Abstract
Study Objectives:
A single traumatic brain injury (TBI), even when mild (ie, concussion), can cause lasting consequences. Individuals with a history of chronic (>1-year prior) mild TBI have an increased risk of mood disturbances (eg, depression, suicide). This population also has lingering sleep alterations, including poor sleep quality and changes in sleep stage proportions. Given these sleep deficits, we aimed to test whether sleep-dependent emotional memory consolidation is reduced in this population. We utilized a mild TBI group (3.7 ± 2.9 years post injury) and an uninjured (non-TBI) population.
Methods:
Participants viewed negative and neutral images both before and after a 12-hour period containing sleep (“Sleep” group) or an equivalent period of time spent awake (“Wake” group). Participants rated images for valence/arousal at both sessions, and memory recognition was tested at session two.
Results:
The TBI group had less rapid eye movement (REM), longer REM latency, and more sleep complaints. Sleep-dependent memory consolidation of nonemotional images was present in all participants. However, consolidation of negative images was only present in the non-TBI group. A lack of differentiation between the TBI Sleep and Wake groups was due to poor performance in the sleep group and, unexpectedly, enhanced performance in the wake group. Additionally, although the non-TBI participants habituated to negative images over a waking period, the TBI participants did not.
Conclusions:
We propose disrupted sleep- and wake-dependent emotional processing contributes to poor emotional outcomes following chronic, mild TBI. This work has broad implications, as roughly one-third of the US population will sustain a mild TBI during their lifetime.
Keywords: Sleep, traumatic brain injury, concussion, memory, emotion.
Statement of Significance.
Mild traumatic brain injury (TBI) was recently noted to be a “silent epidemic” by the Centers for Disease and Control and Prevention. Over 1 million mild TBIs occur each year in the United States, and many individuals have lasting consequences from a single brain injury. Two of the most well-documented long-term sequelae of mild TBI are sleep disturbances (eg, sleep stage alterations) and a high risk for mood disturbances (depression, anxiety). The current work demonstrates both sleep- and wake-dependent emotional processing are disrupted following mild TBI. Future work should probe whether these disruptions are predictive of emotional dysfunctioning and whether sleep intervention can repair or rescue sleep-dependent alterations. These data have broad implications for any population at high risk for mild TBI, from the football field to the battlefield.
INTRODUCTION
Mild traumatic brain injuries (TBIs), or concussions, occur pervasively. It is estimated that 1.4–3 million people experience a TBI each year in the United States, with 70%–80% of these injuries being classified as mild (Glasgow Coma Score ≥13).1,2 Importantly, TBIs classified as mild are not necessarily benign: in 2003, the US Centers for Disease Control and Prevention released a report stating that mild TBI is a “silent epidemic,” the prevalence and severity of which are highly underestimated.3 Moreover, recent evidence from empirical studies suggests just a single, mild TBI can cause lasting, unfavorable consequences. Therefore, the need for exploration of this topic is both critical and broadly impactful.
Emotional Disturbances Following Mild TBI
Mild TBI patients exhibit a wide range of physical, cognitive, and emotional complaints chronically (>1 year since injury). One of the most well-documented, long-term impacts of mild TBI is an increased risk for mood disturbances. Specifically, new-onset depression is common following mild TBI,4,5 and elevated depression rates have been detected as long as 6 years after injury.6 A recent meta-analysis also identified an increased prevalence of anxiety symptoms following TBI, relative to a noninjured, control population.7 Additionally, perhaps as a consequence of post-TBI depression and anxiety, risk for suicide is elevated ~10 years after an individual sustains a concussion, even if mood disorders were not present preinjury.8 Yet, despite clear evidence linking head injury and emotional disturbances, the link between these two factors remains largely unexplored and poorly understood.
Sleep Disturbances Following Mild TBI
Sleep alterations are similarly common after TBI, both immediately9,10 and many years post injury.11 Specifically, chronically injured individuals report poorer sleep quality12 and daytime sleepiness13 relative to healthy controls. Objective recordings of sleep (via polysomnography [PSG]) demonstrate decreased sleep efficiency (time asleep/time in bed14), increased awakenings12, hypersomnia15, and sleep stage proportion changes several years post TBI. Recently, a large meta-analysis, which comprised nine studies, found individuals with a history of TBI tend to have less rapid eye movement (REM) than uninjured individiauls16. However, at present, it remains unclear whether TBI-induced sleep alterations have chronic, deleterious effects on health after brain injury.
Sleep and Emotional Processing
Sleep is a pillar of mental health: every major psychiatric disorder is associated with a high prevalence of sleep disturbances.17 Interestingly, it has been proposed that these sleep disturbances causally impact mood.18,19 That is, sleep alterations are not merely a side effect of the disorder but actively contribute to and maintain dysfunction. Accumulating evidence from the last decade supports this hypothesis.
Sleep, relative to wake, uniquely impacts cognition and emotion. For instance, we and others have shown that sleep, relative to a period spent awake, strengthens emotional memories through a process called sleep-dependent memory consolidation.20–22 In a separate study, we found sleep-dependent memory consolidation may causally impact subsequent (ie, next-day) mood. Specifically, we found sleep staging predicts post-sleep affect/mood but only when sleep-dependent emotional memory consolidation occurred.22 These findings indicate emotional memory consolidation plays a critical role in the relationship between sleep and emotional state. Therefore, a disruption in this underlying mechanism (sleep-dependent memory consolidation), potentially due to sleep stage or sleep quality alterations, can have consequences for emotional well-being.
Further, we21,22 and others23,24 demonstrated sleep and wake differentially impact emotion generation (eg, self-reported valence ratings). Specifically, when individuals are shown the same set of negative stimuli twice (with a period of sleep between viewing sessions), emotionality to negative stimuli is preserved. However, individuals who remain awake between the two sessions (during a normal waking day) habituate to negative stimuli, rating them as more neutral during the second presentation. Similar to memory, sleep-dependent preservation of emotion has been linked to sleep physiology, such that more REM leads to greater emotion preservation over a sleep period.21 Therefore, a disruption of sleep staging, and particularly REM, could disrupt this process.
The Current Study
One aim of the current study is to assess whether sleep-dependent memory consolidation is altered in individuals with chronic, mild TBI with the rationale that disrupted sleep-dependent processes could be a mechanism causing or maintaining mood disturbances in this population. To test this hypothesis, we utilized an emotional memory paradigm.21,22,25 We predicted emotional sleep-dependent memory consolidation would be reduced in a chronic, mild TBI population. Additionally, given the reduction in REM sleep following a mild TBI,16 the second study aim was to assess whether sleep-dependent emotion generation is intact in a mild TBI population. We hypothesized that sleep’s preservation of emotionality might be disrupted in individuals with chronic TBI as well.
MATERIALS AND METHODS
Participants
Eighty-one individuals (59 females and 22 males) between 18 and 30 years (20.01 ± 1.34) participated in the experiment. Participants had normal or corrected-to-normal vision and no history of neurological disease, sleep disorders, psychological disorders, or use of medications known to affect sleep or cognitive functioning (this information was self-reported). All participants were compensated with payment or course credit. Experimental procedures were approved by the University of Massachusetts, Amherst Institutional Review Board, and written informed consent was obtained. Procedures are in accordance with the Declaration of Helsinki.
Participants were assigned to a TBI or non-TBI group. Inclusion criteria for the TBI group included a history of a concussion more than 1 year before testing. Time since injury ranged from 1 to 10 years, with 64.1% of the sample having had a concussion within 1–3 years. Sixty-six percent of the participants had one concussion, 19.3% had a history of two concussions, 5.3% had three concussions, and 10.4% had four concussions. Concussion and symptom diagnosis were self-reported, as in our previous work.11 In the past, self-reported mild TBI has been linked with cognitive, neuropsychological, and physical impairments.26–30 Therefore, we feel participants’ self-reports are a valid measure of having had a concussion. Participants in the non-TBI group were required to have never had a concussion. Individuals unsure of their concussion status were not eligible. The criteria of mild TBI states that (at the very least) an “alternation in brain functioning” (eg, confusion, disorientation, loss of concentration) must occur following head injury.31 Given this, we only included participants with at least one symptom of altered brain functioning. We did not count the presence of a headache as altered brain functioning, as a headache can also come from external pain (eg, superficial injury). Given that participants were not aware of the hypotheses of the study, the sample comprised individuals with a range of symptoms following mild TBI (ie, the sample was not only comprised of individuals with cognitive or sleep complaints). However, the aim of this study was to investigate individuals with chronic, mild TBI, who are often “high functioning” (ie, those who may have little to no ongoing consequences of TBI), and, consequently, our sample comprises individuals on the very mild end of the TBI spectrum.
Participants in each group were further divided into either the Sleep or Wake condition. These conditions differed only in the timing of the performed behavioral tasks (described below).
Materials
Ninety emotionally negative and 90 emotionally neutral pictures were used. The majority of stimuli were obtained from the International Affective Picture System (IAPS).32 The rest were from an in-house set, chosen to match the IAPS pictures in content and emotionality.22,33 Normative data from our lab found negative pictures were moderate to high in arousal, and neutral pictures were low in arousal.
Procedure
This experiment had a between-subjects design with two conditions. Each condition consisted of two sessions. Participants in the “Sleep condition” had session 1 (Encoding) in the evening (between 08:00 pm and 10:00 pm) and session 2 (Recognition) 12 hours later (in the morning). On the other hand, participants in the “Wake condition” had session 1 in the morning (between 08:00 am and 10:00 am) and session 2 12 hours later (in the evening). Individuals in the Wake condition were asked not to nap or consume excessive amounts of caffeine between sessions. All participants were asked not to consume alcoholic beverages.
In the first session, participants provided written informed consent and completed a set of questionnaires including: (1) the Pittsburg Sleep Quality Index (PSQI34), a well-validated measure of self-reported sleep quality during the month prior, (2) the Morningness-Eveningness Questionnaire (MEQ35), an assessment of chronotype, (3) the Epworth Sleepiness Scale (ESS36), which gauges habitual sleepiness, and (4) the Stanford Sleepiness Scale (SSS37), which assesses the participant’s self-reported sleepiness at that given moment. Participants completed the SSS at both session 1 (SSS1) and session 2 (SSS2). Additionally, all participants performed a working memory task, the Digit Span Forward,38 at session 1 to confirm similar encoding ability between TBI and non-TBI groups.
A questionnaire regarding TBI characteristics was administered to the TBI group. This questionnaire probed time since TBI, number of TBIs, and which symptoms they experienced immediately after the most recent concussion occurred.
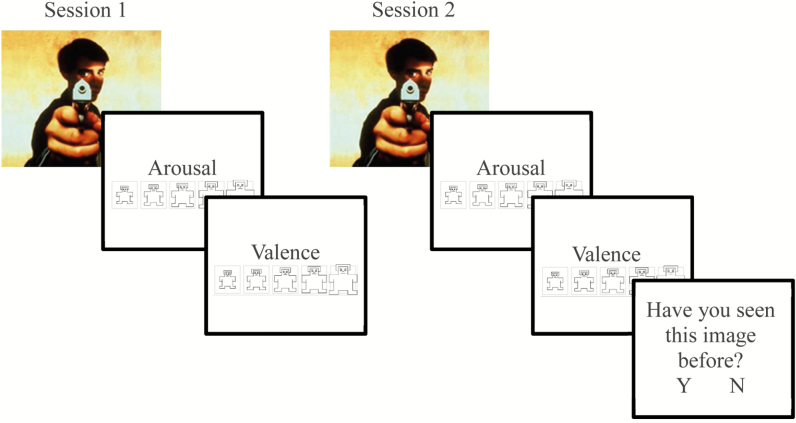
During Encoding, participants viewed 30 negative and 30 neutral target stimuli in pseudorandom order (Figure 1). Each picture appeared on the computer screen for 1000 ms followed by a 1000 ms interstimulus interval. After each picture, participants were prompted to rate the valence of the image on a nine-item self-assessment manikin (SAM) valence scale (1 = negative, 5 = neutral, 9 = positive). Immediately after, participants were prompted to rate the arousability of the image on a nine-item SAM arousal scale (1 = no arousal, 9 = highly arousing). Ratings were entered using numbers on a keyboard without any time limit. Importantly, participants were not informed that memory for the pictures would be subsequently tested. For this reason, each participant could only participate in one condition so that the unanticipated memory test could be preserved.
Figure 1.
Emotional memory consolidation and memory processing task.
During Recognition, which occurred 12 hours later, participants were shown 180 pictures: the 60 images from Encoding (targets) intermixed with 120 novel pictures (foils; 60 neutral and 60 negative). Pictures were displayed for 1000 ms, and participants again rated each for valence and arousal and indicated whether they had seen the picture during session 1 by pressing “Y” for yes and “N” for no.
Polysomnography
PSG was recorded in the Sleep condition participants (20 TBI, 20 non-TBI) in participants’ homes using the Aura PSG ambulatory system (Grass Technologies). Electrodes were applied after completion of the Encoding phase. The electrode montage included two electrooculogram (right and left ocular canthi), two chin electromyogram (EMG), and six cortical electroencephalogram (EEG) leads (O1, O2, C3, C4, F3, F4), with all channels referenced to the contralateral mastoid. Recordings were obtained and scored according to the specifications provided by the American Academy of Sleep Medicine.39 Due to equipment failure and failure of participants to initiate PSG devices before sleep, sleep physiology results are based on 14 participants in the non-TBI group and 16 in the TBI group.
Data Analysis
Statistical analyses were performed on SPSS 22 Software (IBM, Armonk, New York). Participants’ individual valence ratings of the pictures were used to categorize each stimulus for analyses.21,22,40 Targets were categorized based on ratings during the Encoding phase, and foils were categorized based on ratings during the Recognition phase. Negative and neutral pictures were defined as those rated 1–3 and 4–6, respectively. Therefore, the number of items in each emotion category (negative, neutral) varied across participants. Participants were excluded from analyses if they had fewer than 10 negative or neutral targets (n = 3; 2 non-TBI and 1 TBI). On average, participants rated 29.5 ± 8.5 images as neutral and 21.2 ± 4.9 as negative. These numbers did not differ between groups or conditions (p-values > .29).
Hit rate (HR) was defined as the percentage of target pictures correctly identified as previously seen. False alarms (FA) were defined as the percentage of foils that participants indicated as having been seen before. Finally, d’ (recognition memory discriminability) was calculated from these measures (reported in Supplemental Material). Corrected recognition (CR; HR-FA) was used as the main outcome measure, and d’ was used as a supplementary outcome measure.
Changes in valence (Δvalence) and arousal (Δarousal) ratings were calculated separately for negative and neutral target pictures. Specifically: Δvalence = session 2 mean valence rating − session 1 mean valence rating; Δarousal = session 2 mean arousal rating − session 1 mean arousal rating. Thus, a positive Δvalence score for a negative picture indicates a decrease of the initial negative reaction (toward neutrality). A positive Δarousal score indicates an increase in arousal from the first to the second session. All arousal data are reported in the attached Supplemental Material.
Comparisons of means were conducted using two-way analyses of covariance (ANCOVAs). Group (TBI vs. non-TBI) and condition (Sleep vs. Wake) were entered as predictor variables. The outcome variables were those calculated for memory, arousal, and valence (described above). Analyses were performed for neutral and negative emotions separately, resulting in six total ANCOVA tests. PSQI scores and gender were entered as covariates.21 Post hoc comparisons were conducted using planned comparisons when significant interactions between groups were present.
Where behavioral differences were found between groups, we used a linear regression to identify whether head injury factors, sleep characteristics, or both predicted behavioral outcomes in the TBI population. In the first step of the model, we entered head injury factors: number of TBIs, time since most recent TBI (years), and number of symptoms post-TBI. In the second step, we entered sleep factors: total sleep time, percent of the night in non-REM sleep stage 2 (N2), percent of the night in slow wave sleep (SWS), and percent of the night in REM sleep. These three sleep stages were included given that N2 and, more so, SWS have been implicated in sleep-dependent declarative memory consolidation11,41–43 and, more recently, emotional memory consolidation.44–47 REM has also been implicated in sleep-dependent emotional processing.19,21,48 For the analysis of variance, the whole sample, excluding outliers, was included. However, for the follow-up regressions, only participants with PSG were used.
When calculating descriptive statistics, a chi-square test was used to compare categorical variables (eg, gender) between groups, and independent-sample t-tests were used to compare continuous variables. Multivariate outliers were identified and removed using Cook’s Distance with a cutoff of 4/n (n = 2). Univariate outliers were identified and removed using the median absolute deviation (MAD) method with a moderate cutoff criterion (median ± 2.5 × MAD). In the case of univariate outliers, individuals who were an outlier in one domain (eg, memory) were not removed entirely but were removed only from that specific analysis. Therefore, when including multivariate outliers and those removed for having fewer than 10 negative or neutral targets (discussed above), there were six participants without memory data, five participants without valence data, and seven participants without arousal data.
RESULTS
Demographic Information
Demographic information is listed in Table 1. TBI and non-TBI groups did not differ in age, years of education, or gender. Similarly, participants assigned to Sleep and Wake conditions did not differ in age, years of education, or gender. Notably, there was a large proportion of female participants in this sample, representative of a typical psychology undergraduate program from which the sample was drawn.
Table 1.
Participant Demographics.
| Non-TBI (n = 41) | TBI (n = 40) | p-value | Sleep (n = 41) | Wake (n = 40) | p-value | |
|---|---|---|---|---|---|---|
| Age (years) | 20.15 ± 1.18 | 19.87 ± 1.5 | .37 | 19.82 ± 1.04 | 20.2 ± 1.6 | .22 |
| Education (years) | 14.85 ± 1.25 | 14.53 ± 1.28 | .27 | 14.6 ± 1.2 | 14.77 ± 1.34 | .56 |
| Gender (%F) | 70.0 | 75.6 | .37 | 68.3 | 77.5 | .24 |
| MEQ | 44.82 ± 8.11 | 44.91 ± 9.3 | .96 | 46.07 ± 8.85 | 43.6 ± 8.41 | .20 |
| ESS | 8.35 ± 3.56 | 8 ± 3.72 | .66 | 8.06 ± 3.6 | 8.29 ± 3.69 | .75 |
| SSS1 | 2.8 ± 1.38 | 3.17 ± 1.08 | .18 | 2.68 ± 1.03 | 3.3 ± 1.37 | .02 |
| SSS2 | 2.65 ± 1.57 | 2.42 ± 1.37 | .49 | 2.68 ± 1.33 | 2.38 ± 1.61 | .36 |
| PSQI | 4.97 ± 2.52 | 5.87 ± 1.97 | .08 | 5.56 ± 2.33 | 5.28 ± 2.28 | .59 |
| Digit Span | 9.4 ± 2.2 | 10.3 ± 2.6 | .11 | 10.0 ± 2.1 | 9.7 ± 2.7 | .64 |
| Time since TBI (year) | — | — | 4.32 ± 2.97 | 3.19 ± 2.77 | .21 | |
| Concussions (#) | — | — | 1.55 ± 1.05 | 1.73 ± 1.33 | .62 |
ESS = Epworth Sleepiness Scale; MEQ = Morningness-Eveningness Questionnaire; PSQI = Pittsburgh Sleep Quality Index; SSS1 and SSS2 = Stanford Sleepiness Scale at Sessions 1 and 2; TBI = traumatic brain injury.
Bold value indicates p-value < .05.
TBI and non-TBI participants did not differ in terms of MEQ, ESS, SSS1, or SSS2 scores. However, consistent with previous work,14,16,49 the TBI group had marginally higher PSQI scores, which was driven by a significant difference in the sleep latency component of the scale (time to fall asleep; non-TBI = 1.42, TBI = 2.1; t(78) = 2.57, p = .01). The Sleep and Wake conditions did not differ based on MEQ, SSS2, or ESS scores. However, the Wake conditions had higher SSS1 scores, indicating the Wake participants were sleepier during session 1 (which was a morning session) than the Sleep participants (for whom session 1 was an evening session). These results are consistent with work showing high sleepiness in young adults in the morning due to a circadian phase delay.50 For this reason, SSS1 scores were included as a covariate in all analyses. Finally, participants in the non-TBI and TBI groups did not differ in Digit Span performance, indicating no difference in working memory abilities.
Given that PSG data of several individuals were not available in the analyses (as previously discussed), we conducted an additional set of analyses without such participants. Demographic results were unchanged.
Head Injury Characteristics
The average time since the most recent concussion in the TBI group was 3.7 ± 2.9 years. Concussions were sustained from a sports-related injury (68%), fall (17%), car accident (8%), or by other means (6%). No concussions were sustained in a combat-related situation. Participants had an average of 4.5 ± 1.2 symptoms following concussion. As indicated in Table 2, the prevalence of loss of consciousness and post-traumatic amnesia were fairly low, indicating this sample was on the “minor” end of the mTBI spectrum. Sleep and Wake TBI groups did not differ on number of TBIs, time since TBI, and number of symptoms experienced after TBI.
Table 2.
Concussion Parameters.
| Symptom | Sleep (n = 20) | Wake (n = 20) | p-value |
|---|---|---|---|
| LOC (%) | 30.0 | 38.1 | .74 |
| PTA (%) | 10.0 | 4.7 | .60 |
| Nausea (%) | 50.0 | 52.4 | – |
| Vomiting (%) | 25.0 | 14.2 | .45 |
| Dizziness (%) | 80.0 | 85.7 | .69 |
| Headache (%) | 100 | 100 | – |
| Fatigue (%) | 95.0 | 85.7 | .60 |
| Concentration (%) | 80.0 | 66.6 | .48 |
| Sleep issues (%) | 10.0 | 28.5 | .23 |
| Total symptoms (#) | 4.7 ± 1.4 | 4.4 ± 1.0 | .56 |
LOC = loss of consciousness; PTA = post-traumatic amnesia.
Sleep Architecture
Sleep characteristics are listed in Table 3. There were no differences between the TBI and non-TBI Sleep groups for total sleep time, sleep efficiency, or time spent awake after sleep onset. There was a marginal difference for sleep latency, such that the TBI group had longer sleep latency than did the non-TBI group [t(19.3) = 1.85, p = .08 when adjusting for homogeneity of variance]. The TBI group also had significantly longer REM latency [t(28) = 2.37, p = .02]. There were no significant differences between the percent of the night groups spent in N1, N2, or SWS. However, the TBI group had significantly less REM than the non-TBI group [t(28) = −2.7, p = .01].
Table 3.
Sleep Characteristics.
| Sleep parameter | Non-TBI (n = 14) | TBI (n = 16) | p-value |
|---|---|---|---|
| TST (min) | 339.8 ± 61.5 | 365.8 ± 51.2 | .21 |
| Sleep efficiency (%) | 90.2 ± 2.2 | 88.2 ± 6.2 | .25 |
| WASO (min) | 26.9 ± 13.1 | 27.7 ± 15.2 | .87 |
| Sleep latency (min) | 10.4 ± 6.2 | 18.9 ± 17.0 | .08 |
| REM latency (min) | 107.6 ± 33.8 | 141.6 ± 43.2 | .02* |
| N1 (%) | 2.4 ± 1.7 | 2.3 ± 1.9 | .89 |
| N2 (%) | 54.2 ± 6.9 | 56.8 ± 7.6 | .33 |
| SWS (%) | 28.1 ± 7.4 | 30.1 ± 6.2 | .43 |
| REM (%) | 15.4 ± 3.7 | 10.8 ± 5.03 | .01* |
N1 = non-REM stage 1; N2 = non-REM stage 2; REM = rapid eye movement sleep; SWS = slow wave sleep; TBI = traumatic brain injury; TST = total sleep time; WASO = wake after sleep onset.
*p-value < .05.
Baseline Ratings of Stimuli
An ANCOVA test was used to probe whether there were differences in baseline ratings of valence between groups and conditions. For valence of neutral images, there was no main effect of Injury Group [F(1,67) = 0.78; p = .38], no main effect of Condition [F(1,67) = 1.67; p = .20], and no interaction between factors [F(1,67) = 0.72; p = .40]. Similarly, for negative valence, there was no main effect of Injury Group [F(1,67) = 2.34; p = .13], no main effect of Condition [F(1,67) = 0.27; p = .60], and no interaction between factors [F(1,67) = 2.4; p = .12].
Memory Accuracy
Memory was assessed following an interval including sleep or wake. ANCOVA analyses were used to separately gauge memory performance (CR) for negative and neutral stimuli. For neutral stimuli, we identified a main effect of Condition [F(1,66) = 6.64; p = .03], such that the Sleep condition had significantly higher CR than the Wake condition. There was no main effect of Injury Group [F(1,66) = 1.63; p = .20] and no interaction between these two factors [F(1,66) = 0.05, p = .83], indicating that the TBI group did not have a detectable deficit in sleep-dependent memory consolidation of neutral stimuli.
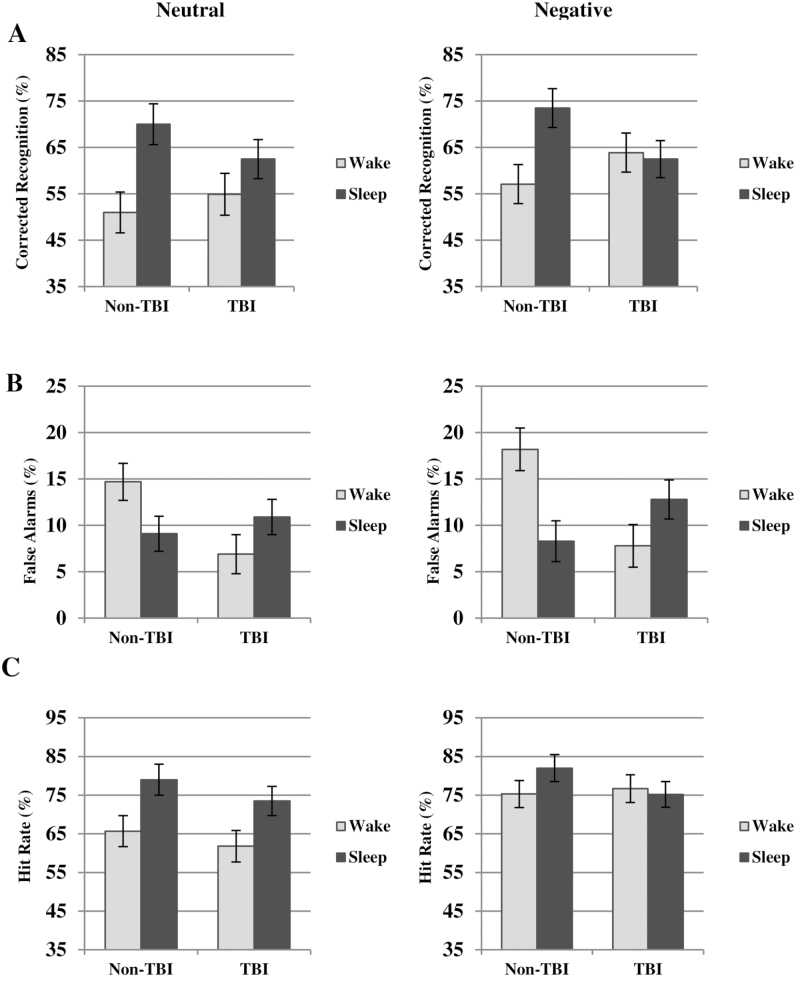
For negative stimuli, there was no main effect of Condition [F(1,66) = 0.90; p = .32] and no main effect of Injury Group [F(1,66) = 0.009; p = .92] for CR. However, there was a significant interaction between these factors [F(1,66) = 9.04, p < .01]. As shown in Figure 2A, the non-TBI Sleep condition performed significantly better than the non-TBI Wake condition [F(1,66) = 4.30; p = .04], but the TBI Sleep and Wake conditions did not differ [F(1,66) = 0.46; p = .49].
Figure 2.
Emotional memory consolidation (A) corrected recognition; (B) false alarms; (C) hit rate for neutral and negative stimuli. Marginal means (not raw values) are plotted. TBI = traumatic brain injury.
The TBI Sleep group had significantly reduced CR for negative stimuli relative to the non-TBI Sleep group [F(1,66) = 4.27; p = .04]. We therefore examined whether differences in FA or HR accounted for this difference. We found the TBI Sleep group had notably (but nonsignificantly) higher FA than the non-TBI Sleep group [F(1,66) = 2.5, p = .11]. There was also a marginally significant reduction in HR in the TBI Sleep group relative to the non-TBI Sleep group [F(1,66) = 2.85; p = .09].
Interestingly, the lack of differentiation in CR between the TBI Sleep and TBI Wake groups also was influenced by alterations in memory processing in the TBI Wake group relative to the non-TBI Wake group. The TBI Wake group had strikingly lower FA than the non-TBI Wake group [F(1,66) = 10.87; p < .01; Figure 2B]. A reduction in FA in this group rendered CR scores higher, thus limiting differentiation from the TBI Sleep group for that measure. On the other hand, there was no difference in HR between the non-TBI and TBI Wake groups [F(1,66) = 0.05; p = .82]. Taking these findings with those above, both the TBI Sleep and TBI Wake groups had irregularities in FA such that the TBI Wake group performed better than the control group and the TBI Sleep group performed worse than the control group.
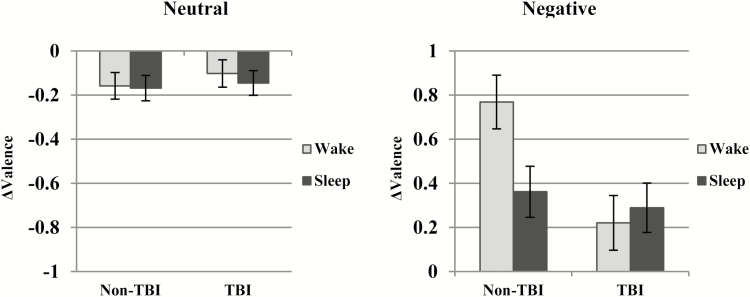
Valence
We next used an ANCOVA to examine whether there were differences between groups or conditions for Δvalence (Table 4). When examining the neutral images, we found no main effect of Injury Group [F(1,67) = 0.519; p = .47], no main effect of Condition [F(1,67) = 0.13; p = .71] and no interaction between factors [F(1,67) = 0.10; p = .75].
Table 4.
Valence Ratings.
| Non-TBI | TBI | |||
|---|---|---|---|---|
| Wake | Sleep | Wake | Sleep | |
| Valence (neg) | 1.69 ± 0.43 | 1.94 ± 0.49 | 1.94 ± 0.46 | 1.82 ± 0.39 |
| Valence (neu) | 4.96 ± 0.15 | 4.94 ± 0.09 | 4.97 ± 0.16 | 5.0 ± 0.13 |
Neg = negative; neu = neutral; TBI = traumatic brain injury.
However, when examining Δvalence for negative images, we found a main effect of injury group [F(1,66) = 7.89; p < .01] such that the non-TBI group had an overall higher change in the positive direction (toward neutrality). There was no main effect of Condition [F(1,66) = 1.37; p = .25], but there was a significant interaction between factors [F(1,66) = 4.84; p = .03]. As shown in Figure 3A, consistent with our previous results21, post hoc simple effect analyses showed the non-TBI Wake group had a significantly higher Δvalence [became more neutral; F(1,66) = 6.82; p = .01], yet negative valence was relatively preserved for the non-TBI Sleep group. On the other hand, there was no significant difference between TBI Sleep and Wake conditions for change in valence [F(1,66)=0.40; p = .53]. As shown in Figure 3, this similarity is present because there is no habituation (ie, movement in the neutral direction) in the TBI Wake condition.
Figure 3.
Change in valence for neutral and negative stimuli. Marginal means (not raw values) are plotted. TBI = traumatic brain injury.
Assessing Factors Predicting Emotional Processing Deficits
Where behavioral differences between Injury Groups and Conditions were detected, we conducted linear regressions to identify which factors predicted behavioral abnormalities in the TBI group. It is notable that the following regressions had relatively low achieved power (ranging from 0.12 to 0.40) due to small sample size and a high number of predictors. Therefore, rejecting predictors based on these analyses is preliminary.
We first probed whether clinical measures or sleep characteristics predicted reduced sleep-dependent memory consolidation. We used regressions to predict two measures: CR and FA. For CR, in the first step of the model, number of TBIs significantly predicted sleep-dependent emotional memory consolidation, such that more concussions predicted poorer memory consolidation (B = −.14, p = .02). Time since most recent TBI and number of symptoms did not predict consolidation (ps > .3). R2 was low (0.34), and the model was not significant [F(3,17) = 2.37, p = .11]. Sleep characteristics were then added to the model. None of the sleep characteristics significantly predicted memory consolidation (ps > .27). Although the R2 value increased (0.55), the model remained not significant [F(7,17) = 1.39, p = .31). Number of TBIs was no longer a significant predictor of memory consolidation nor were the other clinical measures (ps > .32).
Notably, as mentioned above, there was an unexpected reduction in FA in the TBI Wake group for both negative and neutral stimuli. Therefore, head injury factors were used to examine potential changes in the Wake group (sleep characteristics were not available in this group). Given the similarity in pattern between FA for negative and neutral stimuli, these measures were combined (FA negative + FA neutral). None of the aforementioned head injury factors predicted this combined FA score (ps > .30; R2 = .09).
For FA in the TBI Sleep group, in the first step of the model, we found number of TBIs was a significant predictor of FA, such that more TBIs predicted a higher rate of FA (B = 0.05, p = .03). The other clinical predictors were not significant (p-values > .7). The overall model had a low R2 (0.32) and was not significant [F(3,17) = 2.3, p = .12]. When the sleep characteristics were added to the model, number of TBIs remained significant (B = 0.06, p = .02) whereas the other head injury factors did not change. Additionally, FA was predicted by the percent of the night in N2 (B = −0.03, p = .02, change in R2 = 0.02), SWS (B = −0.04, p = .02, change in R2 = .14) and marginally so by REM (B = −0.02, p = .07, change in R2 = 0.13), such that less time in each sleep stage predicted a higher number of FA. Neither total sleep time (p = .55) nor REM latency (p = .98) predicted FA. R2 was improved by the addition of the sleep characteristics (0.64) and the model neared significance [F(7,17) = 2.6, p = .08, change in R2 = 0.32).
When examining valence, we performed a similar linear regression to identify factors predicting the wake-dependent differences identified above. We tested whether clinical injury outcomes predicted Δvalence in the TBI Wake group. Similar to what was found for memory accuracy, number of TBIs significantly predicted Δvalence, such that individuals with a higher number of TBIs had a lower (more negative) Δvalence (B = −0.2, p = .04). Time since most recent TBI and number of symptoms did not predict Δvalence (ps > .17). R2 was low (0.27), and the model was not significant overall [F(3,19) = 2.04, p = .14].
The Influence of Multiple TBIs
Notably, as our results show, the link between the number of TBIs an individual has sustained and their emotional processing is robust. Given this, we reperformed the initial ANCOVA tests using only individuals who had sustained one concussion to ensure that individuals with multiple concussions were not driving these results. For memory measures (HR, FA, CR, d’), results were nearly identical (HR interaction: p < .01; FA main effect of condition: p = .04; FA interaction: p < .01; CR main effect of condition: p < .01; CR interaction: p < .01; d’ main effect of condition p < .01; d’ interaction: p < .01). The Δvalence results were also maintained (main effect of group: p = .04; interaction: p < .01). Overall, these results indicate that a single concussion produces the emotional deficits discussed above, yet multiple concussions worsen emotional outcome.
DISCUSSION
Sleep Quality and Architecture in Chronic TBI
Individuals with a history of TBI had marginally higher PSQI scores (ie, poorer sleep quality) than control participants, in line with several other investigations.14,16,49 In the current sample, the difference in PSQI scores was mainly driven by the sleep latency component of the scale, which queries how long it takes the individual to fall asleep. Participants with a history of TBI reported significantly longer sleep latency. The TBI group also had marginally longer objectively measured sleep latency (PSG). Our results support and add to others’ findings that self-reported sleep quality is poorer several years after a concussion has occurred.
In the current sample, individuals with a history of TBI spent a significantly lower proportion of the night in REM than the non-TBI group. This finding is consistent with a recent meta-analysis that aggregated nine studies to compare sleep architecture between participants with a history of TBI and healthy controls.16 The meta-analysis found no differences between groups for time spent in SWS, N1, and N2, yet the TBI individuals had marginally less REM sleep than healthy controls. Reduced REM in chronic TBI may be the result of a reduction in melatonin production.14 As such, reduced REM seems to be a prominent following chronic, mild TBI.
The TBI group also had significantly longer REM latency than the non-TBI group, similar to what others have reported.51 Interestingly, longer REM latency has been identified in individuals with post-traumatic stress disorder (PTSD),52 and REM latency alterations are a well-established biomarker of major depressive disorder.53 It is possible that longer REM latency is a marker of having had a concussion. However, the meta-analysis described above does not support this hypothesis, as no differences in REM latency were found between aggregated TBI and non-TBI groups in that investigation.16 Alternatively, the TBI individuals could be experiencing a TBI-induced circadian rhythm delay,54 which could delay REM onset.
Emotional Memory Consolidation in Chronic TBI
We found a specific deficit in the consolidation of negative emotional stimuli in individuals with a history of TBI. It is possible that hippocampal injury that occurs during TBI55,56 is the primary cause of reduced consolidation, but this seems unlikely given that consolidation of neutral stimuli is preserved in the TBI population. Alternatively, hippocampal damage may lead to the disruption of the structurally linked limbic system, which modulates emotional processing.57 It is also possible that prolonged frontal lobe damage disrupts downstream emotional processing via disrupted communication with and inhibition of the amygdala. Further work to probe these hypotheses using functional imaging is warranted.
In the TBI group, memory consolidation was affected by lower FA in the TBI Wake group and higher FA in the TBI Sleep group. We probed whether clinical injury factors and sleep characteristics predicted FA rate. For the TBI Wake group, we did not identify any factors predicting FA. However, in the TBI Sleep group, we found individuals with a history of more TBIs had a higher rate of FA. We also found the percent of the night in N2 and SWS significantly predicted FA, such that more time in these stages predicted lower rates of FA. REM marginally predicted FA in the same manner. Importantly, total sleep time did not predict FA, suggesting an active contribution of each sleep stage rather than a passive effect of sleep (ie, a lack of interference caused by less wake time). We propose the dearth of REM in this population contributed to reduced memory consolidation (discussed further below).
In our sample, the TBI Wake group unexpectedly had low FA rates but normal HR rates relative to non-TBI Wake participants. Interestingly, these data are consistent with work demonstrating patients with moderate to severe head injury respond with fewer errors during a reaction time task58 and during a memory recognition task.59,60 The author of these studies suggested that reduced errors may have resulted from either a general increase in hesitancy or a failure to encode. Although assessing the former hypothesis is beyond the scope of this study, we do not feel the latter hypothesis to be true. Specifically, if TBI-related encoding deficits were the sole mechanism underlying reductions in FA, we would expect the TBI Sleep group to also have low FA. However, the TBI Sleep group had marginally higher FA than the non-TBI Sleep group. This leads us to believe either (1) encoding deficits are not responsible for lower FA in the TBI Wake group, or (2) poor encoding indeed affected FA in all TBI participants, but the TBI Sleep group experienced additional, unrelated interference, which led to an increase in FA. We believe the latter to be the case.
We propose that inefficient or incomplete reorganization of memory during sleep may have resulted in higher FA in the TBI Sleep group. According to the synaptic homeostasis hypothesis, global downscaling of synapses during sleep (namely SWS) eliminates weak synaptic connections while strengthening stronger connections.61 An incomplete downscaling could leave weaker connections in place, increasing interference and decreasing the signal-to-noise ratio. TBI induces deficiencies in synaptic function and plasticity (via oxidative stress),62 and it is possible that lingering synaptic damage hinders the downscaling process. Yet it must be stressed that neutral sleep-dependent consolidation is intact in the TBI population, and consolidation deficits must therefore be emotion–dependent. Given this, our results cannot be fully explained by this hypothesis.
Separately, it has been proposed that efficient sleep-dependent memory processing depends on both REM and non-REM episodes.63 That is, sleep-dependent consolidation is optimized when both non-REM and REM are present and also sequential.64–66 The delay in REM onset and general reduction in REM in this sample may have hindered consolidation by reducing such REM/non-REM interactions. Relatedly, it has long been proposed that amygdala activation during REM is critical for sleep-dependent emotional processing.67 Recent evidence suggests activation of the amygdala occurs predominantly after REM bursts during REM sleep.68 Thus, a reduction in REM should limit amygdala activation, reducing the sleep-dependent “boost” for emotional stimuli.
An interesting alternative hypothesis regarding low FA in the TBI Wake group comes from emerging work suggesting brain injury causes sleep and wake to become less dissociated. Specifically, a recent study found more “microsleep” bouts (ie, spontaneous slow waves) occur during wake following mild TBI than controls (in both mice and humans, with humans being ~5 years post injury).69 The authors suggested that sleep-wake states are blurred following TBI, even years after injury. Given the unexpected decrease in FA observed in the TBI Wake group, it is possible that spontaneous slow wave activity during the day facilitated consolidation, which manifested as lower FA.
Emotional Valence Changes in Chronic TBI
The TBI and non-TBI groups did not exhibit differences in baseline valence ratings of either emotional or nonemotional stimuli. This is counter to what we would expect, as an increased risk for mood disturbances could alter initial ratings of stimuli. Nevertheless, groups differed in terms of Δvalence. In the control group, we replicated our previous results21,22 that showed sleep significantly preserves valence ratings (ie, maintains negativity) relative to a period spent awake. That is to say, the Wake group experiences desensitization or habituation to negative images, but the Sleep group does not. In comparison, we unexpectedly found that although the TBI Sleep group preserved negativity, the TBI Wake group did as well. Therefore, both the TBI Sleep and the TBI Wake groups found the negative images to be almost as negative at the second presentation.
These results are compatible with current theories of depression development and maintenance. Depressive thoughts arise and are perpetuated when an individual is unable to use proper emotional regulation strategies (eg, suppression, reappraisal) to habituate when emotional experiences occur.70 In the current sample, a failure to habituate to negative stimuli during a waking period might suggest a prolongation of negative information processing, which could ultimately maintain negative thoughts and facilitate depression. Clinical populations that have experienced trauma, such as individuals with borderline personality disorder and PTSD, show a similar lack of habituation over a waking period.71,72 Therefore, a lack of habituation may either be a “marker” of brain circuitry decrements or simply a reflection of poor mental health. Future work should investigate the effectiveness of emotional regulation strategies in a TBI population relative to uninjured controls.
The Role of Multiple TBIs
We found number of TBIs to be a significant predictor of memory and Δvalence such that more TBIs predicted (1) poorer sleep-dependent memory consolidation (FA) and (2) a greater preservation of valence. Importantly, we did not find any correlations between Δvalence and memory consolidation, suggesting number of TBIs was separately predictive of emotional deficits for valence and memory. These findings indicate individuals who have sustained multiple concussions have poorer emotional processing during sleep, during a waking period, and overall.
These data are consistent with a broad range of literature showing individuals with multiple concussions have poorer recovery outcomes than individuals with no concussions or one concussion. For instance, active duty military members with multiple concussions were found to have greater ongoing emotional distress (eg, PTSD, depression, anger symptoms) than military members with no concussions or one concussion.73 Athletes with a history of two or more concussions demonstrated significant cognition and sleep changes relative to individuals with no concussions or one concussion,74 and in a similar population, number of concussions predicted the incidence for depression.75 Our findings add to this growing body of literature by demonstrating one concussion, relative to none, worsens emotional functioning, but more concussions produce more severe emotional dysfunction.
Strengths and Limitations
There are several limitations to the current study that must be addressed. First, there are no clinical outcome measures (eg, depression and anxiety symptomology questionnaires) available. We are therefore limited in drawing conclusions about how the sleep- and wake-dependent alterations exhibited in this population contribute to negative mental health outcomes or whether sleep- and wake-dependent alterations mediate the relationship between TBI and poor emotional outcomes. Furthermore, this study did not include physiological measurements of arousal (eg, skin conductance). However, we feel that our measures of self-rated arousal are accurate and valid, as our participant ratings of arousal are similar to those found with these same stimuli set.21,22 Nevertheless, future work could include physiological measurements in order to draw more complex conclusions about which mechanisms are disrupted in this population.
Previous work has demonstrated medications may impact both emotional functioning and sleep staging. For instance, antidepressants impact valence perception.76 In the current study, we screened against individuals with an active psychiatric disorder (self-reportedly). Given this, we did not include individuals taking antidepressants. Additionally, over-the-counter antihistamines have been shown to impact the percentage of REM sleep. According to the PSQI, two participants in the TBI Sleep group and three participants in the non-TBI Sleep group reported using over-the-counter sleep aids within the last month. However, we are unaware whether participants used over-the-counter antihistamines, which increase REM latency and reduce REM77, during the day of testing. If a greater proportion of TBI individuals were on antihistamine medication during the day of testing, sleep architecture could have been impacted.
Finally, the use of a retrospective study design with self-reported TBI comes with limitations. If participants incorrectly recalled details (eg, regarding postconcussive symptoms), error variance may have been added to the sample, thereby creating null effects when they are not present. Furthermore, without a formal TBI diagnosis, we are limited in our ability to draw conclusions about injury location or whether participants had complicated mTBI, which is usually identified with magnetic resonance imaging. Ideally, future work could utilize longitudinal data following specific populations (eg, student athletes) before and after brain injury.
Despite these limitations, this investigation also has substantial strengths. This study included a large clinical sample with a range of TBI characteristics (eg, time since TBI, symptoms following TBI). Moreover, the TBI population in this sample was recruited within a university setting, and participants were not aware of the hypotheses of the study before participating. That is, this sample was not comprised of individuals with sleep or emotional complaints, but rather it contained community-dwelling, high-functioning individuals. Given this, we can conclude that our study detected sleep- and wake-dependent emotional deficits in a “high functioning” mild TBI population who have seemingly recovered normally. Finally, we included individuals who have had multiple concussions, allowing us to observe how repeated brain injuries affect sleep- and wake-dependent emotional processing.
CONCLUSIONS
Mild TBI is a major public health issue for a wide range of individuals—from the football field to the battlefield. Individuals who have sustained a concussion are often plagued with both emotional issues and sleep problems. Importantly, healthy sleep-dependent emotional processing is posited to contribute to emotional health. We therefore aimed to test whether chronic sleep issues in a TBI population had an effect on sleep-dependent emotional processing. This study is the first to detect specific sleep-dependent emotional deficits (eg, reductions in memory consolidation) in a TBI population. Given the purported role of emotional memory consolidation in emotional health, this study provides a viable link between poor sleep and mental health in individuals with a history of chronic, mild TBI.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health [grant no. R01 AG040133].
REFERENCES
- 1. Silver JM, McAllister TW, Yudofsky SC. Textbook of Traumatic Brain Injury. Vol 293; 2005. doi:10.1001/jama.293.17.2161-a. [Google Scholar]
- 2. Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006; 21(6): 544–548. [DOI] [PubMed] [Google Scholar]
- 3. Gerberding JL, Binder S. Report to congress on mild traumatic brain injury in the United States: Steps to prevent a serious public health problem. Centers Dis Control Prev. 2003;(September):9–11. [Google Scholar]
- 4. Chi YC, Wu HL, Chu CP, Huang MC, Lee PC, Chen YY. Traumatic brain injury and affective disorder: a nationwide cohort study in Taiwan, 2000–2010. J Affect Disord. 2016; 191: 56–61. [DOI] [PubMed] [Google Scholar]
- 5. Rao V, Bertrand M, Rosenberg P, et al. Predictors of new-onset depression after mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2010; 22(1): 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konrad C, Geburek AJ, Rist F, et al. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol Med. 2011; 41(6): 1197–1211. [DOI] [PubMed] [Google Scholar]
- 7. Scholten AC, Haagsma JA, Cnossen MC, Olff M, van Beeck EF, Polinder S. Prevalence of and risk factors for anxiety and depressive disorders after traumatic brain injury: a systematic review. J Neurotrauma. 2016; 33(22): 1969–1994. [DOI] [PubMed] [Google Scholar]
- 8. Fralick M, Thiruchelvam D, Tien HC, Redelmeier DA. Risk of suicide after a concussion. CMAJ. 2016; 188(7): 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haboubi NH, Long J, Koshy M, Ward AB. Short-term sequelae of minor head injury (6 years experience of minor head injury clinic). Disabil Rehabil. 2001; 23(14): 635–638. [DOI] [PubMed] [Google Scholar]
- 10. Ponsford JL, Parcell DL, Sinclair KL, Roper M, Rajaratnam SM. Changes in sleep patterns following traumatic brain injury: a controlled study. Neurorehabil Neural Repair. 2013; 27(7): 613–621. [DOI] [PubMed] [Google Scholar]
- 11. Mantua J, Mahan KM, Henry OS, Spencer RM. Altered sleep composition after traumatic brain injury does not affect declarative sleep-dependent memory consolidation. Front Hum Neurosci. 2015; 9: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parcell DL, Ponsford JL, Redman JR, Rajaratnam SM. Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch Phys Med Rehabil. 2008; 89(5): 843–850. [DOI] [PubMed] [Google Scholar]
- 13. Kaiser PR, Valko PO, Werth E, et al. Modafinil ameliorates excessive daytime sleepiness after traumatic brain injury. Neurology. 2010; 75(20): 1780–1785. [DOI] [PubMed] [Google Scholar]
- 14. Shekleton JA, Parcell DL, Redman JR, Phipps-Nelson J, Ponsford JL, Rajaratnam SM. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 2010; 74(21): 1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imbach LL, Buchele F, Valko PO, et al. Sleep-wake disorders persist 18 months after traumatic brain injury but remain underrecognized. Neurology. 2016; 86(21): 1945–1949. [DOI] [PubMed] [Google Scholar]
- 16. Grima N, Ponsford J, Rajaratnam SM, Mansfield D, Pase MP. Sleep disturbances in traumatic brain injury: a meta-analysis. J Clin Sleep Med. 2016; 12(3): 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992; 49(8): 651–68; discussion 669. [DOI] [PubMed] [Google Scholar]
- 18. Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002; 159(10): 1696–1701. [DOI] [PubMed] [Google Scholar]
- 19. Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009; 135(5): 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008; 19(8): 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baran B, Pace-Schott EF, Ericson C, Spencer RM. Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012; 32(3): 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones BJ, Schultz KS, Adams S, Baran B, Spencer RM. Emotional bias of sleep-dependent processing shifts from negative to positive with aging. Neurobiol Aging. 2016; 45: 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom Med. 2002; 64(4): 627–634. [DOI] [PubMed] [Google Scholar]
- 24. Lara‐Carrasco J, Nielsen TA, Solomonova E, Levrier K, Popova A. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions. J Sleep Res. 2009; 18(2): 178–187. [DOI] [PubMed] [Google Scholar]
- 25. Pace-Schott EF, Shepherd E, Spencer RM, et al. Napping promotes inter-session habituation to emotional stimuli. Neurobiol Learn Mem. 2011; 95(1): 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Noordt S, Good D. Mild head injury and sympathetic arousal: investigating relationships with decision-making and neuropsychological performance in university students. Brain Inj. 2011; 25(7-8): 707–716. [DOI] [PubMed] [Google Scholar]
- 27. Baker JM, Good DE. Physiological emotional under-arousal in individuals with mild head injury. Brain Inj. 2014; 28(1): 51–65. [DOI] [PubMed] [Google Scholar]
- 28. Bernstein DM. Information processing difficulty long after self-reported concussion. J Int Neuropsychol Soc. 2002; 8(5): 673–682. [DOI] [PubMed] [Google Scholar]
- 29. Vanderploeg RD, Curtiss G, Luis CA, Salazar AM. Long-term morbidities following self-reported mild traumatic brain injury. J Clin Exp Neuropsychol. 2007; 29(6): 585–598. [DOI] [PubMed] [Google Scholar]
- 30. Kerr ZY, Marshall SW, Harding HP, Jr, Guskiewicz KM. Nine-year risk of depression diagnosis increases with increasing self-reported concussions in retired professional football players. Am J Sports Med. 2012; 40(10): 2206–2212. [DOI] [PubMed] [Google Scholar]
- 31. Menon DK, Schwab K, Wright DW, Maas AI; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010; 91(11): 1637–1640.21044706 [Google Scholar]
- 32. Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Vol 77; 1997. doi:10.1016/j.epsr.2006.03.016. [Google Scholar]
- 33. Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging. 2012; 33(5): 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 35. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976; 4(2): 97–110. [PubMed] [Google Scholar]
- 36. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992; 15(4): 376–381. [DOI] [PubMed] [Google Scholar]
- 37. Broughton RJ, Dinges DF. Sleep and Alertness: Chronobiological, Behavioural, and Medical Aspects of Napping. New York, NY; 1989. [Google Scholar]
- 38. Wechsler D. Wechsler adult intelligence scale - Fourth Edition (WAIS-IV). San Antonio: 2008:1–3. [Google Scholar]
- 39. Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007; 3(2): 121–131. [PubMed] [Google Scholar]
- 40. St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol Sci. 2009; 20(1): 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004; 27(8): 1479–1485. [DOI] [PubMed] [Google Scholar]
- 42. Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci USA. 2004; 101(7): 2140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baran B, Mantua J, Spencer RM. Age-related changes in the sleep-dependent reorganization of declarative memories. J Cogn Neurosci. 2016; 28(6): 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benedict C, Scheller J, Rose-John S, Born J, Marshall L. Enhancing influence of intranasal interleukin-6 on slow-wave activity and memory consolidation during sleep. FASEB J. 2009; 23(10): 3629–3636. [DOI] [PubMed] [Google Scholar]
- 45. Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. Contribution of norepinephrine to emotional memory consolidation during sleep. Psychoneuroendocrinology. 2011; 36(9): 1342–1350. [DOI] [PubMed] [Google Scholar]
- 46. Cairney SA, Durrant SJ, Hulleman J, Lewis PA. Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep. 2014; 37(4): 701–7, 707A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Payne JD, Kensinger EA, Wamsley EJ, et al. Napping and the selective consolidation of negative aspects of scenes. Emotion. 2015; 15(2): 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev. 2010; 14(4): 219–226. [DOI] [PubMed] [Google Scholar]
- 49. Gosselin N, Lassonde M, Petit D, et al. Sleep following sport-related concussions. Sleep Med. 2009; 10(1): 35–46. [DOI] [PubMed] [Google Scholar]
- 50. Millman RP; Working Group on Sleepiness in Adolescents/Young Adults; AAP Committee on Adolescence Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005; 115(6): 1774–1786. [DOI] [PubMed] [Google Scholar]
- 51. Arbour C, Khoury S, Lavigne GJ, et al. Are NREM sleep characteristics associated to subjective sleep complaints after mild traumatic brain injury? Sleep Med. 2015; 16(4): 534–539. [DOI] [PubMed] [Google Scholar]
- 52. Cowdin N, Kobayashi I, Mellman TA. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res. 2014; 232(5): 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Giles DE, Biggs MM, Rush AJ, Roffwarg HP. Risk factors in families of unipolar depression. I. Psychiatric illness and reduced REM latency. J Affect Disord. 1988; 14(1): 51–59. [DOI] [PubMed] [Google Scholar]
- 54. Ayalon L, Borodkin K, Dishon L, Kanety H, Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007; 68(14): 1136–1140. [DOI] [PubMed] [Google Scholar]
- 55. Warriner EM, Velikonja D. Psychiatric disturbances after traumatic brain injury: neurobehavioral and personality changes. Curr Psychiatry Rep. 2006; 8(1): 73–80. [DOI] [PubMed] [Google Scholar]
- 56. Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992; 12(12): 4846–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. LeDoux JE. Emotional memory systems in the brain. Behav Brain Res. 1993; 58(1–2): 69–79. [DOI] [PubMed] [Google Scholar]
- 58. Battistone M, Woltz D, Clark E. Processing speed deficits associated with traumatic brain injury: processing inefficiency or cautiousness? Appl Neuropsychol. 2008; 15(1): 69–78. [DOI] [PubMed] [Google Scholar]
- 59. Brooks DN. Recognition memory after head injury: a signal detection analysis. Cortex. 1974; 10(3): 224–230. [DOI] [PubMed] [Google Scholar]
- 60. Brooks DN. Long and short term memory in head injured patients. Cortex. 1975; 11(4): 329–340. [DOI] [PubMed] [Google Scholar]
- 61. Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003; 62(2): 143–150. [DOI] [PubMed] [Google Scholar]
- 62. Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008; 45(4): 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsáki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012; 75(6): 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010; 11(2): 114–126. [DOI] [PubMed] [Google Scholar]
- 65. Ficca G, Lombardo P, Rossi L, Salzarulo P. Morning recall of verbal material depends on prior sleep organization. Behav Brain Res. 2000; 112(1–2): 159–163. [DOI] [PubMed] [Google Scholar]
- 66. Sonni A, Spencer RM. Sleep protects memories from interference in older adults. Neurobiol Aging. 2015; 36(7): 2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004; 27: 1–28. [DOI] [PubMed] [Google Scholar]
- 68. Corsi-Cabrera M, Velasco F, Del Río-Portilla Y, et al. Human amygdala activation during rapid eye movements of rapid eye movement sleep: an intracranial study. J Sleep Res. 2016; 25(5): 576–582. [DOI] [PubMed] [Google Scholar]
- 69. Modarres MH, Kuzma NN, Kretzmer T, Pack AI, Lim MM. EEG slow waves in traumatic brain injury: Convergent findings in mouse and man. Neurobiol Sleep Circadian Rhythm. 2016; 2: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teasdale JD. Cognitive vulnerability to persistent depression. Cogn Emot. 1988; 2(3): 247–274. [Google Scholar]
- 71. Hendler T, Rotshtein P, Hadar U. Emotion-perception interplay in the visual cortex: “the eyes follow the heart”. Cell Mol Neurobiol. 2001; 21(6): 733–752. [DOI] [PubMed] [Google Scholar]
- 72. Hazlett EA, Zhang J, New AS, et al. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol Psychiatry. 2012; 72(6): 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Spira JL, Lathan CE, Bleiberg J, Tsao JW. The impact of multiple concussions on emotional distress, post-concussive symptoms, and neurocognitive functioning in active duty United States marines independent of combat exposure or emotional distress. J Neurotrauma. 2014; 31(22): 1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schatz P, Moser RS, Covassin T, Karpf R. Early indicators of enduring symptoms in high school athletes with multiple previous concussions. Neurosurgery. 2011; 68(6): 1562–7; discussion 1567. [DOI] [PubMed] [Google Scholar]
- 75. Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007; 39(6): 903–909. [DOI] [PubMed] [Google Scholar]
- 76. Rizvi SJ, Salomons TV, Konarski JZ, et al. Neural response to emotional stimuli associated with successful antidepressant treatment and behavioral activation. J Affect Disord. 2013; 151(2): 573–581. [DOI] [PubMed] [Google Scholar]
- 77. Boyle J, Eriksson M, Stanley N, Fujita T, Kumagi Y. Allergy medication in Japanese volunteers: treatment effect of single doses on nocturnal sleep architecture and next day residual effects. Curr Med Res Opin. 2006; 22(7): 1343–1351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.