Abstract
Study Objectives
To replicate and expand upon past research by evaluating sleep and wake electroencephalographic spectral activity in samples of frequent nightmare (NM) recallers and healthy controls.
Methods
Computation of spectral activity for sleep (non-REM and REM) and wake electroencephalogram recordings from 18 frequent NM recallers and 15 control participants.
Results
There was higher “slow-theta” (2–5 Hz) for NM recallers than for controls during wake, non-REM sleep and REM sleep. Differences were clearest for frontal and central derivations and for REM sleep cycles 2-4. There was also higher beta activity during NREM sleep for NM recallers. Findings partially replicate past research by demonstrating higher relative “slow-theta” (3-4Hz) for NM recallers than for controls.
Conclusions
Findings are consistent with a neurocognitive model of nightmares that stipulates cross-state anomalies in emotion processing in NM-prone individuals.
Keywords: nightmares, REM sleep, theta, EEG spectal analysis, parasomnias
Statement of Significance
This research offers new insights into nightmare pathology and REM-dependent emotion processing by comparing EEG spectra across REM, NREM, and wake states in frequent nightmare recallers and healthy controls. Results suggest that nightmare recallers have cross-state EEG alterations, a feature that also occurs in posttraumatic stress disorder, which is characterized by repetitive nightmares among other symptoms. The findings partially replicate and extend previous research and highlight the possible role of the “slow theta” frequency band in nightmare pathology. Slow theta has been associated with fear memory processing in rodents during both wake and REM sleep. Future research could further clarify the altered role of theta activity in the physiology of idiopathic nightmares using other brain imaging methods.
INTRODUCTION
Nightmares (NMs) are a common disturbance of REM sleep, with lifetime prevalences of clinically significant NMs (at least 1/week) estimated to be from 1% to 8% of the population.1–3 NMs are an important clinical concern, having been linked to poor sleep quality4 and shown to be comorbid with several sleep disorders, including insomnia.5 NMs are more prevalent in psychiatric populations5 and have been linked to psychopathologies such as anxiety, depression, schizophrenia, borderline personality disorder, suicide, and posttraumatic stress disorder (PTSD) as well as numerous physical ailments.2,6–10 NMs are also an important risk factor for several of these illnesses, most notably suicide11 and PTSD.12 To illustrate, among Dutch service members, predeployment NMs—but not insomnia—predicted the appearance of PTSD symptoms 6 months postdeployment.12 NMs have also been linked to greater severity of symptoms in different psychiatric populations.13,14 These associations between NMs and psychopathology are often independent from other sleep impairments (e.g.,8,12). Despite such findings, there is a paucity of research on the neurophysiological characteristics of individuals who frequently recall nontraumatic NMs. Yet, such research may provide valuable information on the mechanisms of NM production and how such mechanisms contribute to a variety of pathologies.
Quantitative electroencephalography (qEEG) has been used to document alterations in a variety of sleep disorders, including narcolepsy,15 rapid eye movement (REM) sleep behavior disorder,16 insomnia,17 and somnambulism,18 but only a single study by Simor et al. has used qEEG to assess frequent NM recallers.19 This study found that NM recallers and matched controls differ in relative spectral power during REM sleep; NM recallers have: (1) elevated “high alpha” (10–14.5 Hz) and (2) elevated frontocentral “high delta” (3–4 Hz). The “high delta” difference is of particular interest because it corresponds to what others define as “slow-theta”, for example, 2.78–5.4220 or 2.5–5.0 Hz.21 Slow-theta power, especially in the left temporal region, is associated with the viewing of fearful stimuli22 and with threat-induced anxiety.23 This contrasts with fast-theta power, which accompanies the viewing of social stimuli22 and spatial navigation tasks.23 Slow-theta power is thought to play a role in memory consolidation, a role supported by much animal work.24–26 Findings also suggest that there are functional similarities between 1–4 Hz oscillations in humans and hippocampal 4–10 Hz theta oscillations in rodents.27–30 Bódizs et al.30 identified slow theta oscillations (1.5–3.0 Hz) in patients with epilepsy using both indwelling and surface EEG methods and found them to be highly characteristic of REM sleep. For REM sleep, in particular, power in the standard theta band has been linked to memory consolidation,31 but it remains unknown if this also holds true specifically for the slow-theta band.
Current models of PTSD—for which NMs are a hallmark symptom32,33—emphasize the role of REM sleep in fear memory consolidation and extinction (for review, see Pace-Schott et al.34). A new theory of NM pathogenesis stipulates that idiopathic NMs originate in early childhood adversity just as PTSD nightmares are triggered by traumatic experiences,35 while a neurocognitive model of NM production36 stipulates that both PTSD and idiopathic NMs result from dysfunction in a hippocampal–amygdala–prefrontal neural circuit that controls fear memory formation and extinction. Thus, disturbances in theta power, an EEG oscillation associated with fear memory consolidation in the hippocampus, amygdala, and prefrontal cortex,37,38 might be expected to characterize the EEG of frequent NM recallers. This is the case for some cohorts of patients with PTSD. For example, Cowdin et al.39 found lower right prefrontal theta in REM sleep in participants with PTSD than in trauma-exposed participants without PTSD; further, they suggest that this measure may serve as a biomarker of adaptive emotional memory processing after trauma. In light of the proposed centrality of REM sleep-related fear memory processes in NM pathology, we attempted an independent replication and extension of the basic qEEG findings of Simor et al.19
There is also a dearth of research on waking-state brain activity among frequent NM recallers. Some theoretical models of NM production40 stipulate a cross-state continuity in the pathology of NMs such that NM-centric disorders should show similarly altered brain activity in both sleep and waking states. This seems to be the case in PTSD, for example, as waking state beta power is higher among patients with PTSD than among controls,41 and the REM beta–NREM beta power ratio is also higher among patients with PTSD than among controls.42 This continuity notion is also consistent with the finding that theta activity is a prominent feature of both wake and REM sleep.38 We therefore assessed qEEG comparatively for samples of wake in addition to samples of REM and NREM sleep.
Objectives and Hypotheses
We used qEEG to characterize alterations in brain activity during REM sleep, NREM sleep, and quiet wakefulness among NM recallers and age-matched controls to replicate and extend previous work. We predicted a replication of findings by Simor et al.19 of elevated REM sleep activity in 3.0–4.0 Hz and 10.0–14.5 Hz bands among NM recallers as well as findings consistent with the cross-state continuity notion, that is, theta power for frontal and central derivations will be elevated in all states for frequent NM recallers.
METHODS
Participants
Participants were recruited by word of mouth, advertisement in local university campuses, and through our laboratory’s website. They were aged 18–35 years and were fluent in English or French. Each underwent a telephone screening interview conducted by trained undergraduate and graduate students, assessing (1) frequency and intensity of dream and NM recall (including a 1–9 scale for NM distress); (2) presence of sleep disorders (e.g., sleep paralysis, night terrors, and narcolepsy); (3) usual sleep schedule and quality of sleep, including number of hours slept; (4) consumption of alcohol, drugs, and caffeine; (5) recent (past 6 months) traumatic experiences, and (6) medical and psychiatric conditions.
NM participants were selected according to adapted International classification of sleep disorders—2nd edition criteria for Nightmare Disorder;3 they reported recalling at least two NMs or bad dreams (dysphoric dreaming without awakening) per week for a minimum of 6 months. Clinical significance of the NMs and their impact on daily activity was obtained from questionnaires (see below) but was not used to include or exclude participants. Control participants reported fewer than one NM or bad dream per month and were matched for age. They were drawn from one of our previous studies in which they had undergone the same protocol as the participants with NMs.43 None of the participants reported any sleep disorder, medical or psychiatric condition (past or present), recent trauma, or medication use other than oral contraceptives.
Participants who answered “poor” or “very poor” to the sleep quality item of the telephone screening interview were not included in the study nor were those who reported sleeping fewer than 6 hours per night.
Participants reported no more than 10 consumptions of alcohol per week for women or 15 for men. Some reported using marijuana once per month or less but did not report taking other drugs. Their reported daily caffeine intake was equivalent to 3 cups of coffee or less in all cases.
The study was approved by the Research Center’s ethics and scientific committees. All participants provided written informed consent after being given a complete description of the study protocol. Participants were compensated financially for the night spent in the laboratory, parking or public transit, and breakfast expenses.
The initial sample consisted of 18 controls and 18 participants with NMs. Three control participants scored >14 on the Beck’s Depression Inventory II (BDI-II) and were excluded from the sample. One additional participant in the NM group did not complete the BDI-II and State-Trait Anxiety Inventory (STAI-T) questionnaires but was nonetheless included for EEG analyses. One control participant and two participants with NMs had sleep that was too disturbed for polysomnograhy (PSG) interpretation and were excluded from sleep EEG analyses but included in wake EEG analyses. The final sample thus consisted of 15 CTL and 18 participants with NMs for wake EEG analyses and of 14 CTL and 16 participants with NMs for sleep EEG analyses. The male–female ratio marginally differed between the groups (χ2 = 3.49, p = .06). Participant characteristics are shown in Table 1.
Table 1.
Participant characteristics and sleep architecture.
| Measure | CTL group | NM group | P | t or χ2 | df | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Age (years) | 23.67 | 3.83 | 23.17 | 4.15 | >.20 | ||
| Sex (M:F) | 7:8 | 3:15 | .06a | 3.49 | 3 | ||
| Dreams (#/month)b | 8.67 | 6.40 | 27.44 | 13.61 | ≤.001 | 5.18 | 31 |
| Bad dreams (#/month) | 0.71 | 0.58 | 11.11 | 5.99 | ≤.001 | 7.33 | 31 |
| NM (#/month)b | 0.09 | 0.19 | 5.32 | 6.40 | ≤.001 | 6.69 | 31 |
| STAI_T (raw score) | 39.27 | 6.50 | 36.47 | 7.37 | >.20 | ||
| STAI_S (raw score) | 30.20 | 5.21 | 32.71 | 9.07 | >.20 | ||
| BDI-II (raw score) | 6.07 | 3.62 | 4.12 | 3.39 | >.20 | ||
| NDQ (raw score) | 11.72 | 5.24 | |||||
| PSQI (raw score) | 4.73 | 2.63 | 5.06 | 2.10 | >.20 | ||
| Dreams (#/week) c | 3.14 | 1.83 | 2.90 | 1.51 | >.20 | ||
| Bad dreams (#/week) c | 1.22 | 1.12 | 2.21 | 1.60 | .051 | 2.03 | 31 |
| NM (#/week) c | 0.28 | 0.39 | 0.87 | 0.94 | .031 | 2.26 | 31 |
| Dysphoric dreams (#/week)c |
1.50 | 1.16 | 2.92 | 1.69 | .009 | 2.77 | 31 |
| Total sleep time (min) | 412.25 | 38.67 | 425.66 | 54.72 | >.10 | ||
| Sleep efficiency (%)b | 92.68 | 5.10 | 91.15 | 8.65 | >.10 | ||
| WASO (min)b | 32.93 | 24.49 | 41.31 | 39.68 | >.10 | ||
| Awakenings (#) | 13.00 | 6.71 | 11.44 | 5.99 | >.10 | ||
| Sleep latency (min)b | 11.64 | 6.99 | 14.97 | 13.24 | >.10 | ||
| Stage 1 (%) | 8.45 | 3.75 | 7.22 | 3.11 | >.10 | ||
| Stage 2 (%) | 45.55 | 7.19 | 43.19 | 5.53 | >.10 | ||
| Stage N3 (%) | 26.47 | 9.20 | 28.91 | 4.56 | >.10 | ||
| REM sleep (%) | 19.54 | 4.25 | 20.69 | 4.19 | >.10 | ||
| REM sleep efficiency (%) | 89.15 | 9.41 | 89.82 | 6.07 | >.10 | ||
| REM sleep latency (min) | 82.68 | 21.71 | 71.28 | 12.83 | >.10 | ||
| REM sleep periods (#) | 4.36 | 1.01 | 4.56 | 0.63 | >.10 | ||
BDI-II = Beck Depression Inventory II; REM = rapid eye movement; NM = nightmare; STAI = State-Trait Anxiety Inventory; PSQI = Pittsburgh Sleep Quality Index; NDQ = Nightmare Distress Questionnaire.
The p values are the result of independent samples t-tests; except where indicated; values less than .06 are italicized.
aThe p value is the result of a chi-square (χ2) test.
b Variables log-transformed (value+1) for statistical analysis.
c Prospective measures obtained from sleep-dream log.
MATERIALS
Participants Completed the Following Questionnaires:
State-Trait Anxiety Inventory
The STAI44,45 is a self-report questionnaire assessing state (20 items) and trait (20 items) anxiety. For the state subscale, the participant responds according to his or her present state and for the trait subscale, according to how he or she usually feels. Response scales are from 1 (not at all) to 4 (a lot), resulting in scores between 20 and 80 for both state and trait estimates.
Beck Depression Inventory II
The BDI-II is a 21-item questionnaire with 0–3 response scales commonly used to assess depressive symptoms.46 It provides a total score between 0 and 63 and has four typical cutoff points: under 14 (not depressed), 14–19 (slightly depressed), 20–28 (moderately depressed), and over 29 (severely depressed). Other cutoff values have been suggested: 0–12 (nondepressed), 13–19 (dysphoric), and 20–63 (dysphoric depressed).47 A more conservative approach was chosen and participants who scored above 14 were excluded from the sample.
Nightmare Distress Questionnaire
The Nightmare Distress Questionnaire (NDQ)48 is a 13-item questionnaire assessing waking distress associated with nightmares. The items have 0–4 response scales (from never to always); only items 1 to 11 were used here, yielding a maximum score of 0–44.
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI)49 is a 19-item questionnaire assessing sleep quality over the last month. Response scales are: not in the last month, less than once a week, once or twice a week, and more than 3 times a week. Individual responses are grouped in seven equally weighted components (0–3 for each component), yielding a total score between 0 and 21. These components are subjective sleep quality, sleep latency, sleep duration, sleep efficacy, sleep disorders, medication intake, and diurnal dysfunction. A global PSQI score >5 was proposed to best discriminate between good and poor sleepers, although more recent research suggests a >8 cutoff value depending on the study.50,51
Procedure
Before coming to the sleep laboratory, participants completed home sleep–dream logs using an interactive voicemail system (IVMS; described in43) for 1 week and for a second week after their laboratory stay. The IVMS permitted automated recording of dream mentation and prompted participants to rate sleep (quality, #hours, napping, and #awakenings) and several features of their dream content (recall clarity, emotion, lab incorporations) most on 1–9 Likert-type scales. A 2-week duration for the dream diary is common for studying NMs52–55 although it may underestimate participants’ true NM frequencies.
Participants underwent one whole night of undisturbed PSG (except for the final awakening) with no adaptation night. They arrived at the laboratory around 8:00 pm and filled out several additional questionnaires (not reported here), after which electrodes were fitted by experienced PSG technicians. One hour before target bedtime (between 10 pm and midnight, depending on self-reported bedtimes in the sleep-dream log), participants underwent a series of cognitive tasks including the Tower of Hanoi, Corsi Block Tapping, Rey-Osterrieth Complex Figure, and Mirror-Tracing (results not reported here). They then underwent a biocalibration procedure, during which waking-state EEG (2 min relaxed wakefulness with eyes closed) was recorded. Immediately before lights out, participants completed a modified version of the Differential Emotions Scale,56 which assesses 12 basic emotions. After a minimum of 6.5 hours of sleep and after 20 minutes of REM sleep, participants were awakened using a 500-Hz tone repeated up to 5 times as necessary. They were first asked to complete the sleep-dream log using the IVMS on a cordless phone. They then completed the Differential Emotions Scale for a second time. Another series of cognitive tasks was completed approximately half an hour after awakening (results not reported here).
PSG
A 6-electrode EEG montage (F3, F4, C3, C4, O1, and O2) referenced to A1, including A2 for re-referencing off-line to A1+A2, was fitted according to the 10–20 placement system.57 Participants were also fitted with four EOG electrodes (vertical and horizontal eye movements), three ECG electrodes, and 4 bipolar EMG electrode pairs, including the chin (submentalis). Gold-plated silver EEG cup electrodes were affixed with EC2 Grass Electrode Cream (Grass Technologies, Middleton, Wisconsin) and referred off-line to both ears. Impedances were kept below 5kΩ. For one control participant, A1 was used as a reference instead of A1+A2 because A2 was excessively noisy. Spectral power values for this participant were higher than those of the rest of the sample, but deleting this participant did not change the results in any way.
EEG signals were acquired using Model 12 or 15 Grass Neurodata Acquisition Systems and acquisition software Harmonie v6.2b.58 EEG signals were filtered (−6 dB filters) at 0.3 and 100 Hz and sampled at 256 Hz. Sleep stages were scored according to standard criteria59 by an experienced PSG technician blind to group membership. Standard sleep variables were calculated off-line using in-house software.
Spectral Analyses
Nonoverlapping, artifact-free 5-second epochs from all EEG derivations were Cosine-tapered and Fourier transformed using the Fast Fourier Transformation (FFT) algorithm to calculate average power spectral densities (µV2/0.20 Hz). For sleep EEG, spectral power densities were averaged separately for whole-night NREM (stages 1–3) and REM sleep. For wake EEG, artifact-free, 5-second epochs were selected manually from the biocalibration. For two participants in each group, biocalibration signals were unusable, and epochs were instead selected manually after the lights-out period. All selected epochs’ spectral densities were averaged to produce absolute spectral activity for each 0.2 Hz bin between 0.6 and 32 Hz.
Relative spectral activity for “high alpha” (10–14.6 Hz) and “high delta/slow-theta” (3–4 Hz) was obtained by dividing activity in these frequency bands by total spectral power (0.6–32 Hz), in order to directly replicate the findings of Simor et al.19
Home Sleep-Dream Log Analyses
Dream recall was scored as successful when recall clarity was ≥1 (0 indicated no dream recall). A bad dream was scored as 1 when the negative emotion was ≥5; 0 otherwise. A nightmare was scored as 1 when negative emotion was ≥5 and the dream caused an awakening. The dysphoric dream measure summed the two previous categories. Results were computed to obtain weekly prospective frequencies for dreams, bad dreams, NMs, and dysphoric dreams (see Table 1).
Statistical Analyses
Demographics and PSG
Distributions of all measures were examined manually for normality and log-transformed as needed. Relevant demographic measures, screening interview responses, questionnaire scores, and PSG measures were compared between groups using independent samples t tests.
Quantitative EEG
Spectral density scores were log-transformed (power+1) before statistical analysis. Groups were compared using independent samples t tests for each 0.2 Hz bin, each electrode (F3, F4, C3, C4, O1, O2), and each of three states (REM sleep, NREM sleep, and wake). Spectral density scores were obtained for the whole night as well as for the first four sleep cycles separately.
The same methods were used to assess group differences in relative (as opposed to absolute) EEG activity for REM 3.0–4.0 Hz and 10–14.6 Hz bands in our attempted replication of Simor et al.19
RESULTS
Demographics and PSG
Means, standard deviations, and statistical tests for demographics, questionnaires, home sleep-dream logs, and PSG measures are reported in Table 1. Groups differed on retrospectively estimated recall of dreams, bad dreams, and NMs (all p< .001) and prospectively estimated recall of bad dreams, NMs, and dysphoric dreams (all p < .051) but not on psychopathology or sleep architecture measures (all p > .10).
Full-Spectrum Power
A marginally unbalanced sex ratio may have impacted our results; some past research has shown that women have higher EEG spectral power than men.60,61 To test whether our NM group had higher full-spectrum power because it contained more women than the CTL group, we performed independent samples t tests between groups for all-night full-spectrum (0.6–32 Hz) activity in wake, REM sleep, and NREM sleep. Across all electrode sites, NM recallers at best had marginally more total activity in both REM and NREM sleep (all p < .10) but not in wake.
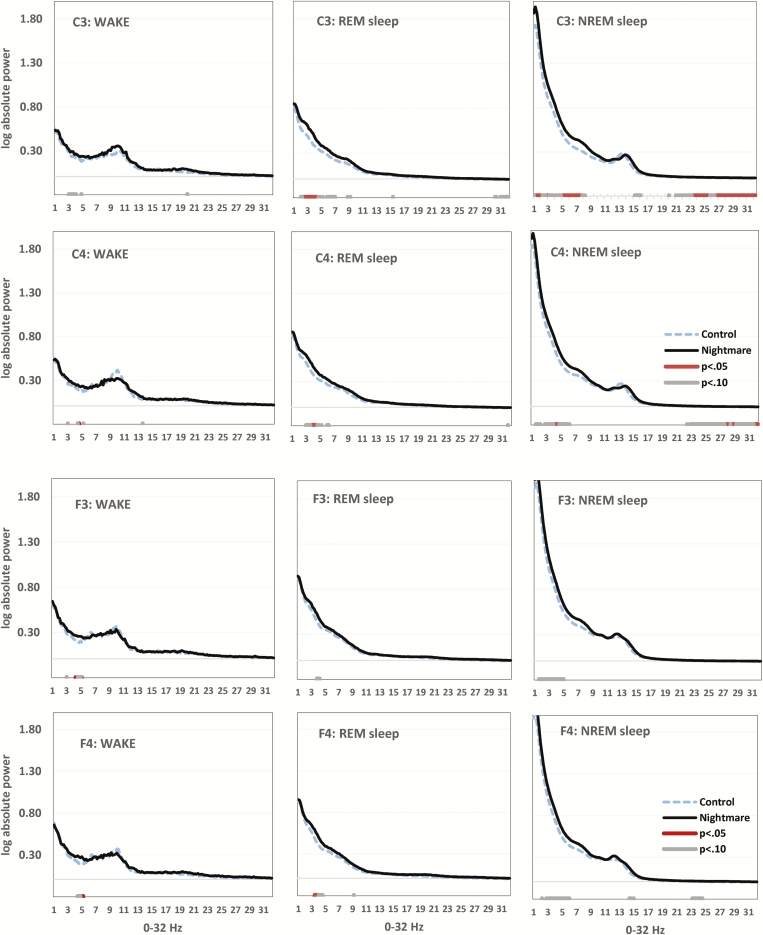
Waking Theta (Absolute Power)
As shown in Figure 1 (left column), group differences in a narrow slow-theta band were observed for C3, C4, F3, and F4, with NM recallers showing higher spectral power values in all cases. The difference was clearest for F3 especially between 3.8 and 5.0 Hz (p < .05) and F4 between 4.0 and 5.0 Hz (p < .05). For C3, the same difference was seen between 2.6 and 4.6 Hz (p < .10) and, for C4, between 4.2 and 4.4 Hz and 4.8 and 5.0 Hz (p < .05). For O1 and O2, the theta difference was not visible at all (not shown).
Figure 1.
Group differences in all-night electroencephalogram (EEG) power for C3, C4 (upper panels) and F3, F4 (lower panels) for Wake (left column), rapid eye movement (REM) sleep (middle column), and non-REM (NREM) sleep (right column). Horizontal axis is spectral frequency for 0–32 Hz by 0.2 Hz bins. Vertical axis is log-transformed spectral activity for each bin. P values (p < .05 and p < .10) for independent samples t-tests on each bin appear in red and gray, respectively. Elevated slow theta (2–5 Hz), especially in C3 during REM sleep, distinguishes the Nightmare group.
REM Sleep Theta (Absolute Power)
The narrow theta difference seen in wake was more apparent in REM sleep and more widespread. For F3, NM recallers showed higher spectral power between 3.0 and 4.4 Hz (p < .10). For F4, the difference was primarily between 3.0 and 4.4 Hz (p < .05). For C3, the difference was seen especially between 2.0 and 4.4 Hz (p < .05). For C4, the difference was between 2.8 and 4.6 Hz (p < .05). For O1, the difference was apparent between 3.2 and 3.6 Hz and 3.8 and 4 Hz (p < .10) and, for O2, between 2.4–2.6 Hz, 2.8–3.0 Hz, 3.2–3.6 Hz, and 3.8–4.2 Hz (p < .10).
REM Sleep Theta and Alpha (Relative Power)
Relative REM 3.0–4.0 Hz activity was higher in NM recallers for F3 (t28 = 2.20, p = .037), F4 (t28 = 2.59, p = .015), C3 (t28 = 1.91, p = .067), and C4 (t28 = 2.07, p = .048) but not for O1 or O2.
For relative REM 10.0–14.6 Hz activity, a trend opposite to that for absolute activity was seen, that is, lower power for NM recallers in O1 (t28 = 1.822, p = .079; all other p > .10).
NREM Sleep Theta (Absolute Power)
The theta difference favoring NM recallers in REM sleep and to a lesser extent in wake appeared also to some degree in NREM. For F3, differences were especially apparent between 1.2 and 3.0 Hz and 3.4 and 4.8 Hz (p < .05). For F4, differences occurred especially between 2.8 and 5.2 Hz (p < .05). For C3, differences appeared between 0.8 and 1.6 Hz and 4.2 and 7.4 Hz (p < .05) and for C4, between 3.4 and 5.8 Hz (p < .05). For O1, differences were not seen while for O2, there were marginal differences between 0.8 and 2 Hz (p < .10).
Differences favoring NM recallers were also seen in and around the high beta band (26.0–32.0 Hz). Although not as consistent as for the theta difference, the high beta pattern was observed for C3 (22.6–32.0 Hz; p < .05), C4 (23.2–25.0 Hz and 26.4–32.0 Hz; p < .05) and to a lesser extent (all p < .10) F3 (23.6–24.2 Hz), F4 (20.6–24.8 Hz), and O1 (27.2–32.0).
Finally, there was a similar pattern of group differences (p < .05) in the high alpha band (12.0–15.0 Hz) in F4 (13.8–15.4 Hz), C3 (14.2–16.4 Hz), C4 (14.2–15.8 Hz), O1 (12.6–13.2 Hz) and, at p < .10, O2 (12.6–13 Hz).
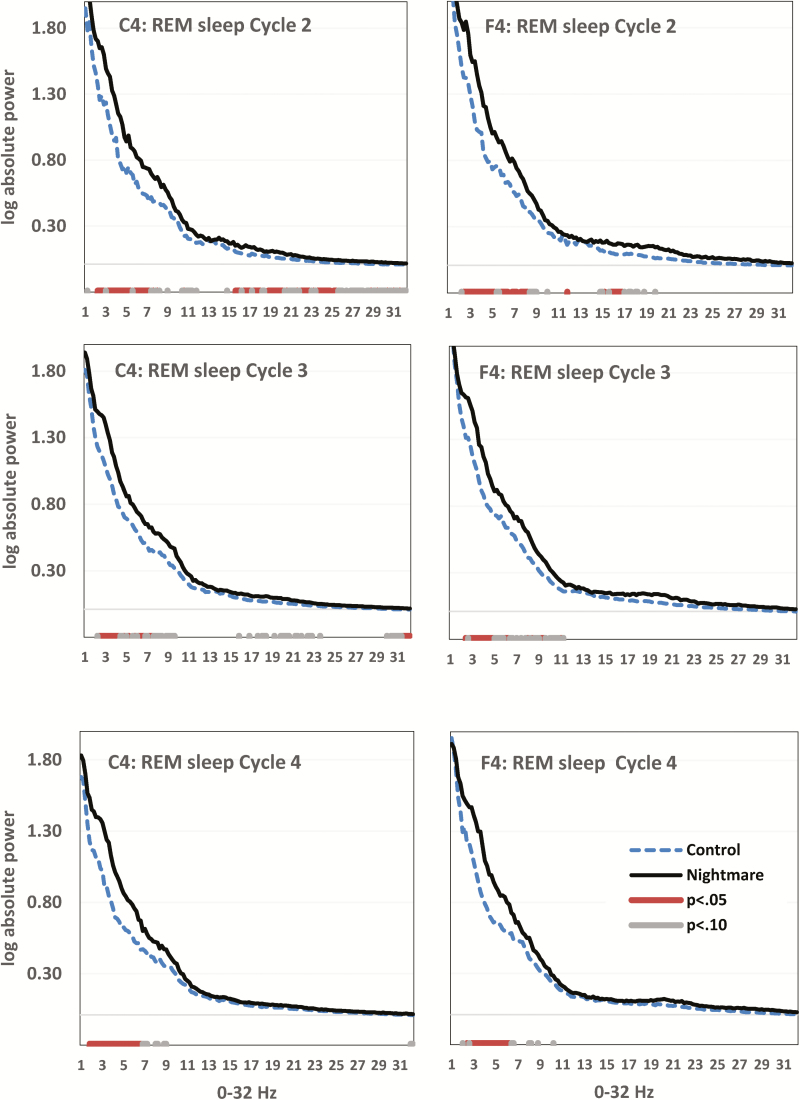
REM Sleep Cycle-by-Cycle Comparisons
The theta difference in REM sleep favoring NM recallers was present when the sleep cycles were examined separately. Very few differences were observed for the first sleep cycle, probably due to its short duration, but were very apparent for cycles 2, 3, and 4, especially in frontal and central electrode derivations. REM sleep spectral plots for cycles 2–4 in derivations F4 and C4 are shown in Figure 2.
Figure 2.
Group differences in rapid eye movement (REM) sleep electroencephalogram (EEG) power for C4 (left column) and F4 (right column) for Cycle 2 (first row), Cycle 3 (middle row), and Cycle 4 (bottom row). Horizontal axis is spectral frequency for 0–32 Hz by 0.2 Hz bins. Vertical axis is log-transformed spectral activity for each bin. P values (p < .05 and p < .10) for independent samples t-tests on each bin appear in red and grey respectively. Elevated slow theta (2–5 Hz) distinguishes the Nightmare group through all REM cycles but is especially distinctive in REM cycle 4.
Cycle 2
For F4, differences occurred especially between 2.0–5.0 and 5.4–8.0 Hz (p < .05). For C4, differences were apparent mainly between 2.0 and 7.0 Hz (p < .05).
Some differences were also found for higher frequencies. For F4, differences were apparent between 15.4 and 16.6 Hz (p < .05) and for C4, between 15.2–15.8, 16.0–16.8, 17.0–17.8, 18.4–20, 21.6–22.6, and 23.2–25.2 Hz (p < .05).
Cycle 3
The theta difference favoring NM recallers was observed for F4 between 2.4–4.6, 5.6–6.0, and 8.8–9.2 Hz (p < .05) and for C4 between 2.0–4.0, 5.8–6.4, and 6.8–7.2 Hz (p < .05). There were also several differences in nonconsecutive bins ranging from 15.4 to 23.4 Hz (p < .10). Finally, there were differences between 29.6 and 32.0 Hz, especially between 31.2 and 32.0 Hz (p < .05).
Cycle 4
The same theta difference was found for F4 between 2.4 and 6 Hz (p < .05) and for several nonconsecutive bins between 7.6 and 10.0 Hz (p < .10). For C4, differences were found between 1.4–6.4 Hz (p < .05) and 6.4–8.2 Hz (p < .10).
DISCUSSION
Results largely support our hypotheses concerning theta power. There was higher absolute power for NM recallers in a spectral band corresponding to slow theta (2.0–5.0 Hz) which was, as expected from a cross-state continuity assumption, evident for all three sleep–wake states but was especially clear for REM sleep. In addition, the REM sleep results were observed in cycles 2–4, suggesting that the difference is relatively consistent and possibly independent from ultradian influences. That the difference also appeared in NREM sleep was not expected but consistent with the possibility that theta anomalies are even more generalized for frequent NM recallers than the literature leads us to expect.38 However, in NREM sleep, there was also evidence of abnormally high activity in a broader theta band that includes fast theta (up to 7.4 Hz).
The expected localization of slow-theta activity to a limbic-prefrontal circuit was supported by evidence that the power difference arose in C3, C4, F3, and F4—but not in O1 and O2—for REM and NREM sleep and in F3 and F4 for wake. Overall, the findings suggest that NM recallers produce more theta activity in a limbic-prefrontal circuit in all sleep–wake stages but especially in REM sleep.
There is considerable evidence that slow-theta rhythms are implicated in the acquisition, extinction, and retrieval of fear memories and that these oscillations are prominent in both wake and REM sleep in humans and rodents (reviewed in38). These oscillations arise from brain regions we previously hypothesized to contribute to NM formation, that is, hippocampus, amygdala, medial prefrontal cortex (mPFC), and anterior cingulate cortex (ACC; Nielsen & Levin36) among others. Thus, one study using source location analyses during fear conditioning found ACC-localized theta power to be associated with the amplitude of responses to conditioned but not to unconditioned stimuli.62 Other studies have found increased coherence of theta oscillations between amygdala and hippocampus during fear memory retrieval63 and increased coupling of theta in amygdala, hippocampus, and mPFC during fear memory retrieval.64 While such findings were made in the waking state, similar theta coherence patterns are observed during REM sleep.65 Thus, our results could reflect some alterations in the treatment of fear memories during REM sleep as proposed by the neurocognitive model of nightmares.40 With our participants, such alterations might have been exacerbated by the extensive presleep neurocognitive testing or by the emotional challenges of sleeping overnight in an unfamiliar laboratory. However, our results cannot be taken to support the more precise fear memory treatment mechanisms proposed by the model. These mechanisms should be studied separately and with other research methods.
Our finding of elevated REM slow-theta power in NM recallers is also coherent with a theoretical model of emotion processing during sleep66 which stipulates that emotional memory loses its affective tone, in part, through its neural reactivation during REM sleep. Theta oscillations in a network comprised of (frontal) cortex, hippocampus, and amygdala are hypothesized by Walker and van der Helm to underlie this neural reactivation and downregulation. To illustrate, the same group37 found that the extent of consolidation of negative emotional memories following a 90-min nap was positively correlated with relative right (vs. left) frontal theta during REM sleep, the correlation being maximal (r = .88, p < .001) at 5.75 Hz. We speculate that NM recallers may have been stimulated to activate emotional memory networks—possibly in response to the pre-sleep neurocognitive testing or the emotional challenge of sleeping in the laboratory—to a greater extent than were control participants.
This possible alteration of memory processing during REM sleep in participants with NM could be related to other REM-dependent functions, such as associative memory processing.67 NMs have often been conceptualized as a pathological phenomenon, triggered by stress and adversity (i.e., as a diathesis-stress phenomenon). However, some recent results fit less well into this kind of conceptualization, suggesting instead that NM recallers may also show more creativity, for example, by demonstrating enhanced associative processes for both positive and negative stimuli.68 Therefore, the proposed enhanced memory processing by patients with NM could have simultaneously adaptive and maladaptive features. While the associative function of REM sleep has been demonstrated,67–69 its EEG correlates remain unclear. However, theta and/or high-frequency bands such as gamma might be involved.70
For NREM sleep, our results indicated increased power for NM recallers in a broad band of activity ranging mainly between 1–8 Hz and 20–32 Hz (beta). Group differences were most prominent for central derivations and only marginally present for frontal derivations. Heightened beta activity during NREM sleep has been regarded as indicating hyperarousal.17 As such, our results converge with other accounts of autonomic arousal in NM recallers during NREM sleep.71 NM participants also displayed trends for enhanced activity in the high alpha band, which could indicate decreased sleep depth and lower threshold for awakening.72
Elevated 25–32 Hz NREM activity in NM recallers could, to some extent, reflect increased gamma band activity. Although gamma is typically considered to range between 30 and 100 Hz, some authors argue that its lower limit is 25 Hz.73 Like theta, gamma is implicated in memory consolidation.73–75 In rodents, hippocampal gamma is modulated by and phase locked to theta activity,76,77 and these mechanisms are involved in long-term potentiation.78 There is not enough evidence to propose a role for theta–gamma interactions in NM production at the moment, but gamma could be involved in the ability to recall dreams.79
One of our study’s long-term objectives is to evaluate how the altered EEG power in NM recallers contributes to the presence of NMs in other psychopathologies. Clinically significant NMs are prevalent in psychiatric populations with various psychopathologies compared to the general population.80 Alterations in EEG have been found in such populations, including alterations in the theta frequency band. Alterations in theta coherence have been found in depression81–83 and are related to positive symptoms in patients with psychosis.84 Furthermore, alterations in theta power have been shown in panic disorder,85 and theta power is correlated with a measure of trait dissociation.86 To summarize, theta alterations seem to be involved in various psychopathologies for which NM prevalence is also high. Other EEG analyses (coherence, asymmetry patterns, etc.) in frequent NM recallers could offer further insight into brain mechanisms underlying NM production in a variety of psychiatric populations.
For the wake EEG, we found fewer differences between groups. NM recallers displayed higher power in several 0.2 Hz frequency bins, ranging from 3 to 5.5 Hz, on frontal and central derivations. While our methods and the size of our sample warrant a cautious interpretation of these results, they are compatible with our hypothesis of altered wake theta power over a limbic-prefrontal circuit. Group differences in wake EEG are also consistent with our cross-state continuity assumption.40 It is nevertheless possible that differences in spectral activity are more evident during REM sleep than during wakefulness; REM sleep has at times been described as a “challenge” state,87 given its features of heightened autonomic arousal and high levels of brain activity. In comparison, our wake EEG sample was obtained during relaxed wakefulness, surely a less challenging state. Additionally, there may have been some emotional arousal during REM sleep related to dream mentation, while relaxed wakefulness was likely an emotionally neutral state.
Our results partially replicate those of a previous study19 in revealing higher REM 3.0–4.0 Hz relative power in NM recallers compared to controls. This result converges with other accounts of enhanced theta activity, which were described earlier. However, results do not replicate Simor et al.’s19 finding of elevated relative REM “high alpha” in this population. If anything, there was a tendency in the direction opposite to what was expected. However, we did find trends for enhanced absolute “high alpha” in NREM sleep.
The enhanced relative REM 10–14.5 Hz activity, obtained in a previous study19 but not in the present one, was interpreted as indicating intensified oneiric processes. Despite the appeal of this explanation, it could also be argued that less alpha activity is related to intensified oneiric processes. Indeed, two of the effects of REM sleep deprivation are decreased alpha during recovery nights88,89 and intensification of dream-like quality of mentation.90 Decrease in REM alpha power has also been related to successful dream recall,91,92 although this result was not replicated in all studies93–95 and may also be region specific.92 The possible role of alpha activity in NM formation remains unclear and, as our results suggest, may not be the only mechanism contributing to it.
LIMITATIONS
It could be argued that our results in EEG absolute activity are due to differences in gender ratio between groups and that relative power measures would be more valid. Indeed, some studies show higher power densities across a wide frequency range in women than in men,60,61 and these results have been attributed to differences in skull thickness. Some other work highlights the complexity of this phenomenon and challenges previous assumptions about gender differences in the EEG.96 Nevertheless, we reanalyzed our main results with a balanced gender ratio (by only keeping three males in the control group and three males in the NM group). For the wake EEG, the results were basically the same but with fewer 0.2-Hz bins reaching either the p < .10 or p < .05 levels. In NREM sleep, the group effect was reduced to statistical trends for derivations F3, F4, and C4 but remained approximately intact for C3. Results for O1 and O2 remained similar. Despite the loss of statistical power, higher theta power was still observable in NM recallers in REM sleep on derivations F4, C3, and, to a lesser extent, C4. A few differences were observable at p < .10 for F3 and no group differences were observed for O1 and O2. The differences in high frequencies were still observable on C3 and C4. Thus, our key results remain intact after controlling for gender.
Our NM recallers may not be entirely representative of samples studied in previous research. For example, NM recallers scored no different than controls on the BDI-II. While we might also expect group differences in trait anxiety, there were none. These results are at odds with studies showing associations between NM frequency, NM distress, and psychopathology7,9,53 and may, in part, be due to our sampling method. The fact that we included participants based on NM frequency rather than NM distress (or a combination of both) combined with the fact that there is a closer association between NM distress and psychopathology than there is between NM frequency and psychopathology9,53 could have resulted in a NM sample with rather low NM distress and hence reduced psychopathology. Simor et al.19 seem to have used a more selective sampling procedure that resulted in their cohort leaning more toward the “high distress” type of idiopathic NM recaller.39 Nevertheless, the reduced psychopathology of our NM recaller sample also increases our confidence that the results are not simply due to differences in trait anxiety, depression, or sleep disturbances.
As pointed out by one reviewer, it is unclear whether our NM group suffers at a clinically significant level. However, our participants were not clinical patients and did not seek treatment for their NMs. Our participants also had a higher retrospective dream recall frequency than did the control group, in addition to recalling more NMs. As we indicate in the Discussion section, it is possible that our NM recallers possess both adaptive and maladaptive features that differentiate them from the control group. Such a possibility would be consistent with Hartmann’s work on personality boundaries, in which he describes individuals with thin boundaries as more sensitive, creative, and prone to recall both dreams and NMs; he did not regard thin boundaries as pathological per se.97 In a recent study from our lab, sleep spindle density was found to be associated with both dream recall and bad dream recall, but not with NM recall, suggesting that bad dreams may have some adaptive or memory function as indexed by spindles.98 Future conceptualizations of nightmares should account for research results which suggest that idiopathic bad dreams and nightmares are not simply pathological phenomena.99
It should be noted that this study did not include an adaptation night, while Simor et al.19 analyzed recordings from the second laboratory night. Some authors call for systematic two-night polysomnographic assessments.100 The absence of an adaptation night in the present study may have exaggerated group differences, since NM recallers have an enhanced first-night effect101 and we cannot entirely discard its possible impact on our results. While in-depth discussion of sleep architecture results is beyond the scope of the present report, it is worth mentioning that our results are independent of changes in sleep macrostructure and self-reported sleep problems (PSQI scores), which was not the case in a previous study.19,102
The present study was designed to be descriptive and exploratory and suffers from low statistical power. The fact that we used 0.2 Hz frequency bands rather than a set of predefined frequency bands led to a considerable number of statistical tests. But it also provides more precision about the location of group differences, circumventing limitations in the traditional EEG band definitions. It is important to add that not all of our results would have survived a conservative correction for multiple comparisons. Thus, interpretation of our results should be made with caution and a consideration for weaknesses in the study design. Nevertheless, our results offer meaningful insights into NM pathology and justify further attempts to replicate and expand them.
FUTURE STUDIES
This study used power spectral analysis to document sleep alterations in frequent NM recallers, but other EEG dynamics (coherence, asymmetry patterns, etc.) could also be useful in assessing NM pathology. In light of literature illustrating the importance of theta coherence between key brain regions in fear memory extinction, it would be informative to use high-density EEG recordings to more precisely localize the source of theta activity.
Future studies could also investigate the effects of REM sleep deprivation on frequent NM recallers. While NM recallers appear to react differently from control participants to REM deprivation,103,104 no study has yet investigated deprivation-induced changes in spectral activity. A study of the effects of REM deprivation in normal participants found diminished power in the alpha band on recovery nights.88,89 If alpha activity (or other markers such as REM density88 or REM latency103) reliably index REM sleep pressure, it is possible that REM deprivation could affect NM and controls’ spectral activities differentially. Another possibility would be to investigate EEG activity in NM-prone individuals during phases of high-REM propensity using a morning nap paradigm.68
CONCLUSION
This study examined EEG spectral activity in frequent NM recallers and controls during wakefulness and sleep. We found higher “slow-theta” EEG activity in the NM group primarily for frontal and central electrodes. These alterations were observable during wakefulness, NREM, and REM sleep but more distinctive for REM sleep. The findings are consistent both with a neurocognitive model of nightmares40 which stipulates that idiopathic NMs result from alterations in a hippocampal–amygdala–prefrontal neural circuit controlling fear memory formation and extinction, and with animal studies of fear conditioning during wakefulness and REM sleep.63,65,105 The results also support a model that emphasizes the role of REM sleep theta in emotion processing.64 Secondarily, we found that frequent NM recallers display elevated NREM beta and possibly gamma power. Finally, the results partially replicate a previous investigation of EEG activity in NM recallers.19 Reasons for the nonreplication of some aspects of the prior study include methodological differences, especially differences in sampling methods and the severity of NM pathology of our participants.
Further investigation is needed to fully understand brain mechanisms underlying NM production and especially how such mechanisms are present in a variety of psychiatric populations. Future studies could use other EEG measures (coherence analyses), more sophisticated tools (high-density EEG with enables source localization), or experimental procedures (REM deprivation, nap paradigms) to uncover these mechanisms. Dream reports could also be used to link subjective experiences (clarity of dream recall, negative emotions, distress) to EEG activity, especially to theta power or coherence. This could provide much needed insight into the possible emotion-processing functions of REM sleep.
Funding
This work was supported by the Canadian Institutes of Health Research grant (Nielsen: Grant # MOP-115125); and the Natural Sciences and Engineering Research Council of Canada (Nielsen: Grant #312277).
Disclosure Statement
None declared.
REFERENCES
- 1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2. Li SX, Zhang B, Li AM, Wing YK. Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep. 2010; 33(6): 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Academy of Sleep Medecine. The International Classification of Sleep Disorders–Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 4. Paul F, Schredl M, Alpers GW. Nightmares affect the experience of sleep quality but not sleep architecture: an ambulatory polysomnographic study. Borderline Personal Disord Emot Dysregul. 2015; 2(1). doi:10.1186/s40479-014-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohayon MM, Morselli PL, Guilleminault C. Prevalence of nightmares and their relationship to psychopathology and daytime functioning in insomnia subjects. Sleep. 1997; 20: 340–348. [DOI] [PubMed] [Google Scholar]
- 6. Berquier A, Ashton R. Characteristics of the frequent nightmare sufferer. J Abnorm Psychol. 1992; 101(2): 246–250. [DOI] [PubMed] [Google Scholar]
- 7. Levin R, Fireman G. Nightmare prevalence, nightmare distress, and self-reported psychological disturbance. Sleep. 2002; 25(2): 205–212. [PubMed] [Google Scholar]
- 8. Tanskanen A, Tuomilehto J, Viinamaki H, Vartiainen E, Lehtonen J, Puska P. Nightmares as predictors of suicide. Sleep. 2001; 24(7): 844–847. [PubMed] [Google Scholar]
- 9. Miro E, Martinez M. Affective and personality characteristics in function of nightmare prevalence, nightmare distress, and interference due to nightmares. Dreaming. 2005; 15(2): 89–105. [Google Scholar]
- 10. Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull. 2007; 133: 482–528. [DOI] [PubMed] [Google Scholar]
- 11. Tanskanen A, Tuomilehto J, Viinamaki H, Vartiainen E, Lehtonen J, Puska P. Nightmares as predictors of suicide. Sleep. 2001; 24: 845–848. [PubMed] [Google Scholar]
- 12. van Liempt S, van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013; 30(5): 469–474. [DOI] [PubMed] [Google Scholar]
- 13. van Schagen A, Lancee J, Swart M, Spoormaker V, van den Bout J. Nightmare disorder, psychopathology levels, and coping in a diverse psychiatric sample. J Clin Psychol. 2017; 73(1): 65–75. [DOI] [PubMed] [Google Scholar]
- 14. Sheaves B, Onwumere J, Keen N, Stahl D, Kuipers E. Nightmares in patients with psychosis: the relation with sleep, psychotic, affective, and cognitive symptoms. Can J Psychiatry. 2015; 60(8): 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mukai J, Uchida S, Miyazaki S, Nishihara K, Honda Y. Spectral analysis of all‐night human sleep EEG in narcoleptic patients and normal subjects. J Sleep Res. 2003; 12(1): 63–71. [DOI] [PubMed] [Google Scholar]
- 16. Massicotte-Marquez J, Carrier J, Decary A et al. Slow-wave sleep and delta power in rapid eye movement sleep behavior disorder. Ann Neurol. 2005; 57(2): 277–282. [DOI] [PubMed] [Google Scholar]
- 17. Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998; 10(5): 1826–1834. [DOI] [PubMed] [Google Scholar]
- 18. Gaudreau H, Joncas S, Zadra A, Montplaisir J. Dynamics of slow-wave activity during the NREM sleep of sleepwalkers and control subjects. Sleep. 2000; 23(6): 755–760. [PubMed] [Google Scholar]
- 19. Simor P, Horvath K, Ujma PP, Gombos F, Bodizs R. Fluctuations between sleep and wakefulness: wake-like features indicated by increased EEG alpha power during different sleep stages in nightmare disorder. Biol Psychol. 2013; 94(3): 592–600. [DOI] [PubMed] [Google Scholar]
- 20. Lanquart JP. Contribution to the definition of the power bands limits of sleep EEG by linear prediction. Comput Biomed Res. 1998; 31(2): 100–111. [DOI] [PubMed] [Google Scholar]
- 21. Lega B, Burke J, Jacobs J, Kahana MJ. Slow-theta-to-gamma phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb Cortex. 2016; 26(1): 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tendler A, Wagner S. Different types of theta rhythmicity are induced by social and fearful stimuli in a network associated with social memory. eLife. 2015; 4: e03614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C. Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus. 2012; 22(9): 1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris Water Maze. J Neurosci. 2008; 28(23): 5983–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat Neurosci. 2013; 16(3): 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen H, Yang L, Chen F et al. Functional inactivation of orexin 1 receptors in the cerebellum disrupts trace eyeblink conditioning and local theta oscillations in guinea pigs. Behav Brain Res. 2013; 250: 114–122. [DOI] [PubMed] [Google Scholar]
- 27. Moroni F, Nobili L, Curcio G et al. Sleep in the human hippocampus: a stereo-EEG study. PLoS One. 2007; 2: e867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobs J. Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Philos Trans R Soc Lond B Biol Sci. 2014; 369(1635): 20130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012; 22(4): 748–761. [DOI] [PubMed] [Google Scholar]
- 30. Bódizs R, Kantor S, Szabo G, Szucs A, Eross L, Halasz P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001; 11: 747–753. [DOI] [PubMed] [Google Scholar]
- 31. Durrant SJ, Cairney SA, McDermott C, Lewis PA. Schema-conformant memories are preferentially consolidated during REM sleep. Neurobiol Learn Mem. 2015; 122: 41–50. [DOI] [PubMed] [Google Scholar]
- 32. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989; 146: 697–707. [DOI] [PubMed] [Google Scholar]
- 33. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013; 170(4): 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pace-Schott EF, Germain A, Milad MR. Sleep and REM sleep disturbance in the pathophysiology of PTSD: The role of extinction memory. Biol Mood Anxiety Disord. 2015; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nielsen T. The stress acceleration hypothesis of nightmares. Front Neurol. 2017; 8: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. 2007; 11(4): 295–310. [DOI] [PubMed] [Google Scholar]
- 37. Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009; 19(5): 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hutchison IC, Rathore S. The role of REM sleep theta activity in emotional memory. Front Psychol. 2015; 6: 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cowdin N, Kobayashi I, Mellman TA. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res. 2014; 232(5): 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levin R, Nielsen T. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull. 2007; 133(3): 482–528. [DOI] [PubMed] [Google Scholar]
- 41. Jokic-Begic N, Begic D. Quantitative electroencephalogram (qEEG) in combat veterans with post-traumatic stress disorder (PTSD). Nord J Psychiatry. 2003; 57(5): 351–355. [DOI] [PubMed] [Google Scholar]
- 42. Woodward SH, Murburg MM, Bliwise DL. PTSD-related hyperarousal assessed during sleep. Physiol Behav. 2000; 70(1): 197–203. [DOI] [PubMed] [Google Scholar]
- 43. Dumel G, Carr M, Marquis L-P, Blanchette-Carriere C, Paquette T, Nielsen T. Infrequent dream recall associated with low performance but high overnight improvement on mirror-tracing. J Sleep Res. 2015; 24(4): 372–382. [DOI] [PubMed] [Google Scholar]
- 44. Spielberger CD, Gorsuch R.. State-Trait Anxiety Inventory for Adults: Manual and Sample: Manual, Instrument and Scoring Guide. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 45. Gauthier J, Bouchard S. Adaptation canadienne-française de la forme révisée du State–Trait Anxiety Inventory de Spielberger. Can J Behav Sci. 1993; 25(4): 559–578. [Google Scholar]
- 46. Beck A, Steer R, Brown G.. Manual for the BDI-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 47. Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory–II. Psychol Assess. 1998; 10(2): 83–89. [Google Scholar]
- 48. Belicki K. The relationship of nightmare frequency to nightmare suffering with implications for treatment and research. Dreaming. 1992; 2(3): 143–148. [Google Scholar]
- 49. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 50. Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998; 45(1): 5–13. [DOI] [PubMed] [Google Scholar]
- 51. Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Stability of the pittsburgh sleep quality index and the epworth sleepiness questionnaires over 1 year in early middle-aged adults: the CARDIA study. Sleep. 2006; 29(11): 1503–1506. [DOI] [PubMed] [Google Scholar]
- 52. Schredl M. Effects of state and trait factors on nightmare frequency. Eur Arch Psychiatry Clin. 2003; 253: 241–247. [DOI] [PubMed] [Google Scholar]
- 53. Blagrove M, Farmer L, Williams E. The relationship of nightmare frequency and nightmare distress to well‐being. J Sleep Res. 2004; 13(2): 129–136. [DOI] [PubMed] [Google Scholar]
- 54. Blagrove M, Fisher S. Trait–state interactions in the etiology of nightmares. Dreaming. 2009; 19(2): 65. [Google Scholar]
- 55. Wood JM, Bootzin RR. The prevalence of nightmares and their independence from anxiety. J Abnorm Psychol. 1990; 99(1): 64–68. [DOI] [PubMed] [Google Scholar]
- 56. Izard CE. The Differential Emotions Scale: DES IV-A; A Method of Measuring the Meaning of Subjective Experience of Discrete Emotions. Newark, DE: University of Delaware; 1993. [Google Scholar]
- 57. Jasper HH. The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol. 1958; 10: 371–375. [PubMed] [Google Scholar]
- 58. Stellate Systems Inc. Harmonie v6.2b Montréal, Québec, Canada:Stellate Systems Inc 2007. [Google Scholar]
- 59. Silber MH, Ancoli-Israel S, Bonnet MH et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007; 3(2): 121–131. [PubMed] [Google Scholar]
- 60. Dijk DJ, Beersma DG, Bloem GM. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989; 12: 500–507. [DOI] [PubMed] [Google Scholar]
- 61. Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology. 2001; 38(2): 232–242. [PubMed] [Google Scholar]
- 62. Mueller EM, Panitz C, Hermann C, Pizzagalli DA. Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J Neurosci. 2014; 34(21): 7059–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seidenbecher T, Laxmi TR, Stork O, Pape H- C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003; 301(5634): 846–850. [DOI] [PubMed] [Google Scholar]
- 64. Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011; 6(6): e21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Popa D, Duvarci S, Popescu AT, Léna C, Paré D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010; 107(14): 6516–6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Walker MP, van Der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009; 135(5): 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A. 2009; 106(25): 10130–10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carr M, Blanchette-Carrière C, Marquis L- P, Ting CT, Nielsen T. Nightmare sufferers show atypical emotional semantic associations and prolonged REM sleep-dependent emotional priming. Sleep Med. 2016; 20: 80–87. [DOI] [PubMed] [Google Scholar]
- 69. Carr M, Nielsen T. Morning rapid eye movement sleep naps facilitate broad access to emotional semantic networks. Sleep. 2015; 38(3): 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miltner WH, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999; 397(6718): 434–436. [DOI] [PubMed] [Google Scholar]
- 71. Simor P, Bodizs R, Horvath K, Ferri R. Disturbed dreaming and the instability of sleep: altered nonrapid eye movement sleep microstructure in individuals with frequent nightmares as revealed by the cyclic alternating pattern. Sleep. 2013; 36(3): 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McKinney SM, Dang-Vu TT, Buxton OM, Solet JM, Ellenbogen JM. Covert waking brain activity reveals instantaneous sleep depth. PLoS One. 2011; 6(3): e17351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Colgin LL. Do slow and fast gamma rhythms correspond to distinct functional states in the hippocampal network? Brain Res. 2015; 1621: 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cornwell BR, Overstreet C, Grillon C. Spontaneous fast gamma activity in the septal hippocampal region correlates with spatial learning in humans. Behav Brain Res. 2014; 261: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lega B, Burke J, Jacobs J, Kahana MJ. Slow-theta-to-gamma phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb Cortex. 2016; 26(1): 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czéh B, Morozov A. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003; 116(1): 201–211. [DOI] [PubMed] [Google Scholar]
- 77. Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003; 37(2): 311–322. [DOI] [PubMed] [Google Scholar]
- 78. Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986; 368(2): 347–350. [DOI] [PubMed] [Google Scholar]
- 79. Ferrarelli F, Smith R, Dentico D et al. Experienced mindfulness meditators exhibit higher parietal-occipital EEG gamma activity during NREM sleep. PLoS One. 2013; 8(8): e73417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Swart ML, van Schagen AM, Lancee J, van den Bout J. Prevalence of nightmare disorder in psychiatric outpatients. Psychother Psychosom. 2013; 82(4): 267–268. [DOI] [PubMed] [Google Scholar]
- 81. Fingelkurts AA, Fingelkurts AA, Rytsälä H, Suominen K, Isometsä E, Kähkönen S. Impaired functional connectivity at EEG alpha and theta frequency bands in major depression. Hum Brain Mapp. 2007; 28(3): 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology. 2003; 40(6): 939–949. [DOI] [PubMed] [Google Scholar]
- 83. Knott V, Mahoney C, Kennedy S, Evans K. EEG power, frequency, asymmetry and coherence in male depression. Psychiat Res Neuroim. 2001; 106(2): 123–140. [DOI] [PubMed] [Google Scholar]
- 84. Morrison PD, Nottage J, Stone JM et al. Disruption of frontal theta coherence by [Delta]9-Tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology. 2011; 36(4): 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Knott VJ, Bakish D, Lusk S, Barkely J, Perugini M. Quantitative EEG correlates of panic disorder. Psychiat Res Neuroim. 1996; 68(1): 31–39. [DOI] [PubMed] [Google Scholar]
- 86. Russ MJ, Campbell SS, Kakuma T, Harrison K, Zanine E. EEG theta activity and pain insensitivity in self-injurious borderline patients. Psychiatry Res. 1999; 89(3): 201–214. [DOI] [PubMed] [Google Scholar]
- 87. Verrier RL, Muller JE, Hobson JA. Sleep, dreams, and sudden death: the case for sleep as an autonomic stress test for the heart. Cardiovasc Res. 1996; 31(2): 181–211. [PubMed] [Google Scholar]
- 88. Endo T, Roth C, Landolt H- P et al. Selective REM sleep deprivation in humans: Effects on sleep and sleep EEG. Am J Physiol Regul Integr Comp Physiol. 1998; 274(4): R1186–R1194. [DOI] [PubMed] [Google Scholar]
- 89. Roth C, Achermann P, Borbély AA. Alpha activity in the human REM sleep EEG: Topography and effect of REM sleep deprivation. Clin Neurophysiol. 1999; 110(4): 632–635. [DOI] [PubMed] [Google Scholar]
- 90. Nielsen T, Stenstrom P, Takeuchi T et al. Partial REM-sleep deprivation increases the dream-like quality of mentation from REM sleep and sleep onset. Sleep. 2005; 28(9): 1083–1089. [DOI] [PubMed] [Google Scholar]
- 91. Esposito MJ, Nielsen T, Paquette T. Reduced alpha power associated with the recall of mentation from Stage 2 and Stage REM sleep. Psychophysiology. 2004; 41(2): 288–297. [DOI] [PubMed] [Google Scholar]
- 92. Chellappa SL, Frey S, Knoblauch V, Cajochen C. Cortical activation patterns herald successful dream recall after NREM and REM sleep. Biol Psychol. 2011; 87(2): 251–256. [DOI] [PubMed] [Google Scholar]
- 93. Marzano C, Ferrara M, Mauro F et al. Recalling and forgetting dreams: theta and alpha oscillations during sleep predict subsequent dream recall. J Neurosci. 2011; 31(18): 6674–6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Siclari F, Baird B, Perogamvros L et al. The neural correlates of dreaming. Nat Neurosci. 2017; 20(6): 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Scarpelli S, D’Atri A, Mangiaruga A et al. Predicting dream recall: EEG activation during NREM sleep or shared mechanisms with wakefulness? Brain Topogr. 2017. [DOI] [PubMed] [Google Scholar]
- 96. Brière M-È, Forest G, Chouinard S, Godbout R. Evening and morning EEG differences between young men and women adults. Brain Cogn. 2003; 53(2): 145–148. [DOI] [PubMed] [Google Scholar]
- 97. Hartmann E. Boundaries in the Mind: A New Psychology of Personality. New York: Basic Books; 1991. [Google Scholar]
- 98. Nielsen T, Carr M, Blanchette-Carrière C et al. NREM sleep spindles are associated with dream recall. Sleep Spindles Cortical Up States. 2017; 1(1): 27–41. [Google Scholar]
- 99. Nielsen T, Carr M. Nightmares and nightmare function. In: Kryger M, Roth BJ, Dement W, eds. Principles and Practice of Sleep Medicine. Vol 6 New York: Elsevier; 2016: 546–554. [Google Scholar]
- 100. Newell J, Mairesse O, Verbanck P, Neu D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res. 2012; 200(2): 795–801. [DOI] [PubMed] [Google Scholar]
- 101. Kis A, Szakadát S, Simor P, Gombos F, Horváth K, Bódizs R. Objective and subjective components of the first-night effect in young nightmare sufferers and healthy participants. Behav Sleep Med. 2014; 12: 1–12. [DOI] [PubMed] [Google Scholar]
- 102. Simor P, Horváth K, Gombos F, Takács KP, Bódizs R. Disturbed dreaming and sleep quality: altered sleep architecture in subjects with frequent nightmares. Eur Arch Psychiatry Clin Neurosci. 2012; 262(8): 687–696. [DOI] [PubMed] [Google Scholar]
- 103. Nielsen T, Paquette T, Solomonova E, Lara-Carrasco J, Popova A, Levrier K. REM sleep characteristics of nightmare sufferers before and after REM sleep deprivation. Sleep Med. 2010; 11(2): 172–179. [DOI] [PubMed] [Google Scholar]
- 104. Nielsen T, Paquette T, Solomonova E, Lara-Carrasco J, Colombo R, Lanfranchi P. Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep. 2010; 33(1): 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo-hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007; 25(6): 1823–1831. [DOI] [PubMed] [Google Scholar]