Abstract
Study Objectives
The present study explored the sleep mechanisms which may support awareness of hidden regularities.
Methods
Before sleep, 53 participants learned implicitly a lateralized variant of the serial response-time task in order to localize sensorimotor encoding either in the left or right hemisphere and induce implicit regularity representations. Electroencephalographic (EEG) activity was recorded at multiple electrodes during both task performance and sleep, searching for lateralized traces of the preceding activity during learning. Sleep EEG analysis focused on region-specific slow (9–12 Hz) and fast (13–16 Hz) sleep spindles during nonrapid eye movement sleep.
Results
Fast spindle activity at those motor regions that were activated during learning increased with the amount of postsleep awareness. Independently of side of learning, spindle activity at right frontal and fronto-central regions was involved: there, fast spindles increased with the transformation of sequence knowledge from implicit before sleep to explicit after sleep, and slow spindles correlated with individual abilities of gaining awareness. These local modulations of sleep spindles corresponded to regions with greater presleep activation in participants with postsleep explicit knowledge.
Conclusions
Sleep spindle mechanisms are related to explicit awareness (1) by tracing the activation of motor cortical and right-hemisphere regions which had stronger involvement already during learning and (2) by recruitment of individually consolidated processing modules in the right hemisphere. The integration of different sleep spindle mechanisms with functional states during wake collectively supports the gain of awareness of previously experienced regularities, with a special role for the right hemisphere.
Keywords: Sleep spindles, slow waves, implicit learning, executive functions, memory consolidation, awareness
Statement of Significance
The offline mechanisms supporting postsleep awareness of hidden task regularities are not known. Such mechanisms may reprocess implicit regularity knowledge, but they may also restructure sensorimotor information or reactivate functional systems involved during presleep learning. The present study shows that explicit awareness after sleep relies on sleep spindle mechanisms which trace the activation of those motor and right-hemisphere regions that were more strongly involved already during learning. Also, sleep spindles in the right hemisphere were a marker of individual abilities to gain awareness. Thus, the integration of different sleep spindle mechanisms with functional states during wake collectively supports the gain of awareness of previously experienced regularities, with a special role for the right hemisphere in mediating learning- and trait-dependent neuroplasticity.
INTRODUCTION
Memories may be reorganized during sleep such that new memories different from original experiences are formed.1 Thereby, novel behaviors may emerge after sleep in humans as reflections of creativity, insightfulness, and decision making.2 An original demonstration of this process was provided by Wagner et al.,3 who showed that the number of participants becoming aware of an abstract rule hidden in an implicitly trained sensorimotor task more than doubled after sleep compared with wake. With this evidence, a major focus of the present study is on the mechanisms by which awareness of hidden regularities is reflected in sleep.
We first sought to see whether awareness-related sleep mechanisms operate on task-specific information encoded before sleep, possibly restructuring sensorimotor task engrams.4 To label sleep activations as task-specific, we used a lateralized variant of a visual serial reaction-time task (SRTT): The relevant stimuli (four different colors) appeared in one hemi-field only, left or right, requiring choice responses with the ipsilateral hand.5 About half of the participants learned the task on the left, and the other half on the right. The major idea of this lateralized design was to induce, by virtue of neuroanatomical projections, the formation of sensory and motor information engrams in the hemisphere contralateral to the side of learning as has been successfully shown for visual short-term memories.6
Second, we accounted for the effects of implicit sequence representations acquired before sleep on subsequent awareness,7–10 which have remained a matter of debate.11,12 To induce implicit sequence representations (i.e., representations of abstract regularity), participants learned the SRTT implicitly before sleep not being told about the presence of a sequence.13 The amount of acquired implicit sequence knowledge (ImK) was measured in each individual by response-time speeding during presleep SRTT training.
Third, we explored whether awareness-related sleep mechanisms depend on the reactivation of functional systems during sleep. Region-specific reactivations during sleep have been found to correspond to functional task activations during wake.14 Likewise, functional inactivations during wake have produced region-specific reductions of sleep activity at previously suppressed cortical areas.15 With this account, we regarded the possibility that individuals who become aware of regularities after sleep process the task differentially already at learning using specific processing strategies16 and activating more attention and executive control processes.5,17–19 We therefore sought to see whether awareness-related sleep mechanisms operate on functionally preactivated cortical regions.
As a relevant neurophysiologic measure of offline processing during sleep, we analyzed sleep spindles, an electroencephalographic (EEG) hallmark of nonrapid eye movement (NREM) sleep.20 Sleep spindles are generally recognized as correlates of offline memory consolidation and neural plasticity.21,22 Both slow (9–12 Hz) and fast (13–16 Hz) spindles have been shown to increase following presleep procedural and declarative learning21,23–25 and to sub-serve the offline generalization and integration of new with existing memory schemes.26 Specifically slow spindles during slow wave sleep (SWS) have been found to support rule awareness after sleep and implicit-to-explicit knowledge transformation.9
We hypothesized that if rule extraction and awareness are related to offline processing of task-specific information during sleep, explicit knowledge (ExK) generation (which was measured here after sleep only) would be accompanied by enhanced sleep-spindles at sensory and motor regions contralateral to the side of training. If sleep-related rule extraction depends on functional reactivations during sleep, gain of awareness would be accompanied by enhanced sleep spindles at those cortical regions that were specifically preactivated more in individuals who generated explicit sequence knowledge. Also, offline transformation of sequence representations from implicit to explicit was expected to correlate with sleep spindles during SWS.9,27 Finally, if increased awareness was determined by individual or trait-like characteristics,20 some participants might have both more explicit knowledge and more spindles, irrespective of presleep task learning.28
METHODS AND MATERIALS
Participants
The participants in the present study are part of a larger study designed to investigate the effects of sleep on hemisphere-specific processing, where one hundred nine participants were included. According to the general study design, the retention periods extended either across a night full of continuous sleep or a day-time wake period. Accordingly, about half of the participants participated in the morning (day group) and the other half in the evening (night group).5 Here, data from the night group only, comprising a total of 53 students from the University of Lübeck (28 females), will be reported. All were between 20 and 30 years of age (mean 23.4 ± 2.2 years) and had normal or corrected to normal vision as well as normal color vision, according to self-report. All participants were right-handed, evaluated by the Edinburgh Handedness Inventory,29 without any history of somatic, neurologic, and psychiatric disturbances. Participants were asked to complete the self-reported questionnaire about sleep disturbances or an irregular sleep-wake cycle during the last 4 weeks. Any current or past sleep disturbance or an irregular sleep-wake cycle was an exclusion criterion. Also, participants were asked to abstain from caffeine 6 hours before the experiment. The study was conducted in an EEG laboratory at the Department of Neurology, University of Lübeck and was approved by the university’s Ethic Committee. Participants received monetary compensation of €60 and gave informed written consent before the study.
Experiment
The SRTT was practiced in an evening session before sleep (presleep learning) and in a morning session after sleep for update (Figure 1). Half of the participants learned the task on the left, and the other half on the right. “Left” and “right” applied both to the responding hand30 and to the side of relevant visual stimulation, always in parallel. All participants had spent an adaptation night in the laboratory with a polysomnographic (PSG) recording which will be denoted here as a nonlearning night. During the nonlearning night, participants were carefully observed for the presence of abnormal respiratory events, periodic leg movements, and other sleep pathology signs. None of them manifested any event implying pathological sleep. After 7–29 days (median 11 days), they spent the learning night preceded by SRTT learning (Figure 1). A self-report was also completed before the learning night to exclude irregular sleep cycle or sleep disturbances between the two nights. For the learning night, participants reported to the laboratory at ~21:00 hours. After the placement of electrodes for PSG recording, they performed three blocks of practice (Learning—parts 1, 2, and 3 in Figure 1) and thereafter went to bed at ~23:00 hours. After 8 hours in bed, participants were awakened at ~07:00 hours. They were only awakened from light sleep stages 1 or 2 to avoid cognitive disturbances that can occur after awakenings from SWS or rapid eye movement (REM) sleep. Finally, participants performed the morning SRTT session with EEG recording starting at ~07:30 hours. Not relevant to the present study, half of the participants crossed the task side (visual stimulation and performing hand) during the SRTT session after sleep, and the other half did not. Subjective levels of sleepiness, activation, boredom, concentration, and motivation were assessed on five-point scales immediately before and after each session before and after sleep. Scores did not deviate for any of the subjects.
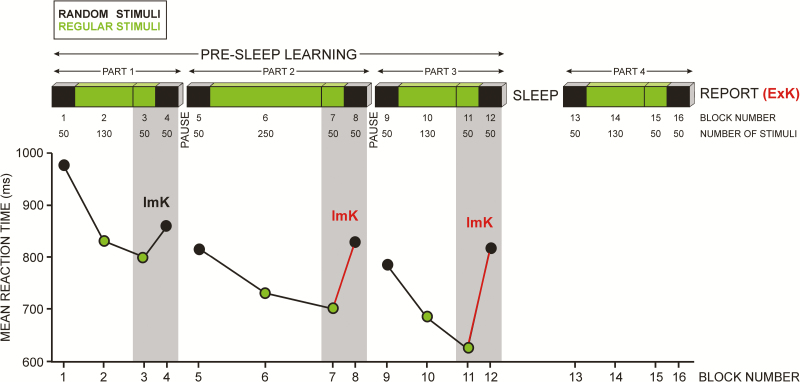
Figure 1.
Graphic illustration of the experiment and parameters. A SRTT was performed before sleep (presleep learning with parts 1, 2, and 3), and after sleep (part 4). Unknown to participants, each part had a “sandwich structure” with two random blocks (indicated in black) surrounding regular blocks (indicated in green). For reasons of analysis, the blocks are numbered consecutively from 1 to 16 (BLOCK NUMBER), with the number of trials in each block indicated below (NUMBER OF STIMULI). Outer blocks with 50 trials (1, 4, 5, 8, 9, 12, 13, 16) followed a quasi-random series. Inner blocks (2 and 3, 6 and 7, 10 and 11, 14 and 15) repeated a fixed sequence of 12 stimuli. Blocks 3, 7, 11, and 15 were separated to simply denote the final 50 trials of these repeated-sequence (“regular”) blocks in each part preceding the final random blocks. For computation of implicit sequence knowledge before sleep (ImK, shaded areas), parts 2 and 3 were used. The bottom part of the figure displays group mean reaction times of all participants in each block. The difference between reaction times in final random and preceding regular blocks of parts 2 and 3 (7 vs. 8 and 11 vs. 12) is marked in red as relevant for the computation of the ImK coefficient. Explicit knowledge (ExK) was probed after block 16 of part 4.
Stimuli and Procedure
The SRTT used in this study was a modification of the standard version introduced by Nissen and Bullemer13 and Willingham et al.31 The task was programmed by means of Presentation Software version 14.5 (Neurobehavioral Systems, Inc., Albany, USA). Stimuli were presented on a 17” CRT monitor. Participants were instructed to maintain their gaze during the whole experiment to the middle of the monitor. A black fixation cross (with a size corresponding to 0.5° × 0.5° visual angle) was always visible at the center of the white screen. After 400 ms, the cross was replaced by two circles of ~1 cm2 each (with a diameter corresponding to 1° visual angle), one in color and the other in grey, with equal displacement of 4.4° visual angle from screen center. For a given participant in the learning session, the colored circles appeared always right or always left, with colors changing across trials between green, blue, red, and yellow, always counterbalanced by a grey circle at the opposite side. The two circles were presented for 200 ms and served as stimulus. If the response was correct, the cross changed after 200 ms for 200 ms to bold, then returned to its normal shape, and after 600 ms the next stimulus appeared. If the response was not correct, the cross did not change to bold until the correct button was pressed.
To control for eye fixation at screen center, an eye tracker was used (Eye-Tracker 600 Series, Eyegaze Edge, LC Technologies, Inc., Fairfax, USA). If fixation deviated from screen center by more than 2.6 cm at trial onset (visual angle larger than 1.3°), a large red exclamation mark appeared for 2 s in the middle of the screen attracting gaze back to the center. Then the trial was restarted.
Throughout any session, responses were given with the same hand, ipsilateral to the constant side of the color stimuli. Participants were instructed to press the respective button on a response pad as quickly and accurately as possible. The response pad was designed in such a way that the position of the four buttons corresponded to the position of the fingers of a relaxed freely placed hand: the blue button (B) was exactly below the index finger, the red button (R) below the middle finger, the yellow button (Y) below the ring finger, and the green button (G) below the little finger.
Task structure followed the design used by Cohen et al.30 As displayed in Figure 1, the learning session before sleep was divided by two short self-terminated breaks into three parts of 280, 400, and 280 trials, altogether 960 trials, and the session after sleep consisted of one part of 280 trials. The three parts before sleep served to enable the computation of the dynamics of implicit knowledge gain (Figure 1, also see below). Untold to participants, each part was a “sandwich” where the outer trials (first 50 and last 50 marked in black in Figure 1) followed a predetermined quasi-random series (but immediate repetitions of the same color did not occur), whereas the inner trials (marked in green in Figure 1) repeated a fixed sequence of 12 stimuli: B-R-Y-B-G-Y-R-B-Y-G-R-G. In the three parts of the learning session, there were 15, 25, and 15 sequence repetitions. Participants were not informed about the existence of this regularity until after the update session in the morning.
SRTT Performance Analysis
Response times (RTs) to correct responses were averaged for each participant in each block of SRTT sessions. As shown in Figure 1, to compute the gain of ImK, the 50 trials in the last random block and the final 50 trials from the preceding regular block were used in each part of presleep learning. ImK was computed as the difference between RT of random and regular trials in order to reflect RT speeding produced by regularity learning and subsequent RT slowing produced by regularity violation in the random block. To achieve normalization, an ImK coefficient was computed as the rate of change: ImK coefficient = 100 × RTrandom/RTregular − 100. Individual gain of implicit knowledge before sleep was quantified using the averaged ImK coefficients of the second and third (last) parts of the presleep learning session (Figure 1). These two parts were used to combine interindividual variations both in speed of implicit learning (possibly more apparent in the second part differentiating individuals with faster and slower ImK gain) and in extent of implicit learning (possibly more apparent in the third part differentiating individuals with large or small ImK gain—Figure 2). Also, continuous regularity practice was most extended in the second part, which may have additionally affected sequence learning. Individual averaged ImK coefficients formed a single variable in the ANCOVA design (see Statistical analysis).
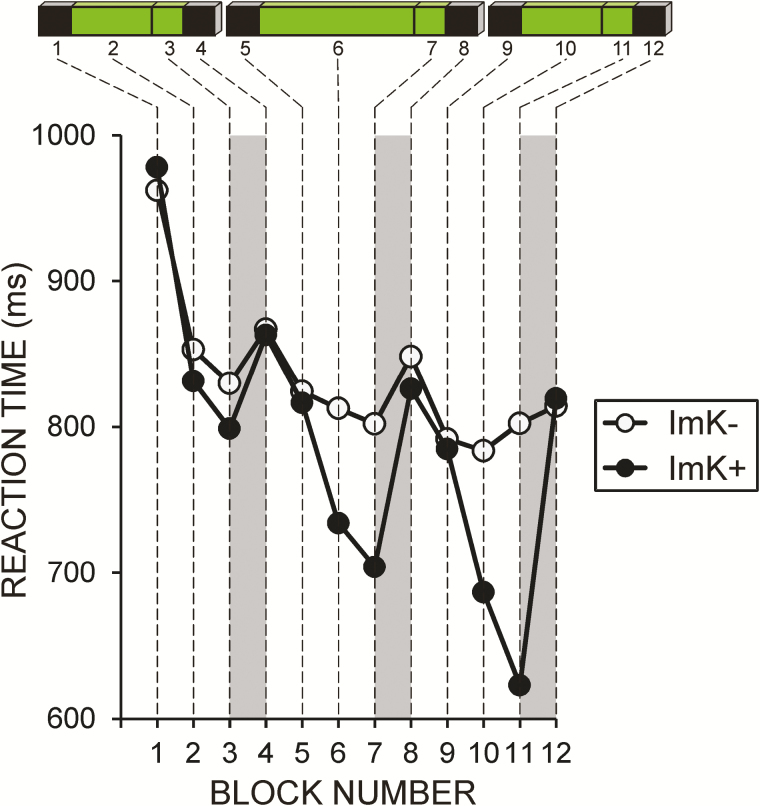
Figure 2.
Illustration of the gain of implicit knowledge during SRTT training. Shown are mean response times for the 12 presleep learning blocks indicated in Figure 1 for participants: (i) who did not reliably gain implicit knowledge during training (ImK−) and (ii) who did accumulate implicit knowledge about the regularity of stimuli (ImK+). Gray rectangles indicate the RT difference between the last regular and the last random blocks in each part of the learning session used for computation of the ImK coefficient.
Explicit knowledge about the regular sequence was tested after the update session after sleep. Participants were asked first whether they had noted anything worth reporting, second whether they had noted any regularity, and third (independent of their previous responses) to write on paper any regular sequence they might have encountered. Gain of explicit knowledge was scored from 1 to 5 in the following way. In case of no regularity being detected or no feeling of any pattern in the stimulation, participants obtained a score of 1. Those who could recall a single sequence of 3–4 items scored 2; those recalling two correct sequences of 3–4 items each scored 3; those recalling a correct sequence of more than 8 items scored 4, and participants who were able to report the whole sequence of 12 items scored 5. Scores from 1 to 5 were used as a continuous predictor variable of sleep parameters. Continuous scoring of the total raw number of recalled items was not applied as not taking into account that recall of two chunks of 3–4 items (i.e., score 3) had a much higher guessing probability than recall of one unitary sequence of 6–8 items. Two control parameters were adopted to control for a possible emergence of explicit knowledge during learning and to guarantee that learning was conducted under implicit conditions (vs. attentive control of explicitly known sequence): (1) a sustained reduction of RT in regular blocks to the level of RT in simple response conditions and (2) the presence and rate of premature (<150 ms) responses in regular blocks. These procedures (details in Supplementary Data 2, SD2.2 and SD2.3) indicated that participants with high ExK scores after sleep might not have gained explicit sequence knowledge during SRTT learning.
Sleep EEG Recording
During the control and experimental nights, EEG was recorded with Ag/AgCl electrodes (EasyCap, http://www.easycap.de) from 25 scalp sites according to the International 10/20 system (F7, F3, F4, F8, FC3, FCz, FC4, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, PO7, PO8, O1, O2) against a reference positioned on Fz, and Fpz serving as a ground electrode. Additionally, bioelectrical signals from both sides of the nose were recorded for offline reference. Horizontal and vertical electrooculograms (EOG) as well as electromyogram (EMG) from left and right musculus masseter were also recorded. Data were amplified with cut-off frequencies DC and 250 Hz by a BrainAmp MR plus (Brain Products GmbH, Gilching, Germany) and stored with a sampling rate of 500/s. EEG was offline re-referenced to the mean value of both nose electrodes. Analyses were performed by means of Brain Vision Analyzer 2.1 (Brain Products GmbH, Germany) and by specially designed software on Matlab R2013b (The MathWorks, Inc.).
Sleep Analysis
Sleep Stage Scoring
Based on EEG/PSG data, manual scoring of sleep stages (S1, S2, S3, S4, and REM sleep), awake time, and movements was done for 30-s periods by two experienced raters who were blind to the hypotheses, following the criteria of Rechtschaffen and Kales,32 with inter-rater agreement > 92%; Cohen’s kappa in the range 92–95. Quantitative analysis of spectral sleep EEG was done separately for the scored S2 and SWS (S3 and S4) epochs.
Spectral Sleep EEG Analysis
For analysis of EEG frequency content, after artifact rejection procedures (automatic threshold of ±200 μV in EOG records and manual inspection of all channels), the artifact-free records for sleep stages SWS and S2 were divided into epochs of 5.12-s duration. After applying a 20% Hanning window, these epochs were fast Fourier transformed with a frequency resolution of 0.195 Hz and averaged separately for each sleep stage. The averaged power spectra were digitally smoothed by a 3-point moving average. For analysis, several neighboring bins were averaged to reduce the frequency resolution to ~1 Hz and provide measures for each ~1-Hz frequency bin from 1 to 25 Hz.
Spindle Analysis
Spindle identification was further performed applying the algorithm reported in the work of Marshall et al.33 (see also Ref. 23). Following the frequency and topography verification of slow and fast sleep spindles (see Results) as well as individual mean spectral peak frequencies (Supplementary Table S1), the EEG signal was filtered in the slow-spindle (9–12 Hz) and fast-spindle (13–16 Hz) frequency bands using infinite impulse response digital filters (24 dB/octave). The root mean square (rms) amplitude of each 100-ms interval was calculated. Spindle events were defined as consecutive intervals of at least 0.5 s and at most 3 s for which the rms amplitude exceeded the individual threshold defined for each participant and channel. The individual threshold was defined as mean rms amplitude + SD of the individual channel. If the interval was longer than 3 s,23,33 the entire period was excluded and search started again from the next below-threshold point. Spindle rms amplitude was measured as the mean value of spindle events in each epoch. Spindle density was defined and measured as the number of the spindle events in 30-s epochs.
Analysis of Pre-Sleep Activation Patterns
Because individual cognitive strategies for sensorimotor processing may generally differ between individuals capable of extracting regularities,5,17,19 we characterized the presleep activation patterns in participants who became aware of the sequence after sleep to control for the contribution of such functional activations to postsleep awareness. It has been demonstrated that the amplitude of fast alpha (10–12 Hz) and beta (15–25 Hz) rhythms is modulated during sensorimotor stimulation, visual processing, and interactions between somatosensory and motor events at functionally relevant motor and sensory regions.34–36 We therefore evaluated α and β event-related synchronization/desynchronization (ERS/ERD) during SRTT performance to control for the presence of specific activation patterns in ExK participants.
ERS/ERD was evaluated for EEG recorded at the same 25 electrodes used for collecting sleep EEG data. To reflect the overall amount of functional activation, the first two blocks in the beginning of training (random and regular) and the last two blocks in the end of training (regular and random) were used, with the first 50 trials of block 2 and the last 50 trials of block 11 being included (Figure 1). Artifact free and EOG corrected event-related single EEG epochs of 1200 ms length were used. Mean number of trials in each of the four blocks was 44 (SD = 3.7). The spatial resolution of EEG was improved by applying current source density (CSD) to EEG.37 After CSD, high-frequency alpha (α2, 10–12 Hz) and beta (β, 15–25 Hz) ranges were analyzed to reflect the activation of task-specific neural circuits with circumscribed representation.35,36,38 ERS/ERD was computed on single-trial basis with reference to activity in a 200-ms period before stimulus in four time windows from stimulus onset to 800 ms afterward, each of 200-ms duration. Epochs within 200–400 and 400–600 ms were taken to reflect sensorimotor and motor activations39 at electrodes relevant to the visuo-motor SRTT processing.
Statistical Analysis of Sleep Spindles
The role of local spindle networks was targeted by using measures from 12 single electrodes forming two topography within-subject factors, Region and Laterality. Region had four levels for fast spindles (frontal, including F3, Fz, F4; fronto-central including FC3, FCz, FC4; central including C3, Cz, C4; and parietal including P3, Pz, P4) and three levels for slow spindles (frontal, fronto-central, and central). Laterality had three levels (left, including F3, FC3, C3, P3; midline, including Fz, FCz, Cz, Pz; and right, including F4, FC4, C4, P4, with parietal electrodes again not used for slow spindle analysis). Greenhouse-Geisser corrected p-values will be reported.
General Analysis
To apply a unitary design and enable the evaluation of the continuous individual prediction of presleep ImK and postsleep ExK, slow spindle rms amplitude during SWS (there were hardly any slow spindles during S2, as verified by spectral sleep EEG analysis, Figure 3) and fast spindle rms amplitude during SWS and S2 were subjected to repeated-measurement analysis of variance with covariates (ANCOVA). Side of learning was included as a between-subject factor with two levels (Side, left vs. right). ImK and ExK factors were included as co-variates, i.e., as continuous predictors of spindle activity, represented by individual ImK coefficients averaged across the second and third parts of training and individual ExK category from 1 to 5, respectively. Two overall ANCOVAs were run on the data, distinguishing between training effects and trait effects. In both cases, the design was Side × Region × Laterality with ImK and ExK as covariates. (A) Learning effects. Learning effects were evaluated using difference values obtained by subtracting values of the nonlearning night from values of the learning night. (B) Trait effects. Trait effects were evaluated using raw (rather than difference) data from both the nonlearning and learning nights, with the night variable, however, not included in the analysis. Reaction times and sleep stage parameters were also analyzed by using Side as a between-subject variable and ExK and ImK as covariates.
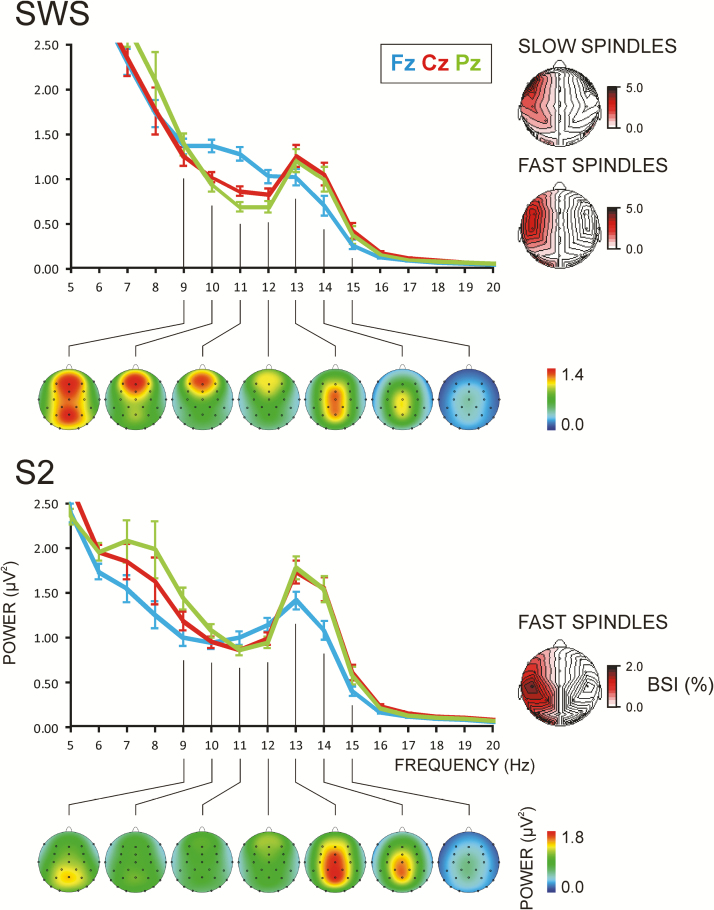
Figure 3.
Spectral analysis of sleep spindles. Mean spectral power for SWS and S2 of all participants (n = 53) at Fz, Cz, and Pz electrodes, presented together with the topographic maps of the respective spectral components from 9 to 15 Hz. Error bars indicate standard error of mean. These topographic patterns were statistically validated by Region (frontal, fronto-central, central, and parietal) × Laterality (left, middle, right) analysis of variance (ANOVA) of 9–12 Hz during SWS and 13–16 Hz activity during SWS and S2, with night (nonlearning and learning) included as an additional within-subjects variable. Slow spindle (9–12 Hz) activity was maximal at frontal regions [F(3/156) = 121.9, p < .001], as compared with any other region [F(1/52) > 69.2, p < .001], and at the midline [F(2/104) = 184.4, p < .001; midline vs. left-/ right-hemisphere electrodes, F(1/52) > 241.1, p < .001]. Fast spindle (13–16 Hz) activity in each of the SWS and S2 stages was maximal at midline centro-parietal regions [Laterality, F(2/104) > 240.8, p < .001; Region × Laterality, F(6/312) > 17.7, p < .001]. Topographic distribution of the BSI for SLOW and FAST SPINDLES is presented on the right. Increasing red scales for BSI indicate larger activity at the left vs. right hemisphere. Topography lines are repeated in the contralateral hemisphere without color.
Whenever significant interactions of ImK and ExK with topography factors (Region × Laterality) were obtained, these interactions were resolved in two ways. First, in order to explain such interactions by effects of the covariates at specific recording sites, effects of these covariates (ImK, ExK, and ImK × ExK) were tested in a multivariate regression model using MANCOVA, where ImK and/or ExK and/or their interactions were used as predictors and all recording sites formed the set of dependent variables, which yielded an F-value of the predictor effect separately for each recording site. When Side was involved in the overall interaction, these MANCOVAs were run separately for left-side and right-side participants. In separate analyses, the number of days between nonlearning and learning nights was included as a covariate to test whether it could have affected sleep quality or sleep spindle parameters. None of the analyses yielded significant effects of this covariate (p > .4) so that this factor was not further considered. Permutation statistics was not used in the present analysis because of the complexity of tested interactions.
Post Hoc Analyses
In order to explain such interactions by differences in topography due to ImK and/or ExK in an illustrative way, t-tests and, for illustration, statistical t-maps were performed between discrete levels of the continuous ImK and ExK variables. For these post hoc comparisons only, participants were grouped, as follows (the number of participants in each of these groups is compiled in Supplementary Table S2).
Implicit Knowledge Groups.
Participants were classified as having or not having gained implicit knowledge on single-trial basis. For each participant, Student’s t-test was applied across single-trial data in order to determine whether RTs were significantly longer in the last random block than in the preceding regular block (Figure 1). Depending on whether this difference was or was not significant (p < .05) at the end of presleep learning (part 3), the participant was classified as having (ImK+), or not having gained (ImK−) implicit sequence knowledge—Figure 2. Notably, this ImK+ and ImK− distinction reflects sensorimotor learning before sleep, independent of the expression of explicit knowledge after sleep. The distribution of participants into ImK groups (Supplementary Table S2A) was not affected by the side of training [χ2(1/53) = 0.8, p = .4].
Explicit Knowledge Groups.
Participants were classified following Nissen and Bullemer (1987) as belonging to groups of no explicit knowledge (ExK−, score 1), partial explicit knowledge (Partial ExK+, scores 2 and 3), and full explicit knowledge (Full ExK+, scores 4 and 5). According to this classification, only 11% of all participants acquired full ExK+, whereas ~57% remained unware of any task regularity. Distribution of ExK participants (Supplementary Table S2B) did not depend on the side of learning [χ2(2/53) = 0.01, p = .99].
Transition (ImK/ExK) Groups.
Transition groups were defined as combinations of presleep ImK with postsleep ExK. Partial and full ExK+ participants were considered together to reflect the expression of explicit knowledge after sleep, to be contrasted with the extreme absence of gain of ExK in the ExK− group. Thus, four transition categories were formed (Supplementary Table S2C). The distribution of participants into combinations of ImK−/ImK+ before sleep and ExK−/ExK+ after sleep was not affected by the side of training [χ2(3/53) = 1.13, p = .8].
Statistical Analysis of Pre-Sleep Activation Patterns During Task Performance
To control for specific presleep activations in individuals with postsleep ExK, electrodes at bilateral cortical regions with relevance to the visuo-motor SRTT were used: sensory (occipital and parieto-occipital), premotor, motor, and sensorimotor (fronto-central, central, and centro-parietal), and associative (frontal, dorsal parietal, and ventral parietal). Accordingly, the set of electrodes included O1/2 and PO7/8; FC3/4, C3/4, and CP5/6; and F3/4, P3/4, and P7/P8. In a repeated measures analysis of variance, there were two topographic within-subjects variables: Region (with eight levels) and Laterality (left vs. right). Side of presleep learning (Side, left vs. right) was the between-subjects variable. Individual ImK coefficients and ExK scores from 1 to 5 were included as independent covariates to control for the presence of specific ImK- and ExK-related region-specific activations. Greenhouse-Geisser corrected p-values will be reported for main and interactive effects of Region. Significant interactions with topography factors (Region × Laterality) were explored by testing covariate effects at specific electrodes using MANCOVA.
RESULTS
Performance
Participants’ performance was evaluated in the first random block of the learning session to test for the presence of individual differences in sensorimotor processing, and in the last random block to test for individual differences in procedural learning, which might have affected sleep EEG patterns (Figure 2). Main and interactive effects of ImK and ExK covariates were not significant [F(1/45) < 0.15, p > .7] in either block. As could be expected in our sample of right-handers, left-side performance was slower than right-side performance in the first block [F(1/45) = 6.9, p = .01], but this was not significant any more in the last block [F(1/45) = 2.6, p = .1]. These observations indicate that the gaining of presleep ImK or postsleep ExK was not associated with differences in individual sensorimotor speed, or in the speed of procedural learning. In contrast with these random blocks, faster RTs in regular blocks at the end of the learning session were, by definition, independently predicted by individual ImK gain [F(1/51) = 19.3, p < .001].
Sleep Stages
ANCOVAs with factor night did not yield significant main effects of Side or of the ExK and ImK covariates for any of the sleep stages or total sleep time [F(1/45) < 2.9, p > .1]. Total sleep time did not differ between nonlearning and learning nights [F(1/45) = 0.2, p > .6], but there was more SWS [F(1/45) = 12.2, p = .001] in the second, learning, than in the first, nonlearning, night and, correspondingly, less time spent in S2 [F(1/45) = 7.9, p = .007]. Also, sleep efficiency was higher in the learning than in the nonlearning night [F(1/49) = 11.5, p = .001]. However, these differences were not modulated by side of learning or knowledge factors as indexed by nonsignificant interactions of Side and of the ImK and ExK covariates with the night variable (p > .6). The distribution of sleep stages in the nonlearning and learning nights is presented in Supplementary Table S3.
Pre-Sleep Functional Activation Patterns
For each frequency band, α2, and β, functional activation indexed by ERD was revealed during sensorimotor SRTT processing. Activation by task-specific sensorimotor processing was overall larger at the left than the right hemisphere as reflected by lateral asymmetry for beta ERD [F(1/49) = 5.3, p = .02]. A pronounced functional asymmetry was observed due to a stronger activation at the hemisphere contra-lateral to the side of learning (Side × Laterality) both for α2 [F(1/49) = 14.3, p < .001] and β [F(1/49) = 12.2, p < .001; Supplementary Figure S1].
Notably, ERD in each frequency band also revealed that those individuals who would become aware of the regularity after sleep manifested differential functional activations already during presleep learning. Gain of awareness was associated with significantly greater functional activation at motor and sensory (occipital) regions and significantly less activation at frontal regions during task training [Region × ExK covariate for α2, F(7/343) = 4.2, p = .004; for β, F(7/343) = 8.7, p = .001], with these effects being most pronounced at the right-hemisphere F4, C4, P8, O2 electrodes and the left-hemisphere C3, CP5 electrodes [Laterality × Region × ExK covariate for α2, F(7/343) = 4.6, p = .004; for β, F(7/343) = 9.4, p = .001], where ExK was a significant continuous predictor [(F1/49) > 4.7, p < .05].
Spectral Sleep EEG Analysis
Slow and fast spindles were first verified by topographic analysis of spectral EEG activity during SWS and stage 2 (S2) of sleep.20Figure 3 shows spectral components at 9–12 Hz with clear frontal distribution during SWS only and spectral components at 13–16 Hz with centro-parietal distribution during both SWS and S2. These frequency-specific topographic patterns of spectral activity were statistically validated by Region (frontal, fronto-central, central, and parietal) × Laterality (left, middle, right) analyses of variance (p< .001, Figure 3). Also, they correspond to individual frequency peaks of slow and fast spindle spectral activity (Supplementary Table S1). Notably, both slow and fast spindle activities were larger over the left than the right hemisphere [F(1/52) > 14.0, p < .001], as further indicated by Brain Symmetry Index (BSI)63 maps in Figure 3, with BSI = (Left − Right)/(Left + Right).
Fast Spindles
Tables 1A and B present statistical results of the learning-related modulations of fast-spindle rms amplitudes. As the first column of Table 1 shows, independently of ExK and ImK neither in SWS nor in S2 were these spindle amplitudes affected by the side of learning, either as a main effect (p > .2) or in a topographically specific manner, in particular, there was no general difference between areas contralateral and ipsilateral to the side of learning (interactions of Side with Laterality and Region, p > .3). However, there were interactive effects of ExK and ImK variables with the topography factors (0.0001< p < .01, rows Region, Laterality, and Region × Laterality in Table 1A and B). Notably, these effects also depended on the side of learning (interactions with Side, 0.0001< p < .005, columns including Side in Table 1A and B). To explore these region-specific complex interactions in each sleep stage and for each side of learning, (1) independent predictions of ExK and ImK covariates and their interaction were tested at specific locations using MANCOVA, and (2) post hoc between-group analyses were conducted at specific locations using Student’s t-test.
Table 1.
Statistical Results From Overall ANCOVAs on Differences Between Learning and Non-Learning Night, Separately for Slow (SS) and Fast (FS) rms Spindle Amplitudes During SWS and S2.
| Df | Side (S) F (p) | ExK F (p) | ImK F (p) | S × ExK F (p) | S × ImK F (p) | ExK × ImK F (p) | S × ExK × ImK F (p) | |
|---|---|---|---|---|---|---|---|---|
| (A) SWS-FS rms amplitude | ||||||||
| Main effect | 1,45 | – | – | – | – | – | – | – |
| Region | 3,135 | – | – | 4.4 (.01) | – | – | 5.4 (.005) | – |
| Laterality | 2,90 | – | – | – | – | – | – | – |
| Reg × Lat | 6,270 | – | 10.4 (.001) | 6.1 (.001) | 3.7 (.004) | 3.1 (.01) | 8.3 (.001) | 4.0 (.002) |
| (B) S2-FS rms amplitude | ||||||||
| Main effect | 1,45 | – | – | – | – | – | 4.9 (.03) | – |
| Region | 3,135 | – | 3.9 (.02) | 3.7 (.03) | 4.4 (.01) | – | 4.6 (.01) | 5.0 (.008) |
| Laterality | 2,90 | – | – | – | 3.3 (.05) | 3.5 (.04) | – | 3.8 (.03) |
| Reg × Lat | 6,270 | – | 4.6 (.001) | 7.1 (.001) | 4.2 (.001) | – | 2.2 (.001) | 4.6 (.001) |
| (C) SWS-SS rms amplitude | ||||||||
| Main effect | 1,45 | – | – | – | – | – | – | – |
| Region | 2,90 | – | – | – | 5.6 (.006) | – | – | – |
| Laterality | 2,90 | – | – | – | – | – | – | – |
| Reg × Lat | 4,180 | – | 3.8 (.01) | – | – | – | – | – |
The trivial main effects of Region and Laterality, and their interaction are not included in the table.
Df = degree of freedom; F = F-value; p = p-value; ExK = explicit knowledge after sleep; ImK = implicit knowledge before sleep; rms = root-mean square; Reg × Lat = Region × Laterality; S = Side.
In the MANCOVA, ExK was a predictor of enhanced fast spindle amplitudes at C4 during both SWS and S2 [4.4 ≤ F(1/52) ≤ 21.2, 0.001 ≤ p ≤ .04] and at FC3 during S2 [F(1/52) = 7.9, p = .007]. Figure 4 demonstrates the consistent increase in fast spindle amplitudes at right central locations after presleep learning in participants who gained ExK after sleep (ExK+) relative to those who did not (ExK−), both in ImK+ and ImK− groups. Figure 5 further shows that for the left-side group, a powerful ExK prediction was obtained at the right central location C4 in both SWS and S2 [8.1 ≤ F(1/27) ≤ 13.4, 0.001 ≤ p ≤ .009], whereas for the right-side group, independent ExK prediction was significant over the left hemisphere at fronto-central and central sites in SWS and S2 [5.0 ≤ F(1/24) ≤ 6.4, 0.02 ≤ p ≤ .03], with C4 being also involved during SWS [F(1/24) = 18.3, p < .001]. These observations indicate an association between the increase in postlearning fast spindle amplitudes at fronto-central areas contralateral to the side of learning during both SWS and S2, and the amount of knowledge about the stimulus sequence. Additionally, they point to an association between this knowledge and postlearning fast spindles at the right central area during SWS.
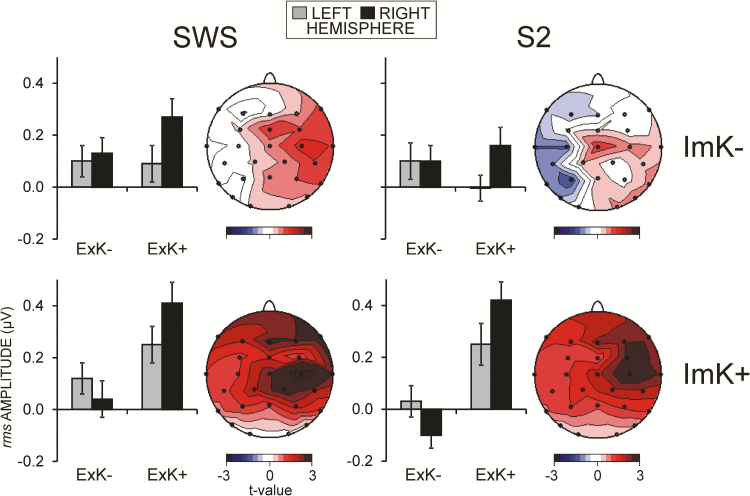
Figure 4.
Effects of explicit knowledge (ExK) after sleep on learning-dependent changes of fast spindle rms amplitudes during SWS and S2, for participants with and without ImK before sleep. For illustration, displayed are group means ± standard errors of subtracted (learning night – nonlearning night) values for left and right hemisphere electrodes and statistical t-maps for the comparison between ExK+ and ExK− for the respective levels of ImK (ImK+ and ImK−). ImK−, participants who did not acquire ImK before sleep; ImK+, participants who acquired ImK before sleep; ExK−, participants with no ExK after sleep; ExK+, participants who gained ExK after sleep. Red color indicates ExK+ > ExK−, whereas blue color indicates the opposite. Note that on the statistical t-maps, maximal t-values (±3) correspond to p = 0.005.
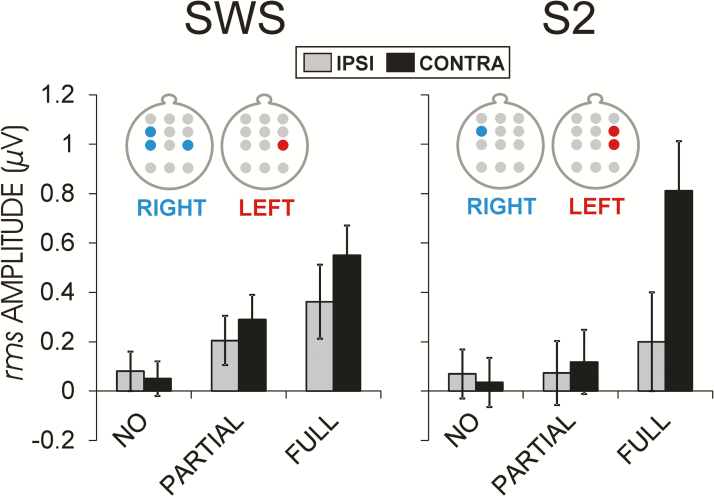
Figure 5.
Predictive effects of ExK after sleep on learning-dependent changes of fast spindle rms amplitudes during SWS and S2. Displayed are group means ± standard errors of subtracted mean values (learning night − nonlearning night) at motor cortical regions (C3, C4) ipsi- and contralateral to the side of presleep training for three groups of participants: without ExK (NO), with partial ExK (PARTIAL), and with full ExK (FULL). On the heads above, the electrode sites are shown at which the independent prediction of ExK covariate was significant, separately for right-side (RIGHT, blue) and left-side (LEFT, red) learning participants. Group values (bars) are for illustration. Independent predictive ExK effects are presented on the maps demonstrating that for the left-side group, a significant independent ExK prediction was obtained at C4 in both SWS and S2, whereas for the right-side group, independent ExK prediction was significant at FC3 and C3 during SWS and S2, and at C4 during SWS (statistical F and p values are given in the text).
In contrast with ExK, the amount of ImK before sleep was not an independent predictor of learning-dependent modulations of fast spindle amplitudes in either SWS or S2 MANCOVAs (p > .4). However, only when variations due to ImK were accounted for (when ImK was included as a simultaneous predictor in the MANCOVAs), did the ExK × ImK interaction affect right-hemisphere fast spindles. In these MANCOVAs, ExK × ImK predicted training-dependent increase in fast spindle amplitudes at F4, FC4, C4 during both SWS and S2 [5.0 ≤ F(1/52) ≤ 6.4, 0.03 ≤ p ≤ .01]. This effect was particularly large during S2 when learning had been contralateral to these right fronto-central areas, i.e., on the left side (Region × Laterality × Side × ExK × ImK, Table 1). The between-group post hoc comparisons confirmed these observations. Statistical t-maps in Figure 4 demonstrate that during both SWS and S2, a significant (p < .005) right-hemisphere enhancement of fast spindles after training was produced by the ImK+/ExK+ combination. This indicates that the increase fast spindles at fronto-central regions contralateral to the side of learning in participants who would express explicit knowledge of the sequence was significantly more pronounced at the right hemisphere if implicit knowledge was acquired before sleep.
Using values from the two nights to explore individual traits yielded nonsignificant main or interactive effects of ImK and ExK covariates (p > .1), revealing that fast spindle amplitudes during SWS and S2 were not associated with individual differences in the ability to learn SRTT implicitly before sleep or explicitly after sleep.
When these analyses of fast-spindle rms amplitudes were conducted on fast-spindle density, as a complementary measure of fast-spindle activity, there was likewise a significant enhancement after learning during S2 in association with the amount of explicit knowledge. Consistent with spindle rms amplitude findings, this enhancement was localized at right central (C4) and midline electrodes [Laterality × Side × ExK, F(6/270) = 2.4, p = .04; ExK effect at C4/Cz, 4.1 ≤ F(1/52) ≤ 6.5, 0.05 ≤ p ≤ .01]. Like with rms amplitudes, no trait effects were found for fast spindle density.
Slow Spindles in SWS
Although slow spindle amplitudes increased after learning at right central regions in association with the amount of explicit knowledge gain, most prominently after learning on the right side (Table 1C), these effects were not supported statistically by MANCOVA where independent ExK prediction did not reach significance at specific electrodes. Hence, slow spindles were not modulated reliably by presleep learning in association with knowledge gain or transition.
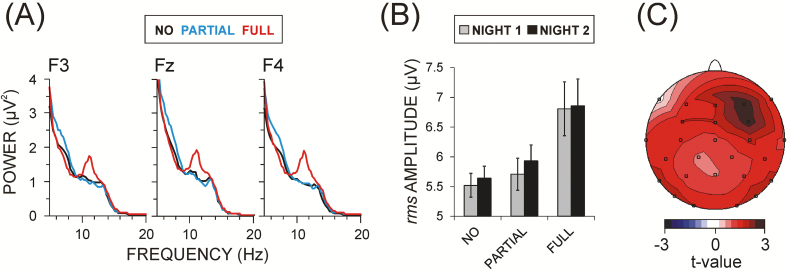
In contrast, slow spindle activity was related in a trait-dependent way to the individual amount of ExK. This association was reflected by a significant Region × Laterality × ExK interaction [F(4/196) = 2.8, p < .05]. MANCOVAs used to test the ExK factor at each electrode in either night confirmed that the ExK effect was significant at FC4 and Cz locations already in the nonlearning night [F(1/52) > 3.9, p < .05], reflecting increased slow spindle amplitudes at right-hemisphere regions as a function of individual capacity to gain explicit knowledge. The between-group post hoc analyses confirmed this observation. Figure 6A demonstrates that slow spindle spectral power was prominently larger in participants who would later acquire full explicit knowledge relative to participants with partial or no knowledge. Consistent with this, Figure 6B shows that slow spindle amplitude increase depended on the amount of later explicit knowledge. The statistical t-map in Figure 6C demonstrates that slow spindle amplitudes were specifically enhanced in participants with full relative to no explicit gain at right frontal-central regions. No significant training or trait effects were found for the density of slow spindles.
Figure 6.
Trait effects related to the amount of ExK gained after sleep. (A) Grand average spectral power at frontal electrodes (F3, Fz, F4) for three groups of participants: without ExK (NO), with partial ExK (PARTIAL), and with full ExK (FULL) for the two nights pooled together. (B) Group means ± standard error of the root mean square amplitude of slow spindles averaged across electrodes (frontal, fronto-central, central at left, midline and right locations) for the nonlearning (NIGHT 1) and learning (NIGHT 2) nights for the same three groups as in (A). (C) Statistical t-map of the comparison of slow spindle rms amplitude between the groups FULL and NO. Red color indicates FULL > NO. Maximal t-values (±3) correspond to p = .005. Group values (bars) are for illustration; statistical effects are presented on the maps.
DISCUSSION
During both SWS and S2, we have the following: (1) fast spindle activity at fronto-central areas contralateral to the side of presleep learning increased with the amount of explicit sequence knowledge; (2) fast spindle activity at right-hemisphere fronto-central regions increased when there was additionally implicit sequence knowledge before sleep manifested in RT speeding; and (3) slow spindle activity at right frontal sites during SWS measured before any learning was larger in participants with later ExK, reflecting some individual predisposition for extracting rules from sensorimotor sequences. These results provide evidence that spindle-related offline mechanisms are related to awareness of previously experienced regularities in several ways: (1) by engaging task-specific, possibly motor, representations activated before sleep, (2) by additionally engaging motor-unspecific areas in relation to the amount of implicit sequence knowledge gained before sleep, possibly related to the transformation of that implicit knowledge to explicit awareness, and (3) by affecting individual traits through offline neuroplasticity. They also reveal that each of these mechanisms recruits unique local spindle activities, with a special role for the right hemisphere.
In our study, learning effects were evaluated by comparing measures from the critical learning night preceded by presleep SRTT practice and the nonlearning night not preceded by learning. Variations in sleep patterns may emerge on the first relative to subsequent nights of PSG sleep registration,40,41 including a reduction of total sleep time, less sleep efficiency, delayed sleep onset and REM sleep latency, and reduced amounts of SWS and REM sleep. Indeed, at least reduced proportions of SWS sleep were evident in our participants’ nonlearning nights. Therefore, the question must be asked whether parts of our results were affected by this unspecific difference between learning and nonlearning nights, the latter represented by the adaptation night. The results specific to the hemisphere contralateral to SRTT performance are most probably independent of the reduced SWS sleep in the adaptation night, because these reductions cannot have been related to the side of following SRTT performance in any way, and indeed, side of learning did not interact with the night variable in either SWS or S2 sleep analyses. Likewise, neither ImK nor ExK, nor their interaction, covaried with the night variable distinguishing the amounts of SWS and S2 stages of sleep in the nonlearning (adaptation) and learning nights. Also, our measure of fast-spindle amplitude was computed here as an average value for each stage, counteracting the effects of different amounts of sleep stages. Thus, the possibility that the smaller amount of SWS in the nonlearning night was already related to later explicit knowledge in such a way that it may account for the relationship of fast right-hemispheric spindle activity to explicit knowledge does not sound very plausible. We have to concede that, of course, the less disputable method would have been to have participants sleep a second night in the lab, after adaptation, and then use this night as a control night for the learning night.
We had expected that sensorimotor engrams in the present lateralized SRTT task would be encoded in the hemisphere contralateral to the side of learning. However, the lack of both global and topography-specific modulations by the side of presleep learning alone (irrespective of sequence knowledge) suggests that sleep spindles may not be directly involved in offline re-processing of task-specific information encoded before sleep in the contralateral hemisphere. Nor do spindles appear to be directly engaged in re-processing of the implicit sequence representations as implied by the lack of independent predictions of implicit sequence gain before sleep.
Notably, however, learning-dependent modulations of fast spindles did exist in association with the gain of explicit sequence knowledge such that the enhancement of fast spindle activity at fronto-central areas contralateral to the side of presleep learning covaried with expression of awareness of the sequence. A plausible interpretation of this localization and its contralateral sensitivity is that this spindle activity is generated by the hand-motor cortex of the hemisphere that was in charge of the responding hand in the training session. In addition to these contralaterally organized spindle activities, we obtained evidence from both density and amplitude analyses for specific roles of right-hemisphere fronto-central spindles. This fits results of previous fMRI studies where learning of several SRTT variants was found to be associated with increased activation of the right premotor cortex irrespective of the side of learning as compared with simple sensorimotor tasks (for review, see Ref. 42), during which contralateral motor networks are engaged.43 The right premotor cortex has been found to be consistently more activated during advanced stages of SRTT practice due to learning of perceptual rather than motor aspects of the task, and storage of sequences.44–46 One interpretation of the present results is that the reactivation of task-specific and sequence representations by local fast spindles might be an endogenous offline promoter of consciously noticing regularities in one’s previous experiences, suggesting a pro-active role of spindles for subsequent rule extraction.
However, analysis of presleep activation patterns during task performance points to an alternative explanation accounting for different processing already at presleep learning in individuals who would subsequently become aware of the regularity. Previously, such individuals have repeatedly manifested behavioral and neurophysiological signs of increased executive control processing from the very beginning of task practice, both in the Number Reduction Task17,19,47 and in the present SRTT condition.5,16 Control analyses (Supplementary Data 2.2 and 2.3) suggest that the relationships between sleep spindle parameters and postsleep ExK reported in the present study are not likely to reflect a presleep change in the mode of processing from implicit to explicit, although intrusion of explicit chunks and related psychological events may not be fully excluded.48 Critically, however, presleep activation during task performance was stronger as a function of explicit knowledge, both at motor regions contra-lateral to the side of learning and at right-hemisphere fronto-central areas. Hence, contralateral postlearning enhancement of fast spindles may reflect a stronger modulation of motor activity by executive control networks during encoding. Likewise, the right-lateralized fast spindle network may reflect an extended recruitment of explicit attentional systems,49,50 especially in the right hemisphere in relation to the load of working memory51 and to increased control of attention shifts,50 or stronger interactions between explicit and implicit processing systems during task encoding16,42 in those individuals who would express the regularity after sleep. Thus, awareness-related fast spindle activity appears as an offline marker of a different mode of presleep functional activations, pointing to a retroactive function of sleep spindles. Region-specific local fast spindles may therefore act to translate task-specific activation patterns from one wake state to another, providing for an increased access by explicit processing systems in the subsequent wake state.
It has been recognized that insightful behaviors are individually specific, with about 15–20% of participants having the cognitive style of abstraction and insightful solutions.3,10,52 Comparisons of the rate of explicit-knowledge gain in a control wake group (Supplementary Data 2.1) demonstrated comparable rates of explicit knowledge generation after wake, confirming a primary role of individual characteristics for insightful abilities. In the present study, slow (but not fast) spindle activity during SWS was enhanced over the right frontal cortex already before training in individuals who would gain explicit knowledge after sleep. Notably, additional analysis of slow-wave activity during S2 and SWS revealed that individual abilities of regularity extraction were also supported by larger slow-wave power at posterior (parietal and parieto-occipital) regions (Supplementary Data 4). Thus, the individual ability to get conscious awareness of implicitly acquired information had notable offline correlates, in accordance with previously found associations between sleep spindles and individual cognitive capacity, intelligence and cognitive development20,22,28,53 reflecting important individual differences in cognitive style of abstraction and neuroplasticity.
This right-hemisphere slow-spindle individual marker of sequence awareness contrasts with the left-hemisphere dominance in absolute size of both slow and fast sleep spindles observed here during the two recording nights (Figure 3). Such left-hemisphere dominance of spindle activity has been described independently of presleep learning54,55 and also following presleep learning,9,24 although in the latter studies, effects of presleep learning of the left hemisphere have not been distinguished from learning-independent effects. Here, α2/β desynchronization indicated that independently of the side of learning, the left hemisphere was overall more activated than the right hemisphere. Hence, left-hemisphere dominance of spindle activity may reflect a leading role of left-hemisphere activations in sensorimotor tasks42 as well as neuroplasticity of networks for offline processing of sensorimotor memories following right handedness in the majority of participants. In particular, left parietal regions have been linked to storage of encoded sensorimotor associations, and the left dorsal premotor cortex has been suggested to play a dominant role for movement selection.56–58 On these grounds, the observations of the right frontal enhancement of slow spindle activity in individuals with insightful abilities are even more conspicuous.
The present results reveal a special role of the right hemisphere for sequence awareness by providing original evidence for distinct types of spindle activations. As described above, the first activity was learning-dependent, was mediated by fast spindles during both SWS and S2, was focused to the right fronto-central region following greater presleep activations during task performance, and was specifically associated with the combination of implicit and explicit knowledge, hence possibly with implicit-to-explicit knowledge transition. The second type of spindle activity was trait-dependent, was mediated by slow spindles during SWS, was focused to the right frontal region, and signified individual capacity of getting conscious access. Considering the features and close localizations of these two clusters in the right hemisphere, inter-frequency spindle dynamics59,60 can be suggested to underlie neural plasticity, which may subsequently shape individual conscious processing of experiences and/or insightfulness52 through right-hemisphere activations during both sleep (current results, see also Ref. 9) and wake.18,47,61,62 With this account, SWS appears as a critical sleep stage which supports the offline translation of newly encoded to existing memories, and the spatially focused interactions of spindle variants appear as a key mechanism of offline neuroplasticity.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
ADDRESS WHERE WORK WAS CONDUCTED
Department of Neurology, University of Lübeck, Lübeck, Germany.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funding granted to RV by the Deutsche Forschungsgemeinschaft within the Collaborative Research Center “Plasticity and Sleep,” SFB 654, project A02. We thank Annemarie Seitz, Petra Diercks, and Jens Rodeck for their help with the EEG recording.
REFERENCES
- 1. Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010; 11(2): 114–126. [DOI] [PubMed] [Google Scholar]
- 2. Landmann N, Kuhn M, Piosczyk H et al. The reorganisation of memory during sleep. Sleep Med Rev. 2014; 18(6): 531–541. [DOI] [PubMed] [Google Scholar]
- 3. Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004; 427(6972): 352–355. [DOI] [PubMed] [Google Scholar]
- 4. Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci. 2011; 15(8): 343–351. [DOI] [PubMed] [Google Scholar]
- 5. Verleger R, Seitz A, Yordanova J, Kolev V. Is insight a godsend? Explicit knowledge in the serial response-time task has precursors in EEG potentials already at task onset. Neurobiol Learn Mem. 2015; 125: 24–35. [DOI] [PubMed] [Google Scholar]
- 6. Fabiani M, Ho J, Stinard A, Grattona G. Multiple visual memory phenomena in a memory search task. Psychophysiology. 2003; 40(3): 472–485. [DOI] [PubMed] [Google Scholar]
- 7. Fischer S, Drosopoulos S, Tsen J, Born J. Implicit learning – explicit knowing: a role for sleep in memory system interaction. J Cogn Neurosci. 2006; 18(3): 311–319. [PubMed] [Google Scholar]
- 8. Yordanova J, Kolev V, Verleger R, Bataghva Z, Born J, Wagner U. Shifting from implicit to explicit knowledge: different roles of early- and late-night sleep. Learn Mem. 2008; 15(7): 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yordanova J, Kolev V, Wagner U, Born J, Verleger R. Increased alpha (8-12 Hz) activity during slow wave sleep as a marker for the transition from implicit knowledge to explicit insight. J Cogn Neurosci. 2012; 24(1): 119–132. [DOI] [PubMed] [Google Scholar]
- 10. Verleger R, Rose M, Wagner U, Yordanova J, Kolev V. Insights into sleep’s role for insight: studies with the number reduction task. Adv Cogn Psychol. 2013; 9(4): 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Sharman A, Siengsukon CF. Time rather than sleep appears to enhance off-line learning and transfer of learning of an implicit continuous task. Nat Sci Sleep. 2014; 6: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meier B, Cock J. Offline consolidation in implicit sequence learning. Cortex. 2014; 57: 156–166. [DOI] [PubMed] [Google Scholar]
- 13. Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol. 1987; 19(1): 1–32. [Google Scholar]
- 14. Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004; 430(6995): 78–81. [DOI] [PubMed] [Google Scholar]
- 15. Huber R, Ghilardi MF, Massimini M et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006; 9(9): 1169–1176. [DOI] [PubMed] [Google Scholar]
- 16. Yordanova J, Kirov R, Kolev V. Increased performance variability as a marker of implicit/explicit interactions in knowledge awareness. Front Psychol. 2015; 6: 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lang S, Kanngieser N, Jaśkowski P, Haider H, Rose M, Verleger R. Precursors of insight in event-related brain potentials. J Cogn Neurosci. 2006; 18(12): 2152–2166. [DOI] [PubMed] [Google Scholar]
- 18. Yordanova J, Kolev V, Wagner U, Verleger R. Covert reorganization of implicit task representations by slow wave sleep. PLoS One. 2009; 4(5): e5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darsaud A, Wagner U, Balteau E et al. Neural precursors of delayed insight. J Cogn Neurosci. 2011; 23(8): 1900–1910. [DOI] [PubMed] [Google Scholar]
- 20. De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003; 7(5): 423–440. [DOI] [PubMed] [Google Scholar]
- 21. Schabus M, Gruber G, Parapatics S et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004; 27(8): 1479–1485. [DOI] [PubMed] [Google Scholar]
- 22. Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011; 35(5): 1154–1165. [DOI] [PubMed] [Google Scholar]
- 23. Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002; 22(15): 6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13–15 Hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008; 31(2): 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007; 2(4): e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010; 30(43): 14356–14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cox R, Hofman WF, Talamini LM. Involvement of spindles in memory consolidation is slow wave sleep-specific. Learn Mem. 2012; 19(7): 264–267. [DOI] [PubMed] [Google Scholar]
- 28. Schabus M, Hödlmoser K, Gruber G et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006; 23(7): 1738–1746. [DOI] [PubMed] [Google Scholar]
- 29. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971; 9(1): 97–113. [DOI] [PubMed] [Google Scholar]
- 30. Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Offline learning of motor skill memory: a double dissociation of goal and movement. Proc Natl Acad Sci USA. 2005; 102(50): 18237–18241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Willingham DB, Nissen MJ, Bullemer P. On the development of procedural knowledge. J Exp Psychol Learn Mem Cogn. 1989; 15(6): 1047–1060. [DOI] [PubMed] [Google Scholar]
- 32. Rechtschaffen A, Kales AA.. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. NIH Publ. No. 204. Baltimore, MD: U.S. Government Printing Office; 1968. [Google Scholar]
- 33. Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006; 444(7119): 610–613. [DOI] [PubMed] [Google Scholar]
- 34. Pfurtscheller G, Klimesch W. Functional topography during a visuoverbal judgment task studied with event-related desynchronization mapping. J Clin Neurophysiol. 1992; 9(1): 120–131. [DOI] [PubMed] [Google Scholar]
- 35. Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999; 110(11): 1842–1857. [DOI] [PubMed] [Google Scholar]
- 36. Babiloni C, Del Percio C, Vecchio F et al. Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clin Neurophysiol. 2016; 127(1): 641–654. [DOI] [PubMed] [Google Scholar]
- 37. Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989; 72(2): 184–187. [DOI] [PubMed] [Google Scholar]
- 38. Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999; 29(2–3): 169–195. [DOI] [PubMed] [Google Scholar]
- 39. Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004; 22(2): 590–602. [DOI] [PubMed] [Google Scholar]
- 40. Agnew HW Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966; 2(3): 263–266. [DOI] [PubMed] [Google Scholar]
- 41. Browman CP, Cartwright RD. The first-night effect on sleep and dreams. Biol Psychiatry. 1980; 15(5): 809–812. [PubMed] [Google Scholar]
- 42. Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013; 67: 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010; 14(6): 277–290. [DOI] [PubMed] [Google Scholar]
- 44. Schubotz RI, von Cramon DY. Predicting perceptual events activates corresponding motor schemes in lateral premotor cortex: an fMRI study. Neuroimage. 2002; 15(4): 787–796. [DOI] [PubMed] [Google Scholar]
- 45. Schubotz RI, von Cramon DY. A blueprint for target motion: fMRI reveals perceived sequential complexity to modulate premotor cortex. Neuroimage. 2002; 16(4): 920–935. [DOI] [PubMed] [Google Scholar]
- 46. Schubotz RI, von Cramon DY. Functional-anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. Neuroimage. 2003; 20 (Suppl 1): S120–S131. [DOI] [PubMed] [Google Scholar]
- 47. Yordanova J, Kolev V, Wagner U, Verleger R. Differential associations of early- and late-night sleep with functional brain states promoting insight to abstract task regularity. PLoS One. 2010; 5(2): e9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frensch PA, Haider H, Rünger D, Neugebauer U, Voigt S, Werg J. Verbal report of incidentally experienced environmental regularity: the route from implicit learning to verbal expression of what has been learned. In: Jiménez L, ed. Attention and Implicit Learning. New York, NY: John Benjamins; 2002: 335–366. [Google Scholar]
- 49. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002; 3(3): 201–215. [DOI] [PubMed] [Google Scholar]
- 50. Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011; 34: 569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998; 55(4): 343–361. [DOI] [PubMed] [Google Scholar]
- 52. Haider H, Rose M. How to investigate insight: a proposal. Methods. 2007; 42(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 53. Bódizs R, Kis T, Lázár AS et al. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res. 2005; 14(3): 285–292. [DOI] [PubMed] [Google Scholar]
- 54. Roth C, Achermann P, Borbély AA. Frequency and state specific hemispheric asymmetries in the human sleep EEG. Neurosci Lett. 1999; 271(3): 139–142. [DOI] [PubMed] [Google Scholar]
- 55. Cajochen C, Di Biase R, Imai M. Interhemispheric EEG asymmetries during unilateral bright-light exposure and subsequent sleep in humans. Am J Physiol Regul Integr Comp Physiol. 2008; 294(3): R1053–R1060. [DOI] [PubMed] [Google Scholar]
- 56. Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. NeuroImage. 2003; 20 (Suppl. 1): S89–S100. [DOI] [PubMed] [Google Scholar]
- 57. Rushworth MF, Nixon PD, Wade DT, Renowden S, Passingham RE. The left hemisphere and the selection of learned actions. Neuropsychologia. 1998; 36(1): 11–24. [DOI] [PubMed] [Google Scholar]
- 58. O’Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007; 54(3): 479–490. [DOI] [PubMed] [Google Scholar]
- 59. Nir Y, Staba RJ, Andrillon T et al. Regional slow waves and spindles in human sleep. Neuron. 2011; 70(1): 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Andrillon T, Nir Y, Staba RJ et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011; 31(49): 17821–17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bowden EM, Jung-Beeman M. Aha! Insight experience correlates with solution activation in the right hemisphere. Psychon Bull Rev. 2003; 10(3): 730–737. [DOI] [PubMed] [Google Scholar]
- 62. Kounios J, Fleck JI, Green DL et al. The origins of insight in resting-state brain activity. Neuropsychologia. 2008; 46(1): 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van Putten MJ. The revised brain symmetry index. Clin Neurophysiol. 2007; 118(11): 2362–2367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.