Abstract
Study objectives
To determine the effect of light exposure on subsequent sleep characteristics under ambulatory field conditions.
Methods
Twenty healthy participants were fitted with ambulatory polysomnography (PSG) and wrist-actigraphs to assess light exposure, rest–activity, sleep quality, timing, and architecture. Laboratory salivary dim-light melatonin onset was analyzed to determine endogenous circadian phase.
Results
Later circadian clock phase was associated with lower intensity (R2 = 0.34, χ2(1) = 7.19, p < .01), later light exposure (quadratic, controlling for daylength, R2 = 0.47, χ2(3) = 32.38, p < .0001), and to later sleep timing (R2 = 0.71, χ2(1) = 20.39, p < .0001). Those with later first exposure to more than 10 lux of light had more awakenings during subsequent sleep (controlled for daylength, R2 = 0.36, χ2(2) = 8.66, p < .05). Those with later light exposure subsequently had a shorter latency to first rapid eye movement (REM) sleep episode (R2 = 0.21, χ2(1) = 5.77, p < .05). Those with less light exposure subsequently had a higher percentage of REM sleep (R2 = 0.43, χ2(2) = 13.90, p < .001) in a clock phase modulated manner. Slow-wave sleep accumulation was observed to be larger after preceding exposure to high maximal intensity and early first light exposure (p < .05).
Conclusions
The quality and architecture of sleep is associated with preceding light exposure. We propose that light exposure timing and intensity do not only modulate circadian-driven aspects of sleep but also homeostatic sleep pressure. These novel ambulatory PSG findings are the first to highlight the direct relationship between light and subsequent sleep, combining knowledge of homeostatic and circadian regulation of sleep by light. Upon confirmation by interventional studies, this hypothesis could change current understanding of sleep regulation and its relationship to prior light exposure.
Clinical trial details
This study was not a clinical trial. The study was ethically approved and nationally registered (NL48468.042.14).
Keywords: circadian rhythms, chronobiology, scoring, sleep/wake mechanisms, actigraphy
Statement of Significance
This research is the first application of ambulatory polysomnography in the assessment of the regulation of sleep. This study demonstrates that laboratory findings of light exposure being related to subsequent sleep characteristics are observable in real life situations for the first time. Through these observations, we form the basis of a hypothesis for the interaction of light exposure and sleep regulation. If supported by intervention studies to be correct, current models of sleep regulation will need to be reassessed.
INTRODUCTION
Light is the primary zeitgeber (entraining cue) for the vertebrate circadian system. In mammals, light information is projected to the circadian pacemaker or synchronizer (located in the hypothalamic suprachiasmatic nucleus, SCN1) via intrinsically photosensitive retinal ganglion cells (ipRGCs).2 Light entrainment is modulated, not only by the duration of light exposure, but also by its intensity and spectral composition.3,4 Additionally, light can influence sleep timing, duration, structure, and quality.5 These sleep characteristics6–8and the intensity of sleep9 are controlled by the circadian drive for wakefulness and the accumulation of homeostatic sleep pressure during preceding wakefulness. We hypothesize that light has an influence on subsequent sleep by either altering the rate of sleep pressure build up or by altering the timing of the circadian-modulated wake drive.
Timing of light exposure has been observed to be linked to clock phase, estimated by the time of dim-light melatonin onset (DLMO), both in the laboratory3,10,11 and in real-life situations.12–16 However, behavioral timing during some highly controlled laboratory studies in animals does not match observations in the field, such as in the cases of House mice,17 Spiny mice,18 Golden hamsters,19 and Tuco tucos20 (e.g., hamsters are nocturnal in the laboratory but diurnal in the wild). These contradictions might be explained by the multiple factors that could affect sleep timing, duration, and structure, which are excluded under controlled laboratory conditions. For humans, social restrictions, environmental influences, eating patterns, artificial light, disturbances by family or pets, and work schedules are just a few examples that could alter circadian entrainment.
In laboratory settings, sleep timing, duration, and structure have been linked to both evening21–26 and morning single-day bright light exposure8,10,25 with morning light advancing sleep timing and leading to a reduction of sleep duration at the expense of rapid eye movement (REM) sleep.25 Some field studies have gone further in assessing how light exposure influences sleep when individuals have the freedom to habitually sleep and conduct their usual daily routines. Objective actigraph and subjective field measurements have shown that earlier sleep timing is related to more daytime light exposure, either with27or without imposed sleep schedules.28–30 Earlier light timing also resulted in earlier sleep phase,15 and repeated bright daytime light exposure has been observed to lead to earlier sleep phase in Alzheimer’s disease patients in real-life settings.31 Consistent with this, later and lower intensity light exposure has also been linked to later sleep phases in subjective field studies.32,33 The relationship between light exposure and sleep duration remains less clear. In an intervention-field study, a reduction in daytime light intensity was linked to a decrease in sleep duration,34 whereas without intervention, the presence of electrical lighting in addition to normal sunlight led to a decrease in sleep duration.35 This paradox might be explained by timing of light. In the evening, additional light seems to decrease sleep duration, whereas during the day it increases sleep duration. There is a lack of real-life modern society observational studies assessing the association of everyday light exposure with subsequent sleep timing and duration.
In subjective reports of sleep in nonintervention field studies, it was observed that sleep quality was considerably reduced when individuals had low exposure to light during the day.36–38 Laboratory electroencephalography (EEG) studies have shown that light directly before24,39,40 or during sleep41 increases sleep disturbance. Increased daytime light exposure through additional lighting in Alzheimer care homes has confirmed laboratory study findings that bright light during the day results in a reduction of sleep disturbances42 and an increase in sleep consolidation.43 These sleep parameters are key experimental targets for improving health44 and therefore require more detailed description under ambulatory conditions.
With the current field study, we sought to simultaneously assess the link between light exposure and subsequent sleep timing, duration, sleep structure, and objective quality without experimental intervention. Although observational field studies have gone far in corroborating laboratory findings, the influence of light exposure on sleep structure and objective quality has not been assessed in the field. Through a multiple-day, comparative analysis of both rest–activity data and polysomnography (PSG)-based sleep data, with preceding light exposure, we can determine whether laboratory findings are transferable to real-life settings for the first time. Furthermore, we seek to assess whether sleep timing, quality, or architecture depends on prior light intensity and timing, and whether these relationships are mediated by the individual’s clock phase. We intend to assess whether current laboratory finding-based sleep regulation models remain valid under ambulatory conditions in the field.
METHODS
Participants
For this study, 23 participants were recruited of which 20 healthy male (n = 8) and female (n = 12) subjects aged 20–29 years (average 23.4 ± 2.2 [±SD]) were used for analysis. Chronotype was assessed via the Munich Chronotype Questionnaire30 (MCTQ) as mid-sleep on free days (MSF) regardless of alarm-clock use. To ensure large variation in chronotypes, the dataset contained eight very early (MSF range 2.75–3.79, 3.50 ± 0.33 [mean ± SD]), nine intermediate (MSF range 4.63–4.83, 4.72 ± 0.09 [mean ± SD]), and three very late (MSF range 7.04–7.75, 7.33 ± 0.37 [mean ± SD]) chronotypes. These cutoffs were aimed at obtaining 10% of the Dutch chronotype distribution in each category, as determined by the updated Dutch MCTQ database45 consisting of 4132 people from the Netherlands aged 20–30 years. Participants were excluded when they had moderate or severe sleep disturbances (Pittsburgh Sleep Quality Index46 [PSQI], ≥10), tendencies for anxiety and/or depression (Hospital Anxiety and Depression Scale47 [HADS], ≥8 and Beck Depression Inventory48 [BDI], ≥8), existing chronic medical conditions, the need for medication use, previous head injury, epilepsy, smoking, excessive use of alcohol or caffeine (>3 and >8 glasses per day, respectively), any use of recreational drugs in the last year, having a BMI of <18 or >27 or a body weight of <36 kg, having travelled across more than one time-zone in the last month or had a history of shift work (in-house general health questionnaire), or color-blindness (Ishihara color blindness test49).
In total, 23 participants were recruited into the study after meeting all inclusion criteria. Of these, three were excluded from both analyses: one dropped out, one was excluded based on inaccurate completion of inclusion questionnaires, and one was excluded due to unreliable light data for both assessment periods. A further six participants were only excluded from PSG analyses either due to malfunction of the actigraph (five) or due to unreliable light data (one; Supplementary Table S1).
The study procedures were approved by the Medical Ethical Research Committee of the University Medical Centre Groningen (NL48468.042.14), The Netherlands and are in accordance with the Declaration of Helsinki (2013). All participants gave written informed consent.
Study Design
The data presented here are a subset of measures from a larger ambulatory assessment of biological rhythms in the field. Here, only rest–activity, light exposure, and PSG measures are described. The study took place between November 2014 and January 2016 in Groningen, The Netherlands.
The study duration totaled 3 weeks, the first 6 days being an ambulatory field assessment of rest–activity (MotionWatch 8™, MW8™, CamNTech Ltd., UK), light intensity monitoring from the same actigraph, followed by a weekend laboratory assessment of salivary melatonin to calculate clock phase under standardized dim light conditions of <10 lux (DLMO; see Figure 1). Eight samples were obtained, one per hour, ending at habitual sleep onset (weighted average of sleep onset for workdays and freedays). To avoid the dim light conditions affecting subsequent measurements, there were no observations for a minimum of 1 week after saliva sampling. After this break, participants undertook two nights of home PSG recordings (Actiwave™, CamNTech Ltd., UK) to measure sleep architecture. The recordings took place on a Wednesday and Saturday night, in randomized order. Electrode placement and removal was conducted at the facility (a visit of maximum 1 hour, at least 3 hours before sleep onset). Concomitant actigraph light intensity monitoring continued throughout this period. There were no interventions throughout the study; however, on two instances of <1 hour, participants were required to attend our facilities briefly during the first week of the study to have batteries replaced and to return other devices.
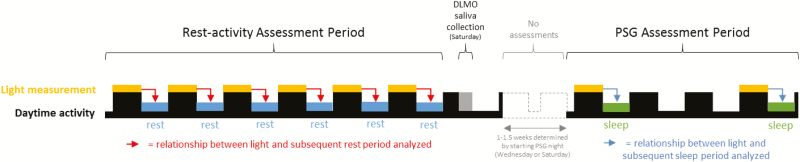
Figure 1.
Study design representation. Timeline shows the two assessment periods (rest–activity; and PSG). Light measurements are shown in yellow with subsequent rest periods (blue) and sleep periods (green). The first half of the timeline indicates a week of rest–activity measurements with light and subsequent sleep comparisons indicated by the red arrows. All daily comparisons of light and subsequent sleep were included in the analyses controlling for subject. After this rest–activity measurement period, a salivary melatonin collection was conducted to determine subject’s DLMO (grey). Following this, there was a period of no assessments lasting at least 1 week dependent on the subject’s randomization of a Wednesday or Saturday first PSG assessment night (dashed lines). The two PSG recordings are shown in green and each was compared with the preceding light exposure (indicated by blue arrows).
Measurements
All time codes were converted to Greenwich mean time (GMT) + 1 hour. All daylight savings time adjustments were removed.
Rest–Activity
Activity counts were collected in 1-minute epochs using a MotionWatch 8™ (MW8™, CamNTech Ltd., UK), worn on the nondominant wrist. Activity timing and rest duration were calculated using Sleep analysis software (version 7, CamNTech Ltd., UK). Activity offsets were determined as the time when activity and light reduced and activity was observed to be less consolidated (assisted by software algorithm from Sleep Analysis 7, CamNTech Ltd., Cambridge, UK) and maintained at a reduced level for at least 10 minutes. Activity onsets were determined using the reverse algorithm of activity offset. Sleep onset and offset were assessed by PSG (see Methods).
DLMO Assessment
Salivary melatonin was collected using Salivette®, Sarstedt™ Ltd., Germany. Samples were centrifuged and stored overnight at approximately 4°C and then stored at −80°C. On completion of the study, a double-antibody radioimmunoassay (RIA) was performed to assess melatonin concentration levels (Bühlmann Direct Saliva Melatonin kit, Bühlmann Laboratories AG, Switzerland; intra-assay variation: 13.97% and 9.11%; interassay variation: 13.99% and 14.64% for low and high concentration controls, respectively). DLMO was marked as the first time where melatonin concentrations exceeded the 4 pg/mL threshold upon linear interpolation of subsequent melatonin values.
PSG Details
PSG was measured using five scalp electrodes (Fpz, Cz, C3, C4, and Oz), a reference electrode on the left mastoid, two electro-oculogram (EOG) electrodes, and one electromyogram (EMG) electrode either under the chin or on eyebrow muscle (when a beard obstructed chin electrode attachment). PSG signals were sampled at 128 Hz, 8 bits and scored in 30-second epochs according to AASM scoring criteria50 with a 50-Hz notch filter, 0.3-Hz high-pass filter, and a 32-Hz low-pass filter (Vitascore software, TEMEC, The Netherlands). Sleep onset was determined as the time of at least 5 consolidated minutes of sleep (stage N1, N2, N3, or REM). First slow-wave sleep (SWS) episode was defined as the first occurrence of an epoch of stage N3 sleep. First REM sleep episode was defined as the first REM epoch occurrence. The number of awakenings, i.e., transitions from sleep to wake epochs, was utilized as a sleep disturbance proxy. Although electrodes were fitted at the Human study facility, University of Groningen, all participants slept at home at a self-chosen time. Sleep stage accumulation data were calculated based on hypnogram cumulative percentage of total sleep time. Division of sleep stage accumulation data by high or low maximum fitted light intensity was defined as individuals with a maximum light intensity on the day of subsequent sleep of either higher or lower than the median of 3.01 log10(lux) (two groups of n = 7). Splitting the group by time of first exposure to more than 10 lux (two groups of n = 7) was at the median of 8.90 hours. Once the groups were split by high or low light intensity, or early or late first exposure to more than 10 lux, the groups were further split by DLMO. This split was conducted on the group as a whole at the median DLMO of 20.07 hours. This resulted in different sample sizes of “early” and “late” clock types; sample sizes are stated in the relevant figure legends. Detailed description of obtained light values and intra-individual distributions of light measurements in this study were described by Woelders and colleagues.51 All DLMO split accumulation results are presented in the text with graphs to be found in Supplementary Figures S1 and S2.
Light Data
Light data from the MotionWatch were cut to end at sleep onset of the sleep/rest period of interest (e.g., beginning of sleep according to PSG recording). The light data were cut to begin at the sleep onset preceding the night of interest. Therefore, light data included light exposure throughout the night and day (approximately 24 hours) preceding the sleep of interest. Because circadian responses to light follow a log-linear relationship,52,53 all light data (measured in lux) were log10 transformed after values below device sensitivity threshold were set to 1 lux. To estimate the time of maximal light exposure, a linear harmonic regression analysis with a single sine wave, fitted per 24 hours due to the entrained nature of the participants’ rhythms (CircWave,54 version 1.4, University of Groningen, The Netherlands), was fitted through the log-transformed lux values for each day separately. We smoothed the data by a local polynomial regression procedure (LOESS55; span of 72 minutes). We then calculated the first and last times at which the smoothed (LOESS) data crossed the 1 log10(lux) threshold on each day, to determine the times of first and last exposure to more than 10 lux. The threshold of 10 lux was chosen based on previous evidence of sleep alterations56 and/or phase shifts57 with lux values above this threshold. Maximal light intensity was calculated per day from the smoothed (LOESS) log10-transformed data. Raw average light intensity was calculated from the nonsmoothed log-transformed data.
Statistical Analysis
Light and sleep comparisons were always confined to a period of light exposure and the directly subsequent rest or sleep period (in rest–activity and PSG measurement periods, respectively). All reliable daily light-rest or light-sleep comparisons were included in statistical analyses while controlling for subject. Linear model fitting was performed in R (R Core Team, 2015; version: 3.2.3), using the most recent shell of Rstudio (version: 0.99.491) and the “lme4” R-package for mixed-effects modeling.58 In all models, participant ID was included as a random effect. A critical p-value of .05 was maintained for all analyses. As a goodness-of-fit parameter for these mixed models, the marginal R2 was reported, using the “MuMIn” R-package. This parameter represents the variance explained by the fixed factors in the model.59 Sleep parameters were included in the model as dependent variables, whereas light parameters were included as fixed effects (see Supplementary Tables S1 and S2). Daylength and DLMO (covariates) were included as additional fixed effects. Daylength was included as the timing and intensity of light exposure might be season-modulated. Daylength was calculated as dusk clock time–dawn clock time on the day of observation. DLMO was included in the analysis to observe the contribution of clock phase to these relationships. When fitting the models, Akaike Information Criteria (AIC) (“drop1” function, “lme4” package) were compared to perform backward selection on the most complex model, dropping the least significant term at each iteration. Insignificant covariates were considered before independent variables of primary interest were dropped from the model. Only in the models where maximal light intensity was significant, timing of maximal light intensity was tested for significance to investigate whether light intensity, independent of timing, could affect sleep. Timing of maximum light intensity was discarded when the model did not improve significantly. For all analyses, a quadratic term of the independent variable was added to the model and discarded when not significant. All sleep and light parameters were regressed against DLMO and its quadratic term to assess the influence of clock phase on these parameters.
For the analysis of the sleep stage accumulation over the sleep period, mixed-effects linear models were fitted using the “lme4” library in R.60 The dependent variable was either REM-sleep, SWS, or wake accumulation since sleep onset (as a percentage of total sleep duration). Participant ID was included in these models as a random factor. The first fixed factor was either the light intensity group, light timing group, or the DLMO group, depending on how the data were split. Time since sleep onset (as a percentage of total sleep duration, rounded to the nearest integer) was included as the second fixed factor. To allow for testing of the difference between two groups (first fixed factor) for each time value since sleep onset (second fixed factor) separately, the latter factor was converted from a numerical factor into a categorical factor (101 categories, range: 0%–100%) before fitting the models. The interaction term was included as the third and final fixed factor. After fitting these models to the data, each model contained 101 interaction coefficients (i.e., the difference between the two groups for each time after sleep onset). For each model, post hoc analysis was then performed on these 101 interaction coefficients (H0: coefficient ≠ 0), via a general linear hypothesis testing procedure (the “glht” function in the “multcomp” R-library). The resulting p-values were corrected for multiplicity using the single-step method.61
Throughout the results section, all results are written as +quadratic term if the quadratic of the independent variable (either light parameter or DLMO) significantly contributed to the model; +daylength if daylength significantly contributed to the model; and +DLMO if DLMO timing significantly contributed to the model; in these cases, details of the sign of the coefficient are provided to explain its interaction with the dependent variable (sleep parameter). If, on testing, only the linear term of the independent variable significantly contributed to explain the variance of the dependent variable, no covariate is stated. Additionally, the R2, chi-square statistic (χ2), associated degrees of freedom and p-value for the final model are provided. Any models where the linear term of the independent variable did not significantly explain dependent variable variance were deemed insignificant regardless of covariate significance. For results of models containing only the linear term or the linear terms and the quadratic term of the independent variable, graphs are shown as raw data with model fit indicated (for PSG graphs, average datapoints ± SE are provided). For results of models containing any additional covariates (i.e., DLMO timing and/or daylength), data are shown as the model prediction ± model SE and raw data are omitted, to facilitate graphical representation.
RESULTS
Light Exposure
The large range of DLMO timing in this dataset (see Table 1) enabled an assessment of the relationship between DLMO timing (considered to represent clock phase) and light exposure. An example of the original data is provided (Figure 2). In general, earlier light exposure was observed to be coupled to exposure to higher intensity light (Table 1).
Table 1a.
Demographic and average light exposure for both assessment periods and DLMO groups.
| Demographic | Assessment period comparison (Mean ±SD) |
DLMO (hh:mm) group comparison
(Mean ±SD; PSG) |
F-test (Sig.) | |||
|---|---|---|---|---|---|---|
|
Rest-activity
Assessment |
PSG Sleep
Assessment |
Early
(<19:00) |
Intermediate
(19:00-21:00) |
Late
(>21:00) |
||
| Gender (m/f) | 8/12 | 8/6 | 1/2 | 4/4 | 3/0 | n.s. |
| Age (yrs.) | 23.4 ± 2.2 | 22.7 ± 1.7 | 23.0 ± 1.7 | 22.6 ± 2.1 | 22.7 ± 0.6 | n.s. |
| Dim-light Melatonin Onset (DLMO; hh:mm) | 20:18 ± 02:00 | 20:12 ± 02:18 | 17:32 ± 00:29 | 20:02 ± 00:30 | 23:25 ± 02:31 | 23.6 (0.0001) |
| Average light intensity (log(lux)) | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | n.s. |
| Maximal intensity of light exposure from fit (log(lux)) | 3.0 ± 0.8 | 2.9 ± 0.5 | 3.5 ± 0.3 | 2.9 ± 0.5 | 2.6 ± 0.5 | n.s. |
| Time of maximal light exposure from fit (hh:mm) | 14:30 ± 01:42 | 15:12 ± 01:54 | 15:19 ± 01:21 | 15:11 ± 01:13 | 17:19 ± 02:27 | n.s. |
| Time of first exposure to >10lux (hh:mm) | 08:00 ± 02:54 | 09:06 ± 02:06 | 08:26 ± 02:02 | 08:40 ± 01:30 | 10:59 ± 03:01 | n.s. |
| Time of last exposure to >10lux (hh:mm) | 21:54 ± 02:00 | 22:24 ± 02:00 | 21:57 ± 00:39 | 22:18 ± 01:35 | 23:11 ± 03:30 | n.s. |
| Sleep onset (hh:mm) | 23:57 ± 01:33 | 00:24 ± 01:20 | 23:11 ± 00:27 | 00:09 ± 00:50 | 02:03 ± 01:30 | 8.0 (0.0071) |
| Sleep offset (hh:mm) | 07:58 ± 01:37 | 07:59 ± 01:15 | 07:04 ± 00:16 | 07:49 ± 00:47 | 09:12 ± 01:57 | n.s. |
| Sleep duration (hrs) | 8.0 ± 1.3 | 7.6 ± 0.7 | 7.9 ± 0.6 | 7.7 ± 0.8 | 7.1 ± 0.5 | n.s. |
| Time from last >10lux to sleep onset (hrs) | 2.0 ± 2.0 | 2.0 ± 1.7 | 1.2 ± 0.7 | 1.9 ± 1.5 | 2.9 ± 2.5 | n.s. |
| Time from sleep offset to first >10 lux (hrs) | -0.1 ± 3.2 | 1.4 ± 1.7 | 1.4 ± 2.1 | 0.9 ± 1.7 | 1.8 ± 1.2 | n.s. |
| Phase angle of Sleep onset- DLMO (hrs) | 3.7 ± 1.7 | 4.1 ± 1.4 | 5.8 ± 0.7 | 4.2 ± 0.9 | 2.4 ± 1.0 | 9.2 (0.0045) |
| PSQI score | 3.5 ± 1.6 | 3.8 ± 1.9 | 3.0 ± 1.7 | 4.0 ± 1.9 | 4.0 ± 2.7 | n.s. |
| BDI score | 1.8 ± 1.9 | 1.7 ± 1.4 | 1.0 ± 1.0 | 1.9 ± 1.7 | 2.0 ± 1.0 | n.s. |
PSQI: Pittsburgh sleep quality index; BDI: Beck’s depression inventory
Table 1b.
Demographic and average light exposure for both light exposure groups.
| Demographic |
First exposure to >10 lux group comparison
(Mean ±SD; PSG) |
t-test (Sig.) |
Maximal light intensity group comparison
(Mean ±SD; PSG) |
t-test (Sig.) | ||
|---|---|---|---|---|---|---|
|
Earlier
(<08:58) |
Later
(>08:58) |
Low intensity exposure
(<2.9 log lux) |
High intensity exposure
(>2.9 log lux) |
|||
| Gender (m/f) | 4/3 | 4/3 | n.s. | 4/3 | 4/3 | n.s. |
| Age (yrs.) | 22.0 ± 1.5 | 23.4 ± 1.6 | n.s. | 22.1 ± 1.2 | 23.3 ± 2.0 | n.s. |
| Dim-light Melatonin Onset (DLMO; hh:mm) | 19:33 ± 00:37 | 20:53 ± 02:46 | n.s. | 21:27 ± 02:23 | 20:07 ± 01:50 | n.s. |
| Average light intensity (log(lux)) | 1.0 ± 0.1 | 0.6 ± 0.1 | 6.2 (0.0001) | 0.8 ± 0.2 | 0.9 ± 0.3 | n.s. |
| Maximal intensity of light exposure from fit (log(lux)) | 3.0 ± 0.4 | 2.8 ± 0.6 | n.s. | 2.5 ± 0.3 | 3.4 ± 0.3 | -6.6 (0.0001) |
| Time of maximal light exposure from fit (hh:mm) | 15:10 ± 00:47 | 16:14 ± 02:12 | n.s. | 16:02 ± 01:55 | 15:13 ± 01:26 | n.s. |
| Time of first exposure to >10lux (hh:mm) | 07:41 ± 00:54 | 10:43 ± 01:58 | -4.2 (0.0014) | 09:47 ± 02:19 | 08:17 ± 01:37 | n.s. |
| Time of last exposure to >10lux (hh:mm) | 22:28 ± 00:51 | 22:22 ± 02:48 | n.s. | 22:39 ± 02:20 | 22:08 ± 01:28 | n.s. |
| Sleep onset (hh:mm) | 00:04 ± 00:59 | 00:45 ± 01:36 | n.s. | 00:34 ± 01:30 | 00:11 ± 01:07 | n.s. |
| Sleep offset (hh:mm) | 07:33 ± 00:39 | 08:28 ± 01:35 | n.s. | 08:13 ± 01:32 | 07:41 ± 00:43 | n.s. |
| Sleep duration (hrs) | 7.5 ± 0.8 | 7.7 ± 0.6 | n.s. | 7.7 ± 0.8 | 7.5 ± 0.7 | n.s. |
| Time from last >10lux to sleep onset (hrs) | 1.6 ± 1.0 | 2.4 ± 2.3 | n.s. | 1.9 ± 1.6 | 2.1 ± 1.9 | n.s. |
| Time from sleep offset to first >10 lux (hrs) | 0.1 ± 1.1 | 2.2 ± 1.5 | -3.3 (0.0069) | 1.6 ± 1.7 | 0.6 ± 1.6 | n.s. |
| Phase angle of Sleep onset-DLMO (hrs) | 4.7 ± 1.2 | 3.6 ± 1.7 | n.s. | 3.3 ± 1.2 | 5.0 ± 1.0 | -2.6 (0.0220) |
| PSQI score | 2.7 ± 1.1 | 4.9 ± 2.0 | -2.5 (0.0267) | 4.4 ± 2.4 | 3.1 ± 1.1 | n.s. |
| BDI score | 1.6 ± 1.9 | 1.9 ± 0.9 | n.s. | 2.0 ± 1.2 | 1.4 ± 1.7 | n.s. |
PSQI: Pittsburgh sleep quality index; BDI: Beck’s depression inventory
Figure 2.
Example of original activity and light data trace. Data were obtained from an intermediate chronotype participant with a DLMO of 19.5 hours. Top panel: Log transformed light intensity data (black lines indicate intensity per minute bin) with harmonic regression sine function (dashed line) plotted for the first week. Bottom panel: black lines indicate activity counts per minute, divided by 1000.
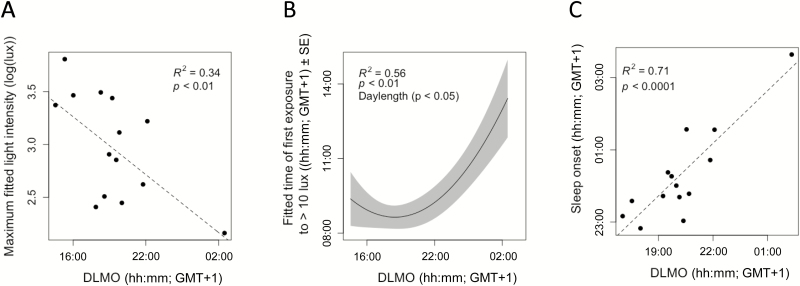
Later DLMO was related to lower maximal light exposure for both the rest–activity (+quadratic term, R2 = 0.23, χ2(2) = 10.01, p < .01) and the PSG assessment periods (no covariate, R2 = 0.34, χ2(1) = 7.19, p < .01; Figure 3A; see Supplementary Table S2 for coefficient information). As well as a lower intensity of light exposure, later DLMO timing was also related to later light exposure. A later DLMO was observed to be associated with later first light exposure (+quadratic term, R2 = 0.14, χ2(2) = 9.82, p < .01; Figure 3B), and a later last light exposure to more than 10 lux during both the rest–activity assessment (no covariate, R2 = 0.07, χ2(1) = 4.24, p < .05) and the PSG period (+quadratic term, +daylength, R2 = 0.56, χ2(3) = 13.03, p < .005, and +quadratic term, R2 = 0.32, χ2(2) = 6.23, p < .05, respectively). Later DLMO was also associated with later time of maximal light exposure (+quadratic term, +daylength, R2 = 0.47, χ2(3) = 32.38, p < .0001) for rest–activity assessment days (see Figure 2 for an example) and PSG days (+quadratic, R2 = 0.58, χ2(2) = 19.39, p < .0001).
Figure 3.
Relationships between dim-light melatonin onset, light exposure, and sleep timing. (A) Higher maximal fitted light intensity exposure was related to earlier DLMO timing. (B) In a curvi-linear fashion, later DLMO was generally related to later time of first exposure to >10 lux when accounting for differences in daylength (model prediction: black line, standard error of mixed model: grey range). (C) Later DLMO timing was related to later sleep onset.
Rest–activity and Sleep Timing
As expected, clock phase (DLMO) correlated positively with behavioral measures of activity and PSG-based measures of sleep. Later DLMO timing was related to a later activity offset (no covariate, R2 = 0.27, χ2(1) = 12.76, p < .001; see Supplementary Table S1 for coefficient information), and in agreement, a later sleep onset (no covariate, R2 = 0.71, χ2(1) = 20.39, p < .0001; Figure 3C). Later DLMO timing was related to a later activity onset (+quadratic term, R2 = 0.50, χ2(2) = 37.02, p < .0001) and likewise to a later sleep offset (no covariate, R2 = 0.53, χ2(1) = 12.37, p < .001). A later DLMO timing was observed to be associated with a longer rest duration (+quadratic term, R2 = 0.08, χ2(2) = 8.19, p < .05), in contradiction to the lack of relationship observed for sleep duration (p > .05). Although it might be expected that a similar relationship for rest and sleep would appear from the data, the rest duration actually consists of a consolidated period of inactivity, whereas sleep duration is the period between sleep onset and offset possibly accounting for this mismatch. In line with these findings, when splitting the study sample by DLMO timing, DLMO group was observed to be related to sleep onset timing and the phase angle between DLMO and sleep onset (see Table 1). This completes an overall picture of a later clock phase (DLMO timing) being related to later sleep timing and a shorter interval between DLMO and sleep onset. Interestingly, individuals classified as having low light exposure also a shorter phase angle between their DLMO and sleep onsets (Table 1).
As DLMO timing was observed to be related to both light exposure and sleep timing, regression analysis was utilized to assess the relationship between light exposure, and activity and sleep timing on the same day. Activity timing was observed to have a quadratic relationship to the time of last exposure to more than 10 lux. A later activity offset was related to both very early and very late last exposure to more than 10 lux, when accounting for the positive relationship DLMO has with activity timing (+quadratic term, +DLMO, R2 = 0.45, χ2(3) = 44.75, p < .0001). This same relationship was observed for activity onset (+quadratic and +DLMO, R2 = 0.44, χ2(3) = 30.93, p < .0001). A similar relationship was observed for sleep onset and offset with last exposure to more than 10 lux, although DLMO timing was not observed to significantly be involved in the relationship (sleep onset: +quadratic term, R2 = 0.68, χ2(2) = 22.09, p < .0001; sleep offset: +quadratic term, R2 = 0.68, χ2(2) = 20.39, p < .0001). Activity onset was also positively associated with first exposure to more than 10 lux and time of maximal light exposure, when controlling for the positive relationship between DLMO timing and activity onset (first exposure: +DLMO, R2 = 0.43, χ2(2) = 28.32, p < .0001 and time of maximal light exposure: +DLMO, R2 = 0.49, χ2(2) = 37.92, p < .0001). Interestingly, longer rest duration was associated with lower maximal light intensity on the preceding day (no covariate, R2 = 0.05, χ2(1) = 4.83, p < .05), and a later timing of maximal light exposure (no covariate, R2 = 0.05, χ2(1) = 4.82, p < .05). In general, individuals who had more time between waking and being exposed to 10 lux for the first time were likely to have that first exposure later in the day (Table 1).
Phase Angle Between Sleep Onset and DLMO
The phase angle between sleep onset and DLMO was also assessed as covariate, but did not contribute significantly to any of the models tested (p > .05).
Light Exposure and Subsequent Sleep Disturbance
Subjects with a late DLMO also showed less sleep stage transitions, except for one included subject, who had a very late DLMO while experiencing more sleep transitions than those with moderate late DLMO timing (+quadratic term, R2 = 0.32, χ2(2) = 6.81, p < .05; Supplementary Table S2).
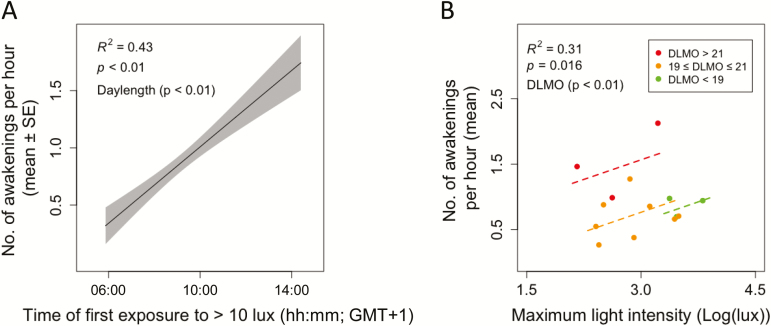
In general, individuals who were first exposed to 10 lux later had significantly more subjectively reported sleep disturbances (Table 1). Objectively recorded sleep disturbances were found to depend on previous light exposure, but time of maximal light exposure did not significantly contribute to this effect. Later time of first exposure to 10 lux was also related to more awakenings, when controlling for daylength (+daylength, R2 = 0.36, χ2(2) = 8.66, p < .05, Figure 4A). Lower maximal light exposure resulted in fewer awakenings, when accounting for variance explained by DLMO timing (+DLMO, R2 = 0.26, χ2(2) = 6.98, p < .05, Figure 4B). Timing of DLMO was found to additively contribute (coefficient: 1.01, p < .05) such that later DLMO timing was related to an increased number of awakenings (Figure 4B and Table 2). Additionally, later time of first exposure to more than 10 lux seemed related to more wake after sleep onset (trend: p = .053). This seems in line with the finding that later first light exposure correlate with more awakenings (Figure 4A), but contrasts with the correlation showing less sleep stage transitions in people with late DLMO (Supplementary Table S2). Sleep characteristics are summarized in Table 2. No differences were found between the two PSG nights (weekday and weekend recordings) in terms of sleep disturbances, architecture, timing, or stage accumulation; or their relationships with preceding light exposure (p > .05; data not shown). No effect of randomization order of starting PSG night was observed (p > .05; data not shown).
Figure 4.
Light exposure and its relationship to subsequent sleep disturbances. (A) Later first exposure to more than 10 lux was related to increased sleep disturbance (number of awakenings per hour), when controlling for daylength (model prediction: black line, standard error of mixed model: grey range). (B) Higher maximal light intensities during the day were followed by more sleep disturbances (number of awakenings per hour TST) within DLMO timing groups (DLMO) timing (red: DLMO > 21 hours, orange: 19 hours ≤ DLMO ≤ 21 hours, green: DLMO < 19 hours).
Table 2a.
Sleep characteristics for PSG assessment and DLMO group comparison.
| Demographic | Group average (Mean ±SD) |
DLMO (hh:mm) group comparison
(Mean ±SD; PSG) |
F-test (Sig.) | ||
|---|---|---|---|---|---|
|
Early
(<19:00) |
Intermediate
(19:00-21:00) |
Late
(>21:00) |
|||
| Sleep duration (hrs) | 7.6 ± 0.7 | 7.9 ± 0.6 | 7.7 ± 0.6 | 7.2 ± 0.5 | n.s. |
| Time in bed (hrs) | 8.0 ± 1.1 | 7.4 ± 1.5 | 7.9 ± 0.6 | 8.7 ± 1.2 | n.s. |
| Sleep onset latency (mins) | 24.2 ± 35.5 | 12.0 ± 2.3 | 25.9 ± 25.4 | 26.0 ± 14.8 | n.s. |
| WASO (mins) | 27.1 ± 16.7 | 24.3 ± 6.5 | 21.6 ± 13.7 | 44.7 ± 22.8 | n.s. |
| % N1 sleep | 4.1 ± 1.7 | 2.7 ± 1.7 | 4.1 ± 1.1 | 4.8 ± 0.7 | n.s. |
| % N2 sleep | 43.6 ± 5.5 | 45.6 ± 11.1 | 44.6 ± 2.0 | 41.1 ± 4.9 | n.s. |
| % N3 sleep | 25.0 ± 4.9 | 25.5 ± 9.2 | 24.6 ± 3.7 | 25.0 ± 2.9 | n.s. |
| % REM sleep | 23.0 ± 3.8 | 22.0 ± 3.4 | 23.0 ± 3.4 | 22.0 ± 2.0 | n.s. |
| REM latency (hrs) | 1.6 ± 0.5 | 1.5 ± 0.6 | 1.8 ± 0.5 | 1.2 ± 0.2 | n.s. |
| N3 latency (mins) | 13.1 ± 5.7 | 11.2 ± 4.3 | 14.1 ± 6.9 | 12.7 ± 4.0 | n.s. |
| Mean awakenings per hour | 0.9 ± 0.5 | 0.9 ± 0.2 | 0.7 ± 0.3 | 1.5 ± 0.6 | 5.91 (0.018) |
| No. of transitions | 142.2 ± 32.3 | 158.7 ± 43.5 | 135.3 ± 30.5 | 144.2 ± 31.9 | n.s. |
WASO: Wake after sleep onset; REM: Rapid-eye movement sleep
Table 2b.
Sleep characteristics for Light exposure group comparisons.
| Demographic |
First exposure to >10 lux group comparison
(Mean ±SD; PSG) |
t-test (Sig.) |
Maximal light intensity group comparison
(Mean ±SD; PSG) |
t-test (Sig.) | ||
|---|---|---|---|---|---|---|
|
Earlier
(<08:58) |
Later
(>08:58) |
Low intensity exposure
(<2.9 log lux) |
High intensity exposure
(>2.9 log lux) |
|||
| Sleep duration (hrs) | 7.6 ± 0.8 | 7.7 ± 0.4 | n.s. | 7.7 ± 0.7 | 7.6 ± 0.6 | n.s. |
| Time in bed (hrs) | 7.8 ± 0.6 | 8.1 ± 1.3 | n.s. | 8.1 ± 0.4 | 7.8 ± 1.4 | n.s. |
| Sleep onset latency (mins) | 15.0 ± 7.4 | 30.9 ± 26.4 | n.s. | 30.9 ± 26.9 | 15.0 ± 5.3 | n.s. |
| WASO (mins) | 22.1 ± 8.0 | 32.1 ± 22.0 | n.s. | 25.1 ± 15.3 | 29.2 ± 19.0 | n.s. |
| % N1 sleep | 3.8 ± 1.4 | 4.2 ± 1.3 | n.s. | 4.7 ± 0.3 | 3.3 ± 1.5 | 2.41 (0.033) |
| % N2 sleep | 44.4 ± 5.7 | 43.7 ± 5.2 | n.s. | 43.9 ± 3.2 | 44.2 ± 7.0 | n.s. |
| % N3 sleep | 25.6 ± 6.3 | 24.1 ± 2.4 | n.s. | 23.5 ± 2.9 | 26.2 ± 5.9 | n.s. |
| % REM sleep | 22.3 ± 2.6 | 22.8 ± 3.4 | n.s. | 23.9 ± 2.9 | 21.2 ± 2.5 | n.s. |
| REM latency (hrs) | 1.9 ± 0.3 | 1.4 ± 0.6 | n.s. | 1.5 ± 0.4 | 1.7 ± 0.6 | n.s. |
| N3 latency (mins) | 11.8 ± 4.2 | 14.5 ± 7.0 | n.s. | 15.2 ± 6.0 | 11.1 ± 5.0 | n.s. |
| Mean awakenings per hour | 0.7 ± 0.3 | 1.1 ± 0.6 | n.s. | 0.8 ± 0.5 | 1.0 ± 0.5 | n.s. |
| No. of transitions | 144.4 ± 34.4 | 140.1 ± 32.7 | n.s. | 140.2 ± 31.9 | 144.2 ± 35.1 | n.s. |
WASO: Wake after sleep onset; REM: Rapid-eye movement sleep
Sleep Architecture
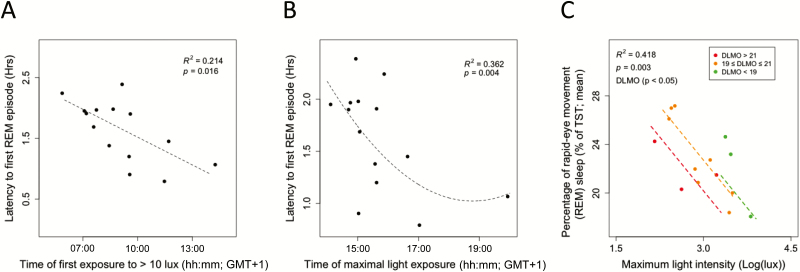
The timing of the DLMO was not related, on its own, to any aspect of subsequent sleep architecture (p > .05). Light exposure variables on their own or combined with DLMO did explain variation in sleep architecture. A later time of first exposure to >10 lux (no covariate, R2 = 0.21, χ2(1) = 5.77, p < .05, Figure 5A) and a later timing of maximal light exposure (+quadratic, +DLMO, R2 = 0.36, χ2(2) = 11.17, p < .01, Figure 5B) were associated with a subsequent shorter latency to first REM episode. The findings might seem paradoxical since REM sleep propensity is thought to be directly influenced by the circadian clock. Hence, at first sight, later DLMO was expected to correlate with the later occurrence of REM sleep. However, the first (or even second) REM sleep bout can be suppressed if non-REM propensity is high. Therefore, we investigated the effects of light intensity on subsequent non-REM and REM sleep. Higher maximal intensity of light on the day before sleeping with PSG was followed by lower percentages of REM sleep, when accounting for variance explained by DLMO timing (+DLMO, R2 = 0.43, χ2(2) = 13.90, p < .001, Figure 5C). The negative coefficient for DLMO timing as a covariate indicates that later DLMO timing was related to a subsequent lower percentage of REM sleep (see Supplementary Table S2 for coefficient information), which may indicate that higher sleep deficit in late sleepers may reduce or eliminate the first REM sleep bout. In the same lines, the percentage of stage 1 sleep was observed to be lower in those individuals who were classified as being exposed to higher light intensity light (Table 2). This could indicate that shorter latencies to non-REM sleep occurring when individuals are exposed to higher maximal light, a pressure for SWS overrides the balance of other sleep stages. This can also be observed with the dominating increase in percentage of subsequent SWS at higher average light intensities over the day (+quadratic, no covariate, R2 = 0.25, χ2(2) = 8.86, p < .05).
Figure 5.
Light exposure and subsequent sleep architecture. (A) Shorter latencies to first REM sleep episode were associated with preceding later timing to first exposure to more than 10 lux (black circles indicate average values of two PSG recordings and of the same 2 days of light recordings and dashed line indicates model fit) and (B) later preceding timing of maximal light exposure. (C) A lower percentage of REM sleep was associated with higher preceding maximal light intensities (model prediction: black line, standard error of mixed model: grey range). This relationship was modulated by DLMO timing, though all DLMO timing categories showed the same relationship (red: DLMO > 21 hours, orange: 19 hours ≤ DLMO ≤ 21 hours, green: DLMO < 19 hours).
Sleep Stage Accumulation
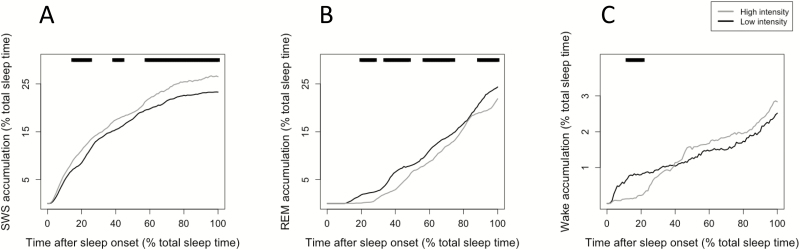
In order to see how light timing and the intensity of light exposure interact with the subsequent accumulation rate of SWS, REM sleep, and wake, the sample was split into low and high maximal intensity exposure, and into early and late first exposure to >10 lux. Those individuals exposed to higher maximal light intensities experienced larger subsequent SWS accumulation (p < .05; Figure 6A), lower subsequent REM sleep accumulation throughout the night (p < .05; Figure 6B), and lower subsequent wake accumulation at the beginning of the night (p < .05; Figure 6C).
Figure 6.
Sleep stage accumulation related to preceding maximal light exposure intensity. (A) SWS accumulation was higher throughout the sleep period in individuals previously exposed to higher maximal light intensities (p < .05). (B) REM sleep accumulation was lower in individuals previously exposed to higher maximal light intensities (p < .05). (C) Wake accumulation was significantly lower at the beginning of the sleep period in individuals previously exposed to higher maximal light intensities (p < .05). In all graphs, line at top indicates a significant difference between groups at that time percentage of total sleep, p < .05.
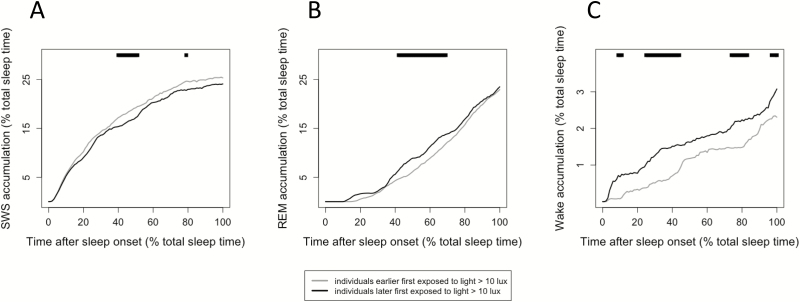
Those individuals who had their first exposure to >10 lux earlier had significantly higher subsequent SWS accumulation (Figure 7A) seemingly at the expense of the accumulation of REM sleep in the middle of the sleep period (Figure 7B), and wake throughout the night (Figure 7C; p < .05).
Figure 7.
Subsequent sleep stage accumulation related to timing of first exposure to more than 10 lux. (A) SWS accumulation was higher in individuals that previously had earlier first exposure to > 10 lux (p < .05). (B) REM sleep accumulation was lower in individuals that previously had earlier first exposure to >10 lux (p < .05). (C) Wake accumulation was significantly lower throughout the sleep period in individuals that previously had earlier first exposure to >10 lux (p < .05). In all graphs, line at top indicates a significant difference between groups at that time percentage of total sleep, p < .05.
DISCUSSION
Our data show that sleep timing, duration, structure, and the number of sleep disturbances observed in the field objectively, for the first time, depended on the aspects of preceding light exposure. In a sample of healthy 20 to 30 years old, selected for a wide range of sleep timing (MSF), we observed large variation in clock phases (DLMO), which optimized the probability to find relationships with preceding light parameters within DLMO timing groups. DLMO was added to our models to assess the impact of clock phase and explain the variance in sleep architecture dependent on the circadian clock to allow for a better analysis of the effect of previous light on subsequent sleep.62 We found that later endogenous clock timing was related to later timing and lower levels of light exposure. Later exposure to light has been observed experimentally to result in a later clock phase.3,10,39 This finding confirms light interventions in the field12,14,15 and the finding that manipulated reduction of blue light in the morning was associated with later clock phases.13 Spectral light composition information would help us to further elucidate this relationship.
Despite the lack of intervention studies, field data indicate that later chronotypes are generally exposed to later light33 and less time outdoors.32 This probably results in lower light exposure,27,33 and probably a reduction in exposure to approximately 460–490 nm photons, which are important for entraining the circadian system.63 In both our dataset and in previous studies, later sleep timing has been shown to be related later clock phase54,55 and later preceding light exposure.33,34 These relationships are most probably due to the influence of light on the entrainment of the circadian clock, which drives process C in sleep regulation, which in turn will shift the timing of sleep.6,7 Because humans have on average an intrinsic circadian period longer than 24 hours (24.2 hours64), they need advancing (morning) light to entrain to the 24-hour day. Exposure to lower light intensities indicates reduced zeitgeber strength and oscillatory theory predicts that this will result in a later (lagging) phase angle of entrainment65,66 when intrinsic period is greater than 24 hours. This later phase angle of entrainment may then be further amplified by artificial light in the evening. In addition, entrainment models would predict a bigger role for parametric entrainment in diurnal animals.67,68 Moreover, according to Aschoff’s rule, diurnal animals will lengthen intrinsic period with lower light intensity.69,70
Although we only found that lower maximal light intensities were associated with subsequent longer rest durations, studies with high intensity light interventions have previously focused on the timing of the light,8,15 rather than the intensity itself. Despite that, shorter subsequent sleep duration has been related to brighter morning light8 and exposure to artificial electric lighting.35 In the current study, however, no relationship between light exposure and sleep duration was found. This is in line with the analysis of sleep behavior of three preindustrial societies that indicated that there were no differences in sleep duration in societies without electric lighting compared with our industrialized sleep habits71 (but see also Ref. 72). Because sleep onset and offset appear gated by the circadian system in a similar way, the reduction in the strength of light as the primary entrainment signal may only play a minimal role in the alteration of sleep duration.
Preceding light exposure, possibly through altering the balance of circadian and homeostatic regulation of sleep, appears to be associated with the amount of subsequent sleep disturbance. Later light was associated with more awakenings, in line with laboratory studies observing evening light being related to more arousals.39,73 It is unclear why late DLMO correlates with less sleep transitions (Supplementary Table S2), but the complex quadratic relationship of this correlation suggests that more data need to be collected to fully understand this. A clearer relationship was found between light parameters, DLMO, and number of awakenings. Late first light exposure and late clock phase correlate with more awakenings (Figure 4A and B). We have described before that people with a late clock phase sleep early relative to DLMO.51 This may indicate that their sleep partially overlaps with the circadian drive for wakefulness, resulting in more awakenings.7
The influence of the circadian drive for wake promotion on sleep offset timing has been reported to result in a reduction of REM sleep, with non-REM sleep appearing unaffected.8,25 Sleep structure appears to be related to preceding light exposure in our dataset. Exposure to higher levels of maximal light was related to less REM in subsequent sleep. In the laboratory, higher levels of blue photons in evening light have been associated with a reduction in REM sleep duration,24,26 whereas light during sleep was associated with an increased prevalence of REM sleep.41 Therefore, influence of light on REM sleep could be due to the circadian regulation of REM sleep. The timing of light during the daytime exposure in our subjects appears to be related also to the timing of the first REM episode. The finding that later light exposure was related to earlier first REM episodes indicates that the timing of light could modulate not only clock phase but in turn also the circadian regulated sleep architecture. There are two alternative explanations, either light is influencing sleep architecture through altering the rate of sleep pressure increase or through the shift of the sleep structure by a shift in the circadian-related drive for wakefulness (and REM sleep). What is known is that the alerting signal of the circadian system decreases throughout the night, rising just before sleep offset.7 Based on the forced desynchrony findings that REM sleep appears clock modulated,74 it can be hypothesized that the influence of later light extending the window for REM sleep episodes could be due to the altered phase of the circadian system and a reduced sleep load build up. Through the sleep stage accumulation analysis, it can be observed that SWS has a direct relationship with the timing and intensity of light. As REM sleep and wake accumulation show the opposite pattern to SWS, it appears that light is influencing the homeostatic pressure shifting other sleep stages in accordance. Therefore, it appears that light, although known to affect the circadian system and in turn REM sleep, additionally increases the build-up of the homeostatic sleep pressure. To further assess the relationship of light exposure with subsequent sleep intensity, an analysis of delta power decay is required. This is beyond the scope of this current article due to the already complex nature of the included analyses. We hypothesize that the underlying biological mechanism for this is the direct light input observed from the retina to the ventrolateral preoptic nucleus, the sleep “switch” alternating sleep stages.75 Upon confirmation of interventional studies, this hypothesis could change the current understanding of the regulation of sleep.
Although there appears in our pilot analysis (in Supplementary Material) to be some influence of DLMO timing on the relationship between light and sleep structure, this influence needs to be further investigated, due to small sample size once participants were grouped by DLMO. In the main analyses of this study, despite the large amount of data obtained per participant, it is possible that this sample is not representative of the broader population. In addition, the majority of participants were university students and therefore may have different lifestyles than the general working population. Although this might be a limitation, it must also be noted that the large variation present in our data (through participant selection based upon chronotype) provides the ability to assess the relationships in a statistically powerful way. Another possible limitation is the assumption that the subsequent sleep is related to the preceding light exposure rather than the sleep having an influence on future light exposure. Through correlational analysis, these relationships cannot be further elucidated here.
In general, our findings corroborate those observed in laboratory settings. Our observations support that despite lacking the many factors that influence sleep in real life, laboratory findings are transferable to how people sleep in naturalistic settings. Although light can be well controlled in the laboratory, it can be difficult to generalize laboratory findings to observations in the field where light quality and quantity show large variation over the day. Our study is the first to assess sleep architecture in the field using ambulatory PSG in the context of chronobiological applications, showing its reliability with the new technological advances utilized here. This study provides further evidence for the link between light exposure and the timing, duration, structure, and quality of subsequent sleep. Not only does this influence have implications for further strategies in the care for patients and those with sleep disturbances, but this study shows that light exposure shapes everyday life. Light technologies and those developing working schedules and living environments could greatly influence the sleep individuals have in real life and its subsequent effects on quality of life, health, productivity, mental performance, and safety. With confirmation from spectral analysis and interventional studies, this study could alter the current understanding of sleep regulation and the role of light history.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
FUNDING
Financial support was obtained from a NWO-STW Program Grant “OnTime” (project 12185).
WORK PERFORMED
Chronobiology unit, groningen institute for evolutionary life sciences, university of groningen, groningen, the netherlands
DISCLOSURE STATEMENT
MCMG reports receiving consultancy fees from Philips Consumer Lifestyle, outside the submitted work. The other authors report no conflicts of interest and all are alone responsible for the content and writing of the paper.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge students Heleen Rinsema and Robin Dennebos for their great assistance in the collection of data and Moniek Geerdink for the melatonin radioimmunoassay analysis.
REFERENCES
- 1. Daan S, Hut RA. Circadian Clock, Program, Oscillator, Pacemaker, Synchroniser? In: Honma K, Honma S, eds. Circadian Clocks. Hokkaido University Press; 2016:21–32. [Google Scholar]
- 2. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002; 295(5557): 1070–1073. [DOI] [PubMed] [Google Scholar]
- 3. Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003; 549(Pt 3): 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010; 2(31): 31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011; 34(11): 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daan S, Beersma DG, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984; 246(2 Pt 2): R161–R183. [DOI] [PubMed] [Google Scholar]
- 7. Dijk D, Edgar DM. Circadian and homeostatic control of wakefulness and sleep. In: Turek F, Zee P, eds. Regulation of Sleep and Wakefulness. New York: Marcel Dekker, Inc; 1999:111–147. [Google Scholar]
- 8. Dijk DJ, Visscher CA, Bloem GM, Beersma DG, Daan S. Reduction of human sleep duration after bright light exposure in the morning. Neurosci Lett. 1987; 73(2): 181–186. [DOI] [PubMed] [Google Scholar]
- 9. Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987; 2(3): 207–219. [DOI] [PubMed] [Google Scholar]
- 10. Gordijn MC, Beersma DG, Korte HJ, van den Hoofdakker RH. Effects of light exposure and sleep displacement on dim light melatonin onset. J Sleep Res. 1999; 8(3): 163–174. [DOI] [PubMed] [Google Scholar]
- 11. Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Acute and phase-shifting effects of ocular and extraocular light in human circadian physiology. J Biol Rhythms. 2003; 18(5): 409–419. [DOI] [PubMed] [Google Scholar]
- 12. Figueiro MG, Plitnick B, Rea MS. The effects of chronotype, sleep schedule and light/dark pattern exposures on circadian phase. Sleep Med. 2014; 15(12): 1554–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Figueiro MG, Rea MS. The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin. Int J Endocrinol. 2010; 2010: 829351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burgess HJ, Molina TA. Home lighting before usual bedtime impacts circadian timing: a field study. Photochem Photobiol. 2014; 90(3): 723–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corbett RW, Middleton B, Arendt J. An hour of bright white light in the early morning improves performance and advances sleep and circadian phase during the Antarctic winter. Neurosci Lett. 2012; 525(2): 146–151. [DOI] [PubMed] [Google Scholar]
- 16. Geerdink M, Walbeek TJ, Beersma DG, Hommes V, Gordijn MC. Short blue light pulses (30 min) in the morning support a sleep-advancing protocol in a home setting. J Biol Rhythms. 2016; 31(5): 483–497. [DOI] [PubMed] [Google Scholar]
- 17. Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. In search of a temporal niche: environmental factors. Prog Brain Res. 2012; 199(1): 281–304. [DOI] [PubMed] [Google Scholar]
- 18. Levy O, Dayan T, Kronfeld-Schor N. The relationship between the golden spiny mouse circadian system and its diurnal activity: an experimental field enclosures and laboratory study. Chronobiol Int. 2007; 24(4): 599–613. [DOI] [PubMed] [Google Scholar]
- 19. Gattermann R, Johnston RE, Yigit N et al. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett. 2008; 4(3): 253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomotani BM, Flores DE, Tachinardi P, Paliza JD, Oda GA, Valentinuzzi VS. Field and laboratory studies provide insights into the meaning of day-time activity in a subterranean rodent (Ctenomys aff. knighti), the tuco-tuco. PLoS One. 2012; 7(5): e37918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santhi N, Thorne HC, van der Veen DR et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012; 53(1): 47–59. [DOI] [PubMed] [Google Scholar]
- 22. Komada Y, Tanaka H, Yamamoto Y, Shirakawa S, Yamazaki K. Effects of bright light pre-exposure on sleep onset process. Psychiatry Clin Neurosci. 2000; 54(3): 365–366. [DOI] [PubMed] [Google Scholar]
- 23. Münch M, Scheuermaier KD, Zhang R et al. Effects on subjective and objective alertness and sleep in response to evening light exposure in older subjects. Behav Brain Res. 2011; 224(2): 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015; 112(4): 1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dijk DJ, Beersma DG, Daan S, Lewy AJ. Bright morning light advances the human circadian system without affecting NREM sleep homeostasis. Am J Physiol. 1989; 256(1 Pt. 2): 106–111. [DOI] [PubMed] [Google Scholar]
- 26. Münch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol Regul Integr Comp Physiol. 2006; 290(5): R1421–R1428. [DOI] [PubMed] [Google Scholar]
- 27. Goulet G, Mongrain V, Desrosiers C, Paquet J, Dumont M. Daily light exposure in morning-type and evening-type individuals. J Biol Rhythms. 2007; 22(2): 151–158. [DOI] [PubMed] [Google Scholar]
- 28. Okudaira N, Kripke DF, Webster JB. Naturalistic studies of human light exposure. Am J Physiol. 1983; 245(4): R613–R615. [DOI] [PubMed] [Google Scholar]
- 29. Savides TJ, Messin S, Senger C, Kripke DF. Natural light exposure of young adults. Physiol Behav. 1986; 38(4): 571–574. [DOI] [PubMed] [Google Scholar]
- 30. Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003; 18(1): 80–90. [DOI] [PubMed] [Google Scholar]
- 31. Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013; 23(16): 1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roenneberg T, Keller LK, Fischer D, Matera JL, Vetter C, Winnebeck EC. Human activity and rest in situ. Methods Enzymol. 2015; 552(1): 257–283. [DOI] [PubMed] [Google Scholar]
- 33. Martin JS, Hébert M, Ledoux E, Gaudreault M, Laberge L. Relationship of chronotype to sleep, light exposure, and work-related fatigue in student workers. Chronobiol Int. 2012; 29(3): 295–304. [DOI] [PubMed] [Google Scholar]
- 34. Stebelová K, Molčan Ľ, Okuliarová M et al. The influence of indoor lighting with low blue light dose on urine 6-sulphatoxymelatonin concentrations and sleep efficiency of healthy volunteers. Biol Rhythm Res. 2014; 46(1): 137–145. [Google Scholar]
- 35. de la Iglesia HO, Fernández-Duque E, Golombek DA et al. Access to electric light is associated with shorter sleep duration in a traditionally hunter-gatherer community. J Biol Rhythms. 2015; 30(4): 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boubekri M, Cheung IN, Reid KJ, Wang CH, Zee PC. Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. J Clin Sleep Med. 2014; 10(6): 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harb F, Hidalgo MP, Martau B. Lack of exposure to natural light in the workspace is associated with physiological, sleep and depressive symptoms. Chronobiol Int. 2014; 1(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 38. Leger D, Bayon V, Elbaz M, Philip P, Choudat D. Underexposure to light at work and its association to insomnia and sleepiness: a cross-sectional study of 13,296 workers of one transportation company. J Psychosom Res. 2011; 70(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 39. Carrier J, Dumont M. Sleep propensity and sleep architecture after bright light exposure at three different times of day. J Sleep Res. 1995; 4(4): 202–211. [DOI] [PubMed] [Google Scholar]
- 40. Cajochen C, Di Biase R, Imai M. Interhemispheric EEG asymmetries during unilateral bright-light exposure and subsequent sleep in humans. Am J Physiol Regul Integr Comp Physiol. 2008; 294(3): R1053–R1060. [DOI] [PubMed] [Google Scholar]
- 41. Cho C, Lee H, Yoon H et al. Exposure to dim light at night increases REM sleep and awakenings. Sleep. 2015; 37(1): 298–299. [DOI] [PubMed] [Google Scholar]
- 42. Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997; 41(9): 955–963. [DOI] [PubMed] [Google Scholar]
- 43. Ancoli-Israel S, Gehrman P, Martin JL et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003; 1(1): 22–36. [DOI] [PubMed] [Google Scholar]
- 44. Cedernaes J, Schiöth HB, Benedict C. Determinants of shortened, disrupted, and mistimed sleep and associated metabolic health consequences in healthy humans. Diabetes. 2015; 64(4): 1073–1080. [DOI] [PubMed] [Google Scholar]
- 45. Zavada A, Gordijn MC, Beersma DG, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne-Ostberg’s Morningness-Eveningness Score. Chronobiol Int. 2005; 22(2): 267–278. [DOI] [PubMed] [Google Scholar]
- 46. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 47. Carroll BT, Kathol RG, Noyes R Jr, Wald TG, Clamon GH. Screening for depression and anxiety in cancer patients using the Hospital Anxiety and Depression Scale. Gen Hosp Psychiatry. 1993; 15(2): 69–74. [DOI] [PubMed] [Google Scholar]
- 48. Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychol Assess. 1998; 10(2): 83–89. [Google Scholar]
- 49. Clark J. The Ishihara test for color blindness. Am J physiol Opt. 1924; 5(1): 269–276. [Google Scholar]
- 50. Silber MH, Ancoli-Israel S, Bonnet MH et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007; 3(2): 121–131. [PubMed] [Google Scholar]
- 51. Woelders T, Beersma DGM, Gordijn MCM, Hut RA, Wams EJ. Daily light exposure patterns reveal phase and period of the human circadian clock. J Biol Rhythms. 2017; 32(3): 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001; 535(Pt 1): 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hut RA, Oklejewicz M, Rieux C, Cooper HM. Photic sensitivity ranges of hamster pupillary and circadian phase responses do not overlap. J Biol Rhythms. 2008; 23(1): 37–48. [DOI] [PubMed] [Google Scholar]
- 54. Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. J Biol Rhythms. 2006; 21(5): 350–361. [DOI] [PubMed] [Google Scholar]
- 55. Cleveland WS, Grosse E, Shyu WM. Local regression models. In: Chambers JM, Hastie TJ, eds. Statistical Models in S. Wadsworth & Brooks/Cole. California, USA: Pacific Grove; 1992. [Google Scholar]
- 56. Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol Int. 2014; 31(6): 779–786. [DOI] [PubMed] [Google Scholar]
- 57. Zeitzer JM, Khalsa SB, Boivin DB et al. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am J Physiol Regul Integr Comp Physiol. 2005; 289(3): R839–R844. [DOI] [PubMed] [Google Scholar]
- 58. Bates D, Mächler M, Bolker B. Fitting linear mixed-effects models using lme4. J Stat Softw. 2012; 51(1): 1. [Google Scholar]
- 59. Johnson PC. Extension of Nakagawa & Schielzeth’s R(2)GLMM to random slopes models. Methods Ecol Evol. 2014; 5(9): 944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bates D, Mächler M, Bolker B. Fitting linear mixed-effects models using lme4. J Stat Softw. 2012; 51(1): 1.23504300 [Google Scholar]
- 61. Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008; 50(3): 346–363. [DOI] [PubMed] [Google Scholar]
- 62. Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995; 15(5 Pt 1): 3526–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gooley JJ, Ho Mien I, St Hilaire MA et al. Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci. 2012; 32(41): 14242–14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Duffy JF, Cain SW, Chang AM et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 2011; 108Suppl 3: 15602–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Daan S, Pittendrigh CS. A functional analysis of circadian pacemekrs in nocturnal rodents. J Comp Physiol A. 1976; 106(1): 253–266. [Google Scholar]
- 66. Floessner T, Hut RA. Basic principles underlying biological oscillations and their entrainment. In: Kumar V, ed. Biological Timekeeping: Clocks, Rhythms and Behaviour. India: Springer; 2017. [Google Scholar]
- 67. Beersma DG, Daan S, Hut RA. Accuracy of circadian entrainment under fluctuating light conditions: contributions of phase and period responses. J Biol Rhythms. 1999; 14(4): 320–329. [DOI] [PubMed] [Google Scholar]
- 68. Roenneberg T, Hut R, Daan S, Merrow M. Entrainment concepts revisited. J Biol Rhythms. 2010; 25(5): 329–339. [DOI] [PubMed] [Google Scholar]
- 69. ASCHOFF J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960; 25: 11–28. [DOI] [PubMed] [Google Scholar]
- 70. Daan S. The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jürgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms. 2000; 15(3): 195–207. [DOI] [PubMed] [Google Scholar]
- 71. Yetish G, Kaplan H, Gurven M et al. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015; 25(21): 2862–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de la Iglesia HO, Fernández-Duque E, Golombek DA et al. Access to electric light is associated with shorter sleep duration in a traditionally hunter-gatherer community. J Biol Rhythms. 2015; 30(4): 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cho JR, Joo EY, Koo DL, Hong SB. Let there be no light: the effect of bedside light on sleep quality and background electroencephalographic rhythms. Sleep Med. 2013; 14(12): 1422–1425. [DOI] [PubMed] [Google Scholar]
- 74. Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999; 516(Pt 2): 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hattar S, Kumar M, Park A et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006; 497(3): 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.