Abstract
Study Objectives
Sleep-disordered breathing (SDB) is prevalent among children and is associated with adverse health outcomes. Worldwide, approximately 250 million individuals reside at altitudes higher than 2000 meters above sea level (masl). The effect of chronic high-altitude exposure on children with SDB is unknown. This study aims to determine the impact of altitude on sleep study outcomes in children with SDB dwelling at high altitude.
Methods
A single-center crossover study was performed to compare results of high-altitude home polysomnography (H-PSG) with lower altitude laboratory polysomnography (L-PSG) in school-age children dwelling at high altitude with symptoms consistent with SDB. The primary outcome was apnea-hypopnea index (AHI), with secondary outcomes including obstructive AHI; central AHI; and measures of oxygenation, sleep quality, and pulse rate.
Results
Twelve participants were enrolled, with 10 included in the final analysis. Median altitude was 1644 masl on L-PSG and 2531 masl on H-PSG. Median AHI was 2.40 on L-PSG and 10.95 on H-PSG. Both obstructive and central respiratory events accounted for the difference in AHI. Oxygenation and sleep fragmentation were worse and pulse rate higher on H-PSG compared to L-PSG.
Conclusions
These findings reveal a clinically substantial impact of altitude on respiratory, sleep, and cardiovascular outcomes in children with SDB who dwell at high altitude. Within this population, L-PSG underestimates obstructive sleep apnea and central sleep apnea compared to H-PSG. Given the shortage of high-altitude pediatric sleep laboratories, these results suggest a role for home sleep apnea testing for children residing at high altitude.
Keywords: Sleep-disordered breathing, obstructive sleep apnea, OSA pathogenesis, home sleep apnea testing, pediatrics, pediatric sleep apnea, altitude, environment, cardiovascular, central sleep apnea
Statement of Significance
This study provides novel insights into the effect of high altitude on sleep and breathing in children, specifically by demonstrating that both obstructive and central sleep apnea are more prevalent and severe in children dwelling at high altitude when measured at the home environment compared to a lower altitude sleep laboratory. Most children residing at high altitude do not have access to a high-altitude sleep laboratory. Therefore, home sleep apnea testing may have a crucial role in achieving accurate diagnosis in children residing at high altitude. Future studies are needed to characterize the risk for health complications within this population and to determine optimal treatment for children with central or obstructive sleep apnea who reside at high altitude.
INTRODUCTION
Sleep-disordered breathing (SDB) represents a continuum of conditions ranging from primary snoring to obstructive sleep apnea (OSA). These conditions are common in childhood, with an overall SDB prevalence of 11% in the United States, including up to 4% of children with OSA.1 Prior studies have identified numerous adverse neurocognitive, cardiovascular, metabolic, behavioral, and quality of life sequelae associated with untreated SDB.2–10 Moreover, recent findings suggest a dose effect of SDB in that more severe disease is associated with more profound neurocognitive impairment.2 Early identification and treatment of SDB can reduce its complications.7–11 However, under-recognition prevents many patients from receiving optimal and timely therapy.
Worldwide, approximately 250 million individuals reside at altitudes higher than 2000 meters above sea level (masl).12 Exposure to high altitude—defined variably in the literature but often as 2500 masl in the context of acute ascent from sea level—directly alters respiratory physiology. As altitude increases, ambient barometric pressure decreases, resulting in a reduction in partial pressure of inspired oxygen and thus decreased partial pressure of arterial oxygen and decreased arterial oxygen saturation. Peripheral chemoreceptors primarily located in the carotid bodies respond to decreased arterial oxygenation by increasing minute ventilation which, in turn, reduces arterial carbon dioxide (CO2). This results in a respiratory alkalosis which acutely blunts the hypoxemic ventilatory response (HVR), creating a temporary negative feedback loop.13
Among the many physiologic adaptations that occur with chronic exposure to high altitude, cerebrospinal fluid (CSF) and blood pH normalize within days of ascent due to movement of bicarbonate out of the CSF and increased renal excretion of bicarbonate, thereby eliminating the negative feedback loop to preserve HVR and increase baseline minute ventilation. Chronic adaptation and even natural selection in humans at high altitude favor hyperventilation.13–16
The pathophysiologic mechanisms underlying OSA, central sleep apnea (CSA), and periodic breathing (PB) suggest that hyperventilation may predispose children dwelling at high altitude to these conditions. However, little is currently known about the impact of high altitude dwelling on sleep-related breathing disorders in children. Healthy 3- to 5-year old children residing in Denver, Colorado (1600 masl) experience subtle alterations to breathing during sleep, including increased frequency of central respiratory events, PB, and arterial oxygen desaturation compared to age-matched controls at sea level.17 Healthy infants residing in Bogotá, Colombia (2640 masl) demonstrate markedly increased frequencies of central respiratory events, obstructive respiratory events, and PB as well as decreased mean and nadir arterial oxygen saturation which gradually improve from birth to 18 months of age.18 Similarly, healthy 7- to 13-year-old Bolivian children dwelling at high altitude (3650 masl) demonstrate increased 3% oxygen desaturation index (ODI) and obstructive hypopnea index (OHI) compared to age-matched controls dwelling at low altitude (500 masl).19 Increasingly, population-level genetic factors are recognized to impact respiratory physiology and ventilatory response to high altitude and hypoxia.14,15,19–21 It remains unknown whether genetic factors within the populations studied in the Andes and the Rocky Mountains impact the applicability of this normative data to pediatric populations in other mountainous regions across the globe.
The impact of chronic high-altitude exposure in children with SDB has not been investigated. In adults with OSA, ascent to altitude causes increased central apnea, reduced arterial and cerebral oxygenation, reduced slow-wave sleep, and increased arousal frequency.22,23 Moreover, complications of OSA are augmented in adults during high altitude sojourn, including increased systolic blood pressure, more frequent cardiac arrhythmias, and impaired driving performance.24
Our center in Denver, Colorado, with sleep laboratories located at approximately 1600–1700 masl, evaluates and treats children with breathing and sleep disorders throughout the Rocky Mountain region, many of whom reside at altitudes ranging from 2000 to 3500 masl. Access to pediatric sleep laboratories is severely limited within the center’s catchment area, particularly in high-altitude mountainous regions. Home sleep apnea testing (HSAT) for the diagnosis of OSA is currently neither recommended in widely implemented practice guidelines25 nor typically reimbursed by payers. Therefore, children who dwell at high altitude and are suspected of having OSA must acutely descend to a lower altitude to undergo attended in-laboratory diagnostic polysomnography (PSG).
The current study aims to determine the effect of chronic exposure to high altitude on frequency of respiratory events, oxygenation, arousal frequency, and sleep architecture in school-aged children with SDB. We hypothesized that participants dwelling at high altitude who have symptoms of SDB would exhibit more frequent respiratory events, more severe oxygen desaturations, and more frequent arousals when undergoing PSG at their high-altitude home residence compared to PSG at a lower altitude pediatric sleep laboratory.
METHODS
Enrollment and Baseline Data
This single-center nonrandomized crossover trial comparing PSG parameters from unattended high-altitude home polysomnography (H-PSG) and attended lower altitude laboratory polysomnography (L-PSG) was approved by the Colorado Multiple Institutional Review Board. Consecutive participants age 4 to 12 years, whose home residence is located at an altitude greater than 2000 masl (approximately 300 meters higher than our center’s highest altitude sleep laboratory) and whose medical provider requested an overnight diagnostic PSG to evaluate for suspected OSA with, at minimum, symptoms including snoring, were enrolled from January 1, 2016 through December 31, 2016. All participants resided at their current home altitude for over 1 year prior to enrollment. Participants with prior diagnosis of OSA, baseline oxygen requirement, baseline positive airway pressure requirement, tracheostomy, intercurrent respiratory illness, travel to lower altitudes within the week prior to H-PSG, or refusal to participate were excluded.
Study personnel reviewed all sleep study requests submitted through our center’s electronic health record to identify participants meeting inclusion criteria. Home residence was cross-referenced with United States Geological Survey topographical data to determine home altitude. Participants whose parents expressed interest during an initial telephone contact were scheduled for a single research visit. During the research visit, baseline data were recorded including participant age, race, body mass index, home altitude, time interval (in days) between L-PSG and H-PSG, environmental tobacco exposure at home, and presence of medical comorbidities including adenotonsillar hypertrophy; obesity; craniofacial abnormalities; genetic or chromosomal syndromes; and underlying pulmonary, cardiovascular, metabolic, and neuromuscular disease.
High-Altitude Home Polysomnography
During the research visit, typically scheduled from 04:00 to 06:00 pm, the H-PSG was set up and initiated in our center’s sleep laboratory by a registered polysomnographic technologist (RPSGT). The H-PSG utilized a Trex portable headbox (Natus Medical, Inc.) and consisted of six electroencephalography (EEG), two electrooculography (EOG), two chin electromyelography (EMG), and two electrocardiography (ECG) leads; nasal pressure (BiNAPS, Salter Labs), oronasal flow (ThermiSense, Salter Labs), abdominal and thoracic effort (SleepSense, Salter Labs), pulse oximetry (Xpod PureSAT SpO2, Nonin), and transcutaneous CO2 (Sentec Digital Monitoring System, SenTec, Inc.). All wires terminated at the portable headbox, which was stored and transported in an appropriately sized children’s backpack. During H-PSG setup, study personnel reviewed written instructions with the participant’s caregiver(s). Upon completion of setup, participants returned to their high-altitude residence, performed their typical bedtime routine, and slept in their usual location within the home. The caregiver was instructed to press an event button connected to the portable headbox at bed time and rise time to identify “lights out” and “lights on” for data collection and scoring purposes. A board eligible sleep physician (BH) was available by telephone overnight to answer caregiver questions and troubleshoot equipment issues.
Lower Altitude Laboratory Polysomnography
Each participant’s lower altitude laboratory polysomnogram (L-PSG) was arranged through our center’s typical scheduling protocol. To reduce period effect, the maximum allowable interval between H-PSG and L-PSG was 30 days. The L-PSG was performed by an RPSGT in accordance with our center’s standard clinical practice, utilizing an Xltek headbox (Natus Medical, Inc.) and consisting of six EEG, two EOG, three chin EMG, two lower extremity EMG, and two ECG leads; nasal pressure (BiNAPS, Salter Labs), oronasal flow (Airflow Sensor, Dymedix Diagnostics, Inc.), abdominal and chest effort (SleepSense, Salter Labs), pulse oximetry (SET Pulse Oximeter, Masimo, Inc.), transcutaneous CO2 (TCM TOSCA Monitor, Radiometer Medical), end-tidal CO2 (Capnocheck Sleep, Smiths Medical), snore microphone, and video.
Scoring and Data Collection
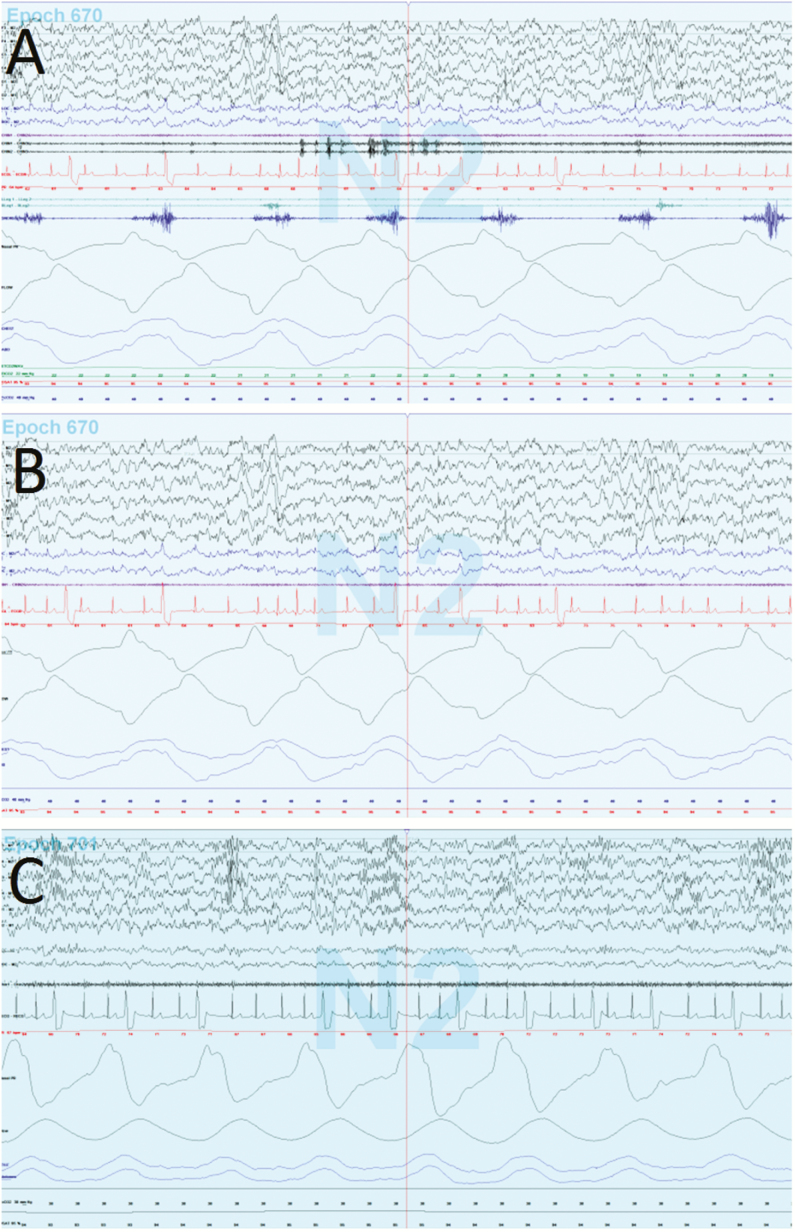
The SleepWorks software application (Natus Medical, Inc.) was utilized for data collection, scoring, and reporting of outcomes measures. Each participant’s L-PSG was pruned to achieve equivalence with the H-PSG montage by removing the end-tidal CO2, snore, and lower extremity EMG channels (Figure 1). For each participant, a total of three studies, including H-PSG, L-PSG, and L-PSG with full laboratory montage (henceforth labeled “L-PSG Full”) were scored in accordance with the AASM Scoring Manual Version 2.2 by an experienced independent research scorer. Once scored, each study underwent analysis using the SleepWorks application, and PSG parameters related to respiratory events, oxygenation, ventilation, sleep architecture, arousals, and heart rate were recorded in REDCap, a secure web-based research database application.
Figure 1.
Representative 30-second epochs from the L-PSG Full (A), L-PSG (B), and H-PSG (C) recorded on a single participant. L-PSG and H-PSG lack snore channel, end-tidal CO2, and lower extremity electromyelography as seen on L-PSG Full. This participant had frequent ectopic beats on two-lead electrocardiography. H-PSG = high altitude home polysomnography; L-PSG Full = lower altitude laboratory polysomnography with full montage; L-PSG = lower altitude laboratory polysomnography.
Each study was assigned an OSA diagnosis using the following criteria: obstructive apnea-hypopnea index (OAHI) 0–1.9 events/hour equals no OSA, OAHI 2.0–4.9 events/hour equals mild OSA, and OAHI greater than or equal to 5.0 events/hour equals moderate to severe OSA. Each study was additionally assigned a CSA diagnosis using the following criteria: central apnea-hypopnea index (CAHI) 0–4.9 events/hour equals no CSA and CAHI greater than or equal to 5.0 events/hour equals CSA.
Statistical Analysis
The analysis considered three sleep studies for each participant: (1) H-PSG, (2) L-PSG, and (3) L-PSG Full, the descriptions for which are included above. Analyses were conducted on individuals who underwent successful H-PSG and L-PSG. Univariate statistics, including frequencies, percentages, ranges, means, standard deviations, medians, and interquartile ranges were used to describe baseline patient information and sleep study measures. Normality assumptions were evaluated using the Anderson-Darling test. Wilcoxon signed rank-sum tests or paired t test were used to compare outcomes between sleep studies. Agreement was assessed with the Kappa statistic for concordance. General linear regression models assessed correlation between test outcomes and altitude difference between PSG conditions. Impact of outliers on regression models were assessed using Cook’s D, and data points were considered extreme at a value of 0.40. All statistical tests were two-tailed tests and considered significant at the 0.05 level. Analyses were conducted using SAS V.9.4 (SAS Institute, Cary, North Carolina, United States).
RESULTS
During the recruitment period, 14 participants met inclusion criteria and expressed interest in participating. Of these, two were lost to follow-up and underwent neither H-PSG nor L-PSG, leaving 12 participants enrolled. Of these, two were withdrawn from final analysis due to noninterpretable H-PSG, both of whom removed all or most recording devices (including EEG and nasal pressure sensor) before sleep was recorded. One of these participants had autism spectrum disorder, and the other was 4.0 years old (the minimum age for inclusion). The remaining 10 participants included in the final analysis ranged in age from 4.9 to 12.7 years. Sleep laboratory altitude ranged from 1595 to 1709 masl, and home altitude ranged from 2108 to 3124 masl. The median time interval between H-PSG and L-PSG was 4 days. Complete demographic and baseline data for participants included in the final analysis and participants excluded due to noninterpretable H-PSG are presented in Table 1.
Table 1.
Demographic and Baseline Data.
| Included in final analysis (N=10) | Excluded due to noninterpretable H-PSG (N=2) | |
|---|---|---|
| Age (years) | 9.1 [5.2–9.8] | 4.0 and 9.3 |
| Sex | ||
| Male | 6 (60%) | 2 (100%) |
| Female | 4 (40%) | 0 (0%) |
| Ethnicity/race | ||
| Hispanic or Latino/white | 1 (10%) | 0 (0%) |
| Non-Hispanic or non-Latino/white | 9 (90%) | 2 (100%) |
| BMI Z-score | 0.34 ± 1.03 | −0.13 and 0.63 |
| Laboratory altitude (masl) | 1644 [1615–1644] | 1644 and 1644 |
| Home altitude (masl) | 2531 [2458–2734] | 2765 and 3083 |
| Altitude difference (masl) | 856 [818–1119] | 1120 and 1439 |
| Baseline sleep characteristics | ||
| AHI | 2.4 [1.6–3.3] | 2.9 and 3.9 |
| Obstructive AHI | 0.65 [0.4–1.9] | 0.5 and 3.6 |
| Central AHI | 1.3 [0.9–2.0] | 0.3 and 2.3 |
| Mean SpO2 | 96.55 [95.5–97.5] | 95.6 and 97.6 |
| Nadir SpO2 | 90 [88–91] | 84 and 93 |
| Underlying diagnosis | ||
| Adenotonsillar hypertrophy | 3 (30%) | 0 (0%) |
| Obese (BMI >95th %ile) | 1 (10%) | 0 (0%) |
| Overweight (BMI 85th–95th %ile) | 1 (10%) | 0 (0%) |
| Prematurity | 1 (10%) | 1 (50%) |
| Lung disease | 2 (20%) | 0 (0%) |
| Pulmonary arterial hypertension | 2 (20%) | 0 (0%) |
| Genetic/chromosomal, metabolic, and neuromuscular disorders | 0 (0%) | 0 (0%) |
Demographic and baseline data including results from the lower altitude laboratory polysomnography with full montage (L-PSG Full) are shown. Summary statistics are presented as median [inner-quartile range], mean ± standard deviation, and count (percent).
AHI = apnea-hypopnea index; BMI = body mass index; H-PSG = high-altitude home polysomnography; masl = meters above sea level.
Differences in study acquisition quality were observed between the attended L-PSG and unattended H-PSG. Specifically, the nasal pressure signal was reliable for greater than 50% of total sleep time for 100% (10/10) of L-PSG studies compared to 80% (8/10) of H-PSG studies. Accounting for the two participants withdrawn from final analysis due to noninterpretable H-PSG, 67% (8/12) of H-PSG studies had reliable nasal pressure signal for greater than 50% of total sleep time.
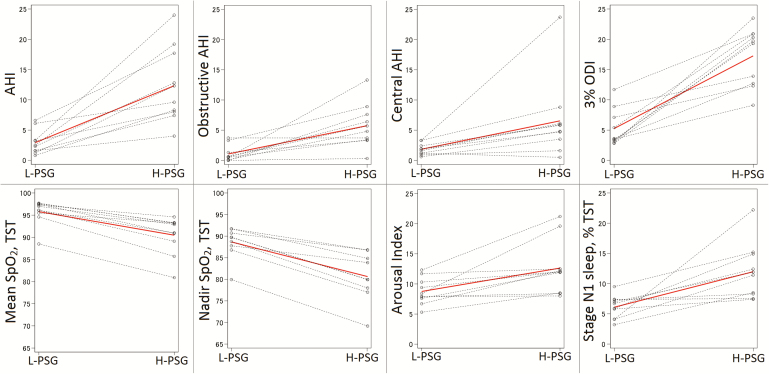
The apnea-hypopnea index (AHI), OAHI, CAHI, 3% ODI, arousal index, proportion of stage N1 sleep, and mean pulse rate were significantly higher on H-PSG compared to L-PSG (Table 2 and Figure 2). Differences in AHI, OAHI, and CAHI were largely driven by increased OHI, central apnea index (CAI), and central hypopnea index at high altitude. Additionally, mean oxygen saturation and nadir oxygen saturation were significantly lower on H-PSG compared to L-PSG. All differences remained clinically meaningful and statistically significant after removing one outlier participant who had very low mean and nadir SpO2. In comparing H-PSG with L-PSG Full, differences in the same outcomes were observed (Supplementary Table S1). In comparing L-PSG with L-PSG Full, there were no statistically significant differences, although trends toward increased central AHI on L-PSG versus L-PSG Full (median 1.75 vs. 1.30, p = .0625) and decreased obstructive AHI on L-PSG versus L-PSG Full (median 0.55 vs. 0.65, p = .0625) were observed (Supplementary Table S1). Reliable transcutaneous CO2 data were available on both H-PSG and L-PSG for one participant, who demonstrated a mean transcutaneous CO2 during sleep of 40 mmHg on L-PSG and 39 mmHg on H-PSG. Respiratory rate was similar between the two altitude conditions (17.1 breaths per minute on L-PSG, 17.2 breaths per minute on H-PSG, p = .22), consistent with prior reports indicating that tidal volume, not respiratory rate, drives the increase in minute ventilation at high altitude.26
Table 2.
Polysomnography Outcomes by Study Condition.
| L-PSG | H-PSG | p-value | |
|---|---|---|---|
| AHI | 2.4 [1.6–3.3; 0.8, 6.6] | 10.95 [8–17.7; 4, 24] | .001 |
| Obstructive AHI | 0.55 [0.1–1.3; 0, 3.7] | 5.25 [3.4–7.6; 0.3, 13.3] | .004 |
| OAI | 0 [0–0.1; 0, 0.3] | 0.15 [0–0.6; 0, 0.8] | .172 |
| OHI | 0.4 [0–1.3; 0, 3.6] | 5.0 [2.3–7.6; 0.3, 11.4] | .006 |
| Central AHI | 1.75 [1.2–2.4; 0.6, 3.3] | 5.3 [3.5–6.1; 0.5, 23.7] | .006 |
| CAI | 0.85 [0.2–1.1; 0, 2.8] | 1.5 [0.7–3.0; 0.5, 15.6] | .004 |
| CHI | 0.75 [0.5–1.2; 0.3, 2.4] | 3.4 [0.9–4.1; 0, 8.1] | .020 |
| 3% ODI | 3.6 [3.2–7.1; 2.8, 11.7] | 19.5 [12.7–20.9; 9.1, 23.5] | .002 |
| Mean SpO2, TST | 96.55 [95.5–97.5; 88.5, 97.7] | 91.95 [89.1–93.2; 80.9, 94.6] | .002 |
| Nadir SpO2, TST | 90 [88–91; 80, 92] | 80 [78–85; 69, 87] | .002 |
| Arousal index | 8.15 [7.6–10.3; 5.3, 12.3] | 12 [8.5–12.5; 8, 21.2] | .009 |
| Stage N1, % TST | 6.3 [4.1–7.3; 3.2, 9.5] | 11.7 [8.3–14.9; 7.4, 22.2] | .006 |
| Total sleep time, minutes | 475 [458–503] | 513 [467–522] | .114 |
| Sleep efficiency, % | 93.9 [92.4–96.1; 84.4, 97.6] | 94.6 [93.3–96; 91.8, 97.4] | .295 |
| Mean pulse rate, bpm | 66.3 [65.3–74.5; 62.2, 86.3] | 78.1 [69.4–80.9; 60.3, 97.9] | .004 |
Polysomnography outcomes for L-PSG and H-PSG are presented as median [inner-quartile range; minimum, maximum] with comparisons between study condition made using the Wilcoxon signed rank-sum test or paired t-test.
AHI = apnea-hypopnea index; bpm = beats per minute; CAI = central apnea index; CHI = central hypopnea index; H-PSG = high-altitude home polysomnography; L-PSG = lower altitude laboratory polysomnography; OAI = obstructive apnea index; ODI = oxygen desaturation index; OHI = obstructive hypopnea index; TST = total sleep time.
Figure 2.
Intrasubject changes in polysomnography outcomes between altitude settings are depicted to demonstrate uniformity of changes. The solid red line indicates median values for each variable. AHI, apnea-hypopnea index; H-PSG, high altitude home polysomnography; L-PSG, lower altitude laboratory polysomnography; ODI, oxygen desaturation index; TST, total sleep time.
There was poor diagnostic agreement between L-PSG and H-PSG, with H-PSG demonstrating more prevalent and severe OSA and CSA compared to L-PSG (Table 3; Kappa = 0.048 for OSA diagnosis, Kappa = 0.0 for CSA diagnosis). If considering H-PSG as the reference diagnostic standard, L-PSG was 22.2% sensitive and 100% specific in detecting OSA of any severity, 0% sensitive and 100% specific in detecting moderate to severe OSA, and 0% sensitive and 100% specific in detecting CSA. In contrast, there was complete diagnostic agreement between L-PSG and L-PSG Full (Kappa = 1).
Table 3.
Prevalence and Severity of OSA and CSA Based on Altitude Setting.
| L-PSG | H-PSG | |
|---|---|---|
| OSA diagnosis | ||
| No OSA | 8 (80%) | 1 (10%) |
| Mild OSA | 2 (20%) | 4 (40%) |
| Moderate to severe OSA | 0 (0%) | 5 (50%) |
| CSA diagnosis | ||
| No CSA | 10 (100%) | 5 (50%) |
| CSA | 0 (0%) | 5 (50%) |
Prevalence and severity of obstructive sleep apnea (OSA) and central sleep apnea (CSA) on lower altitude laboratory polysomnography (L-PSG) compared to high-altitude home polysomnography (H-PSG). Diagnostic thresholds include obstructive apnea-hypopnea index (AHI) 2.0–4.9 = mild OSA, obstructive AHI greater than or equal to 5.0 = moderate to severe OSA; central AHI greater than or equal to 5.0 = CSA.
Altitude difference between study conditions was inversely correlated with mean oxygen saturation (Rho = −0.83, p = .0029), but no other significant correlations between altitude difference and PSG outcomes were observed. Change in obstructive AHI between settings did not correlate with change in central AHI (Supplementary Figure S1).
One participant was incidentally noted to have frequent ectopic beats with morphology consistent with premature ventricular contractions on both the H-PSG and L-PSG (Figure 1). As a post hoc analysis, total ectopic beats were counted in 100 epochs of stage N3 sleep on each study; a 46% increase in ectopic beat frequency was observed on the H-PSG (23.68 events/minute) compared to the L-PSG (16.26 events/minute). Cardiac arrhythmias were otherwise not recorded for the remaining participants.
DISCUSSION
This study extends the existing literature on pediatric sleep at high altitude by investigating the impact of chronic exposure to high altitude on children with SDB. Participants demonstrated an increased frequency of central and obstructive respiratory events and worsened markers of oxygenation and sleep fragmentation while sleeping at their high-altitude residence compared to a lower altitude pediatric sleep laboratory. PSG at the lower altitude consistently underestimated the presence and severity of OSA and CSA compared to PSG at the home altitude. These findings raise important clinical, epidemiologic, and physiologic implications related to SDB in children.
The marked increase in OSA and CSA prevalence and severity at the participants’ high-altitude home compared to the nearest pediatric sleep laboratory highlights the importance of accounting for environmental differences between the sleep laboratory and home. At our center in Denver, Colorado and others across the globe serving populations of high-altitude dwellers, diagnostic testing at or near the patient’s home altitude appears critical in achieving an accurate diagnosis. High-altitude pediatric sleep laboratories are rare, and hypobaric sleep studies are largely unavailable and technically challenging in young children. HSAT offers an alternative strategy to evaluate children at their home altitude. Recent studies have supported the validity of HSAT in specific pediatric populations.27–31 Given the altitude-associated differences noted in the present study, school-age children with SDB who dwell at a significantly higher altitude than the nearest pediatric sleep laboratory (defined in our protocol as a difference of at least 300 meters) may be ideal candidates for HSAT.
The increase in both central and obstructive respiratory events at high altitude reflects overlap in the pathophysiologic mechanisms underlying OSA and CSA. Treatment-emergent CSA and other overlap phenotypes are well described in adults. Boudewyns et al.32 recently published data demonstrating that, within a sample of children with OSA treated with adenotonsillectomy (AT), two-thirds of participants had a preoperative CAI greater than one event/hour with significant decrease in CAI observed following AT. Similarly, Thottam et al.33 reported a high prevalence of CSA in children with Down Syndrome and OSA and a significant reduction in CSA prevalence following AT.
Acclimatized individuals dwelling at high altitude have increased minute ventilation, decreased arterial CO2, and increased arousal frequency.13–18,22,23 Prior physiologic studies have established that hyperventilation causes PB and CSA at high altitude.34 We hypothesize that hyperventilation and increased arousal frequency also initiate a cascade of pathophysiologic consequences that precipitate increased frequency and severity of obstructive respiratory events in at-risk individuals. A model of OSA pathogenesis driven by four physiologic determinants including critical closing pressure, upper airway dilator recruitment threshold, loop gain, and arousal threshold has been proposed.35–37 Baseline hyperventilation and increased arousal frequency among high-altitude dwellers may interact with all four of these dynamic OSA determinants to increase obstructive respiratory event frequency and severity. In addition, with the observed lower baseline SpO2 at high altitude, a lesser change in PaO2 is required to cause a 3% desaturation due to the shape of the oxyhemoglobin dissociation curve.13 This effect likely contributes substantially to the increased frequency and severity of oxygen desaturation episodes at high altitude which, in conjunction with increased arousal frequency, contributes to the increase in obstructive and central hypopneas.
This study does have important limitations, most importantly small sample size. A principal reason for discontinuing enrollment after 12 participants was the significant travel cost for participants’ families, often involving driving over hazardous mountain passes to reach our center. Given the strength of statistical inferences achieved with this small sample, it is unlikely that enrollment of a larger sample would alter the conclusions reached in this study. Acquisition quality was at times inferior on the unattended H-PSG, and reduced nasal pressure signal reliability may have caused underestimation of respiratory event frequency on H-PSG. Lack of snore channel may have caused overestimation of CAHI and underestimation of CAHI; indeed, this was observed in comparing full montage (including snore) and portable montage (excluding snore) on the laboratory PSG. To maximize durability and portability for the H-PSG setup, slightly different equipment was used, including pulse oximeter, which could confound our results. To address this limitation, a supplementary analysis comparing the Nonin and Masimo pulse oximeters was performed by measuring SpO2 using both devices simultaneously in a separate sample of 10 participants age 4–12 years undergoing overnight diagnostic PSG in our center’s sleep laboratory (Supplementary Tables S2 and S3, supplemental materials). Results from this validation sample demonstrated that the Nonin device used for H-PSG underestimated 3% ODI (mean difference −2.26, p = .006) and overestimated nadir SpO2 (mean difference +3.00, p = .027) relative to the Masimo device used for L-PSG; therefore, differences observed between H-PSG and L-PSG in 3% ODI, AHI, OAHI, CAHI, and nadir SpO2 were likely underestimated due to this measurement difference. Conversely, mean SpO2 was slightly lower on the Nonin device relative to the Masimo device (mean difference −0.99, p = .002), and the observed difference in mean SpO2 observed between altitude conditions therefore likely represents an overestimate. However, after adjusting the mean SpO2 values on H-PSG by the largest potential difference attributable to pulse oximeter difference (defined by the upper limit of the 95% confidence interval for difference in mean SpO2 from the validation sample), the difference in mean SpO2 between altitude conditions remained clinically meaningful and statistically significant (p ≤ .002).
The nonrandomized design was implemented to reduce travel cost on participants’ families as participation required up to 12 hours of driving per visit to our center, often involving travel over hazardous mountain passes. Our enrollment workflow involved participants first undergoing H-PSG (with setup at our center’s sleep laboratory), then returning H-PSG equipment—which was not suitable for shipping—at the time of the L-PSG. Had participants been randomized to undergo L-PSG first, an additional trip to our center solely to return H-PSG equipment would have been required. This design, however, does raise the possibility of period effect and PSG order effect as confounders. Period effect was reduced by minimizing time between H-PSG and L-PSG (median interval 4 days). Prior research reveals no “first night effect” to suggest that undergoing one PSG impacts the results of a second PSG.38 Nonetheless, it is theoretically possible that sleep architecture and markers of sleep fragmentation could be impacted by PSG order. Although this is not a blinded study, bias was minimized by having an experienced independent scorer perform scoring on all studies. Despite these limitations, we do feel confident that the observed differences are reproducible.
Further research is required to confirm these results and to explore other questions related to OSA diagnosis, comorbidities, and therapy in this high-altitude pediatric population. The use of a high-altitude sleep laboratory or barometric chamber would allow for this confirmation in a controlled and attended setting. Larger studies may also identify clinical or demographic predictors of altitude-dependent OSA, CSA, and PB. Furthermore, evaluation of neurocognitive and cardiovascular comorbidities in high-altitude participants with SDB will be an important step in ultimately improving outcomes in this population. The increased mean pulse rate observed at high altitude within our sample and the increased frequency of ectopic beats observed at high altitude in one individual do suggest altitude-related cardiovascular morbidity. Finally, two participants had prior diagnosis of idiopathic pulmonary arterial hypertension and demonstrated more severe OSA, CSA, and hypoxemia at the high altitude residence compared to the lower altitude sleep laboratory, further suggesting a role of altitude in cardiovascular morbidity in children with SDB.
The primary environmental differences between H-PSG and L-PSG are ambient barometric pressure and partial pressure of inspired oxygen. We therefore speculate that providing supplemental oxygen in patients who demonstrate obstructive or CSA only at high altitude may be sufficient and effective treatment; studies aimed at testing this hypothesis in children will be useful in optimizing care for this subset of patients. Finally, given the relatively large number of children who dwell at low altitudes but acutely ascend to high altitude for vacation, it will also be important to measure the impact of high altitude sojourn on children with SDB who dwell at or near sea level; we speculate that the effect of altitude will be more severe in these nonacclimatized individuals compared to our high-altitude dwelling participants.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
DISCLOSURE STATEMENT
BHH reports grant funding from the American Medical Association Foundation during the conduct of the current study and access to loaned study equipment from Natus Medical, Inc. (Xltek Trex portable headbox) and SenTec, Inc. (Digital Monitoring System for transcutaneous CO2); ACH reports grant funding from the National Institutes of Health and the National Science Foundation which is unrelated to the current study. DGI and JTB report no relevant disclosures.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge Brad Brainard, Carole Kline, Erin Hill, Alison Essig-Peppard, and Bereket Habte for contributions in data collection, sleep study scoring, and regulatory compliance as well as Natus Medical, Inc. and SenTec, Inc. for loaning study equipment.
REFERENCES
- 1. Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008; 5(2): 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunter SJ, Gozal D, Smith DL, Philby MF, Kaylegian J, Kheirandish-Gozal L. Effect of sleep-disordered breathing severity on cognitive performance measures in a large community cohort of young school-aged children. Am J Respir Crit Care Med. 2016; 194(6): 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottlieb DJ, Chase C, Vezina RM et al. . Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004; 145(4): 458–464. [DOI] [PubMed] [Google Scholar]
- 4. Smith DL, Gozal D, Hunter SJ, Philby MF, Kaylegian J, Kheirandish-Gozal L. Impact of sleep disordered breathing on behaviour among elementary school-aged children: a cross-sectional analysis of a large community-based sample. Eur Respir J. 2016; 48(6): 1631–1639. [DOI] [PubMed] [Google Scholar]
- 5. Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. 2010; 33(11): 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Driscoll DM, Horne RS, Davey MJ et al. . Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011; 12(5): 483–488. [DOI] [PubMed] [Google Scholar]
- 7. Goldbart AD, Levitas A, Greenberg-Dotan S et al. . B-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apnea. Chest. 2010; 138(3): 528–535. [DOI] [PubMed] [Google Scholar]
- 8. Zhang XM, Shi J, Meng GZ et al. . The effect of obstructive sleep apnea syndrome on growth and development in nonobese children: a parallel study of twins. J Pediatr. 2015; 166(3): 646–50.e1. [DOI] [PubMed] [Google Scholar]
- 9. Biggs SN, Vlahandonis A, Anderson V et al. . Long-term changes in neurocognition and behavior following treatment of sleep disordered breathing in school-aged children. Sleep. 2014; 37(1): 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nieminen P, Löppönen T, Tolonen U, Lanning P, Knip M, Löppönen H. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002; 109(4): e55. [DOI] [PubMed] [Google Scholar]
- 11. Teo DT, Mitchell RB. Systematic review of effects of adenotonsillectomy on cardiovascular parameters in children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2013; 148(1): 21–28. [DOI] [PubMed] [Google Scholar]
- 12. Cohen JE, Small C. Hypsographic demography: the distribution of human population by altitude. Proc Natl Acad Sci U S A. 1998; 95(24): 14009–14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. West JB, Luks AM.. West’s Respiratory Physiology: The Essentials. 10th ed Philadelphia, PA: Wolters Kluwer; 2016. [Google Scholar]
- 14. Basang Z, Wang B, Li L et al. . HIF2A variants were associated with different levels of high-altitude hypoxia among native Tibetans. PLoS One. 2015; 10(9): e0137956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu XH, Huang XW, Qun L et al. . Two functional loci in the promoter of EPAS1 gene involved in high-altitude adaptation of Tibetans. Sci Rep. 2014; 4: 7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beall CM, Strohl KP, Blangero J et al. . Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am J Phys Anthropol. 1997; 104(4): 427–447. [DOI] [PubMed] [Google Scholar]
- 17. Burg CJ, Montgomery-Downs HE, Mettler P, Gozal D, Halbower AC. Respiratory and polysomnographic values in 3- to 5-year-old normal children at higher altitude. Sleep. 2013; 36(11): 1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duenas-Meza E, Bazurto-Zapata MA, Gozal D, González-García M, Durán-Cantolla J, Torres-Duque CA. Overnight polysomnographic characteristics and oxygen saturation of healthy infants, 1 to 18 months of age, born and residing at high altitude (2,640 meters). Chest. 2015; 148(1): 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hill CM, Carroll A, Dimitriou D et al. . Polysomnography in Bolivian children native to high altitude compared to children native to low altitude. Sleep. 2016; 39(12): 2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hill CM, Baya A, Gavlak J et al. . Adaptation to life in the high Andes: nocturnal oxyhemoglobin saturation in early development. Sleep. 2016; 39(5): 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beall CM. Tibetan and Andean patterns of adaptation to high-altitude hypoxia. Hum Biol. 2000; 72(1): 201–228. [PubMed] [Google Scholar]
- 22. Pagel JF, Kwiatkowski C, Parnes B. The effects of altitude associated central apnea on the diagnosis and treatment of obstructive sleep apnea: comparative data from three different altitude locations in the mountain west. J Clin Sleep Med. 2011; 7(6): 610–65A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulrich S, Nussbaumer-Ochsner Y, Vasic I et al. . Cerebral oxygenation in patients with OSA: effects of hypoxia at altitude and impact of acetazolamide. Chest. 2014; 146(2): 299–308. [DOI] [PubMed] [Google Scholar]
- 24. Nussbaumer-Ochsner Y, Schuepfer N, Ulrich S, Bloch KE. Exacerbation of sleep apnoea by frequent central events in patients with the obstructive sleep apnoea syndrome at altitude: a randomised trial. Thorax. 2010; 65(5): 429–435. [DOI] [PubMed] [Google Scholar]
- 25. Aurora RN, Zak RS, Karippot A et al. ; American Academy of Sleep Medicine Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011; 34(3): 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Meer K, Heymans HS, Zijlstra WG. Physical adaptation of children to life at high altitude. Eur J Pediatr. 1995; 154(4): 263–272. [PubMed] [Google Scholar]
- 27. Goodwin JL, Enright PL, Kaemingk KL et al. . Feasibility of using unattended polysomnography in children for research—report of the Tucson Children’s Assessment of Sleep Apnea study (TuCASA). Sleep. 2001; 24(8): 937–944. [DOI] [PubMed] [Google Scholar]
- 28. Brockmann PE, Perez JL, Moya A. Feasibility of unattended home polysomnography in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2013; 77(12): 1960–1964. [DOI] [PubMed] [Google Scholar]
- 29. Marcus CL, Traylor J, Biggs SN et al. . Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children. J Clin Sleep Med. 2014; 10(8): 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alonso-Álvarez ML, Terán-Santos J, Ordax Carbajo E et al. . Reliability of home respiratory polygraphy for the diagnosis of sleep apnea in children. Chest. 2015; 147(4): 1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brockmann PE, Damiani F, Nuñez F et al. . Sleep-disordered breathing in children with Down syndrome:usefulness of home polysomnography. Int J Pediatr Otorhinolaryngol. 2016; 83: 47–50. [DOI] [PubMed] [Google Scholar]
- 32. Boudewyns A, Van de Heyning P, Verhulst S. Central apneas in children with obstructive sleep apnea syndrome: prevalence and effect of upper airway surgery. Sleep Med. 2016; 25: 93–97. [DOI] [PubMed] [Google Scholar]
- 33. Thottam PJ, Choi S, Simons JP, Kitsko DJ. Effect of adenotonsillectomy on central and obstructive sleep apnea in children with Downsyndrome. Otolaryngol Head Neck Surg. 2015; 153(4): 644–648. [DOI] [PubMed] [Google Scholar]
- 34. Channer KS, Bastard OG, Vann Jones J, Glover SC. Periodic breathing induced by hyperventilation. Br Med J (Clin Res Ed). 1983; 287(6401): 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005; 172(11): 1363–1370. [DOI] [PubMed] [Google Scholar]
- 36. Owens RL, Edwards BA, Eckert DJ et al. . An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to non positive airway pressure therapy. Sleep. 2015; 38(6): 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deacon NL, Jen R, Li Y, Malhotra A. Treatment of obstructive sleep apnea. prospects for personalized combined modality therapy. Ann Am Thorac Soc. 2016; 13(1): 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katz ES, Greene MG, Carson KA et al. . Night-to-night variability of polysomnography in children with suspected obstructive sleep apnea. J Pediatr. 2002; 140(5): 589–594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.