Abstract
Study Objectives
This study examines the effects of short and long sleep duration patterns in young adults on the levels of C-reactive protein (CRP), as well as the potential effect modification by sex.
Methods
Using data from waves III (age 18–26) and IV (age 24–32) of the National Longitudinal study of adolescent to adult health, we examined the association between sleep trajectories in young adults, and the risk of elevated high sensitivity-CRP (hs-CRP), a marker of systemic inflammation.
Results
Short sleep trajectories were associated with significantly elevated log-transformed hs-CRP (coefficient = 0.11, p-value .03) and with significantly higher odds of having hs-CRP levels > 3 mg/L (OR = 1.86, 95% CI 1.29, 2.67). The association was modified by sex, with the association between short sleep duration and hs-CRP limited to males. Both the continuous (coefficient 0.117, p-value = .0362) and the categorized hs-CRP (OR = 2.21, 95% CI 1.48, 3.30) were significantly elevated with short sleep durations in males, whereas no significant associations were seen in females with short sleep durations. By contrast, log hs-CRP was significantly elevated in females with long sleep durations (coefficient = 0.232, p-value = .0296), with a nonsignificant increase in the odds of having hs-CRP levels greater than 3 mg/L (OR = 1.48, 95% CI 0.75, 2.93), whereas there were no associations with long sleep duration in males.
Conclusions
Systemic inflammation, measured by an elevated level of hs-CRP, is seen with persistent short sleep duration in young adult men and persistent long sleep duration in young adult women.
Keywords: sleep duration, trajectories, inflammation, young adults
Statement of Significance
The results of this study indicate a differential association between sleep duration and systemic inflammation in young adults, where men are affected by short sleep durations while women appear to be more affected by long durations. Given that systemic inflammation has been found to be a risk factor for many chronic illnesses, we think that improving sleep duration in young adults, as part of general lifestyle improvements, may contribute to lowering the risk of cardiovascular disease, depression, and other chronic diseases through the reduction of systemic inflammation. Further studies are needed to verify the association, examine the biological mechanisms underlying these sex differences, and test the impact of consistently optimal sleep duration on levels of high sensitivity–C-reactive protein in young adults.
INTRODUCTION
Sleep loss is an increasingly prevalent public health problem with more than 35% of US adults sleeping less than the recommended 7 hours per night.1 Sleep durations that are very short or very long have been implicated in the risks of obesity, cardiovascular diseases, and depression, among other conditions.2 Recent studies pointed to the production of proinflammatory cytokines, including C-reactive protein (CRP), an acute phase reactant, as a possible pathway that explains these associations.2 Although experimental sleep deprivation studies found an association between acute sleep loss and CRP levels, population-based studies had inconsistent results, with some finding a significant association between short3–5 or long6–9 sleep durations and CRP, whereas others found no significant associations.10–12 Some studies found the association to be limited to women13–15 or men.16 The majority of these studies were cross-sectional. In order to confirm whether systemic inflammation is, indeed, the mediating factor in the association between sleep duration and negative health outcomes, it is imperative to determine first whether sleep duration is associated with systemic inflammation, using large population-based longitudinal studies. These studies are lacking, especially in young adults, and none—to the best of our knowledge—has used trajectories of sleep duration to examine the association between sleep and inflammation in young adults.
This study examines the effects of short and long sleep duration patterns in young adults on the levels of CRP, as well as the potential effect modification by sex.
METHODS
Design
This study uses data from waves III and IV of the National Longitudinal Study of Adolescent to Adult Health (Add health), which is a nationally representative study of adolescent health and behavior in the United States that used clustered sampling design to select adolescents in grades 7–12 and followed them through four waves of interviews into early adulthood (age 24–32 years). Of the 17670 participants who had completed in-home and parental interviews at wave I (1994–1995), 15197 completed interviews in wave III (2001–2002) and 15701 were interviewed in wave IV (2008), with a response rate of 80% in wave IV. Around 0.6% of the cohort had high sensitivity-CRP (hs-CRP) values below the limit of detection and were not included in the analysis. This analysis includes participants who were seen at waves III and IV and have a valid measurement of CRP (n = 10744).
Measures
hs-CRP was collected during wave IV interview using capillary blood spots that were dried, frozen, and assayed using an adapted DBS (Dried Blood Spot) sandwich ELISA procedure. Cross-validation was performed using 87 dried blood spot and paired plasma samples and revealed strong correlation and linear association (Pearson R = 0.98; Plasma CRP = DBS CRP/0.4285). More details are available online (see “Add Health IV Documentation: Measures of Inflammation and Immune Function”).17
Because the distribution of hs-CRP was right-skewed, we used natural log-transformation prior to the analysis. We analyzed log-transformed hs-CRP as a continuous variable, and hs-CRP as a categorical variable, using values ≤3 mg/L (low-to-average risk) as the reference value, and >3 mg/L (high risk) as the main outcome value, in accordance with the American Heart Association and the Centers for Disease Control and Prevention’s classification of hs-CRP concentrations and cardiovascular disease risk in adults.18 For the main analysis, we excluded values of hs-CRP > 10 mg/L, as they are known to be associated with acute infectious or inflammatory conditions and were present in 12.4% of the study sample. We later performed sensitivity analysis including values of hs-CRP > 10 mg/L to check their impact on the results.
Sleep duration was measured in waves III and IV by the participant’s answers to questions about bed-time and wake-up times on work/school nights and nonwork nights. They were asked four questions: (1) On days that you don’t have to get up at a certain time, what time do you usually go to sleep the night or day before? (2) On days when you go to work, school, or similar activities, what time do you usually go to sleep the night (or day) before? (3) On days that you don’t have to get up at a certain time, what time do you usually get up? (4) On days when you go to work, school, or similar activities, what time do you usually wake up?
We calculated a weighted average sleep duration for each participant at each wave by assigning weights of 5/7 to work nights and 2/7 to nonwork night.
Covariates
Body Mass Index (BMI) calculated from measured height and weight in waves III and IV. A participant was considered overweight if he/she had a BMI > 25.0 kg/m2. In order to capture effects of long-standing overweight, we constructed a variable that combined waves III and IV BMIs into one variable with three categories: (0) if normal weight in both waves, (1) if overweight in one of the two waves, and (2) if overweight in both waves.
Demographic variables included age, sex, and race-ethnicity.
Measures of socioeconomic status in wave IV included the following:
Total household income in wave IV before taxes and deductions. We categorized the income variable as follows: (1) less than $50000 per year, (2) $50000−$99999 per year, and (3) more than $100000 per year.
Educational level: Participants were asked about their highest level of education, with choices ranging from (1) eighth grade or less to (2) completed post-baccalaureate professional education. We categorized the education variable into (1) less than high school graduate, (2) high school graduate to some post-secondary education, and (3) college degree or more.
Behavioral factors were measured in wave IV and included the following:
Smoking: Participants were asked, “During the past 30 days, on how many days did you smoke cigarettes?” Those who smoked on one or more days were considered current smokers, as per the definition from the National Survey on Drug Use and Health (NSDUH).19
Alcohol: Participants were asked, “During the past 12 months, on how many days did you drink alcohol?” Those who said they drank once a month or more were considered drinkers, according to the definition of the NSDUH.
Physical activity measure was constructed from a series of seven questions about the frequency of engaging in physical activities of varying intensity in the past seven days. These questions included many types of activities such as walking, gardening, and many types of sports and leisurely activities. Participants who answered yes to any of the moderate to vigorous activity in the last seven days’ questions were coded as “1” on physical activity and those who reported no moderate to vigorous activity in the past week were coded as “0.”
Illnesses and Medications: hs-CRP is sensitive to many illnesses and medications; therefore, we adjusted for these conditions in the analysis using the following variables:
Subclinical symptoms: A count of the number of symptoms the participants reported, including cold or flu symptoms, fever, night sweats, nausea, vomiting, or diarrhea, blood in stool or urine, frequent urination, and skin rash or abscess in the past 2 weeks. Counts range from 0 to ≥3.
Infectious or inflammatory diseases: A count of lifetime diagnoses of conditions including asthma/chronic bronchitis/emphysema, lifetime diagnosis of hepatitis C, in addition to gum disease, active infection, injury, acute illness, surgery, and active seasonal allergies in the past 4 weeks. Counts range from 0 to ≥3.
Other illnesses or conditions: Ever diagnosed and/or current hypertension or depression, current snoring (indicative of obstructive sleep apnea).
Medications that may affect hs-CRP: NSAID/Salicylate, Cox-2 inhibitors, inhaled corticosteroids, corticotropin/glucocorticoids, antirheumatic/antipsoriatic, immunosuppressive, and antiinflammatory medications.
Data Analysis
We identified trajectories of sleep duration using PROC TRAJ in SAS 9.4®, which uses the group-based mixed model described by Nagin.20 This model looks at each individual’s sleep pattern over the two time points and classifies individuals with similar sleep duration patterns into trajectory groups. We tried a number of models identifying two to five trajectory groups. Selection of the optimal number of groups was based on the best fitting model for the data that produced the lowest value for the adjusted Bayesian Information Criterion (BIC),21 while providing an appropriate number of groups with adequate sample sizes that permit a meaningful analysis and facilitate interpretability. Additionally, we used entropy as a measure of classification accuracy to ensure that groups were adequately separated and subjects were placed into the appropriate groups for their sleep patterns.21 Consequently, we found that the model with three trajectory groups was the best fit for our data, based on BIC and entropy, compared with the models with four and five groups, which included trajectory groups with sample sizes too small for meaningful analyses. Therefore, we identified three groups of sleep duration trajectories from waves III to IV. Each participant was assigned into the group into which he or she had the highest probability of belonging.
Descriptive analyses included calculation of means and confidence intervals for continuous variables and frequencies of categorical variables. It was performed using SAS 9.4 procedures SURVEYMEANS and SURVEYFREQ, which take into account the complex sampling design.
Using SURVEYREG procedure in SAS, we ran regression analysis models examining the association between sleep duration trajectories and the natural logarithm of hs-CRP, first unadjusted, then after adjusting for all covariates, obtaining coefficients and p-values. We examined interaction with sex by adding an interaction term to the model and obtaining coefficients and p-values. We then performed the same analysis stratified by sex.
We examined the association with hs-CRP categories using SAS procedure SURVEYLOGISTIC, starting with an unadjusted model, then adding covariates to obtain adjusted odds ratios and 95% confidence intervals. We examined interactions between sleep duration and sex by adding an interaction term to the model. Then, we performed the same analysis above, stratified by sex.
RESULTS
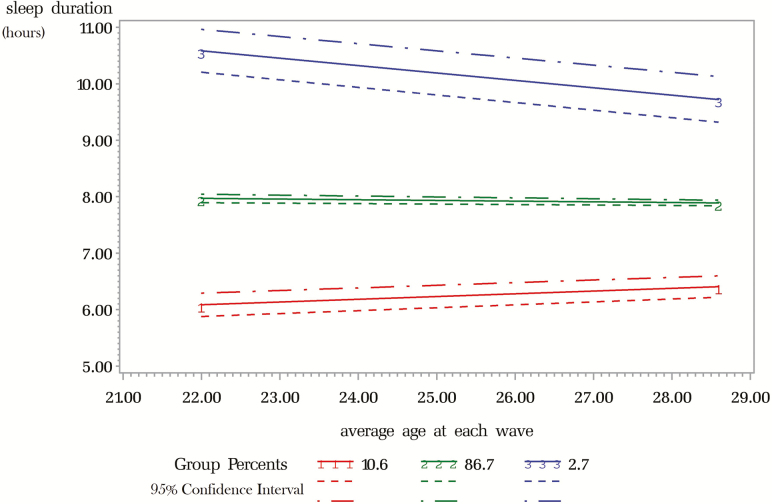
Figure 1 shows the sleep duration trajectories from wave III to wave IV by average age at each wave. The three distinct trajectories represent participant with generally short sleep durations (group 1), those with generally good duration (group 2), and those with generally long duration (group 3). Our model had an entropy of 0.75, and the mean posterior probability for belonging into each of three groups was between 73% and 91%, with a minimum above 50%. Group 1 participants had median sleep durations in waves III and IV of 5.5 and 5.8 hours, respectively. Group 2 had median durations of 7.9 and 7.8 hours, and group 3 had median durations of 10.7 and 10.6 hours in waves III and IV, respectively (Table 1).
Figure 1.
Trajectories of sleep duration between waves III and IV of the Add Health study. Solid lines represent average sleep duration in each trajectory group. Dashed and dashed–dotted lines represent 95% confidence interval.
Table 1.
Selected Characteristics of Participants in Waves III and IV of the National Longitudinal Study of Adolescent to Adult Health, in Total and Stratified by Sleep Duration Trajectory Group.
| Variable | Total (n = 10741) | Short (10.6%) | Good (86.7%) | Long (2.7%) | p |
|---|---|---|---|---|---|
| Age in wave III (mean) (95% CI) | 28.4 (28.2–28.6) | 28.6 (28.3–29.0) | 28.4 (28.2–28.6) | 28.6 (28.1–29.1) | .1790 |
| Log-transformed CRP (mean) (95% CI) | 1.06 (1.04–1.08) | 1.13 (1.04–1.22) | 1.05 (1.03–1.07) | 1.21 (1.07–1.34) | .5637 |
| CRP > 3 mg/L (%) | 32.0% | 40.9% | 31.4% | 35.9% | .0055 |
| Sex (% female) | 50.4% | 30.7% | 51.3% | 64.5% | <.0001 |
| Race/ethnicity (%) | <.0001 | ||||
| White | 66.4% | 57.7% | 67.2% | 46.1% | |
| Black | 15.3% | 23.6% | 14.5% | 41.2% | |
| Hispanic | 11.8% | 11.4% | 11.8% | 10.9% | |
| Other | 6.4% | 7.3% | 6.5% | 1.8% | |
| Overweight | .0390 | ||||
| In one wave (%) | 24.7% | 25.8% | 24.5% | 38.9% | |
| In both waves (%) | 46.8% | 52.6% | 46.5% | 47.2% | |
| Household income (%) | .0002 | ||||
| <$50.000 | 49.5% | 52.6% | 48.9% | 72.4% | |
| $50.000–$100.000 | 36.8% | 36.3% | 37.0% | 23.9% | |
| >$100.000 | 13.7% | 11.1% | 14.1% | 3.8% | |
| Educational level | <.0001 | ||||
| Less than high school | 8.5% | 10.9% | 8.3% | 10.7% | |
| High school diploma | 17.3% | 19.8% | 16.8% | 44.4% | |
| Some post-secondary ed. | 43.8% | 49.0% | 43.5% | 41.6% | |
| College degree or higher | 30.4% | 20.2% | 31.5% | 3.3% | |
| No Physical activity (%) | 15.0% | 12.7% | 14.8% | 30.4% | <.0001 |
| Smoking (%) | 31.0% | 43.7% | 30.4% | 25.8% | <.0001 |
| Alcohol (%) | 58.9% | 58.5% | 59.1% | 39.7% | .0246 |
| Hypertension | 25.3% | 32.3% | 24.8% | 31.6% | .0084 |
| Snoring | 49.7% | 60.5% | 49.2% | 42.4% | <.0001 |
| Depression | 20.7% | 24.2% | 20.4% | 23.3% | .2662 |
| Trouble sleeping or staying asleep (1 or more per week) | 67.8% | 64.3% | 68.3% | 50.6% | .0016 |
* Bold P-values indicate statistically significant differences among sleep trajectory groups.
Characteristics of the study population are presented in Table 1. The analytic sample consisted of 10744 participants, 50.4% of whom were females, 66.4% white, and the mean age at wave IV was 28.4 years. Short sleep duration trajectory included 10.6% and long sleep was present in 2.7%. There were significant differences among people belonging into different trajectory groups in terms of several key variables. Compared with good sleepers, those with short sleep trajectories were significantly more likely to have hs-CRP > 3 mg/L (40.9% vs. 31.4%). They were also more likely to be male (69.3% vs. 49.7%), black (23.6% vs. 14.5%), overweight in both waves (52.6% vs. 46.5%), of lower education and income, and to smoke, have hypertension, depression, or snoring. Long sleepers were the most likely to be female (64.5%), black (41.2%), have income below $50000, high school degree or less, and to have no bouts physical activity. However, they were the least likely to smoke, drink, snore, or have trouble sleeping (Table 1). Both the short and the long sleep duration groups had higher mean Log-transformed hs-CRP (1.13 and 1.21, respectively) compared with the good sleep duration group (1.05); however, the differences were not statistically significant. (Table 1).
Table 2 shows mean hs-CRP and the prevalence of hs-CRP > 3 mg/L by sleep duration categories in waves III and IV. The table shows that 30% of participants slept under 6 hours in wave III and 12% slept under 6 hours in wave IV. Prevalence of sleeping longer than 9 hours was 11% in wave III and 10% in wave IV. Short and long sleepers had higher hs-CRP levels in waves III and IV and higher probability of having hs-CRP greater than 3 mg/L, compared with good sleepers.
Table 2.
Distribution of hs-CRP Values by Sleep Duration Categories in Waves III and IV of the Add Health Study.
| n (%) | Mean CRP (95% CI)a | CRP > 3 mg/L (%) | |
|---|---|---|---|
| Wave III sleep duration | |||
| <6 hours/night | 2834 (30.3%) | 2.62 (2.50, 2.75) | 33.5% |
| 6–9 hours/night | 5448 (58.4%) | 2.45 (2.35, 2.52) | 30.4% |
| >9 hours/night | 1056 (11.3%) | 2.80 (2.60, 3.01) | 36.5% |
| Wave IV sleep duration | |||
| <6 hours/night | 1069 (11.7%) | 2.58 (2.38, 2.77) | 34.4% |
| 6–9 hours/night | 7338 (78.4%) | 2.50 (2.41. 2.58) | 31.2% |
| >9 hours/night | 931 (9.9%) | 2.82 (2.60, 3.04) | 35.2% |
aExcluding CRP values greater than 10 mg/L.
Regression analysis results (Table 3) show that hs-CRP is significantly higher in young adults with short sleep duration patterns compared with those with good sleep durations, after adjusting for relevant covariates (coefficient = 0.111, p-value = .0327). Long duration trajectories were also significant in the unadjusted model (coefficient = 0.154, p-values = .0244) and in the fully adjusted model (coefficient = 0.200, p-value = .0145). Other significant variables included female sex (p < .0001), overweight in one or both waves (p < .0001), low educational level, lack of physical activity, hypertension, and snoring. There was a borderline significant interaction between female sex and short sleep duration (p = .0494), and a significant interaction between long sleep duration and being overweight in both waves (p = .0130).
Table 3.
Results of Multivariate Linear Regression Analysis for Natural Logarithm of CRP and Sleep Duration in Waves III and IV in the Add Health Study (Regression Coefficients and p-Values).
| Unadjusted coefficient (p-value) | Fully adjusteda | |
|---|---|---|
| Sleep duration trajectory group | ||
| Short | 0.08 (.0875) | 0.10 (.0327) |
| Good | Ref | Ref |
| Long | 0.15 (.0244) | 0.20 (.0145) |
| Sex (female) | 0.27 (<.0001) | |
| Race/ethnicity | ||
| Black | −0.02 (.3580) | |
| Hispanic | −0.0001 (.9862) | |
| Other | −0.06 (.1051) | |
| White | Ref | |
| Overweight in both waves | 0.42 (<.0001) | |
| Overweight in one wave | 0.25 (<.0001) | |
| Normal weight in both waves | Ref | |
| Education | ||
| Less than high school | 0.09 (.0291) | |
| High school diploma | 0.12 (<.0001) | |
| Some post-secondary education | 0.08 (.0005) | |
| College degree or higher | Ref | |
| Income | 0.02 (.4805) | |
| <$50.000 | 0.004 (.8563) | |
| $50.000–$100.000>$100.000 | Ref | |
| Alcohol use | −0.08 (.0519) | |
| Smoking | 0.01 (.7227) | |
| Lack of physical activity | 0.06 (.0150) | |
| Hypertension | 0.10 (.0001) | |
| Depression | 0.01 (.5726) | |
| Snoring | 0.08 (<.0001) | |
| Trouble sleeping | 0.01 (.2614) |
aFully adjusted model also included symptoms of infection, subclinical symptoms of inflammatory disease, and medication use (not shown in table).
Statistically significant results are highlighted in bold.
Logistic regression examining categorical hs-CRP as an outcome (Table 4) shows that, similar to linear regression on Ln hs-CRP, short sleep duration raised the odds of having hs-CRP > 3 mg/L in the unadjusted model, and the effect persisted and became more pronounced after adjusting for relevant covariates (OR = 1.86, 95% CI 1.29, 2.67). Long sleep duration was not significantly associated with categorical hs-CRP in any of the models.
Table 4.
Association Between Sleep in Waves III and IV and CRP levels greater than 3 mg/dL in the Add Health Study (Odds Ratios and 95% CI).
| Unadjusted | Fully adjusteda | |
|---|---|---|
| Sleep duration trajectory group | ||
| Short | 1.51 (1.12, 2.04) | 1.86 (1.29, 2.67) |
| Good | Ref | Ref |
| Long | 1.22 (0.82, 1.84) | 1.25 (0.67, 2.35) |
| Sex (female) | ||
| Race/ethnicity | 2.44 (2.09, 2.85) | |
| Black | 0.95 (0.74, 1.22) | |
| Hispanic | 0.98 (0.80, 1.21) | |
| Other | 0.78 (0.57, 1.06) | |
| White | Ref | |
| Overweight in both waves | 3.36 (2.82, 4.02) | |
| Overweight in one wave | 1.97 (1.62, 2.39) | |
| Normal weight in both waves | Ref | |
| Education | ||
| Less than high school | 1.13 (0.85, 1.52) | |
| High school diploma | 1.30 (1.03, 1.66) | |
| Some post-secondary education | 1.13 (0.95, 1.36) | |
| College degree or higher | Ref | |
| Income | ||
| <$50.000 | 1.14 (0.91, 1.43) | |
| $50.000–$100.000 | 1.05 (0.85, 1.29) | |
| >$100.000 | Ref | |
| Alcohol use | 0.86 (0.74, 1.01) | |
| Smoking | 1.03 (0.88, 1.20) | |
| Lack of physical activity | 1.32 (1.08, 1.61) | |
| Hypertension | 1.40 (1.17, 1.68) | |
| Depression | 1.05 (0.85, 1.29) | |
| Snoring | 1.30 (1.13, 1.49) | |
| Trouble sleeping | 1.01 (0.98, 1.04) |
aFully adjusted model also included symptoms of infection, subclinical symptoms of inflammatory disease, and medication use (not shown in table).
Statistically significant results are highlighted in bold.
Other variables that were significantly associated with hs-CRP > 3 mg/L included female sex (adjusted OR = 2.44, 95% CI 2.09–2.85), overweight in one wave OR = 3.36 (2.82, 4.02), and overweight in both waves OR = 1.97 (1.62, 2.39), in addition to low education level (high school degree, compared with college degree or more), hypertension, snoring, and lack of physical activity. Medications and symptoms of infection were not significantly associated with higher levels of CRP, but subclinical symptoms of inflammatory diseases did produce a statistically significant OR with 1, 2, and 3 or more symptoms. Race, smoking, drinking, and trouble sleeping were not significantly associated with CRP in the adjusted model.
Interaction term sex × sleep duration groups were significant for short sleep and female sex, compared with good sleep and male sex (p = .0380). Stratified analysis by sex (Table 5) revealed that the association between short sleep duration and hs-CRP is limited to males. Both the continuous and the categorical hs-CRP were significantly elevated in males who had short sleep durations, whereas no association was found in females. By contrast, log hs-CRP was significantly elevated in females with long sleep durations, and a nonsignificant increase in the odds of having hs-CRP levels greater than 3 mg/L, whereas there were no associations in males.
Table 5.
Results of Sex-Stratified Regression Analysis Examining the Association of Sleep Trajectory Groups and Continuous log hs-CRP as Well as Categorical CRP in the Add Health Study.
| Sleep duration trajectory groups | |||
|---|---|---|---|
| Short | Good | Long | |
| Males | |||
| CRP > 3 mg/L (OR, 95% CI) vs. ≤3 mg/L | 2.21 (1.48, 3.30) | Ref | 0.94 (0.21, 4.11) |
| Ln hs-CRP (coefficient, p-value) | 0.117 (0.0362) | Ref | 0.176 (0.1813) |
| Females | |||
| CRP > 3 mg/L (OR, 95% CI) vs. P ≤ 3 mg/l | 0.998 (0.54, 1.86) | Ref | 1.52 (0.77, 2.95) |
| Ln hs-CRP (coefficient, p-value) | −0.004 (0.9544) | Ref | 0.232 (0.0296) |
Results shown are of the fully adjusted models, which included Race, BMI, education, income, smoking, alcohol, physical activity, depression, hypertension, trouble sleeping, symptoms of infection, subclinical symptoms of inflammatory disease, and medication use.
Statistically significant results are highlighted in bold.
Sensitivity Analysis
Results of multivariate regression including hs-CRP values greater than 10 were not very different. In logistic regression, short sleep trajectory group produced an adjusted OR of 1.74 (1.26, 2.39), which is slightly lower than the one we had while excluding extremely high hs-CRP values. Similar to the original analysis, long sleep trajectories were not significantly associated with hs-CRP > 3 mg/L. Similarly, in the linear regression, short sleep trajectories were associated with an adjusted coefficient of 0.10 and p-value = .0492. Long sleep trajectories were not significant in the adjusted model.
Given that our trajectory groups may include participants whose sleep duration changed significantly between waves, we repeated the analysis after excluding participants whose sleep duration changed by 2 hours or more (17% of the population). The results were very similar to our main analysis in terms of the association between short sleep duration and hs-CRP in the logistic model OR (short sleep vs. normal sleep) = 1.73 (1.15–2.58), and the sex-specific odds ratios: In males: OR = 2.08 (1.24–3.49) vs. females: OR = 1.06 (0.51–2.20). The linear model also showed comparable results regarding short sleep duration (coefficient = 0.11, p = .0402), but long sleep duration was not significant in the adjusted model neither in the combined sample (coefficient = 0.12, p = .3894) nor in females (coefficient = 0.11, p = .4838).
DISCUSSION
This prospective analysis of a nationally representative sample of US young adults revealed different relationships between sleep duration and hs-CRP levels among men and women. Men who sleep less than the recommended seven hours per night have a significant increase in the probability of having systemic inflammation, measured by hs-CRP, whereas no association is detected in females. By contrast, women appear to be more sensitive to long sleep durations, which are associated with elevated hs-CRP in young women but not young men.
Long sleep duration was significantly associated with the high hs-CRP in women, which is consistent with previous studies,9,22,23 but it was not statistically significant when hs-CRP was used as a categorized variable. This may be due to lack of power due to the small number of participants in the long trajectory group (n = 106 females) or may reflect an association with the high hs-CRP, rather than with the probability of having hs-CRP > 3 mg/L, compared with values ≤3 mg/L. Further studies using hs-CRP categories are needed to clarify. Long sleep duration in women was also not statistically significant in the sensitivity analysis that excluded 17% of participants whose sleep duration changed by 2 hours or more between waves III and IV, or in the analysis that included CRP values greater than 10 mg/L.
Our results are somewhat consistent with those found by Ferrie et al.,4 in their longitudinal analysis from the Whitehall II study of middle-aged men and women, using two measures of sleep duration 5 years apart. They found the decrease in sleep duration to be significantly associated with elevated hs-CRP, but the association was no longer significant after adjusting for BMI, hypertension, and diabetes. Unlike Ferrie et al., we adjusted for medication use, symptoms of infectious and inflammatory diseases, and sleep problems. Similarly, the Coronary Artery Risk Development in Young Adults (CARDIA) study12 found a significant association between sleep duration at baseline and CRP levels 5 years later, but similar to the study of Ferrie et al. above, the association was attenuated to nonsignificant after adjusting for covariates. This study used CRP as a continuous variable and, unlike our analysis, had only one measurement of sleep duration at baseline, categorized as short (<6 hours) or usual (6 hours or more). Both studies above had older cohorts than ours, and smaller sample sizes (5003 and 2679, respectively), that may explain the nonsignificant results. Neither of the two studies examined the interaction between sleep duration and sex.
Our finding of significant effect modification by sex requires further study. We found short sleep in men and long sleep in women to be associated with high levels of hs-CRP. In contrast, analysis of Miller et al. from the Whitehall II study11 found the association between short sleep duration and hs-CRP to be limited to females. The difference in results may be due to the fact that their cohort was older with most women at the perimenopause or postmenopause stage, which differ from our young women in terms of hormonal influence on CRP and in terms of sleep patterns. With regards to long sleep duration in women, Miller et al. also found high levels of hs-CRP in women who slept for more than 8 hours, but unlike our results, it was not statistically significant. Their analysis was cross-sectional with a smaller sample size (n = 4677) than ours, which may explain the nonsignificant results. The Nurses’ Health Study,9 which included 935 women aged 43–69 years, also found an association between long sleep duration and hs-CRP (as a continuous variable) in women. Similar sex-related differences have been observed in the relationship between sleep apnea and inflammation, and between sleep duration and each of BMI,24 hypertension,25 and inflammation-linked cardiovascular disease risk.24 These observed differences may be related to menstrual cycles or the use of hormonal contraceptives, which has an effect on CRP levels.10 Studies have shown that CRP levels are higher during menstruation compared with the rest of the menstrual cycle.26,27 Given that many women experience sleepiness and tiredness during their menses, it is possible that the observed association between long sleep duration and CRP levels is affected by the menstrual cycle. We are unable to confirm this given that our data did not include information on menstrual cycle at the time of CRP collection. Additionally, most long sleepers in our study were female (64%) which may also explain why the results were significant in females but not in males as the male group had a much fewer long sleepers.
In a recent study using data from Add health, Mantua and Spencer28 examined the interactive effects of nocturnal sleep and daytime naps in relation to hs-CRP. This study included 2147 participants and measured sleep duration in wave III only. They found that short sleepers had the highest hs-CRP, but did not find a significant effect of sleep duration on hs-CRP in their regression model. The smaller sample size may be responsible for the lack of significant results. Including naps in our model did not change the results of our analysis.
This is the first study to our knowledge that used sleep trajectories to examine the association between sleep duration and CRP. Our sleep trajectories matched those found by Gilmour et al.29 in a study of Canadians during the same time period as our study. Their short duration trajectory included 11.1% of the population, and the long duration trajectory included 2.1%. In comparison, our short and long trajectories included 10.6% and 2.7% of our population, respectively.
Our findings suggest that improved sleep duration in young adults may reduce the risk of systemic inflammation. As recent studies2 suggested that the production of proinflammatory cytokines, including CRP, an acute phase reactant, is a possible pathway that explains the association between sleep duration and many chronic illnesses, we think that improving sleep duration in young adults, as part of general lifestyle improvements, may contribute to lowering the risk of cardiovascular disease, depression, and other chronic diseases.
The main limitation of this analysis was the reliance on self-reported sleep durations in waves III and IV, which raises the question of agreement between subjectively and objectively measured sleep duration. Self-report was found by Lauderdale et al. to over-estimate actigraphy-measured sleep duration.30 By contrast, several studies found self-report to be valid for large-scale studies of habitual sleep duration and correspond well to objective measures such as actigraphy.31–33
Another limitation is the lack of measurement of CRP prior to wave IV, which does not allow for determination of temporality. As with many chronic health outcomes that produce no symptoms, it is very difficult to capture the onset of systemic inflammation. We are cautious in interpreting the results of our analysis and realize the need for further longitudinal studies with multiple measurements of CRP. Lastly, we cannot rule out the possibility of residual confounding which may have affected the results.
Major strengths of this study include the large cohort of US young adults, an under-represented group in epidemiologic research, and the complex sampling design that insures the sample is representative of US population. Additional strengths include the longitudinal design, and utilization of group-based trajectory modeling to predict the impact of sleep duration patterns on levels of hs-CRP. Use of sleep trajectories, or growth curve models, provides great flexibility when dealing with complex study designs and is typically characterized by much higher levels of statistical power than comparable traditional methods applied to the same data.34
CONCLUSION
Accumulating evidence point to sleep loss, as well as excess sleep as modifiable risk factors for many acute and chronic diseases, resulting in significant public health burden. It is therefore very important to understand the mechanism of these associations. The present study supports previous research indicating a link between sleep duration and inflammation, which partially explains the detrimental effects of inadequate sleep. It also indicates that the impact of inadequate sleep duration differs between males and females, which will help guide public health interventions and sleep education efforts. More prospective studies are needed to understand the mediating effect of inflammation on the risk of systemic inflammation and associated chronic diseases.
FUNDING
No direct support was received from grant P01-HD31921 for this analysis.
DISCLOSURE STATEMENT
None declared. This analysis used secondary data and does not contain any studies with human participants or animals performed by any of the authors. This analysis used secondary data with no involvement of human participants. For this type of study formal consent is not required. Furthermore, data did not include any identifying information.
ACKNOWLEDGMENTS
This manuscript uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, The University of North Carolina at Chapel Hill, Carolina Population Center, 206 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth_contracts@unc.edu).
REFERENCES
- 1. Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report.Unhealthy Sleep-Related Behaviors—12 States, 2009.; 2011. http://www.cdc.gov/mmwr/PDF/wk/mm6008.pdf. Accessed March 8, 2015. [PubMed] [Google Scholar]
- 2. Miller MA, Cappuccio FP.. Sleep, Inflammation, and Disease. Aetiology to Public Health; 2010:1–34. doi:10.1093/acprof. [Google Scholar]
- 3. Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S et al. ; AFINOS Study Group. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Med. 2011; 12(10): 997–1002. [DOI] [PubMed] [Google Scholar]
- 4. Ferrie JE, Kivimäki M, Akbaraly TN et al. . Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol. 2013; 178(6): 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013; 36(5): 769–779E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011; 21(11): 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013; 5: 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel SR, Zhu X, Storfer-Isser A et al. . Sleep duration and biomarkers of inflammation. Sleep. 2009; 32(2): 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007; 30(5): 1233–1240. [DOI] [PubMed] [Google Scholar]
- 10. Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009; 23(3): 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller MA, Kandala NB, Kivimaki M et al. . Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009; 32(7): 857–864. /pmc/articles/PMC2706900/?report=abstract. Accessed July 31, 2014. [PMC free article] [PubMed] [Google Scholar]
- 12. Cho HJ, Seeman TE, Kiefe CI, Lauderdale DS, Irwin MR. Sleep disturbance and longitudinal risk of inflammation: Moderating influences of social integration and social isolation in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Brain Behav Immun. 2015; 46(46): 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cappuccio FP, Miller MA, Lockley SW.. Sleep, Health, and Society: The Contribution of Epidemiology. Aetiology to Public Health series. Oxford, New York: Oxford University Press; 2010. [Google Scholar]
- 14. Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008; 22(6): 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang S, Liu X, Kim JS et al. . Association between short sleep duration and the risk of sensitization to food and aero allergens in rural Chinese adolescents. Clin Exp Allergy. 2011; 41(4): 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liukkonen T, Räsänen P, Ruokonen A et al. . C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007; 69(8): 756–761. [DOI] [PubMed] [Google Scholar]
- 17. Whitsel EA, Cuthbertson CC, Tabor JW et al. . Add Health Wave IV Documentation: Measures of Inflammation and Immune Function — Add Health 2012. http://www.cpc.unc.edu/projects/addhealth/documentation/guides/add-health-wave-iv-documentation-measures-of-inflammation-and-immune-function/view. Accessed May 19, 2016.
- 18. Pearson TA, Mensah GA, Alexander RW et al. . Markers of inflammation and cardiovascular disease application to clinical and public health practice a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation. 2003;107(3):499–511. [DOI] [PubMed] [Google Scholar]
- 19. Stanton CA, Keith DR, Gaalema DE et al. . Trends in tobacco use among US adults with chronic health conditions: National Survey on Drug Use and Health 2005–2013. Prev Med (Baltim). 2016. doi:10.1016/j.ypmed.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001; 29(3): 374–393. [Google Scholar]
- 21. Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model A Multidiscip J. 2007; 14(4): 535–569. http://eric.ed.gov/?id=EJ780617. Accessed May 17, 2016. [Google Scholar]
- 22. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016; 80(1): 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prather AA, Vogelzangs N, Penninx BW. Sleep duration, insomnia, and markers of systemic inflammation: results from the Netherlands study of depression and anxiety (NESDA). J Psychiatr Res. 2015; 60 (50): 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5(2):93–102. http://www.ncbi.nlm.nih.gov/pubmed/17430213. Accessed May 23, 2016. [DOI] [PubMed] [Google Scholar]
- 25. Cappuccio FP, Miller MA, Lockley SW.. Sleep, Health and Society. Oxford: Oxford University Press; 2010. http://www.oxfordscholarship.com.ezproxy.lib.usf.edu/view/10.1093/acprof:oso/9780199566594.001.0001/acprof-9780199566594. [Google Scholar]
- 26. Gursoy AY, Caglar GS, Kiseli M, Pabuccu E, Candar T, Demirtas S. CRP at early follicular phase of menstrual cycle can cause misinterpretation for cardiovascular risk assessment. Interv Med Appl Sci. 2015; 7(4): 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008; 136(2): 138–146. [DOI] [PubMed] [Google Scholar]
- 28. Mantua J, Spencer RM. The interactive effects of nocturnal sleep and daytime naps in relation to serum C-reactive protein. Sleep Med. 2015; 16(10): 1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilmour H, Stranges S, Kaplan M et al. . Longitudinal trajectories of sleep duration in the general population. Heal reports. 2013; 24(11): 14–20. [PubMed] [Google Scholar]
- 30. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008; 19(6): 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biddle DJ, Robillard R, Hermens DF, Hickie IB, Glozier N. Accuracy of self-reported sleep parameters compared with actigraphy in young people with mental ill-health. Sleep Heal. 2015; 1(3): 214–220. [DOI] [PubMed] [Google Scholar]
- 32. Tremaine RB, Dorrian J, Blunden S. Subjective and objective sleep in children and adolescents: Measurement, age, and gender differences. Sleep Biol Rhythms. 2010; 8(4): 229–238. [Google Scholar]
- 33. Wolfson AR, Carskadon MA, Acebo C et al. . Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003; 26(2): 213–216. http://www.ncbi.nlm.nih.gov/pubmed/12683482. Accessed April 19, 2016. [DOI] [PubMed] [Google Scholar]
- 34. Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. J Cogn Dev. 2010; 11(2): 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]