Abstract
Study Objectives
There remains an important and unmet need for fully effective and acceptable treatments in obstructive sleep apnea (OSA). At present, there are no approved drug treatments. Dronabinol has shown promise for OSA pharmacotherapy in a small dose-escalation pilot study. Here, we present initial findings of the Phase II PACE (Pharmacotherapy of Apnea by Cannabimimetic Enhancement) trial, a fully blinded parallel groups, placebo-controlled randomized trial of dronabinol in people with moderate or severe OSA.
Methods
By random assignment, 73 adults with moderate or severe OSA received either placebo (N = 25), 2.5 mg dronabinol (N = 21), or 10 mg dronabinol (N = 27) daily, 1 hour before bedtime for up to 6 weeks.
Results
At baseline, overall apnea–hypopnea index (AHI) was 25.9 ± 11.3, Epworth Sleepiness Scale (ESS) score was 11.45 ± 3.8, maintenance of wakefulness test (MWT) mean latency was 19.2 ± 11.8 minutes, body mass index was 33.4 ± 5.4 kg/m2, and age was 53.6 ± 9.0 years. The number and severity of adverse events, and treatment adherence (0.3 ± 0.6 missed doses/week) were equivalent among all treatment groups. Participants receiving 10 mg/day of dronabinol expressed the highest overall satisfaction with treatment (p = .04). In comparison to placebo, dronabinol dose-dependently reduced AHI by 10.7 ± 4.4 (p = .02) and 12.9 ± 4.3 (p = .003) events/hour at doses of 2.5 and 10 mg/day, respectively. Dronabinol at 10 mg/day reduced ESS score by −3.8 ± 0.8 points from baseline (p < .0001) and by −2.3 ± 1.2 points in comparison to placebo (p = .05). MWT sleep latencies, gross sleep architecture, and overnight oxygenation parameters were unchanged from baseline in any treatment group.
Conclusions
These findings support the therapeutic potential of cannabinoids in people with OSA. In comparison to placebo, dronabinol was associated with lower AHI, improved self-reported sleepiness, and greater overall treatment satisfaction. Larger scale clinical trials will be necessary to clarify the best potential approach(es) to cannabinoid therapy in OSA.
Keywords: obstructive sleep apnea, pharmacotherapy, cannabinoid, clinical trial
Statement of Significance
Untreated or insufficiently treated obstructive sleep apnea (OSA) is associated with significant independent risks for incidence and progression of cardiovascular, metabolic, and behavioral morbidities. Positive airway pressure, the current frontline therapy, is efficacious but difficult to tolerate and long-term adherence is poor. There are no approved drug treatments for OSA. Here, we report initial results from the largest and longest randomized controlled trial to date of any putative drug treatment for OSA. The findings suggest the therapeutic potential of dronabinol, a nonselective cannabinoid agonist, in OSA. Future trials will be necessary to clarify the best approach(es) to cannabinoid therapy in OSA.
INTRODUCTION
The burden of untreated obstructive sleep apnea (OSA) syndrome in the general population is immense.1 Longitudinal studies with up to 20 years of follow-up demonstrate OSA to be an independent risk factor for both incidence and progression of hypertension, diabetes, stroke, and depression, as well as mortality.1–4 This burden is amplified by the fact that health care utilization and costs among people with OSA are double those of age, body mass index (BMI), and gender matched controls.2 The first-line treatment, positive airway pressure (PAP), improves quality of life, reduces daytime sleepiness, lowers blood pressure, diminishes hospital admissions and health care costs, and increases survival in people with OSA.2 Unfortunately, PAP is cumbersome and difficult for many patients to tolerate, yielding poor long-term adherence rates.5–7 Thus, there remains a critical unmet medical need for novel OSA therapies.
Drug treatments for OSA have been sought for many years, but effective agents remain to be identified.8–10 Based on a series of animal investigations, we proposed that drugs which dampen afferent vagal feedback to the medulla may be effective in stabilizing respiratory pattern generation and increasing activation of upper airway dilating muscles during sleep (for a review, see Ref.11). Nodose ganglion cells express a variety of somatic receptors including excitatory serotonin type 3 (5-HT3) and inhibitory cannabinoid type 1 (CB1) receptors.12,13 We previously demonstrated that dronabinol, a nonselective CB1 and CB2 receptor agonist, reduced the frequency of spontaneous central apneas in a rodent model of sleep-related breathing disorder.14 In an initial small scale dose-escalation pilot study, we further demonstrated that dronabinol significantly reduced the apnea–hypopnea index (AHI) in 17 patients with moderate-to-severe OSA.15
Here, we report initial findings from the PACE (Pharmacotherapy of Apnea by Cannabimimetic Enhancement) study; a Phase II multisite, fully blinded, parallel groups, randomized placebo-controlled clinical trial of dronabinol in participants with moderate or severe OSA. We hypothesized that dronabinol would dose-dependently reduce OSA severity and daytime sleepiness in comparison to placebo.
METHODS
Participants
Participants aged 21 to 65 years with known or suspected OSA were drawn from the clinical populations of two tertiary-care sleep centers (University of Illinois at Chicago and Northwestern University). Additional participants were recruited from the community via print and radio advertising. Participants with AHI ≥ 15 and ≤50 documented by screening polysomnography (PSG) were randomized to treatment group subject to the following exclusion criteria: Epworth Sleepiness Scale (ESS) score < 7 (to exclude nonsleepy participants); BMI > 45; motor vehicle accident or “near-miss” due to sleepiness (self-report) within 2 years; arterial oxygen saturation < 75% for more than 5% of total sleep time on baseline (screening) PSG; severe OSA that in the investigator’s judgment precluded delaying (re)institution of PAP treatment; prior upper airway surgery for snoring or OSA as an adult; significant defect in nasal patency due to anatomical abnormality or uncontrolled rhinitis; bariatric surgery within 2 years; medically managed weight-loss program within 6 months; noninvasive treatment for OSA within 1 month (self-report); history of shift work or rotating shifts within 1 month; any clinically significant uncontrolled cardiopulmonary, gastrointestinal, pancreatic, hepatic, renal, hematologic, endocrine (including type 1 diabetes), neurological, urogenital, psychiatric, or sleep disorder (other than OSA); seizure disorder; use of CNS active drugs; pregnancy; recreational drug use or positive urine drug screen; or clinically significant abnormality on complete blood count or liver function tests. This study was approved by the Institutional Review Boards of the University of Illinois at Chicago and Northwestern University.
Study Protocol
The overall protocol included six laboratory visits spanning 7–8 weeks. During the initial visit, after providing written informed consent, all participants underwent general and detailed sleep histories, physical examination, and laboratory evaluation. After a 5–10-day placebo run-in period, visit 2 completed the screening process, including baseline overnight laboratory PSG, maintenance of wakefulness testing (MWT), and completion of the ESS and Treatment Satisfaction Questionnaire for Medication (TSQM)—a well-established and validated instrument for use in individuals with chronic disease.16 Participants meeting all inclusion and exclusion criteria were randomized to a treatment group. Randomized participants completed daily self-administration of study drug for 6 weeks, returning to the laboratory every 2 weeks for overnight PSG, MWT, physical examination, and questionnaire completion. A final exit visit was completed 2–5 days after final drug administration.
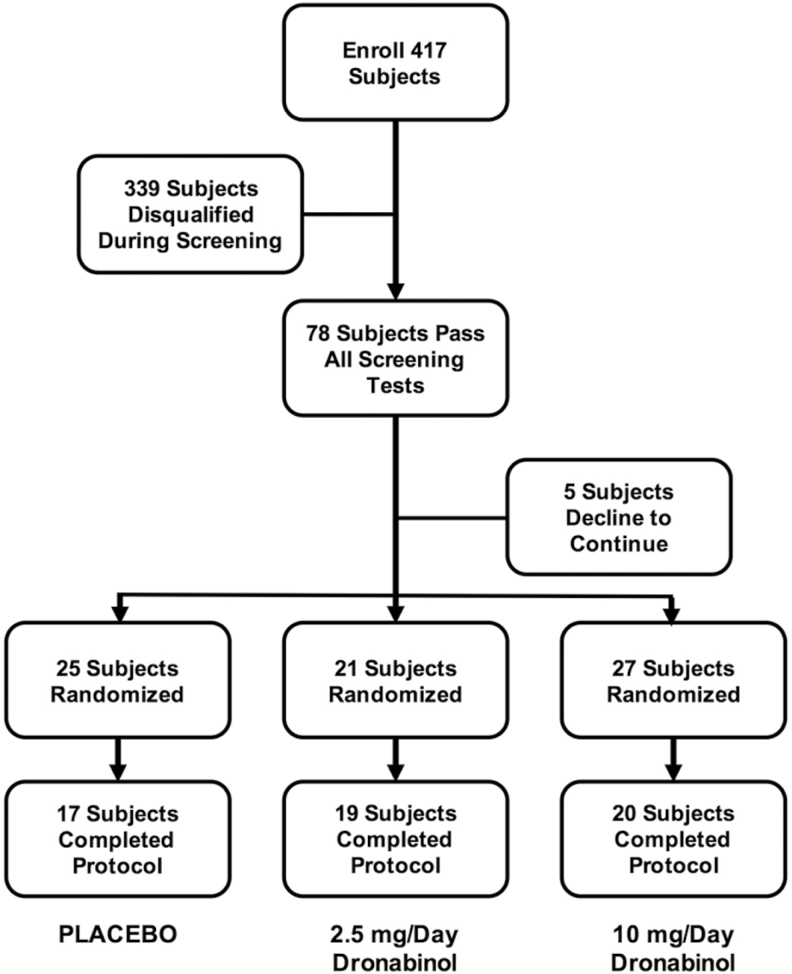
Figure 1 depicts the disposition of all enrolled participants. Four hundred seventeen participants provided initial informed consent to participate and were enrolled. Seventy-eight of the 417 enrolled participants met all inclusion/exclusion criteria and 73 of these participants agreed to continue and were randomized to specific treatment groups. Fifty-three of these participants had moderate OSA (15 ≤ AHI < 30) and 20 had severe OSA (30 ≤ AHI ≤ 50). The randomization schedule was prepared and maintained by the investigational pharmacy at the University of Illinois at Chicago. The most frequent reasons for participant exclusion during the screening process were AHI out of range (97), reported recreational drug use or positive urine screen (60), ESS < 7 (24), use of CNS active medications (16), and BMI out of range (15). An additional 34 participants declined to continue their participation after initial enrollment but prior to final screening. Although the randomization schedule was balanced, the number of participants who completed the study per-protocol ranged from 17 to 20 per treatment group.
Figure 1.
Workflow diagram illustrating the disposition of 417 enrolled in the protocol.
Randomized participants initiated treatment the night immediately following their baseline screening PSG. Each randomized participant was provided blinded study agent in white capsules prepared on coded blister cards labeled with the date for each pill to be taken as well as storage and administration instructions. Participants self-administered one capsule by mouth, 60 minutes before bedtime, and completed a sleep/activity/drug-administration log daily throughout their participation. Participants were randomized to one of the three treatment groups: Placebo, 2.5 mg/day dronabinol or 10 mg/day dronabinol. These doses were selected based upon the previous pilot study of dronabinol in OSA15 and on the FDA-approved clinical dosing for appetite stimulation in AIDS-related wasting (2.5 to 20 mg per day).17 Participants maintained daily dosing for 42 days with repeated laboratory visits on days 14, 28, and 42. Each 14-day card supply carried four labeled reserve doses, allowing a ±2-day window for scheduling of repeat visits and also to replace lost or damaged dose units. They returned all unused study medication and were provided a new supply at each laboratory visit. Participants randomized to the Placebo or 2.5 mg/day groups received their assigned doses each day throughout the 42-day treatment period. In order to comply with FDA-approved dronabinol labeling, participants randomized to the 10 mg/day group completed an initial dose escalation phase, receiving 2.5 mg/day for 7 days, followed by 5 mg/day for 7 days, and then 10 mg/day dronabinol for the final 14 days of the 28-day treatment period. Treatment adherence was assessed by self-report (logged) study drug administration and by return of unused dosage units. At the initial screening visit, participants were instructed to maintain a regular schedule and sleep/wake/activity pattern throughout their participation. They were given sleep/activity/drug-administration logs, instructed in their use, and asked to return completed logs at each subsequent visit. They were cautioned that the study medication may cause drowsiness and that they should refrain from driving or operating equipment until they determined their reaction to the medication. Adverse events (AEs) were monitored by scheduled phone calls from study coordinators, by study logs, and by physician evaluation at laboratory visits.
Participants underwent overnight PSG/MWT at either University of Illinois at Chicago or Northwestern University. Participants reported to the sleep center at 20:30 and were instrumented for electroencephalogram (EEG; C3/A2, C4/A1, F3/A2, F4/A1, O1/A2, O2/A1), bilateral electrooculogram, electromyogram (submental, bilateral anterior tibial), electrocardiogram, oronasal airflow (thermistor and nasal pressure transducer), thoracoabdominal motion (piezo crystals), arterial oxygen saturation by pulse oximetry, snoring, and body position. All signals, including infrared digital video, were acquired and stored using Alice5 (Respironics) or Polysmith 8 (Nihon Kodon) digital PSG systems. All biopotential signals were digitized at 500 samples per second. Records were securely transferred to a centralized data coordinating center using the European Data Format. Participants were administered their study medication 60 minutes before lights out when in the laboratory and were given an 8-hour time in bed sleep opportunity.
Sleep was scored by a single registered polysomnographic technologist according to standard criteria on 30-second epochs, and respiratory events were scored according to American Academy of Sleep Medicine guidelines.18 Specifically, hypopneas were scored when a reduction in airflow of >50% was associated with either an oxygen desaturation of >3% or an arousal. EEG arousals were scored according to American Academy of Sleep Medicine guidelines.19 Time in bed (TIB) was computed as the number of minutes in bed between lights out and lights on, total sleep time (TST) as the number of minutes of sleep between lights out and lights on, and sleep efficiency as the ratio of 100 × TST/TIB. The distribution of sleep stages was separately computed as percentages of total sleep time for stage N1 or N2, stage N3, and stage rapid eye movement (REM).
Commencing 2 hours after completion of each overnight PSG, a 4-trial 40-minute MWT protocol was conducted according to guidelines published by the American Academy of Sleep Medicine.20 Specifically, trials were separated by 2 hours, and for each trial, participants were seated comfortably in the same room used for overnight testing and instructed to sit still and remain awake for as long as possible, without looking directly into the light. Indirect illumination was provided with an intensity at the participant’s eyes of approximately 0.1–0.15 lumens. Each trial was ended after 40 minutes of wakefulness, or after 10 consecutive epochs of sleep. For each trial, sleep latency was defined as three consecutive epochs of stage 1 sleep or one epoch of any other sleep stage, based on central and occipital EEG derivations. A latency of 40 minutes was assigned if sleep was not observed. A single board-certified polysomnographer, blinded to participant and treatment, scored all PSG and MWT studies reported here.
Statistical Analysis
All analyses reported here are based on the intent to treat (ITT) population, comprising all participants who received at least one dose of study medication (N = 73). Safety was assessed by tabulation of the frequency and nature of AEs in relation to treatment group, as well as other biometric variables. Tolerability was assessed by tabulation of TSQM scores, again in relation to treatment group and biometric variables. Adherence to the medication regimen (quantified by missed doses per 2-week treatment interval) was used as a secondary tolerability measure. Measures of association were tested by the likelihood ratio (χ2).
The primary efficacy measure was changed from baseline in the AHI at the end of treatment. The changes in AHI in the active treatment groups were compared with the Placebo group. Additional primary endpoints included changes in ESS score and MWT mean sleep latency. Changes in AHI, ESS, and MWT were assessed using direct likelihood estimation of linear mixed effect models. Because the individual participants were assessed at multiple time points during the trial, these models included participant-specific random effects to account for the correlation between these measurements. We chose the direct likelihood estimation method, as “likelihood methods are in principle the most efficient because they do not involve simulation at all.”21 This approach carried the additional advantage that data imputation methods were un-necessary.22 This modeling approach also allowed specific contrasts between active treatment groups and the Placebo group to be assessed at each available time point.22 Baseline values of age, race, ethnicity, gender, and AHI scores were introduced into the models as potential explanatory covariates. Similar models were employed to conduct secondary endpoint analyses of change in AHI stratified according to event type (apnea/hypopnea) and sleep stage (non-rapid eye movement [NREM]/REM), as well as to measures of sleep architecture and oxygenation. Assessment of these secondary endpoints was viewed as exploratory and p values are reported without adjustment.
A priori power calculation suggested that 37 participants per treatment group would be sufficient to detect simultaneous effects in three primary endpoints, with an α level of 0.05 and a power for each endpoint of 80%. This estimate was based on the effect size for dronabinol on AHI (Cohen’s d = 0.59) estimated from our previous pilot study of dronabinol in OSA.15 In all cases, p < .05 was considered statistically significant.
RESULTS
Table 1 presents baseline characteristics of the full ITT population. There were no significant differences between treatment groups in polysomnographic characteristics, blood pressure, BMI, or gender, race, or ethnicity distributions. Age did differ between treatment groups with placebo participants being oldest and low dose dronabinol participants being youngest. These age differences were not clinically meaningful. Additionally, there were trends toward differences in ESS score and MWT mean sleep latency at baseline between treatment groups. Participants’ characteristics did not differ between the two recruiting sites in terms of age, gender, ethnicity, BMI, or baseline AHI, ESS, or MWT scores. The majority of participants randomized at the University of Illinois at Chicago were African American, whereas the majority of participants randomized at Northwestern University were white (p = .06).
Table 1.
Baseline Characteristics of Participants Randomized to Treatment.
| Baseline | N | Placebo | Dronabinol 2.5 mg/day | Dronabinol 10 mg/day | p |
|---|---|---|---|---|---|
| ESS, mean (SD) | 73 | 11.1 (3.8) | 10.2 (3.5) | 12.8 (3.8) | .052 |
| MWT, mean (SD) | 73 | 22.7 (12.9) | 20.6 (12.2) | 15.0 (9.4) | .051 |
| Age, mean (SD) | 73 | 58.8 (6.1) | 52.7 (7.7) | 54.7 (7.0) | .04 |
| Gender | 73 | 25 | 21 | 27 | .77 |
| Male | 52 | 18 | 16 | 18 | |
| Female | 21 | 7 | 5 | 9 | |
| Race | 73 | 25 | 21 | 27 | .08 |
| White | 37 | 9 | 16 | 12 | |
| Black or African American | 32 | 14 | 5 | 13 | |
| Asian | 3 | 1 | 0 | 2 | |
| Unknown/undisclosed | 1 | 1 | 0 | 0 | |
| Ethnicity | 73 | 25 | 21 | 27 | .25 |
| Hispanic or Latino | 9 | 4 | 4 | 1 | |
| Not Hispanic or Latino | 63 | 21 | 17 | 25 | |
| Unknown/not reported | 1 | 0 | 0 | 1 | |
| BMI, mean (SD) | 73 | 33.7 (14.8) | 33.1 (10.8) | 33.5 (4.8) | .94 |
| Systolic pressure, mean (SD) | 73 | 133.5 (14.8) | 129.3 (10.8) | 129.9 (15.0) | .53 |
| Diastolic pressure, mean (SD) | 73 | 84.5 (11.4) | 82.0 (9.5) | 81.2 (9.9) | .51 |
| Polysomnographic variables | |||||
| AHI, mean (SD) | 73 | 23.9 (9.6) | 28.2 (12.5) | 26.0 (11.9) | .46 |
| Total sleep time (min) | 73 | 363.6 ± 60.8 | 383.9 ± 54.0 | 397.5 ± 59.0 | .12 |
| Sleep efficiency (%) | 73 | 75.9 ± 12.6 | 80.2 ± 11.3 | 82.7 ± 12.2 | .13 |
| Arousal index | 73 | 35.9 ± 23.2 | 31.9 ± 21.4 | 30.3 ± 16.3 | .60 |
| Mean nadir SpO2 | 73 | 91.7 ± 2.5 | 90.9 ± 2.8 | 91.2 ± 2.4 | .57 |
| Minimum SpO2 | 73 | 79.0 ± 9.3 | 79.4 ± 6.7 | 80.0 ± 6.9 | .90 |
SD = standard deviation; ESS = Epworth Sleepiness Scale; MWT = multiple sleep latency test mean sleep latency; BMI = body mass index; AHI = apnea–hypopnea index; SpO2 = oxygen saturation by pulse oximetry.
p-Values are for one-way ANOVA.
Safety
Most (88%) participants experienced one or more AEs during their participation. The likelihood of reporting one or more AEs did not differ according to treatment group (p = .16), gender (p = .35), race (p = .49), or ethnicity (p = .72). The average number of AEs reported by the 73 participants in the ITT group was 4.1 ± 4.0 and this did not differ from Placebo (3.4 ± 2.9) among participants receiving either 2.5 mg/day (2.8 ± 3.6) or 10 mg/day (5.8 ± 4.7) of dronabinol. The number of AEs reported by women (5.5 ± 5.6) tended to be higher than the number reported by men (3.4 ± 3.1; p = .057 by one-way ANOVA); and the number reported by black participants (5.4 ± 5.1) was significantly higher than reported by white participants (2.9 ± 2.3; p = .03 by Scheffe test).
The most frequently reported verbatim AEs included sleepiness/drowsiness (N = 25; 8% of total AEs reported), headache (N = 24; 8%), nausea/vomiting (N = 23; 8%), and dizziness/lightheadedness (N = 12; 4%). The frequency of these AEs did not differ according to treatment group (p > .1 for each).
As shown in Table 2, severity was rated as mild for 73% of AEs, moderate for 25%, and severe for only 2%; the distribution of AE severity did not differ by randomization group (p = .47). Similarly, AE severity did not differ by ethnicity (p = .16), but women versus men tended to report more severe AEs (p = .06), as did black versus white participants (p = .02). Half of all AEs were judged to be unrelated to the study agent, with 44% judged as possibly related and 5% as probably related.
Table 2.
AE Characteristics.
| Action | Outcome | Severity | Relation to study agent | Expected | SAE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agent withdrawn | 7 | Resolved | 283 | Mild | 218 | Unrelated | 152 | Yes | 104 | Yes | 2 |
| Dose reduced | 0 | Resolving | 1 | Moderate | 76 | Possibly Related | 132 | No | 196 | No | 298 |
| Dose increased | 0 | Persisting | 0 | Severe | 6 | Probably related | 16 | ||||
| Dose not changed | 279 | Resolved w/ Sequelae | 0 | Life threatening | 0 | Related | 0 | ||||
| Unknown | 0 | Fatal | Fatal | 0 | |||||||
| Not applicable | 14 | Unknown | 16 | ||||||||
AE = adverse event; SAE = serious adverse event.
Of the 73 participants randomized to treatment, two participants experienced serious adverse events: one participant was struck by a car while riding a bicycle during the placebo run-in period and discontinued participation; one participant experienced diarrhea and vomiting while on treatment and was admitted to the hospital for hydration and monitoring. This participant was released after 24 hours and continued to complete the study—the hospital admission was not reported to the study team until after discharge. This AE was judged to be possibly related to study medication. Six other participants withdrew or were withdrawn by investigators due to adverse events. One participant experienced lactose intolerance during the placebo run-in period and withdrew; one participant reported flu-like symptoms that were disclosed to have started before randomization and withdrew; one participant experienced dizziness and vision changes possibly related to study medication and was withdrawn; one participant reported vertigo probably related to study medication and withdrew; one participant experienced ECG arrhythmias detected during PSG after 4 weeks of treatment, which were judged to be possibly related to study medication and was withdrawn; one participant reported headache, dizziness, and vomiting during the first 2 weeks of treatment judged to be possibly related to study medication and was withdrawn.
Tolerability
As illustrated in Table 3, the TSQM allowed the participant to rate overall treatment satisfaction on a 7-point Likert scale from “extremely dissatisfied” to “extremely satisfied.” The distribution of scores on this scale did not differ (p = .26) between randomization groups at the end of the placebo run-in period. In contrast, there was a significant association between treatment satisfaction and randomization group at the end of 6-weeks treatment (Table 3; p = .04), with participants receiving 10 mg/day dronabinol expressing the greatest overall satisfaction.
Table 3.
End of Treatment Satisfaction Questionnaire for Medication—Per Protocol Participants.
| Tx response | Placebo | 2.5 mg/day dronabinol | 10 mg/day dronabinol | Total |
|---|---|---|---|---|
| Extremely dissatisfied | 3 | 2 | 1 | 6 |
| Very dissatisfied | 1 | 2 | 0 | 3 |
| Dissatisfied | 0 | 3 | 0 | 3 |
| Somewhat satisfied | 5 | 6 | 4 | 15 |
| Satisfied | 1 | 4 | 4 | 9 |
| Very satisfied | 5 | 1 | 5 | 11 |
| Extremely satisfied | 1 | 1 | 6 | 8 |
| Total | 16a | 19 | 20 | 55 |
p = .04. Tx = treatment.
aTSQM data missing from one participant randomized to receive placebo medication
As noted above, 7 of the 73 participants randomized to treatment discontinued participation prior to completing the protocol due to a variety of adverse events. An additional 10 participants elected to discontinue prior to completing the protocol due to a range of reasons such as taking a new job, family illness, unexpected travel requirements, or participant burden of participation. Thus, 56 participants completed participation per-protocol. Randomized participants who completed the protocol did not differ from participants discontinuing early according to treatment randomization group (p = .15), BMI (p = .28), baseline AHI (p = .97), TSQM scores (p = .29), race (p = .21), or ethnicity (p = .26), but there was a trend for a greater fraction of men (15/52) to discontinue than women (2/21) (p = .07). The average number of AEs reported by randomized participants who discontinued participation early (4.12 ± 3.10) did not differ (p = .99 by ANOVA) from those who completed the protocol (4.11 ± 4.31).
Another metric of tolerability is adherence to treatment. Table 4 summarizes the number of “missed doses” for each 2-week treatment interval. Overall, nearly two-thirds of participants took all scheduled doses and returned the correct number of dosage units across all treatment intervals. The maximum number of missed doses in any 2-week treatment interval was 7. As shown in Table 4, the distribution of missed doses was equivalent during all three treatment intervals (p = .22) and was equivalent for all three treatment groups during each 2-week interval (p > .33 for each).
Table 4.
Number of Doses Missed.
| Missed doses | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Interval | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total |
| Escalation | 49 (74%) | 10 | 4 | 3 | 0 | 0 | 1 | 1 | 66 |
| Wks 2–4 | 37 (64%) | 15 | 3 | 1 | 0 | 0 | 2 | 0 | 58 |
| Wks 4–6 | 32 (57%) | 13 | 3 | 4 | 2 | 0 | 0 | 0 | 56 |
| Total | 118 | 38 | 10 | 8 | 2 | 0 | 3 | 1 | 180 |
p = .22 for association between missed doses and time on treatment.
p > .33 for association between missed doses and treatment group at each time interval.
Wks = weeks.
Neither placebo treatment (−0.2 ± 2.6 kg) nor dronabinol at 2.5 mg/day (−0.5 ± 3.9 kg) or 10 mg/day (0.5 ± 0.7 kg) caused any weight change among study participants (p ≥ .4 for each).
Efficacy—Primary
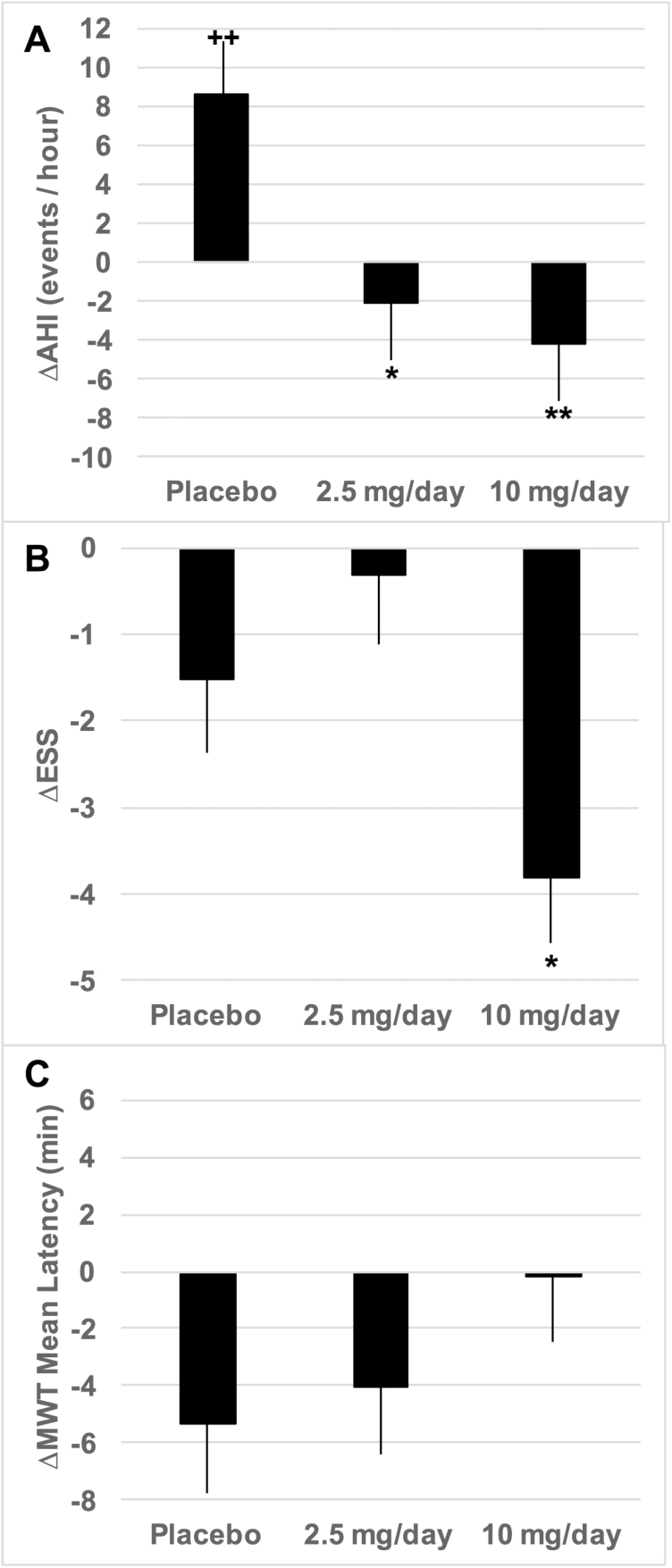
By design, the primary efficacy endpoint for this study was change from baseline in AHI after 6-weeks of treatment. Figure 2A depicts the end-of-treatment change from baseline AHI for each of the treatment groups. With respect to baseline, the AHI increased significantly among Placebo participants (p = .01), whereas it decreased among participants receiving dronabinol. ∆AHI was significantly negative in comparison to Placebo for participants receiving dronabinol at either 2.5 (p = .02) or 10 (p = .003) mg/day. ∆AHI did not differ significantly between the 2.5 and 10 mg/day dronabinol groups. We repeated this analysis entering age, race, ethnicity, and baseline AHI as covariates. After adjusting for these covariates, the main effect of treatment group remained significant (Table 5), whereas none of the other covariates was significant. After adjustment, the increase in AHI from baseline in the Placebo-treated group was smaller and not statistically significant (p = .45). Conversely, the decrease from baseline AHI in the group treated with 10 mg/day dronabinol was greater than without adjustment. Again, adjusted ∆AHI was significantly negative in comparison to Placebo for participants receiving dronabinol at either 2.5 (p = .02) or 10 (p = .004) mg/day. Adjusted ∆AHI did not differ significantly between the 2.5 and 10 mg/day dronabinol groups (Table 5).
Figure 2.
Efficacy of dronabinol after 6 weeks of treatment. Bars depict mean ± SE for each treatment group. (A) Change from baseline in AHI. *p = .02 versus Placebo. **p = .003 versus Placebo. Mean values for 2.5 and 10 mg/day did not differ significantly (p = .60). ++p = .01 versus no change from baseline AHI. (B) Change from baseline in ESS total score. *p = .05 versus Placebo; p = .002 versus 2.5 mg/day and p < .0001 versus no change from baseline. ESS score for Placebo and 2.5 mg/day groups did not differ from baseline values. (C) Change from baseline in MWT mean sleep latency. ∆MWT sleep latency was equivalent among all treatment groups and the mean change was not different from zero in any treatment group.
Table 5.
Treatment Effects Stratified by Event Type and Sleep Stage—Adjusted for Baseline AHI, Age, Gender, Race, and Ethnicity.
| Treatment parameter |
Placebo | 2.5 mg/day | 10 mg/day | p for Tx effect |
|---|---|---|---|---|
| AHI | 4.1 ± 5.5 | −6.6 ± 5.9 | −8.5 ± 5.2 | .05 |
| p vs Placebo | — | 0.02 | 0.004 | — |
| p vs 2.5 mg/day | — | — | 0.65 | — |
| AHI NREM | 3.0 ± 6.0 | −8.7 ± 6.4 | −10.2 ± 5.7 | .07 |
| p vs Placebo | — | 0.02 | 0.005 | — |
| p vs 2.5 mg/day | — | — | 0.74 | — |
| AHI REM | 7.7 ± 7.7 | −4.5 ± 8.3 | −5.5 ± 7.3 | .11 |
| p vs Placebo | — | 0.05 | 0.02 | — |
| p vs 2.5 mg/day | — | — | 0.86 | — |
| AI NREM | 1.0 ± 5.2 | −11.6 ± 5.6 | −8.1 ± 4.9 | .02 |
| p vs Placebo | — | 0.004 | 0.03 | — |
| p vs 2.5 mg/day | — | — | 0.70 | — |
| AI REM | 3.6 ± 7.3 | −11.8 ± 7.9 | −13.7 ± 7.0 | .001 |
| p vs Placebo | — | 0.01 | 0.002 | — |
| p vs 2.5 mg/day | — | — | 0.28 | — |
| HI NREM | 2.1 ± 3.2 | 2.8 ± 3.4 | −2.1 ± 3.0 | .01 |
| p vs Placebo | — | 0.76 | 0.09 | — |
| p vs 2.5 mg/day | — | — | 0.04 | — |
| HI REM | 4.2 ± 4.7 | 7.2 ± 5.1 | 8.1 ± 4.5 | .04 |
| p vs Placebo | — | 0.44 | 0.28 | — |
| p vs 2.5 mg/day | — | — | 0.79 | — |
| Supine AHI | 5.7 ± 6.9 | −10.0 ± 7.9 | −11.4 ± 5.9 | .001 |
| p vs Placebo | — | 0.01 | 0.005 | — |
| p vs 2.5 mg/day | — | — | 0.87 | — |
| Nonsupine AHI | 1.0 ± 5.0 | 4.5 ± 3.3 | −6.6 ± 3.6 | .02 |
| p vs Placebo | — | 0.60 | 0.03 | — |
| p vs 2.5 mg/day | — | — | 0.01 | — |
Values are mean ± standard deviation.
AI = apnea index (apneas/h within sleep stage); HI = hypopnea index (hypopneas/h within sleep stage); Tx = treatment. Bold text indicates statistical significance at p < .05.
Self-reported daytime sleepiness, assessed by the ESS, was a second primary efficacy endpoint. In comparison to baseline, participants receiving Placebo treatment and those receiving 2.5 mg/day dronabinol showed no change in ESS score (Figure 2B). In contrast, participants receiving 10 mg/day dronabinol showed significantly decreased sleepiness in comparison to baseline (p < .0001), and this decrease was also significant with respect to the changes observed with treatment by Placebo (p = .05) or 2.5 mg/day dronabinol (p = .002). Conversely, objective sleepiness measured by the MWT mean sleep latency, the third primary efficacy endpoint, did not change from baseline in any treatment group (Figure 2C).
Efficacy—Secondary
Table 5 delineates the effects of treatment on disordered breathing events in the ITT population stratified by sleep stage, event type, and body position, showing values adjusted for age, gender, race, ethnicity, and baseline AHI. As for overall AHI, both NREM AHI and REM AHI decreased significantly in comparison to Placebo for both 2.5 and 10 mg/day dronabinol treatment groups, which did not differ significantly from each other.
As shown in Table 5, these effects on AHI were driven primarily by changes in the expression of apneas. As for overall AHI, both the NREM apnea index (AI) and the REM AI were decreased significantly with respect to Placebo by both doses of dronabinol. In fact, the impact of 10 mg/day dronabinol on REM AI was the largest treatment effect size for any event type in any sleep stage. In contrast, dronabinol had little net effect on the expression of hypopneas, with only the higher dronabinol dose tending to decrease NREM hypopnea index (HI) in comparison to Placebo (p = .09).
Table 6 demonstrates the impact of treatment on sleep architecture, sleep position, and oxygenation measures, providing baseline and end of 6-week treatment values for each parameter. Statistical assessments of significance for overall treatment effects and contrasts between treatment groups were again derived from linear mixed models applied to within participant changes from baseline to end of treatment using the per-protocol dataset. As can be seen from the table, there were no statistically significant changes in sleep architecture or oxygenation observed for any of the treatment groups, with the exception of a slight increase in REM% among participants treated with dronabinol and a concomitant decrease in REM% among Placebo-treated participants. In addition, there were no significant changes in the duration of supine sleep from baseline to end of treatment in any treatment group.
Table 6.
Treatment Effects on Sleep Architecture.
| Treatment | Placebo | 2.5 mg/day | 10 mg/day | p for Tx effect | |||
|---|---|---|---|---|---|---|---|
| Parameter | Base | EOT | Base | EOT | Base | EOT | |
| TST (min) | 364 ± 61 | 384 ± 59 | 384 ± 54 | 401 ± 55 | 398 ± 61 | 423 ± 42 | .98 |
| p vs Placebo | — | 0.85 | 0.77 | — | |||
| p vs 2.5 mg/day | — | — | 0.64 | — | |||
| Supine (min) | 204 ± 162 | 252 ± 144 | 216 ± 114 | 186 ± 102 | 222 ± 132 | 234 ± 132 | .93 |
| p vs Placebo | — | 0.65 | 0.98 | — | |||
| p vs 2.5 mg/day | — | — | 0.76 | — | |||
| Efficiency | 76 ± 13 | 80 ± 14 | 80 ± 11 | 83 ± 11 | 83 ± 12 | 88 ± 10 | .91 |
| p vs Placebo | — | 0.80 | 0.92 | — | |||
| p vs 2.5 mg/day | — | — | 0.71 | — | |||
| SOL (min) | 14.3 ± 17.2 | 18.8 ± 33.6 | 16.8 ± 17.1 | 16.0 ± 18.7 | 8.6 ± 8.5 | 20.8 ± 31.8 | .14 |
| p vs Placebo | — | 0.54 | 0.39 | — | |||
| p vs 2.5 mg/day | — | — | 0.12 | — | |||
| REML (min) | 98 ± 52 | 114 ± 71 | 109 ± 60 | 89 ± 42 | 109 ± 69 | 83 ± 57 | .16 |
| p vs Placebo | — | 0.38 | 0.18 | — | |||
| p vs 2.5 mg/day | — | — | 0.91 | — | |||
| WASO (min) | 100 ± 52 | 71 ± 46 | 78 ± 48 | 61 ± 47 | 74 ± 58 | 42 ± 33 | .85 |
| p vs Placebo | — | 0.46 | 0.84 | — | |||
| p vs 2.5 mg/day | — | — | 0.32 | — | |||
| NREM% | 78.7 ± 6.8 | 81.3 ± 7.7 | 81.0 ± 4.7 | 76.9 ± 5.8 | 78.9 ± 4.7 | 77.0 ± 6.0 | .04 |
| p vs Placebo | — | 0.006 | 0.06 | — | |||
| p vs 2.5 mg/day | — | — | 0.33 | — | |||
| SWS% | 18.0 ± 9.0 | 14.6 ± 9.5 | 15.4 ± 7.4 | 15.9 ± 9.1 | 17.6 ± 7.8 | 17.4 ± 8.1 | .71 |
| p vs Placebo | — | 0.17 | 0.25 | — | |||
| p vs 2.5 mg/day | — | — | 0.78 | — | |||
| REM% | 21.3 ± 6.8 | 18.7 ± 7.7 | 19.0 ± 4.7 | 23.1 ± 5.8 | 21.1 ± 4.7 | 23.0 ± 6.0 | .04 |
| p vs Placebo | — | 0.006 | 0.06 | — | |||
| p vs 2.5 mg/day | — | — | 0.33 | — | |||
| Arousal index | 35.9 ± 23.2 | 37.8 ± 19.5 | 31.9 ± 21.4 | 42.7 ± 23.4 | 30.3 ± 16.3 | 33.8 ± 19.5 | .28 |
| p vs Placebo | — | 0.19 | 0.83 | — | |||
| p vs 2.5 mg/day | — | — | 0.24 | — | |||
| SpO2 < 85% (min) | 3.0 ± 7.4 | 4.2 ± 4.1 | 3.6 ± 7.3 | 3.4 ± 7.8 | 4.7 ± 10.0 | 4.5 ± 10.1 | .53 |
| p vs Placebo | — | 0.36 | 0.35 | — | |||
| p vs 2.5 mg/day | — | — | 0.99 | — | |||
| Mean SpO2 nadir | 91.6 ± 2.5 | 91.2 ± 2.7 | 90.9 ± 2.8 | 90.7 ± 2.8 | 91.2 ± 2.4 | 90.3 ± 2.9 | .47 |
| 0.79 | 0.46 | — | |||||
| p vs 2.5 mg/day | — | — | 0.30 | — | |||
Values are mean ± standard deviation.
Tx = treatment; EOT = end of treatment; Base = baseline value; TST = total sleep time; Supine = duration of supine sleep; Efficiency = sleep efficiency; SOL = sleep onset latency; REML = REM latency from sleep onset; WASO = wake after sleep onset; NREM% = percent total sleep time spent in NREM sleep; REM% = percent total sleep time spent in REM sleep; SpO2 = oxygen saturation by pulse oximetry.
Efficacy—Responder Analysis
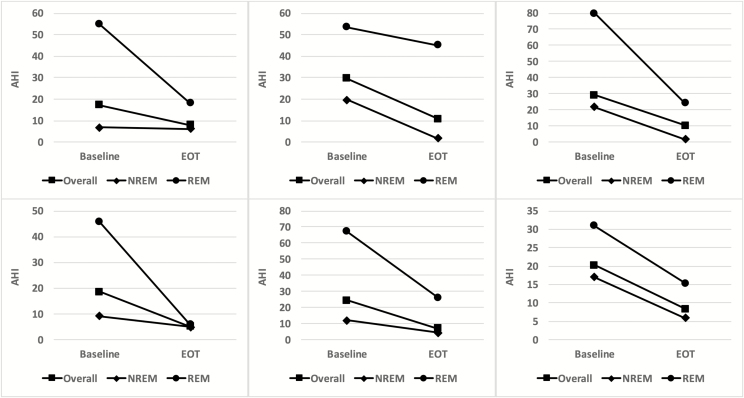
No clear consensus exists regarding what is a clinically meaningful response to OSA treatment. If we arbitrarily consider a final on-treatment AHI of ≤15 plus a reduction from baseline AHI of ≥50% to represent a clinically meaningful treatment response, 6 of 39 participants randomized to receive dronabinol were treatment “responders,” in contrast to 0 of 17 participants randomized to receive Placebo treatment (p = .03 for the association between active versus placebo treatment and responder status). Figure 3 presents the individual treatment responses of these six individuals in terms of AHI (mean decrease = 15.0 ± 4.0), NREM AHI (mean decrease = 10.3 ± 7.6), and REM AHI (mean decrease = 33.0 ± 17.8). Responders exhibited a threefold greater decrease in ESS score (−4.8 ± 4.3) than did nonresponders (1.6 ± 4.1), but this did not achieve statistical significance (p = .08), perhaps due to the small number of responders. There was no difference between responders (−2.0 ± 8.1 minutes) and nonresponders (−1.5 ± 12.0 minutes) in treatment-related change in mean sleep latency on the MWT.
Figure 3.
Treatment-related changes in AHI individual “responders.” Each panel depicts the AHI measured at baseline and at the end of treatment for a single responder (square symbols). In addition, AHI stratified according the NREM sleep (diamond symbols) and REM sleep (circle symbols) are depicted for each of these participants.
Because there were no “responders” among the Placebo-treated group, we focused on exploratory analysis of differing characteristics between responders and nonresponders on the 39 individuals who completed the protocol and were randomized to receive active treatment.
Treatment responders were slightly younger (47.8 ± 11.6 years) in comparison to nonresponders (54.8 ± 5.9 years; p = .03 by ANOVA), but responders and nonresponders were equivalent in terms of BMI, change in BMI, baseline AHI, baseline ESS, number of missed treatment doses, and number of AEs reported (Table 7). Sleep architecture did not differ between responders and nonresponders in terms of sleep efficiency, sleep onset latency, REM latency, arousal index, or percentages of slow-wave, NREM, or REM sleep (p > .10 for each). However, responders did achieve more total sleep than nonresponders during baseline PSG recordings (Table 7).
Table 7.
Responder Versus Non-Responder Characteristics.
| Nonresponder | Responder | p | |
|---|---|---|---|
| N | 33 | 6 | — |
| Age | 54.8 ± 5.9 | 47.8 ± 11.6 | 0.03 |
| BMI | 33.8 ± 4.9 | 34.9 ± 4.0 | 0.61 |
| Change in BMI | −0.1 ± 3.39 | 0.13 ± 1.10 | 0.92 |
| Baseline AHI | 28.3 ± 13.0 | 23.3 ± 5.4 | 0.36 |
| Baseline ESS | 11.7 ± 4.2 | 13.3 ± 3.5 | 0.37 |
| Missed doses/14 D | 0.94 ± 1.46 | 1.17 ± 1.47 | 0.73 |
| No. of AEs reported | 4.5 ± 4.8 | 5.5 ± 4.8 | 0.65 |
| Baseline TST | 374.1 ± 59.5 | 423.4 ± 29.6 | 0.05 |
| Baseline REM AHI | 37.0 ± 19.7 | 55.5 ± 17.3 | 0.04 |
| Baseline NREM AHI | 26.2 ± 16.6 | 14.5 ± 6.0 | 0.1 |
| Base REM/total AHI | 1.6 ± 1.0 | 2.4 ± 0.6 | 0.05 |
| Base mean event Dur | 26.9 ± 5.4 | 22.1 ± 3.2 | 0.04 |
Values are mean ± standard deviation.
BMI = body mass index; AHI = apnea–hypopnea index; ESS = Epworth Sleepiness Scale; D = days; AE = adverse event; TST = total sleep time; AHI = apnea–hypopnea index; Base = baseline; Dur = duration. Bold text indicates statistical significance at p < .05.
In contrast to overall sleep architecture, the characteristics of disordered breathing did differ significantly, with responders exhibiting a greater preponderance of REM-related apnea/hypopnea and a shorter average event duration in comparison to nonresponders. Both the REM AHI and the ratio of REM-AHI/overall-AHI were significantly higher among responders (Table 7).
We also employed logistic regression to model treatment-responder status. Age and race (using white as the index race) showed significant effects on the odds of being a treatment responder, with increasing age decreasing those odds (OR = 0.87 ± 0.06; p = .046) and black or Asian race increasing those odds (OR = 3.22 ± 1.90; p = .047).
DISCUSSION
This randomized, fully blinded, placebo-controlled trial supports that dronabinol is safe and well-tolerated in people with moderate or severe OSA. Moreover, dronabinol dose-dependently reduced AHI from baseline in comparison to Placebo during both NREM and REM sleep. This effect was accompanied by a dose-dependent reduction in self-reported but not objective daytime sleepiness. Furthermore, participants receiving the higher dose of dronabinol expressed significantly greater overall treatment satisfaction. We found no evidence for improved sleep architecture with dronabinol treatment.
The safety and tolerability of dronabinol use by people with OSA are supported by the present findings. Nearly 90% of all participants reported at least one adverse event while on treatment, but this proportion did not differ between Placebo and active treatment groups. Furthermore, neither the number nor severity of AEs differed between Placebo and either dronabinol treatment groups. There was a single serious adverse event involving diarrhea and vomiting that required overnight hospitalization. This serious AE was judged as possibly related to the study drug, but was transient and did not recur. The participant went on to complete the study. All of these observations argue that dronabinol, at doses from 2.5 to 10 mg/day, is safe for use by medically stable patients with moderate or severe OSA. Participants also tolerated and adhered well to daily self-administration of dronabinol. The average number of “missed” doses reported during each 2-week treatment interval was less than one, and this did not differ between the three treatment groups (p = .93). Conversely, overall treatment satisfaction did differ significantly among the groups, with those receiving 10 mg/day dronabinol expressing the greatest treatment satisfaction on the TSQM. Although not strictly a tolerability measure, weight gain is undesirable for most individuals with OSA and dronabinol is clinically indicated for appetite stimulation in AIDS-related wasting syndrome.17 Therefore, it is important to note that dronabinol did not cause weight gain over a 6-week treatment period in individuals with moderate or severe OSA.
This study also supports the potential efficacy of dronabinol, demonstrating improvements in AHI and ESS score in comparison to placebo. The potential mechanisms of these effects therefore have clinical relevance. Although the mechanisms of dronabinol’s action on respiratory pattern and upper airway stability cannot be determined from the present study, increased vagal afferent activity has been hypothesized to play a role in OSA. For example, apnea exacerbation has been associated with use of vagus nerve stimulation for epilepsy.23 Afferent vagal neurons express a range of somatic receptors, including both inhibitory CB112,13 and excitatory 5-HT324 subtypes. Under this hypothesis, activation of nodose ganglion 5-HT3 receptors would be expected to increase apnea propensity, whereas activation of CB1 receptors would be expected to attenuate apnea expression. Using an animal model for spontaneous sleep-related central apneas, both of these effects have been demonstrated.14,25 Intraperitoneal serotonin, which does not cross the blood brain barrier, increased apnea frequency,25 whereas both dronabinol and oleamide, an endocannabinoid modulator,26,27 dose-dependently reduced apnea frequency.14 Furthermore, pretreatment with dronabinol completely blocked the apnea exacerbation produced by intraperitoneal serotonin.14
The present findings extend those of our previous pilot study, expanding the treatment duration from 3 to 6 weeks, adding multiple additional outcome measures and employing a fully blinded, placebo-controlled, parallel groups randomized design. In the present study, the mean change in AHI for participants treated with 10 mg/day dronabinol was −12.9 in comparison to the Placebo group, with a Cohen’s d effect size of 0.80. This is in line with the findings of the pilot study, which demonstrated a mean treatment effect on AHI of −11.3 with a Cohen’s d effect size of 0.56 for eight participants receiving 1 week of dronabinol at a dose of 10 mg/day. Collectively, these results suggest that dronabinol may meaningfully affect breathing control in OSA, at least in individuals with mild or moderate disease severity. These findings further argue that the impact of dronabinol on AHI is durable, at least for 6 weeks. This is an important observation because the endocannabinoid system is thought to play important roles in synaptic plasticity,28,29 which could cause dronabinol effects to change over time. Sukys-Claudino et al. reported an average decrease in AHI of 13.9 (effect size = 0.23) following 1 month of treatment by 10 mg/day donepezil, suggesting that cholinergic signaling may be an important aspect of breathing control in people with OSA.30 In contrast, a single 10 mg dose of donepezil yielded no change in AHI.31 It is not possible from the present study to determine to what extent effects of dronabinol on sleep disordered breathing may be mediated by changes in cholinergic neurotransmission.
Most people with OSA experience a significant number of disordered breathing events during both NREM and REM sleep. Thus, for any therapeutic intervention to be generally effective, it should be able to control disordered breathing throughout all stages of sleep. Here, we observed significant dronabinol-related decreases of disordered breathing events during both NREM and REM sleep. This is in contrast to our previous pilot study in which REM AHI was not decreased by dronabinol at doses between 2.5 and 10 mg/day.15 This discrepancy may reflect the smaller scale and/or shorter treatment duration of the pilot study in comparison to the present PACE trial. Consistent with this possibility, in the present study, the magnitude of the 10 mg/day dronabinol effect on REM AHI was only −3.6 with an effect size of 0.10 after 2 weeks of treatment, increasingly significantly to −11.9 with an effect size of 0.34 after 4 weeks of treatment.
The only statistically significant treatment-related change in sleep architecture associated with dronabinol treatment was a slight shift toward increased REM sleep percentage (Table 6). However, the mean baseline sleep efficiency for participants randomized to receive 10 mg/kg dronabinol was 83%, which is considered in the normal range for the laboratory environment; a nonsignificant improvement to 88% was observed, suggesting a possible ceiling effect. Arousal index did not decrease, but many disordered breathing events are not associated with EEG-observable arousal. Furthermore, scoring guidelines for EEG arousals place duration constraints on detection of arousals and on the duration of preceding sleep necessary for arousal detection.19 These constraints may collectively contribute to the observation that the frequency of arousals was reduced less than the frequency of apneas and hypopneas in the present study. Percentages of slow-wave sleep were normal at baseline for all treatment groups, making it unsurprising that these percentages did not change significantly with treatment.
OSA is recognized as a multifactorial disorder, and increasing attention has been paid to defining various OSA phenotypes based on the expectation that such phenotypes may respond differently to various treatment interventions (for a recent review, see Ref.32). From this perspective, it is unlikely that any drug treatment could be fully effective in all people with OSA. Here, despite clinically meaningful and statistically significant effects of dronabinol in reducing AHI and increasing daytime alertness, we observed considerable interparticipant variability in the magnitude of these effects. Although the study size was too small to make any firm characterization of “responders,” we note that participants with predominantly REM-related apnea appeared to exhibit the greatest treatment responses, as did younger and non-white individuals.
Limitations
To the best of our knowledge, the PACE clinical trial is the largest and lengthiest randomized controlled trial of any putative primary drug treatment for OSA. Despite this fact, the study may remain underpowered to detect simultaneous clinically meaningful effects in multiple endpoints. Despite randomizing only 21 to 27 participants per treatment group, we did in fact detect significant differences between Placebo and 10 mg/day dronabinol treatments for changes in AHI and ESS, but the observed effect sizes for these responses were greater than or equal to the estimate used for the power calculation. Considering the change with treatment in MWT scores, we observed a slight improvement in mean sleep latency for participants receiving 10 mg/day dronabinol and a reduction in sleep latency (greater sleepiness) for those receiving Placebo. However, the observed effect size for this difference was only 0.31 (Cohen’s d). Thus, based on the powering of the PACE study, it is possible that dronabinol may provide a small improvement in objective alertness measured by the MWT which can only be revealed by larger future studies.
Another study limitation arises from the fact that only a single night of in-laboratory polysomnography was employed to assess the baseline severity of OSA in each participant. Although the “first-night effect” may substantially be the quantity and quality of sleep, this effect may have been minimized in the present study participants for at least two reasons: (1) all of our participants were sleepy at baseline (ESS score of 8–19) and (2) most of our participants had previously undergone clinical polysomnography. Nonetheless, significant night-to-night variability of AHI has been reported. Although this random variability would lead to AHI measurement imprecision, based on the study design, this imprecision was most probably evenly distributed among all groups and did not contribute meaningfully to the observed average treatment effects.
In view of the numerous inclusion/exclusion criteria, there are also limitations to the generalizability of findings from the PACE trial. Considering the effect size on AHI, dronabinol might prove to be most appropriate for individuals with mild-to-moderate OSA, but individuals with mild disorder were excluded from the present study. The present PACE trial included only participants with daytime sleepiness. Although there was no correlation between baseline ESS score and treatment response assessed by change in AHI (p = .38), it remains unknown how patients without significant daytime sleepiness might respond to dronabinol. Additionally, individuals with OSA are often taking other CNS active drugs which could have impact on the efficacy of dronabinol, and these were excluded from the present study. Identifying the safety, tolerability, and efficacy of dronabinol in the broader clinical population of individuals with OSA will require larger future studies with fewer exclusion criteria.
Another effect of failing to fully achieve randomization targets is the fact that the group sizes were not fully balanced. This fact may have contributed to the slight differences in age as well as baseline scores for the ESS and MWT. We do not believe that these differences materially affected the analysis or interpretation of findings, however, because introducing age as well as baseline values of ESS and MWT into the linear mixed models for efficacy analyses did not change the findings.
Another potential limitation is the fact that participants randomized to Placebo treatment experienced a worsening of their OSA during participation in the protocol. It is not immediately obvious why this should occur, and we note that AHI did not exhibit any significant treatment-related increase after adjustment for participant age, gender, race, ethnicity, and baseline AHI in the models. All participants exhibited moderate or severe OSA upon randomization, with AHI between 15 and 50. Although fully documented clinical histories were not generally available, it is probably that all of these participants had developed OSA months or years prior to enrollment. It would therefore not be expected that the natural progression of their disease over a period of 6 weeks would account for the observed increases in AHI among participants in the Placebo treatment group. Nor did clinically significant progression of comorbid medical conditions probably contribute to the observed increase in AHI, given that participants were medically stable upon randomization, and were closely monitored and evaluated by a physician every 2 weeks during their participation. AHI often is higher during supine than nonsupine sleep. If participants randomized to placebo treatment slept mostly in nonsupine positions during baseline evaluation but supine during subsequent evaluations, this may have contributed to the observed increase in AHI over time on treatment. Conversely, if participants randomized to receive 10 mg/day dronabinol spent less time in supine sleep during treatment than at baseline, it may have contributed to the observed decrease in AHI. However, such positional effects do not account for our observations, because the minutes spent in supine sleep did not differ over time in any treatment group nor between treatment groups at any time point (Table 6). Moreover, the treatment effect of dronabinol specific to supine sleep was similar to the overall effect on AHI (Table 5).
In our view—and this would apply to participants randomized to any of the treatment groups—the mostly likely contributing factor to worsening OSA during the treatment period is the fact that potential participants were allowed to discontinue use of noninvasive treatments for OSA in order to participate. Participants were asked to verify that they had not used any treatment for their OSA within 1 month prior to randomization; it was not possible to objectively confirm this attestation.
Little is known regarding the long-term time course of OSA following withdrawal of noninvasive treatment. There is good evidence regarding the immediate or short-term impact of PAP withdrawal. Kribbs et al. noted that even a single night of PAP withdrawal caused AHI to increase significantly, but AHI remained at only 66% of the pretreatment baseline level.33 This finding was replicated by Phillips et al. who observed an increase in AHI to 59% of baseline level after 1 night of PAP withdrawal34 and a further increase to ~80% of baseline level after 7 days of withdrawal.34,35 Kohler et al. demonstrated an increased AHI to 75% of baseline levels after 2-weeks of PAP withdrawal in people with moderate-to-severe OSA.36 Thus, according to available evidence, AHI does not return to pretreatment level within 2 weeks of PAP withdrawal. We are unaware of any studies documenting the time course of the return of AHI to pretreatment level (or higher) beyond 2 weeks of PAP withdrawal. Still, it is physiologically plausible that full return to pretreatment AHI may take many weeks or even months after PAP withdrawal. We speculate that such an extended time course may reflect immediate exacerbation of AHI due to the acute loss of airway splinting by the positive airway pressure, followed by a much slower worsening due to return of upper airway edema consequent to the recurrence of airway traumatizing disordered breathing events during sleep. Indeed, Corda et al. showed that pharyngeal dimensions continued to increase between 1 week and 6 months after institution of PAP therapy in people with severe OSA, suggesting that this reflects ongoing reduction in soft tissue edema.37 If the converse is true, then withdrawal of PAP treatment may result in an initial acute increase in AHI followed by a slower recurrence of upper airway tissue edema and concomitant increase in AHI over a period of weeks to months. Collectively, these findings provide a basis to expect that some participants randomized into the PACE study would demonstrate progressively increasing AHI during their participation over a period of 4–10 weeks after PAP withdrawal. This effect would then be directly observable among those participants randomized to receive Placebo treatment.
CONCLUSION
This fully blinded, parallel groups, randomized placebo-controlled trial demonstrates that dronabinol is safe and well tolerated in participants with moderate-to-severe OSA. Dronabinol dose-dependently reduced AHI and improved self-reported but not objective sleepiness in comparison to Placebo over 6 weeks of oral administration. Dronabinol treatment may be a viable alternative or adjunctive treatment in selected people with OSA. Confirming these observations and defining the phenotypic characteristics of the most appropriate candidates for dronabinol therapy will depend on larger future trials.
FUNDING
This study was funded by National Institutes of Health, National Heart Lung and Blood Institute Grant Number UM1-HL112856 and National Center for Advancing Translational Sciences, Grant Numbers UL1TR001422 and UL1TR002003.
CLINICAL TRIAL REGISTRATION
Title: “Safety and Efficacy of Dronabinol to Treat Obstructive Sleep Apnea (PACE)
URL: https://clinicaltrials.gov/ct2/show/NCT01755091?term=PACE
Identifier: NCT01755091
DISCLOSURE STATEMENT
DWC reports grants from National Institutes of Health during the conduct of the study. DWC is an inventor of intellectual property assigned to the University of Illinois at Chicago, including US patent 7,705,039; US patent 8,207; US patent application 20130281523; and US patent application 20120231083. Collectively, these patents and applications relate to treatment of sleep-related breathing disorders by cannabinoid drugs. The University of Illinois has granted an exclusive license to these and related international patents to RespireRx Pharmaceuticals. RespireRx pays annual fees to the University of Illinois to maintain this license and plans to develop drug treatments for sleep-related breathing disorders. Upon commercialization of cannabinoid drug(s) for this purpose, RespireRx will pay royalties to the University of Illinois at Chicago. In addition, DWC holds shares of common stock in RespireRx Pharmaceuticals. He has not been paid by, nor does he advise RespireRx Pharmaceuticals.
ACKNOWLEDGMENTS
The authors gratefully acknowledge technical support provided by Julie Law, Kaitlyn Jeffries, Henry Arantes, Rosemary Ortiz, Gary Pearson, Natalie Pace, and James Sbarboro. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Heart Lung and Blood Institute Grant Number UM1HL112856 and National Center for Advancing Translational Sciences, Grant Numbers UL1TR001422 and UL1TR002003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009; 108(5): 246–249. [PMC free article] [PubMed] [Google Scholar]
- 2. Pagel JF. Obstructive sleep apnea (OSA) in primary care: evidence-based practice. J Am Board Fam Med. 2007; 20(4): 392–398. [DOI] [PubMed] [Google Scholar]
- 3. Hale CS. Obstructive sleep apnea and cardiovascular disease and mortality: the argument for causality. J Insur Med. 2005; 37(4): 272–282. [PubMed] [Google Scholar]
- 4. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007; 3(5): 467–472. [PMC free article] [PubMed] [Google Scholar]
- 5. Weaver TE, Kribbs NB, Pack AI et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997; 20(4): 278–283. [DOI] [PubMed] [Google Scholar]
- 6. Means MK, Edinger JD, Husain AM. CPAP compliance in sleep apnea patients with and without laboratory CPAP titration. Sleep Breath. 2004; 8(1): 7–14. [DOI] [PubMed] [Google Scholar]
- 7. Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016; 45(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith I, Lasserson TJ, Wright J. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006; (2):CD003002 doi:10.1002/14651858.CD003002.pub2. [DOI] [PubMed] [Google Scholar]
- 9. Kohler M, Bloch KE, Stradling JR. Pharmacological approaches to the treatment of obstructive sleep apnoea. Expert Opin Investig Drugs. 2009; 18(5): 647–656. [DOI] [PubMed] [Google Scholar]
- 10. Calik MW. Treatments for obstructive sleep apnea. J Clin Outcomes Manag. 2016; 23(4): 181–192. [PMC free article] [PubMed] [Google Scholar]
- 11. Carley DW, Radulovacki M. Pharmacology of vagal afferent influences on disordered breathing during sleep. Respir Physiol Neurobiol. 2008; 164(1-2): 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004; 24(11): 2708–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol. 2010; 299(1): G63–G69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carley DW, Paviovic S, Janelidze M, Radulovacki M. Functional role for cannabinoids in respiratory stability during sleep. Sleep. 2002; 25(4): 391–398. [PubMed] [Google Scholar]
- 15. Prasad B, Radulovacki MG, Carley DW. Proof of concept trial of dronabinol in obstructive sleep apnea. Front Psychiatry. 2013; 4: 1 doi:10.3389/fpsyt.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atkinson MJ, Sinha A, Hass SL et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004; 2: 12 doi:10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badowski ME, Perez SE. Clinical utility of dronabinol in the treatment of weight loss associated with HIV and AIDS. HIV AIDS (Auckl). 2016; 8: 37–45. doi:10.2147/HIV.S81420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep; 1999; 22(5): 667–689. [PubMed] [Google Scholar]
- 19. Members of Task Force EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992; 15(2): 173–184. [PubMed] [Google Scholar]
- 20. Littner MR, Kushida C, Wise M et al. ; Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005; 28(1): 113–121. [DOI] [PubMed] [Google Scholar]
- 21. Schafer JL. Analysis of Incomplete Multivariate Data. Boca Raton, UK and New York, Washington DC: CRC Press; 1997. 1 [Google Scholar]
- 22. Sitlani CM, Heagerty PJ, Blood EA, Tosteson TD. Longitudinal structural mixed models for the analysis of surgical trials with noncompliance. Stat Med. 2012; 31(16): 1738–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parhizgar F, Nugent K, Raj R. Obstructive sleep apnea and respiratory complications associated with vagus nerve stimulators. J Clin Sleep Med. 2011; 7(4): 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhuo H, Ichikawa H, Helke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol. 1997; 52(2): 79–107. [DOI] [PubMed] [Google Scholar]
- 25. Carley DW, Radulovacki M. Role of peripheral serotonin in the regulation of central sleep apneas in rats. Chest. 1999; 115(5): 1397–1401. [DOI] [PubMed] [Google Scholar]
- 26. Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic?Curr Med Chem. 1999; 6(8): 757–773. [PubMed] [Google Scholar]
- 27. Mendelson WB, Basile AS. The hypnotic actions of oleamide are blocked by a cannabinoid receptor antagonist. Neuroreport. 1999; 10(15): 3237–3239. [DOI] [PubMed] [Google Scholar]
- 28. Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004; 47Suppl 1: 345–358. [DOI] [PubMed] [Google Scholar]
- 29. Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004; 3(9): 771–784. [DOI] [PubMed] [Google Scholar]
- 30. Sukys-Claudino L, Moraes W, Guilleminault C, Tufik S, Poyares D. Beneficial effect of donepezil on obstructive sleep apnea: a double-blind, placebo-controlled clinical trial. Sleep Med. 2012; 13(3): 290–296. [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Owens RL, Sands S et al. The effect of donepezil on arousal threshold and apnea-hypopnea index. A randomized, double-blind, cross-over study. Ann Am Thorac Soc. 2016; 13(11): 2012–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eckert DJ. Phenotypic approaches to obstructive sleep apnoea–new pathways for targeted therapy. Sleep Med Rev. 2016. doi:10.1016/j.smrv.2016.12.003. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33. Kribbs NB, Pack AI, Kline LR et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993; 147(5): 1162–1168. [DOI] [PubMed] [Google Scholar]
- 34. Phillips CL, Yang Q, Williams A, Roth M. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea – PHILLIPS – 2007 – Journal of Sleep Research – Wiley Online Library. J Sleep Res. 2007; 16(2): 217–225 [DOI] [PubMed] [Google Scholar]
- 35. Phillips CL, Yee B, Yang Q et al. Effects of continuous positive airway pressure treatment and withdrawal in patients with obstructive sleep apnea on arterial stiffness and central BP. Chest. 2008; 134(1): 94–100. [DOI] [PubMed] [Google Scholar]
- 36. Kohler M, Stoewhas AC, Ayers L et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011; 184(10): 1192–1199. [DOI] [PubMed] [Google Scholar]
- 37. Corda L, Redolfi S, Montemurro LT, La Piana GE, Bertella E, Tantucci C. Short- and long-term effects of CPAP on upper airway anatomy and collapsibility in OSAH. Sleep Breath. 2009; 13(2): 187–193. [DOI] [PubMed] [Google Scholar]