwent overnight attended sleep studies at the University of Wisconsin Clinical Research Unit. After informed consent and completion of anthropometric assessments and questionnaires, an 18-channel polysomnographic recording system (model 78, Grass Instruments, Quincy, MA) was used to monitor sleep stage, respiration, body movements, and cardiac variables as previously published.12 The recording montage was as follows: electroencephalography, electrooculography, and submental electromyography to determine sleep state; continuous pulse oximetry (model 3740, Ohmeda, Englewood, CO) to assess arterial oxygen saturation; nasal–oral thermocouple (ProTec, Hendersonville, TN) to assess nasal and oral airflow; nasal pressure transducer (Validyne Engineering, Northridge, CA) for nasal airflow; and respiratory inductance plethysmography (Respitrace, Ambulatory Monitoring, Ardsley, NY) to detect body abdominal and chest wall excursion. Sleep studies were staged and respiratory events scored by trained sleep technicians and then reviewed by an expert polysomnographer. Note that between 1988 and 2000, sleep studies were scored using a paper-based system; since 2000, studies have been scored on a computer. All statistical modeling adjusts for the scoring changes; this removes instrumentation-related influences on SA assessments after the year 2000.
Because we did not exclude participants with a diagnosis of SA at baseline (first measurement of kidney function with PSG), a small subset of our cohort was using positive airway pressure (PAP) therapy at the first PSG of this analysis. Participants were allowed to use their PAP during the sleep study if they desired. Use of PAP whether in the sleep laboratory or at home was recorded
SA was defined using the apnea–hypopnea index (AHI). Apneas were defined as cessation of airflow for at least 10 seconds. Central and obstructive apneas were distinguished during scoring; the vast majority of apneas were obstructive or mixed. Hypopneas were defined as a discernible reduction in the sum amplitude of the rib-cage plus the abdominal excursions on respiratory inductance plethysmography that lasted at least 10 seconds and that was associated with a reduction in the oxyhemoglobin saturation of at least 4%. The AHI was defined as the average number of episodes of apnea and hypopnea per hour of objectively measured sleep. For the purpose of this analysis, we defined SA as AHI ≥ 15 events per hour or use of PAP (in lab or at home).
Estimation of Renal Function
Serum creatinine levels were measured on average every 4 years between 1993 and 2015. Fasting blood was collected the morning after completion of the overnight sleep study and in 2004–2008 during a daytime cardiovascular protocol visit (not coordinated with an overnight PSG). The blood samples were sent to the University of Wisconsin Hospital and Clinics Laboratory for analysis. The analyzers used to measure serum creatinine levels varied over the years and, therefore, the traceability of the assays to the isotope-dilution mass spectrometry (IDMS) standard varied. Only assays performed from April 2011 were IDMS-traceable.
We calculated the estimated glomerular filtration rate (eGFR) based on the Chronic Kidney Disease-Epidemiology (CKD-EPI) formula which incorporates serum creatinine, age, race, and gender.14 This formula is recommended by the Kidney Disease Improving Global Outcomes 2012 guidelines and improves upon the previously recommended Modification of Diet in Renal Disease (MDRD) equation with respect to accuracy, precision, and bias particularly among those with preserved renal function.15 The CKD-EPI equation was developed using IDMS-traceable serum creatinine assays. We applied a calibration factor of 5% to non-IDMS-traceable serum creatinine values, so they may be used in the CKD-EPI equation, according to the calibration of the MDRD Study laboratory and per prior published literature.16,17
Other Measurements
We collected additional key data from the visit at which the first PSG coincident with a serum creatinine was performed. Such variables included demographic and anthropometric data (age, race/ethnicity, sex, body mass index [BMI]); self-reported comorbid conditions including DM (self-reported and/or use of antihyperglycemic medications), HTN (self-reported and/or use of antihypertensive medications), any cardiovascular disease (CVD) (defined as myocardial infarction, coronary artery disease, atherosclerosis, congestive heart failure, coronary artery bypass grafting, angioplasty, pacemaker, and other heart surgery), stroke (including transient ischemic attack), congestive heart failure, Zung depression scale score, sleepiness (Epworth Sleepiness Scale > 10), and use of renin-angiotensin system blocking agents (ace-inhibitors or angiotensin-receptor blockers).
Statistical Analysis
We used Kruskal–Wallis nonparametric tests and Pearson chi-square tests to compare continuous and categorical variables, respectively, between participants with and without SA at the first PSG visit.
We calculated eGFR trajectory using ordinary least squares regression for each participant regressing observation time (years) on their individual eGFR data to determine the rate of eGFR change (mL/minute/1.73 m2/year). We compared these slopes using generalized linear models. We repeated this analysis using generalized linear mixed models with a compound symmetry covariance structure to account for the correlation for repeated within-person measurements of renal function over time. We then looked at the time × sleep apnea interaction term to see whether there was a time interaction effect with SA in predicting eGFR. Because results were similar to that of least square regression analysis, we present the former for ease of presentation.
In order to examine the impact of SA on extremes of eGFR decline, we performed logistic regression to determine the odds of a rapid decline in eGFR. Extremes of eGFR decline were limited in this generally healthy cohort, so we selected the lowest decile of eGFR decline, >2.2 mL/minute/1.73 m2/year of observation time, as our cut-point for “rapid decline.” For both the eGFR change and odds of rapid decline analyses, we present an unadjusted model, and then a model a priori minimally adjusted for age, sex, BMI, baseline eGFR, and baseline PAP use and a final model further adjusting for DM and HTN. If the point estimate of the SA coefficient remained statistically significant (p < .05) thereafter, we further adjusted other factors that were associated with both our predictor and outcomes in univariate analyses. Because factors such as baseline eGFR, sex, and DM may modify the association between SA and kidney function trajectory, we tested for interaction between SA and each factor (all dichotomous except eGFR which was continuous). If p < .1 for interaction, we then stratified by the given factor. In sensitivity analyses, we repeated the analyses excluding those who were on PAP at baseline (n = 17). We also repeated analyses adjusted for ever diagnosis of DM or HTN or ever use of renin–angiotensin system blockade because these factors may change over time and could affect our outcome. Finally, we repeated our analyses excluding those on renin–angiotensin blockade because use of these medications may affect renal function trajectory.
Finally, to examine the impact of SA on practical renal outcomes, we calculate the risk of CKD development or progression for those with and without SA at baseline. Follow-up time began at the first visit with both the PSG and creatinine and was right-truncated at CKD progression or development (or last creatinine visit if no CKD development or progression). We defined CKD development or progression as follows: two consecutive measurements of eGFR < 60 if first eGFR > 60, eGFR < 45 if first eGFR 45–59, eGFR < 30 if first eGFR 30–44, or development of treated kidney failure with dialysis or transplant. Kaplan–Meier curves depict the probability of development or progression of CKD over the follow-up time by group and we compared these two curves using the log-rank test.
We performed sensitivity analyses using alternative definitions of SA based on AHI ≥ 10 and AHI ≥ 30. All analyses were performed using SAS version 9.4 (Cary, NC).
RESULTS
Baseline Characteristics
The mean age of participants was 50.4 ± 7.6 years. Ninety-seven percent were white, 55% male, and mean BMI was 30.5 ± 6.6 kg/m2. Average follow-up time was 13.9 ± 3.4 years with an average of 4.7 ± 1.1 serum creatinine measurements per participant. Mean baseline eGFR was 89.3 ± 13.8 mL/minute/1.73 m2. Participants with SA were older, more obese, more likely to be sleepy and to be male when compared with those without SA (Table 1). Also, those with SA were more likely to be hypertensive, use renin–angiotensin system blocking medications, and have lower baseline eGFR.
Table 1.
Comparison of Baseline Characteristics Between Those With and Without Sleep Apnea in the Sleep Apnea and Kidney Function Analytic Cohort.
| Baseline Characteristics | No sleep apnea (n = 765) | Sleep apneaa (n = 90) | p |
|---|---|---|---|
| Age (years), mean (SD) | 50.0 (7.5) | 54.1 (7.1) | <.001 |
| Sex, n (%) | <.001 | ||
| Female | 362 (47%) | 25 (28%) | |
| Male | 403 (53%) | 65 (72%) | |
| Race/ethnicity, n (%) | .88 | ||
| White | 743 (97.1%) | 86 (96%) | |
| African American | 6 (0.8%) | 1 (1%) | |
| Asian | 4 (0.5%) | 1 (1%) | |
| Hispanic/Latino | 2 (0.3%) | 0 | |
| Native American | 3 (0.4%) | 1 (1%) | |
| Other | 7 (0.9%) | 1 (1%) | |
| Education status | .89 | ||
| Did not complete high school | 6 (1%) | 1 (1%) | |
| High school graduate | 177 (23%) | 20 (22.5%) | |
| Some college | 228 (30%) | 28 (33.5%) | |
| Bachelor’s degree | 169 (22%) | 20 (22.5%) | |
| Post-graduate work | 180 (24%) | 29 (22.5%) | |
| Body mass index (kg/m2), mean (SD) | 29.9 (6.1) | 35.8 (8.1) | <.001 |
| Diabetes mellitus, n (%) | 23 (3%) | 6 (7%) | .07 |
| Ever diagnosed with diabetes mellitus, n (%) | 125 (16%) | 39 (43%) | <.001 |
| Hypertension, n (%) | 170 (22%) | 36 (40%) | <.001 |
| Ever diagnosed as hypertensive, n (%) | 447 (58%) | 66 (73%) | .007 |
| Renin–angiotensin system blocker use, n (%) | 41 (5%) | 11 (12%) | .010 |
| Ever used renin–angiotensin system blocker, n (%) | 250 (33%) | 43 (48%) | .005 |
| Any cardiovascular disease, n (%) | 27 (3.5%) | 5 (5.6%) | .34 |
| Stroke, n (%) | 4 (0.5%) | 2 (2%) | .07 |
| Congestive heart failure, n (%) | 2 (0.3%) | 0 | .62 |
| Zung depression score, mean (SD) | 32.0 (6.5) | 33.1 (6.1) | .09 |
| Average number of eGFR measurements, mean (SD) | 4.8 (1.1) | 4.6 (1.2) | .16 |
| eGFR (CKD-EPI) (mL/minute/1.73 m2), mean (SD) | 89.8 (13.8) | 85.7 (12.9) | .007 |
| AHI (first PSG), median [IQR] |
NA | ||
| No PAP |
1.1 [0.3–3.34] |
(n = 73): 24.4 [18.2–30.3] |
|
| PAP user (in lab) |
NA |
(n = 7): 1.0 [0.2–3.5] |
|
| PAP user (at home, not in lab) | NA | (n = 10): 32.5 [16.6–55.9] | |
| ESS >10, n (%) | 266 (35%) | 44 (49%) | .008 |
| Deaths, n (%) | 38 (5%) | 7 (8%) | .26 |
| Follow-up time (years), mean (SD) | 13.9 (3.4) | 13.6 (3.5) | .32 |
AHI = apnea–hypopnea index; eGFR = estimated glomerular filtration rate; CKD-EPI = Chronic Kidney Disease-Epidemiology Collaboration; PAP = positive airway pressure; IQR = interquartile range; ESS = Epworth Sleepiness Scale.
aDefined as AHI ≥ 15 or on PAP use.
SA and Kidney Function Trajectory
Overall, the mean annualized eGFR slope for the cohort was −0.88 ± 1.12 mL/minute/1.73 m2/year. The annualized eGFR slope for those with SA was −0.70 ± 1.32 mL/minute/ 1.73 m2/year and for those without SA was −0.90 ± 1.16 mL/minute/1.73 m2/year. Figure 2 depicts eGFR decline per year of age by category of SA.
Figure 2.
Trajectory of estimate glomerular filtration rate by age for those with and without sleep apnea at baseline.
Compared with those without SA, those with SA had a slower rate of eGFR decline (0.2 mL/minute/1.73 m2/year slower), though not statistically significant (β = 0.20 [−0.06–0.45], p = .138, Table 2). However, when we excluded those using PAP therapy at baseline (n = 17), eGFR decline was even slower 0.36 mL/minute/1.73 m2/year slower) for those with SA than those without SA, and the difference was statistically significantly different from zero (β = 0.36 [0.08–0.63], p = .012). This association persisted despite adjustment for age, sex, BMI, and baseline eGFR.
Table 2.
Annualized Change in eGFR by Baseline Sleep Apnea Status (n = 855).
| β: mL/minute/1.73 m2/year (SE) [95% CI] | |||||
|---|---|---|---|---|---|
| Sleep Apnea Category | Unadjusted | Age-adjusted | Age, sex, BMI-adjusted | Age, sex, BMI-adjusted, baseline eGFR | Age, sex, BMI, diabetes, HTN-adjusted, baseline eGFR |
| No sleep apnea, N = 765 | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) | 0.0 (referent) |
| Sleep Apnea (All), N = 90 | 0.20 (0.13) [−0.06–0.45] | 0.23 (0.13) [−0.03–0.49] | 0.24 (0.14) [−0.03–0.52] | 0.23 (0.13) [−0.03–0.48] | 0.24 (0.13) [0.00–0.49] |
| p | .138 | .086 | .078 | .082 | .055 |
| Sleep apnea (excluding PAP users) N = 73 |
0.36 (0.14) [0.08–0.63] | 0.39 (0.14) [0.11–0.67] | 0.39 (0.15) [0.10–0.68] | 0.34 (0.14) [0.07–0.61] | 0.36 (0.13) [0.10–0.63] |
| p | .012 | .007 | .009 | .014 | .007 |
eGFR = estimated glomerular filtration rate; PAP = positive airway pressure; BMI = body mass index; HTN = hypertension.
Compared with participants without SA, those with SA had a 14% lower odds of rapid eGFR decline in unadjusted analyses but this did not reach statistical significance (OR [95% CI]: 0.86 [0.40–1.84], p = .70, Table 3). When we excluded those participants on PAP at baseline, those with SA had even lower odds of rapid eGFR decline in unadjusted analyses but the confidence interval was wide and crossed 1.0 (OR [95% CI]: 0.51 [0.18–1.44], p = .20). Results were unchanged when we adjusted for ever diagnosis of DM, HTN, or ever use of renin–angiotensin system blockade and when we excluded those on renin–angiotensin system blockade (data not shown). Furthermore, there was no evidence of interaction between SA and baseline eGFR, sex, or DM to predict eGFR trajectory (p for interaction .17, .71, and .99, respectively).
Table 3.
Logistic Regression for Odds of Rapid Decline as Defined as Lowest Decile (10%, >2.2 mL/minute/1.73 m2/year) in Annualized Change in eGFR by Baseline Sleep Apnea Status (n = 855).
| Odds ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Sleep apnea category | Unadjusted | Age-adjusted | Age, sex, BMI-adjusted | Age, sex, BMI, baseline eGFR-adjusted | Age, sex, BMI, Diabetes, HTN-adjusted, baseline eGFR |
| No sleep apnea N = 765 |
1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Sleep apnea (all) N = 90 |
0.86 (0.40–1.84) | 0.81 (0.37–1.75) | 0.69 (0.30–1.59) | 0.73 (0.32–1.69) | 0.73 (0.31–1.71) |
| p | .70 | .59 | .39 | .47 | .47 |
| Sleep apnea (excluding PAP users) N = 73 |
0.51 (0.18–1.44) | 0.47 (0.17–1.34) | 0.41 (0.14–1.24) | 0.46 (0.15–1.38) | 0.42 (0.14–1.31) |
| p | .20 | .16 | .11 | .17 | .14 |
eGFR = estimated glomerular filtration rate; PAP = positive airway pressure; BMI = body mass index; HTN = hypertension.
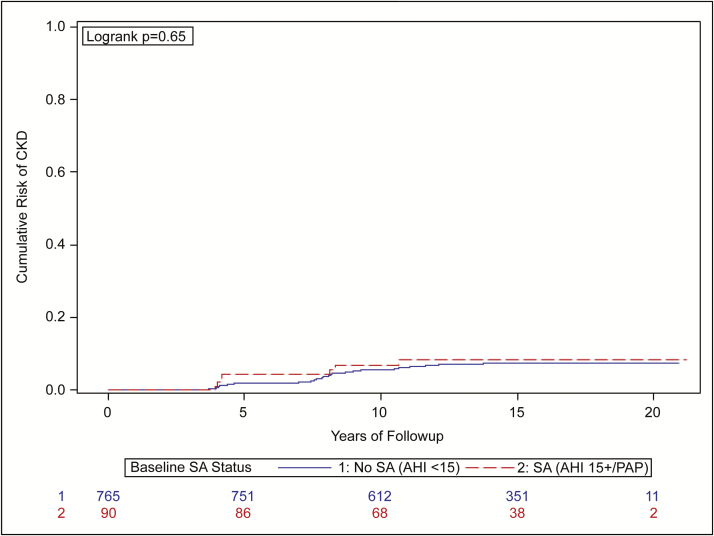
SA and Incidence of CKD Development or Progression
Over the follow-up time, 8% of those with SA developed or had progressive CKD versus 7% of those without SA. Kaplan–Meier curves are shown in Figure 3 and reflect no difference over the follow-up time in the incidence of CKD development or progression (p = .65 log-rank test). When we excluded those on PAP therapy at baseline, the results did not materially change (8% of SA without PAP developed or had progressive CKD vs 7% without SA) (log-rank test p = .58).
Figure 3.
Time to development or progression of CKD by presence or absence of sleep apnea at baseline.
Alternative Definitions of SA and Kidney Trajectory
When we repeated all analyses above alternatively defining SA as AHI ≥ 10, results were unchanged (data not shown). When SA was defined as AHI ≥ 30, results were also materially unchanged though the point estimates for SA to predict eGFR trajectory were lower suggesting less difference in trajectory between those with SA and those without, but the sample size was limited for this analysis (n = 36 with AHI ≥ 30) and results were not statistically significant (β = 0.02 mL/minute/1.73 m2 95% CI [−0.37–0.42], p = .91, unadjusted, for those with SA defined as AHI ≥ 30).
DISCUSSION
In this longitudinal, population-based study, middle-aged men and women with moderate-to-severe SA did not have a faster rate of kidney function decline when compared with those with no or mild SA. Furthermore, when we excluded the small number of participants already being treated for SA at baseline, those with SA had a slower rate of kidney function decline compared with those without SA, despite adjustment for key confounders of this association. Finally, there was no difference in time to development or progression of CKD between those with and without SA.
Our findings contradict previously published longitudinal studies showing that SA, across a variety of definitions and populations, may lead to faster decline in renal function.8–11,18 It is notable, however, that studies published to date were performed in clinically based populations and the definitions of SA and prevalence of CKD differed from our study. For example, one retrospective cohort study examined the impact of SA defined by a 4% oxygen desaturation index on CKD progression in nephrology clinic enrollees with moderate-to-severe CKD (mean eGFR 31 mL/minute/1.73 m2) at two Japanese tertiary care centers.11 Over 1-year follow-up time, the investigators found that patients with a 4% oxygen desaturation index of at least 15 experienced a 3–4 fold faster decline in eGFR (estimating equation not defined) over time, despite multivariate adjustment.11 Another study of 858 patients referred for sleep studies (mean eGFR = 70.8 mL/minute/1.73 m2) to a single sleep center found that, over 2.1 years follow-up, AHI ≥ 15 and AHI ≥ 30 were associated with 2- and 4-fold greater odds of rapid decline in renal function (MDRD eGFR decline > 3 mL/minute/1.73 m2/year), respectively.9 However, in multivariate analysis, only nocturnal hypoxemia defined by % total sleep time < 90% oxygen saturation ≥ 12% was associated with greater odds of rapid decline, independent of AHI and other key factors.9 Finally, Molnar et al. performed a large retrospective study of Veterans Health Administration enrollees without CKD (by CKD-EPI eGFR > 60 mL/minute/1.73 m2) or obstructive SA (by codes) at their first encounter in the inclusion period (fiscal years 2004–2006).8 Veterans who developed obstructive SA code during the inclusion period were 2.3 times more likely to later develop a diagnosis of CKD (by code) and 30% more likely to develop a rapid decline in renal function over a median of 7-year follow-up time, despite multivariate adjustment.8 To sum, despite heterogeneity in populations and definitions of SA and prevalence of CKD, all longitudinal studies to date suggest some association between SA defined by AHI, nocturnal hypoxemia or diagnosis codes, and renal function decline.
Reasons for the divergence of our study results from that of the published literature are unclear. One possible explanation is that SA does indeed negatively affect kidney function but differences in the population we studied or the way we defined our predictor and outcome affected our ability to detect this association. With respect to our population, the prevalence of SA in our cohort (11%) was less than that of other studies that utilized PSG to diagnose SA (46% and 64%).9,10 In addition, our cohort may have been a healthier cohort in general—for example, only 3% of our cohort was diabetic compared with 10%–100% of other cohorts that studied SA and kidney function decline.8–11,18 As such, with a low prevalence of SA and a generally healthy cohort, meaningful decline in renal function may require even longer follow-up in such a healthy cohort. Furthermore, the racial distribution in our cohort was predominantly white; however, other studies had a spectrum of racial distribution from mostly white (like our population) to 54% white to 100% Japanese.8–11 As such, it is unclear what role race may have played in the divergence of our results from other studies. Also, we defined SA using the AHI—a standard definition of SA.3 However, there may be various phenotypes of dysregulated breathing during sleep including phenotypes with more significant hypoxemia which we could not explore in the analytic cohort as most of the baseline PSG studies used for analysis did not have event-level desaturation data available. Finally, the discordance between our findings and those published to date may be because among generally healthy middle-aged adults with preserved renal function, SA as we have defined it does not lead to accelerated decline in kidney function. It may be that SA negatively affects kidney function only in the setting of other diseases such as more advanced CKD with impaired autoregulation or inability to maintain kidney perfusion at extremes of blood pressure, DM, or other potentially nephrotoxic disease states.
It is noteworthy that the rate of kidney function decline was comparable with that reported for typical age-related decline in kidney function.19 Interestingly, however, the decline in kidney function was slower among those with SA. Although this difference was small in magnitude (3 mL/minute/1.73 m2 lower eGFR over 10 years for those without SA compared with those with SA), it was statistically significant after excluding those with PAP therapy at baseline. One possible explanation may be that those identified as having SA would be referred for care and thus have more attention to their health care (e.g., more attention to effective HTN control) and thus slower decline in renal function. Another possibility is that undocumented use of PAP therapy during the follow-up period among those diagnosed with SA may bias results toward the null. However, in retrospective studies of SA and PAP therapy, the impact of PAP therapy on kidney function decline was variable.8,20,21 Also, in our cohort, removal of those with known PAP therapy at baseline (n = 17) resulted in even slower decline in renal function for those with SA, suggesting that those on PAP therapy may have represented a sicker population. It may also be that participants in the no SA group were later diagnosed with SA which would also bias our results to a null finding. Although follow-up PSGs were available for some participants, we did not have this information on all participants in our study.
Our study is the first to examine the association between SA and kidney function decline over time in a population-based sample unselected for SA or kidney disease. Other strengths of our study include extended follow-up time, ascertainment of SA using PSG and serial estimation of kidney function using a standard serum creatinine-based measure. However, our study has important limitations. First, we lacked information on degree of nighttime hypoxia or arousals which may affect kidney function trajectory. Our baseline sleep studies were performed during an era in which output was paper-based and scoring was performed by manual review this output. Although desaturations associated with a SA event were scored, values such as minimum oxygen desaturation, time spent at less than 90% oxygen saturation, and arousals were not scored. In addition, we did not have information regarding an earlier marker of renal injury, albuminuria. Although this may be important to detect early kidney injury in this generally healthy population, given the very low proportion with DM, it is probably that we would have had very few participants with albuminuria. Finally, although some participants had subsequent PSGs or documentation of later PAP therapy during follow-up, we lacked this information consistently for all our participants.
In this cohort of generally healthy middle-aged adults, the presence of SA at baseline did not lead to accelerated kidney function decline compared with those without SA over an average of 13 years’ follow-up. Although SA did not negatively affect kidney function in this cohort, future research should examine whether certain phenotypes of SA may be more nephrotoxic than others. Also, additional studies should determine which populations if any may be most vulnerable to SA-related kidney function decline, particularly given existing literature in distinct cohorts suggesting SA indeed negatively affects kidney function over time. Ultimately, future trials of PAP therapy to slow progression of kidney function decline could be targeted to populations vulnerable to SA-related kidney function decline.
FUNDING
This work was supported by US National Institutes of Health (NIH) grants 1R01AG036838, R01HL62252, 1UL1RR02501, and R21DK103104-01. In addition, Dr. Canales’s time is supported in part by VA CSR&D Career Development Award (CX000533-01A1) and by support from the Division of Nephrology, Hypertension and Renal Transplantation, Department of Medicine, University of Florida. The funding agencies had no role in the study design, collection, analysis or interpretation of the data, the writing of this report, or the decision to submit this manuscript.
WORK PERFORMED
The statistical analysis of the data herein submitted for review was performed at the Department of Population Health Sciences, University of Wisconsin – Madison, WARF Building #611A, 610 Walnut St., Madison, WI 53726, USA. The synthesis of the data and drafting of the manuscript was performed at the Malcom Randall VA Medical Center (corresponding author’s location).
AUTHOR CONTRIBUTIONS
Design and conception of the study: MTC, EWH, JHB, PEP, and SFD. Data acquisition: EWH, JHB, and PEP. Data analysis and interpretation: MTC, EWH, JHB, PEP, and SFD. Drafting of the article, revision for important intellectual content, and final approval of the manuscript: MTC, EWH, JHB, PEP, and SFD. Responsible for accountability for overall work by ensuring the accuracy or integrity of any portion of the work is appropriately investigated and resolved: MTC, EWH, JHB, PEP, and SFD.
DISCLOSURE STATEMENT
JHB, MTC, EWH, PEP, and SFD declare that they have no conflicts of interest to disclose according to the ICMJE Uniform Disclosure Form for Potential Conflicts of Interest. In addition, the results presented in this paper have not been published previously in whole or part, except in abstract format.
ACKNOWLEDGMENTS
We thank the following people for their valuable assistance: Amanda Rasmuson, Dr. Terry Young, Dr. Mari Palta, Robin Stubbs, and Laurel Finn.
REFERENCES
- 1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013; 177(9): 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adeseun GA, Rosas SE. The impact of obstructive sleep apnea on chronic kidney disease. Curr Hypertens Rep. 2010; 12(5): 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 4. U S Renal Data System, USRDS 2016 Annual Data Report: Epidemiology of Kidney Diseases in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 5. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351(13): 1296–1305. [DOI] [PubMed] [Google Scholar]
- 6. Kanbay A, Buyukoglan H, Ozdogan N et al. . Obstructive sleep apnea syndrome is related to the progression of chronic kidney disease. Int Urol Nephrol. 2012; 44(2): 535–539. [DOI] [PubMed] [Google Scholar]
- 7. Nicholl DDM, Ahmed SB, Loewen AHS et al. . Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest. 2012; 141(6): 1422–1430. [DOI] [PubMed] [Google Scholar]
- 8. Molnar MZ, Mucsi I, Novak M et al. . Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax. 2015; 70(9): 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed SB, Ronksley PE, Hemmelgarn BR et al. . Nocturnal hypoxia and loss of kidney function. PLoS One. 2011; 6(4): e19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tahrani AA, Ali A, Raymond NT et al. . Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care. 2013; 36(11): 3718–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakaguchi Y, Hatta T, Hayashi T et al. . Association of nocturnal hypoxemia with progression of CKD. Clin J Am Soc Nephrol. 2013; 8(9): 1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993; 328(17): 1230–1235. [DOI] [PubMed] [Google Scholar]
- 13. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009; 108(5): 246–249. [PMC free article] [PubMed] [Google Scholar]
- 14. Inker LA, Schmid CH, Tighiouart H et al. ; CKD-EPI Investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012; 367(1): 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kidney disease: improving Global Outcomes (KDIGO) CKDWork Group. KDIGO 2012 Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Kidney International Supplement. 2013;3(3):1–150. [Google Scholar]
- 16. Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011; 162(3): 548–554. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Coresh J, Greene T et al. ; Chronic Kidney Disease Epidemiology Collaboration Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007; 53(4): 766–772. [DOI] [PubMed] [Google Scholar]
- 18. Lee YC, Hung SY, Wang HK et al. . Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep. 2015; 38(2): 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009; 120: 419–428. [PMC free article] [PubMed] [Google Scholar]
- 20. Puckrin R, Iqbal S, Zidulka A, Vasilevsky M, Barre P. Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. Int Urol Nephrol. 2015; 47(11): 1839–1845. [DOI] [PubMed] [Google Scholar]
- 21. Koga S, Ikeda S, Yasunaga T, Nakata T, Maemura K. Effects of nasal continuous positive airway pressure on the glomerular filtration rate in patients with obstructive sleep apnea syndrome. Intern Med. 2013; 52(3): 345–349. [DOI] [PubMed] [Google Scholar]