Abstract
Study Objectives:
Both restless legs syndrome (RLS) and periodic leg movements in sleep (PLMS) may be associated with incident cardiovascular disease (CVD). However, the individual contributions of these factors to adverse CVD outcomes are unknown.
Methods:
During the MrOS Sleep Study, 2823 men (mean age = 76.3 years) participated in a comprehensive sleep assessment from 2000 to 2002. RLS was identified by self-report of a physician diagnosis of RLS. A periodic limb movement of sleep index (PLMI) was derived from unattended in-home polysomnography. Incident cardiovascular events were centrally adjudicated during 8.7 ± 2.6 years of follow-up. The primary outcome was all-cause CVD; secondary outcomes included incident myocardial infarction (MI) and cerebrovascular disease. Cox proportional hazards regression models were adjusted for multiple covariates, including PLMI, to examine if there were independent associations of RLS and PLMI to the outcomes.
Results:
Physician-diagnosed RLS was reported by 2.2% and a PLMI ≥ 15 was found in 59.6% of men. RLS was not associated with the composite CVD outcome. RLS was significantly associated with incident MI (Hazard ratio [HR] = 2.02, 95% CI, 1.04–3.91) even after adjustment for multiple covariates. Results were only modestly attenuated when PLMI was added to the model. PLMI also was found to predict incident MI (per SD increase in PLMI, HR = 1.14, 95% CI, 1.00–1.30, p = .05), and was materially unchanged after addition of RLS.
Conclusions:
The independent risk that RLS confers for MI suggests a role for non-PLMS factors such as sleep disturbance, shared genetic factors, or PLM-independent sympathetic hyperactivity.
Keywords: restless legs syndrome, periodic limb movements, coronary artery disease, insomnia.
Statement of Significance
The primary proposed mechanism for a causal association between restless legs syndrome and incident cardiovascular disease is the repetitive autonomic arousal associated with periodic limb movements of sleep. Our data confirm that RLS is a risk factor for incident myocardial infarction events and that periodic limb movements of sleep may not be solely responsible for such an association. Other potential mechanisms include shared genetic factors, PLM-independent sympathetic nervous system hyperactivity, metabolic mechanisms related to sleep disturbance, inflammatory mechanisms, impaired vascular function associated with nitric oxide abnormalities or sleep disturbance or central or peripheral iron deficiency.
INTRODUCTION
Evidence from both cross-sectional1–3 and longitudinal4,5 studies suggests that restless legs syndrome (RLS) is associated with hypertension and cardiovascular disease (CVD). Other studies have not substantiated these findings, and whether there is a causal association, and the direction of causation, remains controversial. Mechanisms for this potential association include common genetic risk, obesity, sleep disruption, and periodic limb movements of sleep (PLMS). PLMS are present in the majority of those with RLS6 but are also common in the general population.7 PLMS are associated with dramatic increases in heart rate8 and blood pressure9 and are often associated with sleep disruption. PLMS have also been shown to independently contribute to CVD and mortality outside of the RLS population.10–12
The individual contributions of RLS and PLMS to adverse cardiovascular outcomes are unknown. Complicating efforts to distinguish these two contributions to cardiovascular outcomes in RLS is the fact that prospective CVD outcome studies require large populations followed over extended periods of time, and that RLS diagnoses and objective recording of PLMS are often not obtained in such studies. The Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep Study)13,14 provides a unique opportunity to study the association of RLS status and PLMS with incident CVD in a large cohort of community-dwelling older men who are well-characterized for sleep exposures and potentially important covariates, allowing for examination of possible mechanisms (sleep disruption, PLMS, hypertension, inflammation, and medication use) that could explain this association. We hypothesized that RLS diagnosis and PLMS each would predict CVD events, and associations would persist after adjusting each condition for the alternative.
METHODS
Study Sample
During the Osteoporotic Fractures in Men Study (MrOS) baseline examination from 2000 to 2002, 5994 community-dwelling men 65 years or older were enrolled at six clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California.13,14 In order to participate, men needed to be able to walk without assistance and must not have had a bilateral hip replacement.
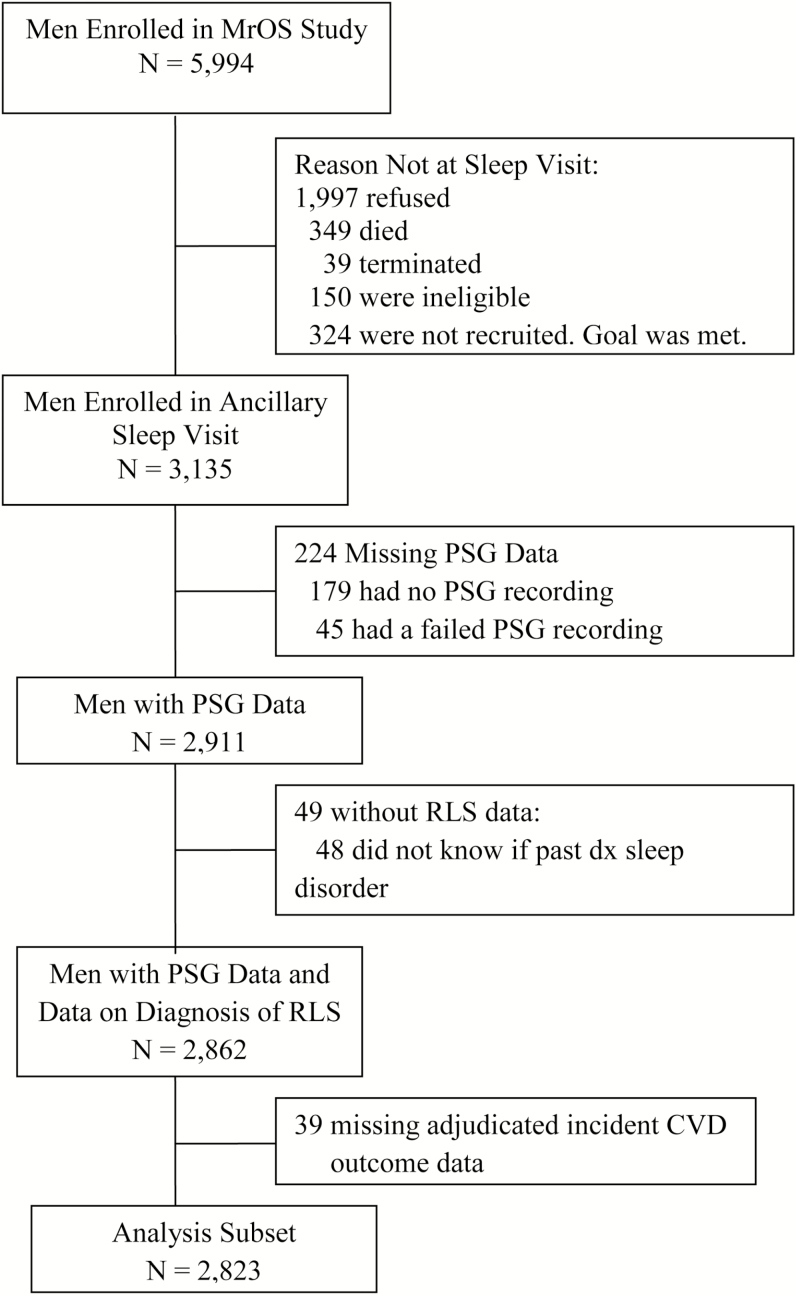
The MrOS Sleep Study, an ancillary study of the parent MrOS cohort, recruited 3135 participants for a comprehensive sleep assessment. Men were screened for nightly use of mechanical devices during sleep including pressure mask for sleep apnea (continuous positive airway pressure or bi-level positive airway pressure), oral appliances, or nocturnal oxygen therapy and were excluded if they could not forgo use of these devices during a polysomnography (PSG) recording. Of the 2859 men who did not participate in this ancillary study, 349 died before the sleep visit, 39 had already terminated the study, 324 were not asked because recruitment goals had already been met, 150 were ineligible, and 1997 refused. Of the 3135 men in the study, 2956 underwent PSG, with 2911 having usable PSG data. Of these 2911 men, 2862 also had self-reported RLS data. Of these 2862, 39 men were missing data on incident CVD, leaving 2823 men in this analytic cohort (Figure 1). Compared to this analysis subset, those 312 men who were not included in the analysis were older by an average of 1.2 years, were more likely to be a minority (18.9% vs. 9.2%), were more likely to have depression (13.3% vs. 6.0%), and more likely to be taking antihypertensive medication (64.3% vs. 58.3%) (p < .05). The two groups had similar levels of body mass index (BMI), rates of comorbid conditions, and similar lifestyle factors.
Figure 1.
Flowchart of participants.
All men provided written informed consent, and the study was approved by the Institutional Review Board at each site.
RLS Symptoms
RLS was identified from responses to two questions asked as part of MrOS sleep study. The RLS identifier was worded as “Has a doctor or health care provider ever told you that you have a sleep disorder other than sleep apnea?”. If the participant answer yes, they were asked a further question, “what other sleep disorder?”. Among the five choices was “restless legs”. If answered affirmatively they were identified as having RLS. Another potential answer to the second question was “periodic leg movements,” which limits the possible misidentification of PLMS as RLS.
Subjective Sleep Measures
Subjective sleep quality and daytime alertness were assessed using the Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS), respectively, which were obtained at the time of the sleep visit.15–17 The PSQI ranges from 0 to 21 and measures reported sleep patterns and sleep problems, including sleep quality, sleep latency, sleep efficiency, and daytime dysfunction. Higher scores on the PSQI represent poorer sleep quality, with a score of >5 indicative of poor sleep. The ESS measures daytime sleepiness, using a scale from 0 to 24, with higher scores representing more daytime sleepiness and a score >10 indicating excessive daytime sleepiness.
Other Measures
At the Sleep Visit examination, MrOS participants completed a questionnaire regarding age, race/ethnicity, education, smoking status, alcohol use, and medical history. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE).18 Depression was assessed using the number of depressive symptoms from the Geriatric Depression Scale (GDS), with a standard cutpoint of six or more symptoms defining evidence of depression.19 Height (centimeters) was measured on Harpenden stadiometers and weight (kilograms) on standard balance beam or digital scales, with participants wearing light clothing without shoes. BMI was calculated as kilograms per square meter. Blood pressure was measured using a conventional mercury sphygmomanometer on the right arm and an appropriate cuff size. Participants were asked to bring all medications used within the past 30 days with them to the clinic. All prescription and nonprescription medications were entered into an electronic medications inventory database. Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).20 C-reactive protein (CRP) was measured using the ELISA assay kit from ALPCO (CRP sensitive ELISA); Interleukin-6 (IL-6) was assayed using the Human ProInflammatory I 4-Plex Ultra-Sensitive Kit by MSD (catalog #K15009C-4). Measures of renal function were performed on previously frozen (−70°C) stored serum samples. Serum creatinine was analyzed using the Roche Modular P chemistry analyzer (Enzymatic/Roche Diagnostics Corp., Indianapolis, IN). Serum cystatin-C was measured using the Roche Modular P chemistry analyzer (Turbidimetric/Gentian AS, Moss, Norway). Glomerular filtration rate was estimated (eGFR) using the Chronic Kidney Disease Epidemiology (CKD-EPI) formula using both serum creatinine and cystatin-C.21
Polysomnography
Sleep testing was conducted with unattended in-home PSG (Safiro, Compumedics, Inc, Melbourne, Australia). The recording montage included the following: C3/A2 and C4/A1 electroencephalography (EEG), bilateral electrooculography, a bipolar submental electromyography, thoracic/abdominal respiratory inductance plethysmography, naso-oral thermistry, nasal pressure transduction, finger pulse oximetry, lead I ECG, body position, and bilateral anterior tibialis piezoelectric movement sensors. Centrally trained staff performed home visits for unit setup and impedance value verification for each channel as previously described.22 Data were downloaded to a central server at the Central Sleep Reading Center (Cleveland, OH) and scored by certified research polysomnologists using standard criteria.23,24 Apnea was defined as complete or near complete reduction in thermistor amplitude for at least 10 seconds; hypopnea was defined as at least 30% reduction in nasal pressure signal or summed inductance bands for at least 10 seconds. Apneas and hypopneas were only scored as such if they were associated with ≥4% desaturation.25 The apnea-hypopnea index (AHI) was calculated as the total number of apneas and hypopneas per hour of sleep. Arousals were scored according to the American Academy of Sleep Medicine (AASM) criteria.24 The arousal index was calculated as the number of arousals per hour of sleep. Wake after sleep onset (WASO) was defined as the minutes scored awake during the sleep period after sleep onset. Sleep efficiency was defined as the percent of time scored as sleep during the sleep period.
PLMS were scored according to ASDA criteria in which individual leg movements were scored if the duration was between 0.5 and 5 seconds and there was a clear amplitude increase from baseline in leg channels.26 To be considered periodic, at least 4 movements needed to occur in succession no less than 5 seconds and no more than 90 seconds apart. The periodic leg movement index (PLMI) was the total number of periodic leg movements per hour of sleep. Leg movements after respiratory events were excluded unless they were part of a ≥4 movement cluster with at least 2 movements occurring independently of respiratory events. The periodic leg movement arousal index (PLMAI) was the total number of periodic leg movements per hour of sleep in which EEG arousal occurred within 3 seconds of movement termination. The PLM parameters were expressed as both continuous and categorical variables (PLMI ≥15 vs. <15;PLMAI ≥5 vs. <5).
Incident CVD Events
Participants were surveyed for potential incident cardiovascular events by postcard and/or phone contact every 4 months with a 99% response rate. Relevant medical records and supporting documentation from any potential incident event, including death certificates for fatal events, were obtained by the clinical center and forwarded to the San Francisco coordinating center for centralized adjudication. When no medical records were available for out-of-hospital deaths, proxy interviews with the next of kin were obtained. For both nonfatal and fatal cardiovascular events, all documents were adjudicated by a board-certified cardiologist using a pre-specified adjudication protocol developed with methods that had been used successfully at the coordinating center for both prior randomized trials and epidemiological studies of CVD. Interrater agreement was periodically evaluated by ≥1 expert adjudicators in a random subset of events to ensure quality control in the outcomes adjudication process.
The cardiovascular outcomes examined were similar to those from prior cross-sectional2,3 or longitudinal4,27,28 studies examining the association of RLS and CVD. These included stroke, myocardial infarction (MI, combining acute, ST-segment or non-ST-segment elevation), and mechanical coronary revascularization (CRV). Cardiovascular events were also grouped as follows: coronary heart disease (CHD) events (MI, CRV, sudden coronary heart disease death, coronary artery bypass surgery, hospitalization for unstable angina, ischemic congestive heart failure, or other CHD event not described here); cerebrovascular disease (CBD) events: stroke or transient ischemic attack (TIA); and all-cause CVD events: CHD, CBD, and peripheral arterial disease events combined (acute arterial occlusion, rupture, dissection, or vascular surgery for arterial disease).
Statistical Analysis
Characteristics of participants were compared across RLS status using chi-square tests for categorical variables, t tests for normally distributed continuous variables, and Wilcoxon rank sum tests for continuous variables with skewed distributions. Similar comparisons were made by PLMI categories (data shown in Supplementary Tables 1 and 2).
Cox proportional hazards regression was used to assess the association between RLS or PLMI and risk of incident CVD, and results are presented as hazard ratios (HR) with 95% confidence intervals (CI). Models were minimally adjusted for age, clinic, BMI, alcohol use and smoking (Model 1). Model 2 included those covariates in Model 1 plus depression, prevalent diabetes mellitus, renal function (eGFR), physical activity, antidepressant use, and benzodiazepine use. Fully adjusted models (Model 3) were also adjusted for AHI. To assess whether significant associations of RLS or PLMI (as a continuous variable) and the CVD outcomes were independent, models were further adjusted for both these variables. Secondary analysis was performed for those outcomes where a significant association was found with PLMI or RLS. Model 3 was further adjusted for those sleep characteristics found to be associated to RLS status (p < .05), hypertension, dopaminergic use, inflammation markers, and history of the outcome to examine if the associations of RLS status or PLMI and incident CVD were independent of these factors. A separate model was performed for each factor, then a model including all these factors. Multicollinearity of the covariates was examined and found to be at an acceptable level (variance inflation factors ≤ 2.4 for all).
All significance levels reported were two-sided and all analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
The mean age of the study sample was 76.3 years (SD = 5.5) (Table 1). Physician-diagnosed RLS was reported by 2.2% of the sample. Notably, those with RLS were more likely to be Caucasian (98.4% vs. 90.6%), have less education, be slightly heavier (BMI 28.1 vs. 27.1 kg/m2), have worse renal function (eGFR 65.2 vs. 70.2 kg/m2 mL/min/1.73m2), have more depressive symptoms, and more commonly use benzodiazepines, antidepressants, dopaminergics, anticonvulsants and antihypertensive medications (p < .05). Further, they more commonly had a self-reported history of diabetes mellitus, coronary heart disease, stroke/TIA, peripheral vascular disease and hypertension (p < .05). Systolic blood pressure measurements were similar in those with and without RLS. Over half of all men had a PLMI ≥ 15 (59.6%) while 27.3% had PLMAI ≥ 5 (Table 2). Of those with RLS, 67.7% had PLMI ≥ 15 (p = .19) and 41.9% had PLMAI ≥ 5 (p = .009). Compared to men with lower PLMI, those with PLMI ≥ 15 were on average older by 1.3 years, had more depressive symptoms and higher levels of inflammation as measured by IL-6, had worse renal function, consumed less alcohol, were more likely to be Caucasian, more likely to take antihypertensives, and had higher rates of comorbid conditions (diabetes mellitus, MI, CHD, CVD). (p < .05, Supplementary Table 1).
Table 1.
Baseline Characteristics by Self-Reported Diagnosis of Restless Legs Syndrome (RLS).
| No RLS diagnosis | RLS diagnosis | ||
|---|---|---|---|
| Characteristic | (N = 2761) | (N = 62) | p |
| Age, years | 76.30 ± 5.48 | 76.40 ± 5.87 | .89 |
| Non-white | 259 (9.38) | 1 (1.61) | .04 |
| Education | <.0001 | ||
| < High school | 138 (5.00) | 7 (11.29) | |
| High school | 434 (15.72) | 20 (32.26) | |
| Some college or more | 2189 (79.28) | 35 (56.45) | |
| Body mass index, kg/m2 | 27.12 ± 3.75 | 28.11 ± 4.03 | .04 |
| Smoking | .41 | ||
| Never | 1098 (39.77) | 20 (32.26) | |
| Past | 1610 (58.31) | 40 (64.52) | |
| Current | 53 (1.92) | 2 (3.23) | |
| Alcohol intake, drinks/week | .08 | ||
| <1 | 1275 (46.41) | 37 (59.68) | |
| 1–13 | 1320 (48.05) | 24 (38.71) | |
| ≥14 | 152 (5.53) | 1 (1.61) | |
| Physical activity scorea | 146.27 ± 71.73 | 134.09 ± 48.70 | .06 |
| Current medication use: | |||
| Benzodiazepines | 111 (4.02) | 12 (19.35) | <.0001 |
| Antidepressants | 201 (7.28) | 15 (24.19) | <.0001 |
| Antihypertensives | 1600 (57.95) | 47 (75.81) | .005 |
| Dopaminergics | 26 (0.94) | 7 (11.29) | <.0001 |
| Anticonvulsants | 83 (3.01) | 8 (12.90) | <.0001 |
| Depression, Geriatric depression scale score ≥ 6 | 159 (5.76) | 11 (17.74) | <.0001 |
| Geriatric depression scale score (range 0 to 15) | 1.70 ± 2.06 | 3.07 ± 2.92 | <.0001 |
| History of: | |||
| Diabetes mellitus | 350 (12.68) | 17 (27.42) | .0006 |
| Myocardial infarction | 474 (17.17) | 12 (19.35) | .65 |
| Stroke or transient ischemic attack | 295 (10.69) | 18 (29.03) | <.0001 |
| Coronary heart diseaseb | 810 (29.39) | 26 (41.94) | .03 |
| Peripheral vascular disease | 263 (9.68) | 14 (23.73) | .0004 |
| Cardiovascular diseasec | 1065 (38.66) | 34 (54.84) | .01 |
| Hypertension | 1354 (49.04) | 41 (66.13) | .008 |
| Parkinson’s disease | 34 (1.23) | 0 | .38 |
| Systolic blood pressure, mmHg | 126.78 ± 16.25 | 127.31 ± 17.41 | .80 |
| Estimated glomerular filtration rate, mL/min/1.73m2 | 70.12 ± 16.95 | 65.19 ± 18.12 | .02 |
| C-reactive protein, ug/ml | 2.74 ± 5.26 | 3.47 ± 3.78 | .06 |
| Interleukin-6, pg/ml | 1.90 ± 6.94 | 1.62 ± 1.51 | .18 |
Results shown as mean ± SD or n (%).p-values for continuous variables are from a t test if normally distributed, a Wilcoxon rank-sum test if skewed. p-values for categorical data are from a chi-square test.
aPhysical Activity Scale for the Elderly (PASE).
bSelf-reported history of coronary heart disease includes myocardial infarction, angina, bypass surgery, or angioplasty.
cCoronary heart disease, peripheral vascular disease, stroke or transient ischemic attack.
Table 2.
Baseline Sleep Characteristics by Self-Reported Diagnosis of Restless Legs Syndrome (RLS).
| No RLS diagnosis | RLS diagnosis | ||
|---|---|---|---|
| Characteristic | (N = 2761) | (N = 62) | p |
| Polysomnography data: | |||
| Apnea-hypopnea index | 11.49 ± 12.77 | 16.76 ± 16.35 | .005 |
| Periodic leg movements/hour of sleep (PLMI) | 35.37 ± 37.21 | 46.21 ± 42.95 | .04 |
| PLMI ≥ 15 | 1641 (59.43) | 42 (67.74) | .19 |
| Periodic leg movements with arousals/hour of sleep (PLMAI) | 4.02 ± 5.70 | 5.81 ± 6.65 | .03 |
| PLMAI ≥ 5 | 733 (26.93) | 26 (41.94) | .009 |
| Total sleep time, h | 5.93 ± 1.15 | 5.88 ± 1.38 | .77 |
| Sleep efficiency, % | 76.25 ± 11.88 | 72.78 ± 14.38 | .06 |
| Arousal Index | 23.51 ± 11.60 | 27.06 ± 15.41 | .08 |
| Wake after sleep onset, min | 114.35 ± 65.91 | 135.06 ± 80.16 | .047 |
| Pittsburgh Sleep Quality Index (PSQI) Data: | |||
| Sleep latency, min | 18.35 ± 19.48 | 25.82 ± 27.31 | .01 |
| Sleep efficiency, % | 86.85 ± 13.53 | 80.20 ± 16.92 | .003 |
| Hours of actual sleep | 6.99 ± 1.18 | 6.71 ± 1.38 | .07 |
| PSQI score (range 0 to 21) | 5.53 ± 3.21 | 8.16 ± 4.34 | <.0001 |
| PSQI score > 5 (poor sleep quality) | 1186 (42.96) | 41 (66.13) | 0.0003 |
| Component 1: Sleep quality | <.0001 | ||
| Very good | 844 (30.57) | 8 (12.90) | |
| Fairly good | 1515 (54.87) | 32 (51.61) | |
| Fairly bad | 341 (12.35) | 20 (32.26) | |
| Very bad | 61 (2.21) | 2 (3.23) | |
| Component 2: Sleep latency | .001 | ||
| Falls asleep quickly | 1192 (43.17) | 20 (32.26) | |
| Falls asleep fairly quickly | 1067 (38.65) | 19 (30.65) | |
| Does not fall asleep quickly | 344 (12.46) | 14 (22.58) | |
| Falls asleep slowly | 158 (5.72) | 9 (14.52) | |
| Component 3: Sleep duration | .09 | ||
| >7 h | 949 (34.38) | 19 (30.65) | |
| ≥6 to <7 h | 1546 (56.01) | 31 (50.00) | |
| ≥5 to <6 h | 194 (7.03) | 9 (14.52) | |
| <5 h | 71 (2.57) | 3 (4.84) | |
| Component 4: Sleep efficiency | <.0001 | ||
| >85% | 1740 (63.04) | 29 (46.77) | |
| ≥75 to 85% | 614 (22.25) | 16 (25.81) | |
| ≥65 to <75% | 240 (8.70) | 4 (6.45) | |
| <65% | 166 (6.01) | 13 (20.97) | |
| Component 5: Sleep disturbances | <.0001 | ||
| Score 0 | 67 (2.43) | 2 (3.23) | |
| Score 1–9 | 1772 (64.18) | 26 (41.94) | |
| Score 10–18 | 892 (32.31) | 28 (45.16) | |
| Score 19–27 | 30 (1.09) | 6 (9.68) | |
| Component 6: Sleep medication use | 0.0001 | ||
| Not in past month | 2200 (79.68) | 38 (61.29) | |
| Less than once/week | 189 (6.85) | 3 (4.84) | |
| 1–2 times/week | 93 (3.37) | 5 (8.06) | |
| ≥3 times/week | 279 (10.11) | 16 (25.81) | |
| Component 7: Daytime dysfunction | <.0001 | ||
| None | 1027 (37.20) | 15 (24.19) | |
| Some | 1442 (52.23) | 27 (43.55) | |
| Moderate | 260 (9.42) | 15 (24.19) | |
| Severe | 32 (1.16) | 5 (8.06) | |
| Epworth sleepiness scale data: | |||
| Epworth sleepiness scale (range 0 to 24) | 6.11 ± 3.64 | 7.79 ± 4.87 | .009 |
| Epworth sleepiness scale >10 (excessive daytime sleepiness) | 349 (12.64) | 15 (24.19) | .007 |
Results shown as mean ± SD or n (%). p-values for continuous variables are from a t test if normally distributed, a Wilcoxon rank-sum test if skewed. p-values for categorical data are from a chi-square test.
Men with physician-diagnosed RLS had significantly worse measures of self-reported sleep in multiple domains: longer sleep latency, lower sleep efficiency, lower sleep quality, worse daytime dysfunction as a result of sleep disturbance, greater levels of daytime sleepiness, and more sleep medication use (Table 2). On the overnight home PSG recording of sleep, those with RLS had greater WASO (135 minutes vs. 114 minutes) (Table 2). Total sleep time was no different between groups. AHI (16.8 ± 16.4 vs. 11.5 ± 12.8, p = .005), PLMI (46.2 ± 43.0 vs. 35.4 ± 37.2, p = .04) and periodic leg movement with arousal index (5.8 ± 6.7 vs. 4.0 ± 5.7, p = .03) were all significantly higher in the RLS than the non-RLS group (Table 2). Compared to men with lower PLMI, those with PLMI ≥ 15 had on average 7 minutes more sleep time as measured by PSG, and had a higher average arousal index (p < .05, Supplementary Table 2).
During the follow up period of 8.7 ± 2.6 years, there were 826 men with at least one CVD event in the analysis cohort, with 140 men having had a fatal CVD event. The rate of men with at least one incident CHD event was 19.9%, CBD was 9.3%, MI was 7.1% and CRV was 8.7%. The multivariate adjusted association of RLS with any incident CVD event was not significant (Model 3: HR = 1.15, 95% CI, 0.76–1.74). (Table 3) There were no significant associations observed between RLS and CHD, CBD, CRV or fatal CVD. The relationship of RLS and all incident myocardial infarction events was significant after multivariable adjustment (Model 3: HR = 2.02, 95% CI, 1.04–3.91). This result was driven by the association of RLS status with non-ST elevated MI events (which were the majority all incident MI events) (Model 3: HR = 2.25, 95% CI, 1.11–4.58). When PLMI was added to Model 3, the HRs fell only modestly to 1.93 for the outcome of all MI and 2.17 for the non-ST elevated MI outcome. (Table 3) The addition of PLMI decreased the beta coefficients for the RLS predictor by 6.5% for the all MI outcome and 4.8% for the non-ST elevated MI outcome. The relationship of RLS and incident MI was strengthened when a history of MI was added to the model (HR = 2.17, 95% CI, 1.12–4.19). (Table 5) The significant associations of RLS status to incident MI and incident non-ST elevated MI remained, with little attenuation of effect size, after adding those sleep characteristics that were related to RLS status (WASO, PSQI variables of sleep latency and sleep efficiency, PSQI score; Epworth Sleepiness Scale score), dopaminergic use (of those with RLS, 11% were using a dopaminergic agent), inflammatory markers, or history of hypertension to Model 3. (Table 5) For example, the largest reduction in effect size of the RLS predictor for the MI outcome occurred with the addition of PSQI score, reducing the odds ratio from 2.01 to 1.93, with a decrease in RLS beta coefficient of 6.8%. Similarly, the largest reduction in effect size for the non-ST elevated MI outcome was also caused by the addition of PSQI score, reducing the odds ratio from 2.25 to 2.16, with a decrease in RLS beta coefficient of 5.0%.
Table 3.
Association of Self-Reported Diagnosis of Restless Legs Syndrome with Incident Cardiovascular Outcomes.
| Hazard ratio (95% Confidence interval) | |||||
|---|---|---|---|---|---|
| Incident event | N (%) of Events | Model 1 | Model 2 | Model 3 | Model 3 + PLMI |
| Cardiovascular disease | 826 (29.26) | 1.31 (0.87, 1.97) | 1.15 (0.76, 1.74) | 1.15 (0.76, 1.74) | 1.14 (0.75, 1.73) |
| Fatal cardiovascular disease | 140 (5.02) | 1.21 (0.45, 3.31) | 1.29 (0.46, 3.59) | 1.25 (0.45, 3.48) | 1.22 (0.44, 3.40) |
| Coronary heart disease | 560 (19.89) | 1.44 (0.90, 2.30) | 1.21 (0.75, 1.97) | 1.21 (0.74, 1.96) | 1.19 (0.73, 1.93) |
| Stroke or transient ischemic attack | 261 (9.30) | 1.42 (0.70, 2.89) | 1.41 (0.69, 2.90) | 1.41 (0.69, 2.90) | 1.44 (0.70, 2.95) |
| Stroke | 181 (6.46) | 1.75 (0.82, 3.75) | 1.80 (0.83, 3.91) | 1.81 (0.83, 3.94) | 1.83 (0.84, 3.97) |
| Myocardial infarction | 199 (7.10) | 2.32 (1.22, 4.40) | 2.05 (1.06, 3.97) | 2.02 (1.04, 3.91) | 1.93 (0.99, 3.75) |
| Acute myocardial infarction | 20 (0.71) | 2.57 (0.34, 19.54) | 1.39 (0.17, 11.47) | 1.05 (0.12, 9.09) | 0.91 (0.10, 8.34) |
| Non-ST elevated myocardial infarction | 143 (5.10) | 2.72 (1.37, 5.37) | 2.30 (1.14, 4.67) | 2.25 (1.11, 4.58) | 2.17 (1.06, 4.43) |
| ST-elevation myocardial infarction | 48 (1.71) | 2.33 (0.56, 9.72) | 2.52 (0.59, 10.72) | 2.57 (0.60, 10.91) | 2.49 (0.58, 10.61) |
| Mechanical coronary revascularization | 243 (8.67) | 1.81 (0.96, 3.42) | 1.54 (0.80, 2.96) | 1.53 (0.79, 2.95) | 1.49 (0.77, 2.88) |
PLMI = periodic leg movement index. Model 1: age, clinic, BMI, alcohol use and smoking. Model 2: Model 1 + depression, prevalent diabetes mellitus, renal function, physical activity, antidepressant use, benzodiazepine use. Model 3: Model 2 + apnea-hypopnea index. Results in bold are p < .05.
Table 5.
Association of Restless Legs Syndrome or Periodic Leg Movement Index and Cardiovascular Outcomes Further Adjusting for Potential Mechanisms.
| Hazard ratio (95% Confidence interval) | ||||
|---|---|---|---|---|
| Predictor | Model | Coronary heart disease | Myocardial infarction | Non-ST elevated myocardial infarction |
| Self-report of diagnosis of restless legs syndrome vs. no diagnosis | Model 3a | 1.21 (0.74, 1.96) | 2.02 (1.04, 3.91) | 2.25 (1.11, 4.58) |
| Model 3a + wake after sleep onset | 1.21 (0.75, 1.97) | 2.04 (1.05, 3.97) | 2.27 (1.11, 4.62) | |
| Model 3a + sleep latencyb | 1.20 (0.74, 1.95) | 2.01 (1.03, 3.89) | 2.24 (1.10, 4.56) | |
| Model 3a + sleep efficiencyb | 1.17 (0.72, 1.90) | 1.98 (1.02, 3.83) | 2.21 (1.09, 4.49) | |
| Model 3a + Pittsburgh Sleep Quality Index score | 1.14 (0.70, 1.84) | 1.93 (0.99, 3.73) | 2.16 (1.07, 4.38) | |
| Model 3a + Epworth Sleepiness Scale Score | 1.19 (0.73, 1.94) | 1.98 (1.02, 3.83) | 2.20 (1.08, 4.48) | |
| Model 3a + dopamenergic use | 1.24 (0.76, 2.03) | 2.04 (1.04, 4.01) | 2.52 (1.23, 5.14) | |
| Model 3a + hypertension | 1.19 (0.73, 1.93) | 1.99 (1.03, 3.86) | 2.20 (1.08, 4.48) | |
| Model 3a + c-reactive protein | 1.42 (0.85, 2.36) | 2.58 (1.33, 4.99) | 2.93 (1.44, 5.96) | |
| Model 3a + IL-6 | 1.41 (0.85, 2.36) | 2.55 (1.32, 4.93) | 2.91 (1.43, 5.90) | |
| Model 3a + history of disease (CHD or MI) | 1.29 (0.80, 2.09) | 2.17 (1.12, 4.19) | 2.51 (1.24, 5.08) | |
| Model 3a + all potential mechanisms | 1.47 (0.88, 2.46) | 2.52 (1.28, 4.94) | 3.06 (1.49, 6.29) | |
| Periodic limb movement index per SD (37.37) increase | Model 3a | 1.09 (1.00, 1.18) | 1.14 (1.00, 1.30) | 1.10 (0.94, 1.29) |
| Model 3a + wake after sleep onset | 1.09 (1.00, 1.18) | 1.14 (1.00, 1.30) | 1.10 (0.94, 1.29) | |
| Model 3a + sleep latencyb | 1.09 (1.00, 1.17) | 1.13 (0.99, 1.29) | 1.09 (0.93, 1.28) | |
| Model 3a + sleep efficiencyb | 1.08 (1.00, 1.18) | 1.14 (1.00, 1.30) | 1.09 (0.94, 1.28) | |
| Model 3a + Pittsburgh Sleep Quality Index score | 1.08 (1.00, 1.17) | 1.13 (0.99, 1.29) | 1.10 (0.94, 1.29) | |
| Model 3a + Epworth Sleepiness Scale Score | 1.09 (1.00, 1.18) | 1.14 (1.00, 1.30) | 1.09 (0.93, 1.28) | |
| Model 3a + dopamenergic use | 1.09 (1.00, 1.18) | 1.14 (1.00, 1.30) | 1.10 (0.94, 1.28) | |
| Model 3a + hypertension | 1.09 (1.00, 1.18) | 1.14 (1.00, 1.30) | 1.10 (0.94, 1.28) | |
| Model 3a + c-reactive protein | 1.10 (1.01, 1.19) | 1.15 (1.01, 1.32) | 1.09 (0.92, 1.28) | |
| Model 3a + IL-6 | 1.10 (1.01, 1.20) | 1.16 (1.01, 1.33) | 1.10 (0.93, 1.29) | |
| Model 3a + history of disease (CHD or MI) | 1.07 (0.99, 1.16) | 1.14 (1.00, 1.30) | 1.10 (0.94, 1.30) | |
| Model 3a + all potential mechanisms | 1.08 (0.99, 1.17) | 1.15 (1.00, 1.32) | 1.07 (0.90, 1.27) | |
CHD = coronary heart disease; IL-6, interleukin-6; MI = myocardial infarction; SD = standard deviation. Results in bold are p < .05.
aModel 3: age, clinic, body mass index, alcohol use, smoking, depression, prevalent diabetes mellitus, renal function, physical activity, antidepressant use, benzodiazepine use and apnea-hypopnea index.
bfrom the Pittsburgh Sleep Quality Index.
A higher PLMI was associated with an increased risk of an incident MI event (Model 3: per SD increase, HR = 1.14, 95% CI, 1.00–1.30, p = .05) and risk of an incident CHD event (Model 3: per SD increase, HR = 1.09, 95% CI, 1.00–1.18). (Table 4) There was virtually no change in the point estimate after further adjustment for RLS (PLMI per SD increase, MI: HR = 1.13, 95% CI, 0.99–1.29; p = .07; CHD: HR = 1.08, 95% CI, 1.00–1.18, p = .05), with a decrease in PLMI beta coefficient of 2.5% for MI, <1% for CHD. The associations of PLMI to incident MI and incident CHD remained, with no attenuation of effect size, after adding all potential mechanisms to Model 3. (Table 5)
Table 4.
Association of Periodic Leg Movement Index with Incident Cardiovascular Outcomes.
| Hazard ratio (95% Confidence interval) | ||||
|---|---|---|---|---|
| Incident event | Model 1 | Model 2 | Model 3 | Model 3 + RLS |
| PLMI predictor, per SD increase (37.37) | ||||
| Cardiovascular disease | 1.04 (0.97, 1.11) | 1.03 (0.96, 1.10) | 1.03 (0.96, 1.10) | 1.03 (0.96, 1.10) |
| Fatal cardiovascular disease | 1.13 (0.97, 1.32) | 1.16 (0.99, 1.35) | 1.16 (0.99, 1.35) | 1.16 (0.99, 1.35) |
| Coronary heart disease | 1.10 (1.01, 1.19) | 1.08 (1.00, 1.18) | 1.09 (1.00, 1.18) | 1.08 (1.00, 1.18) |
| Stroke or transient ischemic attack | 0.92 (0.81, 1.04) | 0.90 (0.79, 1.03) | 0.90 (0.79, 1.03) | 0.90 (0.79, 1.03) |
| Stroke | 0.98 (0.84, 1.13) | 0.97 (0.83, 1.13) | 0.97 (0.83, 1.13) | 0.97 (0.83, 1.12) |
| Myocardial infarction | 1.15 (1.01, 1.31) | 1.14 (1.00, 1.30) | 1.14 (1.00, 1.30) | 1.13 (0.99, 1.29) |
| Acute myocardial infarction | 1.27 (0.85, 1.90) | 1.20 (0.80, 1.81) | 1.21 (0.82, 1.80) | 1.22 (0.82, 1.82) |
| Non-ST elevated myocardial infarction | 1.11 (0.95, 1.30) | 1.10 (0.94, 1.28) | 1.10 (0.94, 1.29) | 1.08 (0.92, 1.27) |
| ST-elevation myocardial infarction | 1.16 (0.89, 1.52) | 1.16 (0.88, 1.52) | 1.16 (0.88, 1.52) | 1.15 (0.88, 1.51) |
| Mechanical coronary revascularization | 1.11 (0.98, 1.25) | 1.10 (0.97, 1.24) | 1.10 (0.98, 1.25) | 1.10 (0.97, 1.24) |
PLMI = periodic leg movement index; RLS = restless legs syndrome. Model 1: age, clinic, BMI, alcohol use and smoking. Model 2: Model 1 + depression, prevalent diabetes mellitus, renal function, physical activity, antidepressant use, benzodiazepine use. Model 3: Model 2 + apnea-hypopnea index. Results in bold are p < .05.
DISCUSSION
In this large community-based sample of older men, we demonstrated that both physician-diagnosed RLS and PSG measured periodic leg movements were associated with incident MI, ascertained over 8 years of average follow-up time. Furthermore, our analyses indicated that the hazard ratio for each exposure (RLS; PLMI) was not, or only modestly, attenuated when both RLS and PLMS were considered concurrently. These data, suggesting that RLS and PLMS each contribute to risk of incident MI, add to a growing body of evidence examining the association of RLS with CVDs based on longitudinal outcomes (Table 6). Some of these studies have identified RLS as an independent risk factor for CVD, whereas others have not confirmed this. Multiple methodological issues potentially contribute to these discrepant findings including method of RLS ascertainment, sex of the subjects, duration of illness and duration of follow-up.
Table 6.
Association of RLS with Cardiovascular Diseases (CVD) Based on Longitudinal Outcomes.
| Cohort | N | RLS (%) | Mean age (years) | Duration of follow-up (years) | RLS information (duration, frequency, 1o/2o) | CVD outcomes |
|---|---|---|---|---|---|---|
| Study of Health in Pomerania (SHIP)1 | 4308 | 10.1 | 50.3 | 5.0 | None; no change without DM cases | –MI –CVA |
| Women’s Health Study3 | 29 756 | 11.7 | 63.4 | 6.0 | None; no change without ESRD/PVD | –first CVD event |
| –MI –CVA | ||||||
| Physician’s Health Study2 | 19 182 | 7.2 | 66.6 | 7.3 | None; no change without ESRD/PVD | –first CVD event |
| –MI –CVA | ||||||
| Nurse’s Health Study4 | 70 977 | 2.1 | 67.0 | 5.6 | Duration > 3 years; no change without DM/ESRD | +(fatal) CHD |
| +first MI event | ||||||
| Kaiser Permanente5 | 10 646 | 1o = 58.1 2 o = 66.5 | 3.9 | Physician diagnosis; 2 o = many comorbidities | 1o: –CVD/MI/CVA | |
| 2o:+CVD/MI/CVD | ||||||
| Veterans Administration35 | 7392 | 0.1 | 59.8 | 8.1 | “incident RLS” | +CHD/CVA |
| MrOS | 2823 | 2.2 | 76.3 | 8.7 | Physician diagnosis | –CVD/CVA |
| +MI events |
36 CHD = coronary heart disease; MI = myocardial infarction; RLS = restless legs syndrome. +: positive association. –: not associate.
The distinctive feature of the current study is the ability to independently assess the contributions of RLS diagnosis, PLMS, and both polysomnographically-recorded and self-reported sleep measures to cardiovascular risk. Our data suggest that RLS is not associated with all CVD events but rather with a subset of these, MI events. The hazard ratios for both Non-ST- and ST- segment elevation were above 2.0, though the small numbers of those with the latter led to a non-significant result. The addition of both RLS and PLMI predictors to these models only modestly attenuated the relationship of RLS or PLMI to MI events suggesting that both the sensory component of RLS as well as the previously documented10 motor component of PLMS contribute to CVD risk. Further adjustment for sleep disruption parameters did not lessen the association of RLS status or PLMI and incident MI. On the other hand, neither the association of RLS or PLMI with cerebrovascular events (stroke, TIA) nor with mechanical coronary revascularization was significant. We confirm and extend prior work by Koo, et al. in this same cohort showed significant associations of PLMI with incident CVD and CHD over 4 years of follow-up.10 After longer follow-up of 8.7 years, the significant association of PLMI and an increased risk of incident coronary heart disease remained. The effect size was not attenuated after further adjustment for potential mechanisms that might explain the association.
Previous longitudinal studies have examined the association of RLS specifically with myocardial infarction events. In the Study of Health in Pomerania (SHIP) cohort,1 there was no association of RLS with incident MI, controlling for other CVDs, over five years of follow-up. Important differences between the MrOS and SHIP cohorts were the younger age of the participants in the latter group (76 vs. 51 years old), the higher prevalence of RLS in SHIP (10.1% vs. 2.2%), the shorter duration of follow up in the latter and the predominance of primary (as opposed to secondary) incident MI events (as only 3% of participants had a history of MI at baseline) in SHIP. Similar results were obtained from the Women’s Health and Physician’s Health Studies2,3 in which RLS was not a risk factor for incident primary MI events at 6.0 and 7.3 years of follow-up respectively. Again, these cohorts were younger (mid-60’s) than those in the current study and the RLS prevalence was substantially higher (11.7% and 7.2%), as it was based on questionnaire diagnosis. Different results were obtained from the Nurses’ Health Study,4 in which women who reported having RLS by physician diagnosis, without a history of CVD (age = 67 years), were followed for 5.6 years. Stratification of the RLS group into those with self-reported diagnoses greater than 3 years led to a clear association of RLS with incident MI. Women with RLS for greater than 3 years had an OR = 1.80 (95% CI, 1.07–3.01) for non-fatal MI in multivariate analysis; those with RLS < 3 years had a non-significant risk of incident MI. Thus, the two studies in which RLS was predictive of incident MI were based on physician, rather than questionnaire, diagnosis of RLS. This led to much lower prevalence rates of RLS (2.2–2.6% vs. 7.2–11.7%) and probably selected for the most severely affected individuals. Another recent study5 followed patients with physician-diagnosed RLS and without prevalent CVD for a mean of 3.9 years. Although MI events were not specifically examined, “primary” RLS was not associated with incident coronary artery disease, which included not only MI but coronary revascularization and angina. On the other hand, “secondary” RLS (ie in individuals with a wide range of medical and neurological illnesses) did predict incident coronary events (HR = 1.40, 95% CI 1.25–1.56). We were not able to identify the wide range of comorbid disorders in our cohort which classified participants as having “secondary” RLS in that study and thus could not confirm whether this distinction was relevant for the RLS-MI association in our data.
Previous studies of the relationship of RLS and CVD did not examine the unique contributions of RLS symptoms and PLMS. In the current analysis, the positive association observed between RLS and incident MI was not materially altered by the addition of PLMS to the model of incident disease. Further, PLMS was also an independent predictor of incident MI in agreement with an earlier analysis of this cohort.10 Thus, both a diagnosis of RLS and polysomnographically measured PLMS are independent risk factors for incident MI in this elderly male population. The relationship between RLS and PLMS is a complicated one. On the one hand, the majority of those with RLS have frequent PLMS: in the current study 67.7% of participants with physician-diagnosed RLS had a PLMI ≥ 15. However, PLMS is also common in the general population, particularly in the elderly.7,29 In this cohort, 59.4% of those without physician-diagnosed RLS had a PLMI ≥ 15 and the mean PLMI of that group was 35.4. Disentangling the roles of these two potential contributors to cardiovascular morbidity may be of value in assessing whether treatment of RLS or PLMS is indicated as a means to reduce future complications. For example, some medications (eg, alpha two delta ligands) are more effective in treating RLS symptoms than reducing PLMS30 whereas others (dopamine agonists) are equally good in treating RLS symptoms and reducing PLMS.31
A number of potential mechanisms exist by which RLS could promote CVD. These include PLMS, effects on sleep quality or quantity, use of medications to control RLS symptoms, inflammatory pathways, or through central nervous system iron-related pathways. Adjustment for some of these, including PLMI, both subjective and objective sleep measures, dopaminergic use, inflammatory markers, and hypertension did not reduce the association of RLS status and incident MI. Nevertheless, we cannot conclude from such results that these factors are unrelated to any deleterious effects of RLS on cardiovascular health. Each of these covariates were recorded at only one time point, and variation in their levels and/or previous contribution to MI risk could have been relevant.
There are a number of strengths to this study. The study had a large population of community-dwelling older men who were not selected for inclusion based on RLS status or history of CVD. Adjustments for multiple potential confounding factors were made, suggesting these associations were not explained by other covariates including depression, medication use, or lifestyle. Results were also robust to adjustment for sleep disruption. There are also limitations to this study. Our data are limited to older, primarily Caucasian men and may not be generalizable to other populations. Due to the low number of minorities, we were unable to adjust our results by race. Adjustment for numerous covariates was performed, but the possibility of residual confounding remains. There was a small number of men with RLS in this sample, potentially limiting the power to identify relationships of RLS to CVD. Previous studies investigating such relationships have identified 200–400 individuals with RLS. On the other hand, given the older age of the MrOS population, the number of incident MIs in this study (n = 199) was substantially larger than in the SHIP cohort (n = 37)1 and roughly the same as in the Women’s Health Study (n = 176),3 though less than in the Physician’s Health Study (n = 431).2 RLS identification based on physician diagnosis may have included RLS mimics as false positive diagnoses of RLS. Conversely, the requirement for those in the RLS group to have had a physician diagnosis depends upon participant symptom reporting, and this limitation probably led to under diagnosis and further misclassification of true RLS diagnoses. Both of these sources of diagnostic imprecision probably produced an underestimate of the association of RLS with CVD. On the other hand, physician diagnosis may have led to identification of only more severe RLS, biasing the results towards an overestimate of the RLS-CVD association. For this reason, future studies should include expert interviews for RLS diagnoses. We did not have information on duration of RLS or frequency of RLS symptoms. Piezoelectric sensors, and not the standard anterior tibialis electromyography, were used to measure PLMS. However, PLMI scored using our approach (ie, 1993 scoring criteria with piezoelectric sensors) is highly correlated (r = 0.83) with the PLMI derived using the 201332 scoring criteria and use of leg electromyography (EMG) sensors.7 Further demonstrating the similarity of leg movements recorded by these two methods, PLMS scored by piezoelectric sensors have the same genetic associations as those scored by anterior tibialis EMG.33 The assessment of PLMS was performed on a single night, and given night to night variability of leg movements,34 we may have misclassified some participants.
In summary, these analyses suggest that RLS and PLMS each contribute to an increased risk of MI in elderly men which is not explained by co-morbidities such as sleep apnea, multiple medical illnesses, multiple indices of disrupted sleep or poor sleep quality, inflammation, dopaminergic use or history of MI. These findings suggest a need to monitor those with RLS and PLMS more closely for MI and examine the role that interventions for these sleep-related movement disorders may play in reducing cardiac risk.
SUPPLEMENTARY MATERIAL
Supplementary data are available at SLEEP online.
FUNDING
The Osteoporotic Fractures in Men Study (MrOS) is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Szentkirályi A, Völzke H, Hoffmann W, Happe S, Berger K. A time sequence analysis of the relationship between cardiovascular risk factors, vascular diseases and restless legs syndrome in the general population. J Sleep Res. 2013; 22(4): 434–442. [DOI] [PubMed] [Google Scholar]
- 2. Winter AC, Berger K, Glynn RJ, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in men. Am J Med. 2013; 126(3): 228–235, 235.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winter AC, Schurks M, Glynn RJ, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in women. Am J Med. 2013; 126(3):220–227, 227 e221-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012; 126(14): 1689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Den Eeden SK, Albers KB, Davidson JE, et al. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a Retrospective Cohort Study from Kaiser Permanente Northern California. Sleep. 2015; 38(7): 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997; 12(1): 61–65. [DOI] [PubMed] [Google Scholar]
- 7. Claman DM, Ewing SK, Redline S, Ancoli-Israel S, Cauley JA, Stone KL; Study of Osteoporotic Fractures Research Group Periodic leg movements are associated with reduced sleep quality in older men: the MrOS Sleep Study. J Clin Sleep Med. 2013; 9(11): 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winkelman JW. Clinical and polysomnographic features of sleep-related eating disorder. J Clin Psychiatry. 1998; 59(1): 14–19. [DOI] [PubMed] [Google Scholar]
- 9. Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007; 68(15): 1213–1218. [DOI] [PubMed] [Google Scholar]
- 10. Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S; Osteoporotic Fractures in Men (MrOS) Study Group Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011; 124(11): 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yumino D, Wang H, Floras JS, et al. Relation of periodic leg movements during sleep and mortality in patients with systolic heart failure. Am J Cardiol. 2011; 107(3): 447–451. [DOI] [PubMed] [Google Scholar]
- 12. Benz RL, Pressman MR, Hovick ET, Peterson DD. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis. 2000; 35(6): 1052–1060. [DOI] [PubMed] [Google Scholar]
- 13. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005; 26(5): 569–585. [DOI] [PubMed] [Google Scholar]
- 14. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005; 26(5): 557–568. [DOI] [PubMed] [Google Scholar]
- 15. Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 16. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992; 15(4): 376–381. [DOI] [PubMed] [Google Scholar]
- 17. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991; 14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 18. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993; 46(2): 153–162. [DOI] [PubMed] [Google Scholar]
- 19. Sheikh J. YJ. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986; 5(1/2):165–173. [Google Scholar]
- 20. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994; 10(4): 405–411. [DOI] [PubMed] [Google Scholar]
- 21. Inker LA, Shaffi K, Levey AS. Estimating glomerular filtration rate using the chronic kidney disease-epidemiology collaboration creatinine equation: better risk predictions. Circ Heart Fail. 2012; 5(3): 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998; 21(7): 759–767. [PubMed] [Google Scholar]
- 23. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington, DC: National Institutes of Health; 1968. [Google Scholar]
- 24. ASDA. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992; 15:173–184. [PubMed] [Google Scholar]
- 25. Berry RB, Budhiraja R, Gottlieb DJ, et al. ; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012; 8(5): 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ASDA. Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993; 16:748–759. [PubMed] [Google Scholar]
- 27. Elwood P, Hack M, Pickering J, Hughes J, Gallacher J. Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J Epidemiol Community Health. 2006; 60(1): 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winter AC, Schürks M, Glynn RJ, et al. Restless legs syndrome and risk of incident cardiovascular disease in women and men: prospective cohort study. BMJ Open. 2012; 2(2): e000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991; 14(6): 496–500. [DOI] [PubMed] [Google Scholar]
- 30. Winkelman JW, Bogan RK, Schmidt MH, Hudson JD, DeRossett SE, Hill-Zabala CE. Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome. Mov Disord. 2011; 26(11): 2065–2072. [DOI] [PubMed] [Google Scholar]
- 31. Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study–the PRELUDE study. Sleep Med. 2006; 7(5): 407–417. [DOI] [PubMed] [Google Scholar]
- 32. Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0.2. Darien, IL: American Academy of Sleep Medicine; 2013. [Google Scholar]
- 33. Winkelman JW, Blackwell T, Stone K, Ancoli-Israel S, Tranah GJ, Redline S; Osteoporotic Fractures in Men (MrOS) Study Research Group Genetic associations of periodic limb movements of sleep in the elderly for the MrOS sleep study. Sleep Med. 2015; 16(11): 1360–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trotti LM, Bliwise DL, Greer SA, et al. Correlates of PLMs variability over multiple nights and impact upon RLS diagnosis. Sleep Med. 2009; 10(6): 668–671. [DOI] [PubMed] [Google Scholar]
- 35. Molnar MZ, Lu JL, Kalantar-Zadeh K, Kovesdy CP. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J Sleep Res. 2016; 25(1): 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gottlieb DJ, Somers VK, Punjabi NM, Winkelman JW. Restless legs syndrome and cardiovascular disease: a research roadmap. Sleep Med. 2016; S1389-9457(16)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.