Abstract
Background
Patients who receive immunotherapeutics may develop an atypical response pattern, which warrants further investigation into the potential benefits and risks for patients who continue immunotherapy beyond RECIST-defined disease progression.
Methods
A pooled analysis including all submissions to U.S. Food and Drug Administration (FDA) in support of marketing applications for anti-PD-1 antibodies and approved by FDA for treatment of patients with unresectable or metastatic melanoma (MM) was conducted to evaluate the potential benefits and safety of treatment beyond progression (TBP). Trials had to allow for continuation of the antibody beyond RECIST-defined progression (RECISTPD) in the anti-PD-1 arm. Any patient receiving the anti-PD-1 antibody after their RECISTPD date were included in the TBP cohort and analyzed descriptively at baseline and at time of progression with the cohort not receiving treatment beyond progression (noTBP). Patients in the TBP cohort had target lesion (TL) response after progression analyzed relative to PD and baseline TL burden.
Findings
Of 2624 pooled patients receiving immunotherapy, 52% (1361/2624) had progressive disease (PD); of these, 51% (692/1361) received continued anti-PD-1 antibody beyond RECIST-defined progression. Nineteen percent (95/500) of patients in TBP cohort with evaluable assessments experienced a ≥ 30% decrease in tumor burden, when considering burden at RECISTPD as the reference, representing 14% (95/692) of those TBP and 3·6% (95/2624) of all immunotherapy treated patients. Overall survival (OS) was greater in the TBP cohort compared with the noTBP cohort. One of the pooled trials was a double-blind, randomized, active-controlled trial evaluating an anti-PD-1 antibody vs. chemotherapy in which OS appeared similar in both arms for patients treated beyond progression and longer than the noTBP cohorts. Immune-related adverse events (irAE) up to 90-days from discontinuation were similar between the TBP cohort and the noTBP cohort.
Interpretation
Continuation of TBP in the product labeling of these immunotherapies has not been recommended as the clinical benefit remains to be proven. TBP with anti-PD-1 antibody therapy may be appropriate for select patients with MM, identified by specific criteria at the time of progression, based on the potential for late responses in the setting of the known toxicity profile.
Funding
none
Introduction
Unique clinical and regulatory issues have arisen in the treatment of unresectable and metastatic melanoma (MM) with the approval of multiple immunotherapies and numerous ongoing and planned clinical trials with these products. One issue concerns the adequacy of conventional response criteria, i.e., Response Evaluation Criteria in Solid Tumors v1·1 (RECIST), to characterize the clinical activity of immunotherapeutics and whether to continue treatment beyond progression (TBP) per conventional response criteria to maximize the potential for benefit from an immunotherapeutic.1 Patients who receive immunotherapy may develop an atypical response pattern, initially meeting conventional response criteria for progressive disease, followed by decreases in tumor burden.2–4 This pattern of response may relate to a delayed anti-tumor effect based on timing of an effective anti-tumor immune response, or to increases in the radiographic appearance of tumor lesions not based on tumor growth, but on inflammatory processes within the lesion due to transient immune cell infiltration (i.e., “pseudoprogression”).5–8 Responder analyses suggest that patients who progress by RECIST, but not by criteria accounting for potential delayed effects of immunotherapy, such as immune-related response criteria (irRC), may have clinical outcomes more closely approximating patients who do not progress by RECIST.2–4,9
Given the uncertainty regarding whether treatment discontinuation based on disease progression per RECIST could be premature in the context of immunotherapies, clinical trials of immunotherapies commonly allow for treatment beyond RECISTPD.1 However, it remains difficult to assess the clinical benefit of TBP, as these trials rarely allowed for continuation of treatment in the control arm beyond progression, the number of patients who developed significant tumor responses after progression was generally small in the recently reported studies of TBP, and associations with TBP and overall survival previously reported in patients with MM did not include similar analyses of the control arm where TBP was also permitted.8–11 To investigate the impact of TBP in MM, and to better characterize which MM patients may benefit from TBP, we conducted a pooled analysis of eight clinical trials that evaluated the safety and efficacy of nivolumab and pembrolizumab—both anti-programmed death receptor-1 (PD-1) antibodies—in this population and permitted selected patients to continue therapy after RECISTPD. To our knowledge, this is the first report of overall survival (OS) in patients TBP in a randomized, double-blind trial comparing nivolumab with dacarbazine, which allowed for the unique situation of TBP in both arms with continued blinding.12,13 These analyses are important to inform recommendations regarding regulatory endpoints and clinical protocol specifications for continuing a therapy after disease progression by conventional criteria.
Methods
Search Strategy and Selection Criteria
Trials were eligible for pooled analysis if they were submitted to the U.S. Food and Drug Administration (FDA) in support of a marketing application that was ultimately approved prior to January 2017 for treatment of patients with MM, included an anti-PD-1 antibody-alone or in combination arm, and allowed for the anti-PD-1 antibody to be continued beyond RECISTPD. Table 1 summarizes the eight multicenter clinical trials that met these criteria and were pooled for this analysis; the designs of these trials have been reported previously.12–21
Table 1.
Summary of Anti-PD-1 Antibody Trials in Melanoma Included in Pooled Analyses
| Study | Anti-P D-1 antibody | Trial Design | N | Control Arm (if applicable) | Primary Endpoint | Key Secondary Endpoint(s) | P otocol Criteria for TBPa |
|---|---|---|---|---|---|---|---|
| KEYNOTE P00116,18 | Pembrolizumab 2 or 10 mg/kg IV Q2W or Q3W | Open-label, randomized, expansion cohort | 416 | -- | ORRb | ORRc | Absence of symptoms and signs indicating clinically significant disease progression , No decline in ECOG performance status, and absence of rapid progression of disease or progressive tumor at critical anatomic sites requiring urgent medical intervention |
| KEYNOTE P00219 | Pembrolizumab 2 mg/kg IV Q3W | Randomized, partially-blinded | 540 | Chemotherapy | PFS, OS | ORRb | Clinical judgment of a patient’s overall clinical condition, including performance status, clinical symptoms, and laboratory data. Absence of clear deterioration of performance status (decrease of two points; from ECOG 0 to ECOG 2 for example), symptoms and signs indicating disease progression |
| KEYNOTE P00625 | Pembrolizumab 2 mg/kg IV Q3W | Randomized, open-label, | 834 | Ipilumumab 3 mg/kg IV Q3W | OS, PFS | ORRb | If imaging at Week 12 shows PD, investigator-assessed based on clinical judgment of a patient’s overall clinical condition, including performance status, clinical symptoms, and laboratory data in absence of clinical deterioration |
| CheckMate 003d21 | Nivolumab 0.1 mg/kg, 0.3 mg/kg, 1 mg/kg, 3 mg/kg or 10 mg/kg IV Q2W | Open-label, dose-escalation | 107 | Safety and tolerability | ORRb, DoR | Absence of clinical deterioration | |
| CheckMate 03714 | Nivolumab 3 mg/kg IV Q2W | Randomized, open-label | 405 | Investigator’s choice chemotherapy (dacarbazine 1000 mg/m2 IV Q3W or carboplatin AUC6 IV and paclitaxel 175 mg/m2 IV Q3W) | OS (ORRb for initial approval) | PFS | Experiencing investigator-assessed clinical benefit and tolerating study drug |
| CheckMate 06612,13 | Nivolumab 3 mg/kg IV Q2W | Randomized Double-Blind | 418 | Dacarbazine 1000 mg/m2 IV Q3W | OS | PFS, ORRb | Experiencing investigator-assessed clinical benefit, and tolerating study therapy |
| CheckMate 06720 | Nivolumab 3 mg/kg IV Q2W; Nivolumab 1 mg/kg IV Q3W + Ipilimumab 3 mg/kg IV Q3W for 4 doses, followed by nivolumab 3 mg/kg Q2W | Randomized, double-blind | 945 | Ipilimumab 3mg/kg IV Q3W for 4 doses | OS, PFS | ORRb | Experiencing Investigator-assessed clinical benefit and tolerating study drug |
| CheckMate 06915 | Nivolumab 1 mg/kg IV Q2W + Ipilimumab 3 mg/kg IV Q3W for 4 doses, followed by nivolumab 3 mg/kg Q2W | Randomized, double-blind | 142 | Ipilimumab 3 mg/kg IV Q3W for 4 doses | ORRb | PFS | Experiencing investigator-assessed clinical benefit and tolerating study therapy |
Abbreviations: DoR, duration of response; IV, intravenous infusion; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; Q2W, every 2 weeks; Q3W, every 3 weeks; TBP , treatment beyond progression.
subjects must discontinue therapy when further progression is documented
by RECIST v1·1
by IrRC
study was not randomized; safety evaluable population used
Procedures
Individual patient data (IPD) from the 2624 patients receiving at least one dose of pembrolizumab or nivolumab were pooled. Investigator evaluated tumor assessments for target, non-target, and new lesions were reviewed to assure each time point had all tumors assessed, that new lesions found at those time points were evaluated as new lesions and not in target lesion totals, and that non-target response was captured. Assessments of tumor response at individual time points and overall were re-derived and analyzed in a manner consistent with RECIST v1·1 across all studies. Target lesion (TL) tumor burden was calculated as the sum of longest diameter for non-nodal lesions and short axis for nodal lesions. Dates of non-protocol therapy, to include radiotherapy, oncologic surgery, or systemic anti-cancer treatments provided a censor for evaluation of response or progression across all studies. For all trials, the assessment schedule for TBP patients was the same as was defined in the pre-RECISTPD phase.
Outcomes
Patients were categorized as being treated beyond progression if the date of last drug exposure was after their progression date, where date of progression was determined by observing one or more of the following: an increase in TL sum of ≥ 20% from nadir along with a relative increase of at least 5mm in tumor burden, unequivocal progression in non-target lesions, or the appearance of a new lesion. The duration of TBP was defined as the length of time from RECISTPD date to the date of last dose of anti-PD-1 antibody treatment or receipt of non-protocol anti-cancer treatment, whichever occurred earlier. A TBP response was defined as a decrease in TL tumor burden (sum of the reference diameters) of at least 30% from the TL tumor burden at the time of RECISTPD, which did not require confirmation at a subsequent assessment. Additionally, patients achieving a 30% decrease in TL tumor burden—relative to baseline—during their post-RECISTPD TBP period were considered responders.
Time point response was defined as complete response (CR) when the disappearance of all target lesions (nodal lesions decreased to < 10mm) and non-target lesions was observed, partial response (PR) was established by ≥ 30% decrease from baseline TL sum, and stable disease (SD) was based on exclusion from CR, PR or progressive disease (PD) categories and having a minimum duration of 8 weeks (this varied by protocol between 6, 9, or 12 weeks or not being defined). Assignment of a CR or PR required confirmation on a subsequent imaging assessment performed at least 4 weeks after the initial documentation of this response. These time point assessments were used to derive a confirmed best overall response and duration of response. Overall Survival (OS) was defined as the time from randomization to death from any cause and was censored at date of last-follow up for patients still alive at data cutoff.
Immune-related AE’s (irAEs) were defined as those select AE categories commonly recognized as being immune-related (per anti-PD-1 package inserts), events occurring after the start of treatment and within 90 days post discontinuation of therapy, and requiring use of corticosteroids for management. Serious adverse events (SAE) were defined as per 21 Code of Federal Regulations 312·32 as those adverse events whose outcome was death, life-threatening, required initial or prolongation of hospitalization, resulted in a incapacity or substantial disruption of the ability to conduct normal life functions, or a congenital anomaly/birth defect, or those adverse events jeopardizing the patient and potentially requiring medical or surgical intervention to prevent one of the prior listed outcomes. AEs (common) and irAE’s among the TBP cohort were analyzed pre- and post-PD, with the post-PD period including 30 days of follow-up (a longer follow-up was not evaluated, to allow for appropriate comparison of the two groups). Analysis of TBP vs. noTBP cohorts included all adverse events experienced after treatment and within 90 days post-discontinuation.
Statistical Analysis
Descriptive statistics were used to characterize demographics, disease characteristics, tumor measurements, progression, treatment duration, confirmed best overall response, and adverse events (AEs) by TBP cohort. Kaplan-Meier methods provided OS estimates and corresponding 95% confidence intervals.22 The analysis for this paper was generated using SAS software version 9·4. Copyright © 2002–2012 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. Survival figures were generated using version 3·4·2 of R: a language and environment for statistical computing and version 1·0·153 of RStudio © 2009–2017 RStudio, Inc.
Role of the funding source
There was no funding source. All authors as FDA employees had full access to the data.
Results
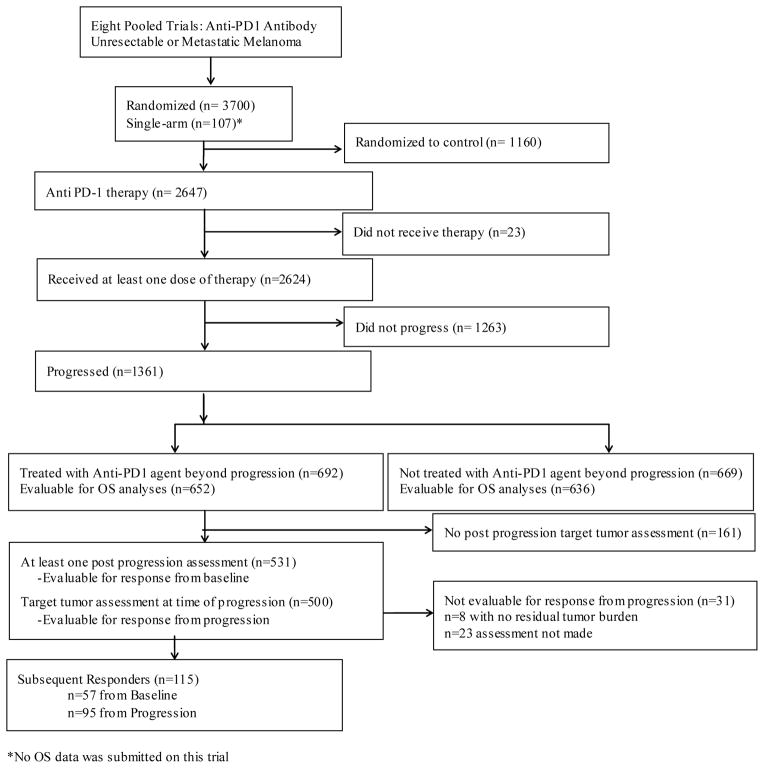
Overall, 2624 patients with MM who received pembrolizumab or nivolumab in clinical trials were included in the pooled analysis; of those, 1361 (52%) met criteria for progression (PD) per RECIST, and 692 (26%) received an anti-PD-1 antibody beyond RECISTPD (TBP cohort) (Figure 1). The majority of patients with RECISTPD progressed at the first assessment after study entry (range: Week 1–Week 24): 66% (459/692) and 63% (422/669) of the TBP and noTBP cohorts, respectively (appendix p2). The median duration of TBP was 1·41 months (m) (IQR: 0·69–4·86) (appendix p3). Patients in the TBP cohort were followed for a median of 15·7 months (IQR: 11·7–23·6) and those in the noTBP cohort had 14·1 months (IQR:11·2–22·2) of follow-up.
Figure 1. Consort Diagram Pooled Population.
Consort Diagram detailing patient inclusion for various analyses from the pooled studies of patients receiving Anti-PD-1 therapy for unresectable metastatic melanoma.
Patient Characteristics at Baseline and Time of Disease Progression
Patient characteristics at baseline appeared similar between the TBP and noTBP cohorts with the exception of a higher proportion of patients in the TBP cohorts with an ECOG performance status (PS) of 0 (77% (533/692) vs. 65% (437/669)) and normal LDH (65% (451/692) vs. 55% (366/669)) (Table 2). At the time of RECISTPD, the differences in ECOG PS of 0 (67% (463/692) vs. 34% (227/669)) and normal LDH (63% (435/692) vs. 31% (205/669)) in the TBP and noTBP cohorts, respectively, were more pronounced. The confirmed best overall response (cBOR) prior to RECISTPD was similar between the TBP and noTBP cohorts—a cBOR of partial or complete response in 14% and PD in approximately two-thirds of patients in both cohorts. However, at the time of RECISTPD, a higher proportion of TBP patients had a reduction in TL tumor burden from baseline than the noTBP cohort, 41% (284/692) vs. 26% (174/669), with a median change in TL tumor burden relative to baseline of 5·8% (IQRL-28 to 29%) in the TBP cohort and 20·7% (IQR: −8 to 47%) in the noTBP cohort.
Table 2.
Demographics and Disease Characteristics of Patients Treated with Anti PD-1 Antibody
| Variable | Category Value | TBP Patients (n=692) N (%) |
noTBP Patients (n=669) N (%) |
TBP, Subsequent Response from PD (n=95) N (%) |
TBP, No Subsequent Response from PD (n=597) N (%) |
|---|---|---|---|---|---|
|
| |||||
| Prior to Anti-PD-1 Therapy | |||||
|
| |||||
| Age | Median (IQR), years | 61 (50, 69) | 60 (50, 69) | 62 (54, 68) | 61 (49,69) |
|
| |||||
| Sex | Female | 266 (38%) | 286 (43%) | 38 (40%) | 228 (38%) |
| Male | 426 (62%) | 383 (57%) | 57 (60%) | 369 (62%) | |
|
| |||||
| AJCC Stage at Study Entry | Stage III | 28 (4%) | 30 (4%) | 7 (7%) | 21 (4%) |
| Stage IV | 556 (80%) | 545 (81%) | 71 (75%) | 485 (81%) | |
| Missing | 108 (16%) | 94 (14%) | 17 (18%) | 91 (15%) | |
|
| |||||
| M Stage at Study Entry | M0/M1A/M1B | 174 (25%) | 167 (25%) | 29 (31%) | 145 (24%) |
| M1C | 367 (53%) | 370 (55%) | 43 (45%) | 324 (54%) | |
| Missing | 151 (22%) | 132 (20%) | 23 (24%) | 128 (21%) | |
|
| |||||
| Prior Lines of Systemic Therapy for Advanced Disease | 0 | 332 (48%) | 331 (49%) | 51 (54%) | 281 (47%) |
| 1 | 175 (25%) | 139 (21%) | 20 (21%) | 155 (26%) | |
| 2 | 116 (17%) | 110 (16%) | 18 (19%) | 98 (16%) | |
| ≥3 | 69 (10%) | 89 (13%) | 6 (6%) | 63 (11%) | |
|
| |||||
| PD-L1 Expression | Negative/Indeterminate | 213 (31%) | 254 (38%) | 23 (24%) | 190 (32%) |
| Positive | 312 (45%) | 259 (39%) | 48 (51%) | 264 (44%) | |
| Missing | 167 (24%) | 156 (23%) | 24 (25%) | 143 (24%) | |
|
| |||||
| BRAF V600 Mutation Status | Mutant | 185 (27%) | 193 (29%) | 27 (28%) | 158 (26%) |
| Wild Type | 486 (70%) | 457 (68%) | 64 (67%) | 422 (71%) | |
| Missing | 21 (3%) | 19 (3%) | 4 (4%) | 17 (3%) | |
|
| |||||
| Baseline ECOG Performance Status | 0 | 533 (77%) | 437 (65%) | 80 (84%) | 453 (76%) |
| 1 | 158 (23%) | 231 (35%) | 15 (16%) | 143 (24%) | |
| 2 | 1 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | |
|
| |||||
| Baseline LDH > ULN Category | ≤ULN | 451 (65%) | 366 (55%) | 67 (71%) | 384 (64%) |
| >ULN | 232 (34%) | 296 (44%) | 27 (28%) | 205 (34%) | |
| Missing | 9 (1%) | 7 (1%) | 1 (1%) | 8 (1%) | |
|
| |||||
| At Time of RECIST-Defined Progression | |||||
|
| |||||
| ECOG Status Within 28 Days Prior to Progression | 0 | 463 (67%) | 227 (34%) | 67 (71%) | 396 (66%) |
| 1 | 205 (30%) | 263 (39%) | 26 (27%) | 179 (30%) | |
| 2 | 7 (1%) | 53 (8%) | 0 (0%) | 7 (1%) | |
| 3 | 7 (1%) | 7 (1%) | 0 (0%) | 7 (1%) | |
| Missing | 10 (1%) | 119 (18%) | 2 (2%) | 8 (1%) | |
| Improvement from baseline | 39 (6%) | 29 (4%) | 5 (5%) | 34 (6%) | |
| Worsening from baseline | 108 (16%) | 183 (27%) | 16 (17%) | 92 (15%) | |
|
| |||||
| LDH Category Within 28 Days Prior to Progression | ≤ULN | 435 (63%) | 205 (31%) | 73 (77%) | 362 (61%) |
| >ULN | 232 (34%) | 337 (50%) | 20 (21%) | 212 (36%) | |
| Missing | 25 (4%) | 127 (19%) | 2 (2%) | 23 (4%) | |
| Improvement to ≤ULN | 72 (10%) | 26 (4%) | 13 (14%) | 59 (10%) | |
| Worsening to >ULN | 72 (10%) | 101 (15%) | 6 (6%) | 66 (11%) | |
|
| |||||
| Reason for Progression | Target | 208 (30%) | 143 (21%) | 30 (32%) | 178 (30%) |
| Non-Target | 66 (10%) | 59 (9%) | 9 (9%) | 57 (10%) | |
| New Lesion | 248 (36%) | 179 (27%) | 43 (45%) | 205 (34%) | |
| Non-Target and New Lesion | 50 (7%) | 71 (11%) | 9 (9%) | 41 (7%) | |
| Target + Other | 120 (17%) | 217 (31%) | 4 (4%) | 116 (19%) | |
| Target/Non-Target | 40 (6%) | 51 (8%) | 1 (1%) | 39 (7%) | |
| Target/New Lesion | 39 (6%) | 69 (10%) | 1 (1%) | 38 (6%) | |
| Target/Non-Target/New Lesion | 41 (6%) | 97 (14%) | 2 (2%) | 39 (7%) | |
|
| |||||
| Confirmed BOR prior to Progression | CR | 8 (1%) | 15 (2%) | 0 (0%) | 8 (1%) |
| PR | 88 (13%) | 81 (12%) | 16 (17%) | 72 (12%) | |
| SD | 123 (18%) | 131 (20%) | 18 (19%) | 105 (18%) | |
| PD | 473 (68%) | 442 (66%) | 61 (64%) | 412 (69%) | |
|
| |||||
| Duration of Response Prior to Progression | Median (IQR), months | 5·5 (3·9, 8·5) | 6·8 (4·4,11·1) | 4·4 (3·5, 6·8) | 5·9 (4·0, 10·5) |
|
| |||||
| Timing of RECIST PD | First Assessment (range week 0–24) | 459 (66%) | 422 (63%) | 59 (62%) | 400 (67%) |
| Second Assessment (range week 6–27) | 58 (8%) | 74 (11%) | 13 (14%) | 45 (8%) | |
| Third Assessment (range week18–50) | 56 (8%) | 47 (7%) | 7 (7%) | 49 (8%) | |
| 12 weeks or less | 443 (64%) | 408 (61%) | 54 (57%) | 389 (65%) | |
| 13 to 16 weeks | 43 (6%) | 41 (6%) | 13 (14%) | 30 (5%) | |
| 17 to 20 weeks | 23 (3%) | 42 (6%) | 5 (5%) | 18 (3%) | |
| 21 to 24 weeks | 55 (8%) | 37 (6%) | 7 (7%) | 48 (8%) | |
| greater than 24 weeks | 128 (18%) | 141 (21%) | 16 (17%) | 112 (19%) | |
At RECISTPD, the determinants of disease progression appeared to differ between cohorts. In the TBP and noTBP cohorts, respectively, 30% (208/692) vs. 21% (143/669) progressed due to TL progression, 36% (248/692) vs. 27% (179/669) progressed due to new lesion(s), 10% (66/692) vs. 9% (59/669) progressed due to non-target lesions, and 25% (170/692) vs. 43% (288/669) progressed based on a combination of TL progression, appearance of new lesions, or non-target lesion progression. In patients progressing solely due to new lesions, the new lesions more frequently involved a non-visceral site (50% (124/248) vs. 36% (64/179)) but less frequently involved the CNS (7·7% (19/248) vs. 21·2% (38/179)) in the TBP cohort than in the noTBP cohort (appendix pp 15–16).
Response after Progression Analysis
Among the 692 patients in the TBP cohort, 500 (72%) were evaluable for a TBP response based on documentation of TL tumor burden at the time of RECISTPD and on at least one post-progression tumor burden assessment. The analysis demonstrated TBP responses (i.e., ≥30% decrease in TL tumor burden) in 95/500 (19%) and increases in TL tumor burden meeting the threshold of ≥20% in 64/500 (13%) of evaluable patients (appendix p4 and p17). Of the 95 patients with a TBP response, eight had documentation of new lesion(s), three had documentation of a change from equivocal to unequivocal progression status in non-target disease, and one had documentation of both during the interval from RECISTPD to first determination of a TBP response. The median time from RECISTPD to TBP response was 2·8m (IQR: 1·4–5·5m). TBP responders were treated beyond progression a median 9·4m (IQR: 5·4–13·7m) and TBP non-responders were treated beyond progression for a median of 1·3m (IQR: 0·7–3·0). An assessment of differences in patient characteristics at the time of RECISTPD within the TBP cohort based on TBP response status reveals that patients with a TBP response more frequently had new lesions as the reason for RECISTPD [(43/95) 45% vs. (205/597)34%] as well as normal LDH [(73/95) 77% vs (362/597) 61%).
In addition to performing an analysis of response in patients TBP as assessed from progressive disease we also performed an analysis of response in patients TBP as assessed relative to TL burden at baseline. Of the 57 patients TBP with a subsequent response on imaging (≥30% reduction in tumor burden) as assessed from baseline after RECIST-defined progression, the timing of initial PD occurred at a median of 12 weeks (IQR: 9–12) with most occurring at the first tumor assessment (demographics and additional information is reported in appendix p23). The criterion met for initial RECIST-defined PD was solely the appearance of new lesion(s) in the majority (34 of the 57) of TBP patients with a subsequent response; moreover, 30 of the 57 patients had already experienced some decrease in tumor burden at the time of progression (appendix p4 and p23). Median duration of TBP among these 57 patients was 7·5 months (IQR: 4·5–12.7) and median time to post-progression response was 2·7 months (IQR: 1·4–4·2). Altogether, there were 115 patients who experienced either a subsequent response (≥30% reduction in tumor burden) in reference to baseline and/or progressive disease during their TBP period; these patients are further described and shown in (appendix p8 and p17).
Analysis of response after RECISTPD in the noTBP cohort is limited to 76/669 (11%) patients with additional TL tumor assessments while in observation prior to starting subsequent therapy, among whom 64/669 (10%) had TL tumor burden levels recorded at time of initial RECISTPD. Sixteen percent (10/64) exhibited post-progression response (≥30% reduction in TL tumor burden) as assessed relative to TL tumor burden at RECISTPD. This represent 1·5% (10/669)of noTBP cohort and 0·4% (10/2624) of all immunotherapy treated patients. In the noTBP cohort, the median time from RECISTPD to start of subsequent therapy was 0·5 months (IQR: 0·2, 1·2). Further analysis was performed examining percent change in tumor burden from baseline by treatment visit for patients progressing due to target lesion increase and continuing treatment beyond progression (appendix pp 12–14).
Overall Survival Analysis
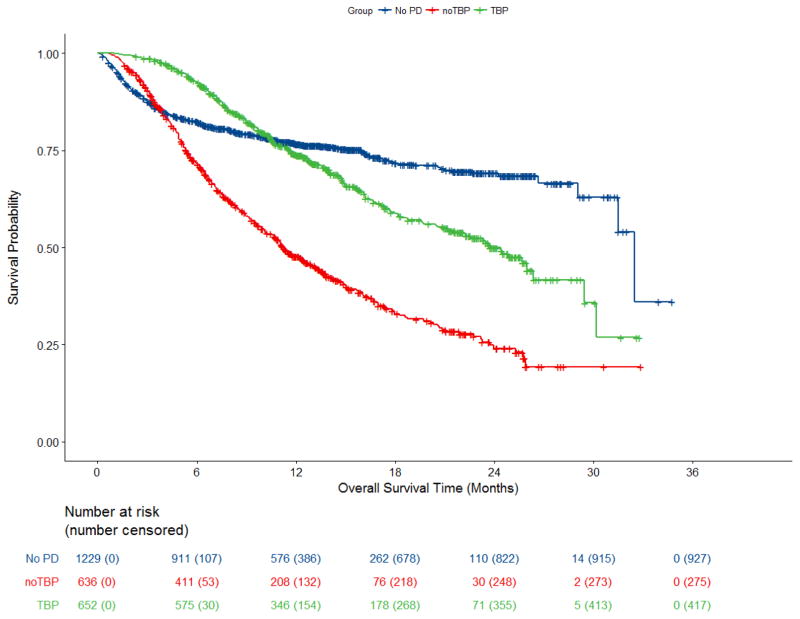
In the OS analysis of anti-PD-1 antibody-treated patients with RECIST-defined PD by TBP status, median OS was 24·4m (95% confidence interval [CI]: 21·2, – 26·3) and 11·2m (95% CI: 10·1, 12·9) in the TBP and noTBP cohorts, respectively (Figure 2). Thirty-six percent (235/652) of the TBP cohort had died from any cause as compared to 57% (361/636) of the noTBP cohort at the time of data cut-off.
Figure 2. Overall Survival Pooled Analysis of Patients Treated with anti-PD-1 Antibody.
Kaplan-Meier estimates of overall survival in pooled patients treated with anti-PD-1 antibody by treatment beyond progression group. Includes all patients receiving at least one dose of anti PD-1 antibody, excluding the n=107 patients on Trial CA209003 who did not have OS follow up. The cohort of patients who did not have PD demonstrated an initial decline in this curve as a result of early mortality prior to an assessment for progression or response.
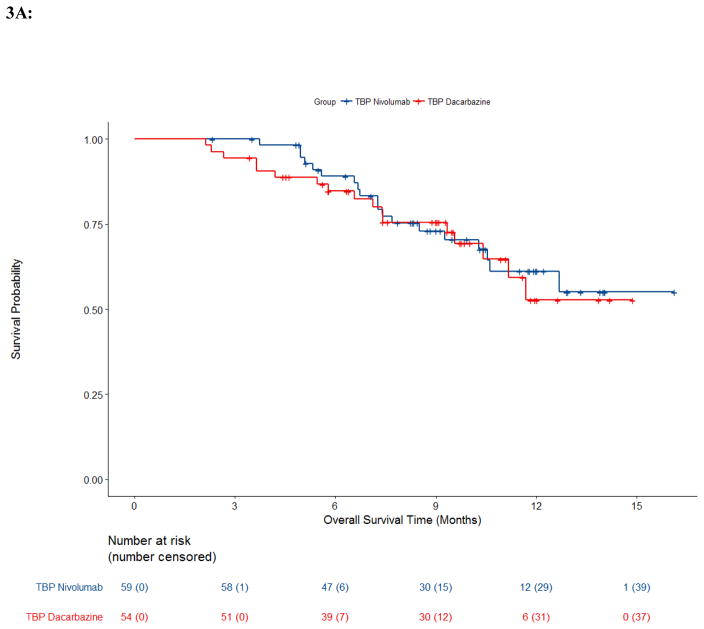
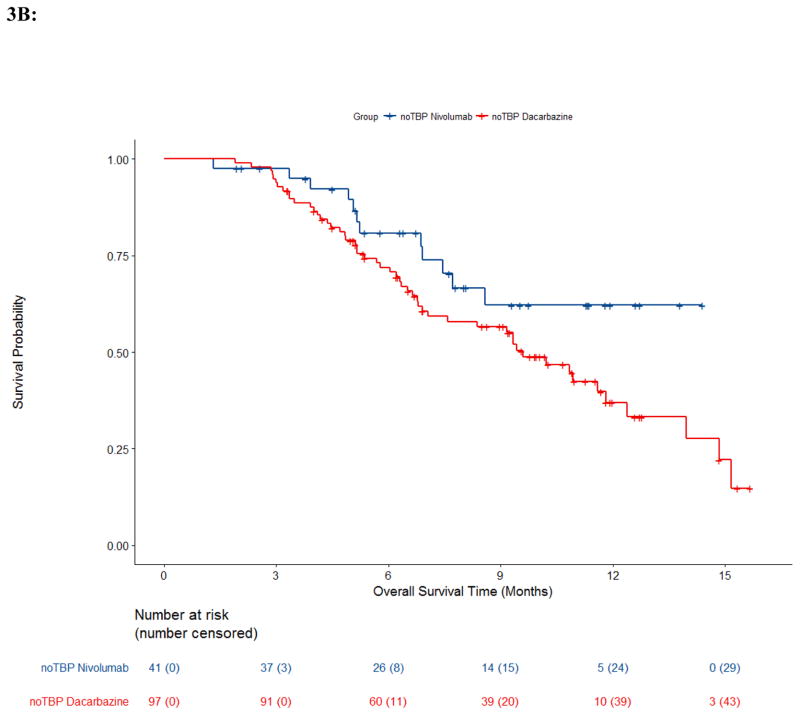
Trial CA209066 provides a rare opportunity to evaluate TBP in a double-blind, randomized, active-controlled clinical trial where patients with RECISTPD could continue assigned treatment, i.e., the anti-PD-1 antibody (nivolumab) or the control chemotherapy (dacarbazine) along with the respective matched placebo infusions to maintain the blind.12,13 Of 250 patients with RECIST-defined PD, 59 received TBP on the nivolumab arm and 54 received TBP on the dacarbazine arm. These numbers differ slightly within each TBP cohort compared to those previously described due to the re-derivation of progression events and recoding for this pooled analysis as described in the methods section.12,13 Demographics between arms in Trial CA209066 and best overall response prior to PD are described in appendix pp 25–26. The median duration of treatment beyond progression in study CA209066 was 1·3 months (IQR: 0·3–2.8) in the nivolumab arm and 0·4 months (IQR: 0·2–1.4) in the dacarbazine arm. The OS Kaplan-Meier curves for patients with RECISTPD are shown in Figure 3. While the dacarbazine arm noTBP demonstrated median OS of 9·6m (95% CI: 6·9, 11·8), no other arms reached median survival. However, survival probabilities at 1 year within the nivolumab arm in noTBP cohort was 62% (95% CI: 42, 77), and was 61% (95% CI: 45, 74) in TBP cohort; within the dacarbazine arm one year survival probability in the TBP cohort was 53% (95% CI: 32, 70), and was 37% (95% CI: 25,49) in noTBP cohort. The percentage of deaths was similar across the TBP (nivolumab 32% (19/59), dacarbazine 31% (17/54)) and nivolumab noTBP arms, 29% (12/41), though higher in the dacarbazine noTBP arm 54% (52/97).
Figure 3. Overall Survival by Arm and Treatment Beyond Progression Trial CA209066.
Kaplan-Meier estimates of overall survival from Trial CA209066 by arm and treatment beyond progression status. A, Treatment beyond progression (TBP); B, No Treatment beyond progression (noTBP).
Safety Analysis
Among 1361 patients with RECISTPD, 362/669 patients (54%) in the noTBP cohort experienced an SAE up to 90 days post treatment discontinuation compared to 295/692 patients (43%) in the TBP cohort. In the TBP cohort, 18% (121/692) experienced a serious adverse event (SAE) in the period of time prior to progression, and 24% (163/692) in the period of time post progression up to 30 days after treatment discontinuation. The only AE with a >2% difference occurring in the post progression time period compared to the treatment prior to progression time period was vitiligo (8% to 5%) (appendix pp 27–28). Of patients not treated beyond progression, 15% (99/669) received another immunotherapy in their next line of therapy, compared to 20% (136/692) of those TBP. In study CA209066, 44% (26/59) TBP went on to receive another immunotherapy, compared to 41·5% (17/41) noTBP in the nivolumab arm, and 50% (27/54) of patients TBP did not receive another immunotherapy compared to 45% (44/97) noTBP in the dacarbazine arm (appendix p29).
Overall, 11% (78/692) of the TBP cohort and 16% (106/669) of the noTBP cohort experienced an irAE within 90 days of receiving the anti-PD-1 antibody (appendix p30). Within the TBP cohort, 4·3% (30/692) experienced an irAE prior to RECISTPD, and 4·0% (28/692) in the time post progression up to 30 days after treatment discontinuation. Given that patients selected for TBP may have had improved tolerability of anti-PD-1 antibody we also evaluated the AEs in the TBP and noTBP cohorts prior to progression (appendix p31). AEs with a ≥ 5% difference were anemia, abdominal pain, diarrhea, nausea, vomiting, pyrexia, decreased appetite, and dyspnea; all of these were increased in the noTBP cohort compared to the TBP cohort.
Discussion
This pooled analysis describes clinical outcomes for the 2624 patients with MM receiving an anti-PD-1 antibody in trials submitted to FDA in support of marketing applications and approved by FDA for treatment of MM patients. While the majority of patients with RECISTPD (51% [692/1361] of patients) received continued anti-PD-1 antibody beyond RECISTPD, a modest proportion of these patients (14% [95/692]) appeared to have subsequent decreases in their TL tumor burden reaching the level of a response (≥30% decrease) in our analysis. We determined that post-progression responses were not limited to the TBP cohort as 1·5% (10/669) of patients in the noTBP cohort experienced a subsequent response. The difference between the TBP and noTBP cohorts in the proportion of patients with post-progression responses is less pronounced when considering only the subset of patients evaluable for a subsequent response, i.e., 19% (95/500) and 16% (10/64), respectively. Overall survival (OS) was greater in the TBP cohort with a median OS of 24·4m (95% confidence interval [CI]: 21·2, – 26·3) compared to the noTBP cohort with a median OS of 11·2m (95% CI: 10·1, 12·9) in the pooled analysis. However, in our exploratory subgroup analyses of overall survival in the CA209066 trial, which permitted TBP in both the nivolumab arm and the dacarbazine arm in this double-blind randomized controlled trial, OS appeared similar in both arms for the subgroup of patients treated beyond progression. Overall, in our pooled analyses the safety profile of anti-PD-1 antibody treatment in the TBP period appears consistent with the safety profile observed in the period prior to disease progression.
The strengths of these analyses lie in the number of trials pooled, representing the largest analysis to date describing outcomes of patients TBP. In addition, the analysis of the double-blind, randomized, controlled trial of nivolumab vs. dacarbazine provided the unique ability to explore survival outcomes for patients within the anti-PD-1 antibody and standard chemotherapy arms based on continuation of TBP. A limitation of these exploratory analyses is that trials included in the pooled analyses used different criteria for TBP continuation and, therefore, the pooled populations differed. Nevertheless, demographic and disease characteristics data demonstrated that patients who received TBP more often had progression with new lesions in non-visceral/non-CNS disease sites, a favorable ECOG performance status (i.e., 0 vs. 1 or 2), and normal LDH compared to patients noTBP. This appears consistent with protocol patient selection criteria intended to limit risks to patients who continue anti-PD-1 antibody therapy after initial documentation of RECISTPD. Typical patient selection criteria for continuation of TBP include absence of signs and symptoms or laboratory values indicating clinically significant PD, absence of a decline in ECOG performance status, and absence of rapid PD at critical anatomical sites requiring urgent medical intervention. Our pooled analyses suggest that disease characteristics and demographics were similar between patients TBP with and without subsequent responses with the exception that patients TBP with subsequent responses more commonly progressed due to new lesions and had a normal LDH at time of progression as compared with those TBP who did not subsequently respond.
Similar to other uncontrolled reports, our pooled analysis shows that among the patients receiving anti-PD-1 antibody therapy who experienced progressive disease by conventional response criteria, the TBP cohort has an increased median OS compared to the noTBP cohort.2,3,10 It is unclear if patients in the TBP cohort who were selected by the investigator to continue anti-PD-1 antibody therapy after investigator-determined PD may have had a better outcome due to differences in known prognostic factors, which was observed in our analyses as well as TBP analyses by others, or in unknown prognostic factors, rather than due to continued TBP with the anti-PD-1 antibody.8,10,11 To account for known and unknown prognostic factors, a trial randomizing patients to receive TBP or not would be necessary to demonstrate the clinical benefit, if any, with TBP. However, OS analysis from trial CA209066 suggests that patients receiving TBP in the nivolumab and dacarbazine arms had similar outcomes despite a clear improvement in efficacy with nivolumab in the overall trial population. While these post-hoc survival analyses are limited by absence of a second randomization at the time of progression, the findings support that those being selected for TBP may have a different natural history of disease as opposed to a delayed immunotherapeutic benefit.
Clinical protocols studying immunotherapies may allow patient management based on immune-related response criteria such as irRC or iRECIST. However, the assessment method for evaluating response intended to support efficacy in a regulatory submission has been based on conventional criteria, typically RECIST.24 While a subset of patients may exhibit tumor shrinkage after continuation of anti-PD-1 antibody beyond RECISTPD, overall this represents 3·6% (95/2624) of the total treated population. Although reference time point to calculate relative tumor burden changes may differ as well as the definition of TBP across studies of TBP with anti-PD-1 antibodies, the rate of response following TBP reported here of 11% (57/500) to 19% (95/500) using a reference time point of baseline or RECISTPD, respectively, is consistent with those reported in MM and across cancer types, including non-small cell lung and renal cell carcinoma trials.2,8–11 In our pooled analysis, we defined patients who were TBP as those who received an anti-PD-1 antibody at any time after RECISTPD albeit any retrospective cutoff to identify TBP is arbitrary. The cutoff for patients treated for greater than 6 weeks was evaluated in two other trials and was in the range seen with our pooled analysis: of the 1361 progressed patients, 359 (26%) received therapy for greater than 6 weeks post initial RECISTPD and among those 24% (85/359) exhibited response from PD.23 While the relatively few additional patients that would be classified as objective responders by tumor response criteria accounting for the potential delayed responses with anti-PD-1 therapy is unlikely to meaningfully alter a benefit-risk analysis, the assessment of time-to-event, tumor-measurement based endpoints (e.g., progression-free survival) using response criteria modified for immunotherapeutics may be more impactful. Another limitation of our pooled analysis is that we were unable to explore associations between survival and tumor measurement based endpoints using alternative response criteria (e.g., irRC and iRECIST) since not all of the trials recorded the necessary lesion measurements. As more combination therapies emerge in clinical trials with the potential to increase tumor inflammation, the need for evaluation of non-classical responses may increase. Our analysis of AEs does not appear to indicate a detrimental safety impact from continuation of anti-PD-1 antibody beyond progression. This is consistent with the typical patient selection criteria for continued treatment which excludes signs and symptoms or laboratory values indicating clinically relevant PD. Examination of irAEs did not cause concern that substantially more irAEs resulted from continuing therapy beyond progression.
In summary, the risks of continued treatment beyond progression—both the safety risks of the anti-PD-1 antibody and the risks of continuing an inefficacious agent with delay of alternative therapy–should be balanced with the modest potential of a subsequent reduction in tumor burden or prolonged stability. Although FDA does not consider costs of drugs in making regulatory decisions, costs associated with continuation of treatment beyond progression may be an additional consideration for other stakeholders, including health care insurers and patients. Further evaluation is needed to identify those who may benefit from continued treatment, with potential incorporation of biomarkers and patient characteristics. From a regulatory standpoint, there does not appear to be an obvious safety reason to recommend against allowing TBP in clinical protocols evaluating immunotherapies. However, we recommend that well-defined patient selection criteria be pre-specified in all clinical protocols and patients receive informed consent documents detailing what is known about TBP in the development program of the immunotherapeutic to ensure the potential benefits outweigh the potential risks. The development of standardized response criteria that account for atypical response patterns with immunotherapeutics for use in defining clinical benefit of these therapies in clinical trials remains an active area of investigation.3,4,24 Comparisons of conventional response criteria such as RECIST with criteria that account for the potential for patients to experience delayed responses such as iRECIST are required not only to assess whether such immune-modified criteria more fully capture the clinical benefit with the use of continued treatment in the presence of progression of existing lesions and appearance of new tumor lesions, but also to identify patients refractory to the immunotherapy for enrollment in subsequent trials. As the survival of patients TBP appears to be similar in the chemotherapy arm and the anti-PD-1 antibody arm in the comparative trial, the absolute number of patients with a “delayed response” during TBP with anti-PD-1 antibodies is low relative to the total population in the pooled analysis, responses after RECISTPD were observed in patients in the noTBP cohort, and none of the trials randomized patients to receive TBP vs. no TBP, the overall clinical benefit of TBP remains unclear at this time. Thus, continuation of TBP in the product labeling of anti-PD-1 antibody therapies has not been recommended. However, the practice of medicine is outside FDA’s purview, and the continuation of an immunotherapeutic in clinical practice to maximize the potential for a patient to experience a late response would continue to weigh clinical criteria, such as those used in clinical trials based on extent of progression of disease and subjective patient status, to inform the risk-benefit analysis of this approach.
Supplementary Material
Research in Context Panel.
Evidence before this study
Patients receiving immunotherapy may develop an atypical response pattern, initially meeting conventional response criteria for progressive disease, followed by decreases in tumor burden. Given the uncertainty regarding whether treatment discontinuation based on disease progression per standard criteria could be premature in the context of immunotherapies, clinical trials of immunotherapies commonly allow for treatment beyond RECISTPD. However, it remains difficult to assess the clinical benefit of TBP, as these trials rarely allowed for continuation of treatment in the control arm beyond progression, the number of patients who developed significant tumor responses after progression was generally small in the recently reported studies of TBP, and associations with TBP and overall survival previously reported in patients with metastatic melanoma did not include similar analyses of the control arm where TBP was also permitted.
Added Value of this study
This is a large pooled-analysis of patients with unresectable or metastatic melanoma treated beyond progression which shows response information at various timepoints and perspectives within the same pooled-group with accompanying safety information. In addition, overall survival data is presented from a randomized trial allowing for continuation of treatment beyond progression in both the anti-PD-1 antibody and chemotherapy control arms.
Implications of all the available evidence
FDA perspective on this issue based on our analysis is described. Continuation of TBP in the product labeling of anti-PD-1 antibody therapies has not been recommended. However, the continuation of an immunotherapeutic in clinical practice to maximize the potential for a patient to experience a late response would continue to weigh clinical criteria, such as those used in clinical trials based on extent of progression of disease and subjective patient status, to inform the risk-benefit analysis of this approach.
Footnotes
Note: This is a U.S. Government work. There are no restrictions on its use.
Contributors
JAB, MH, FM, SM, HC, MRT conceived and designed the study and performed the data analysis. All authors interpreted the data and contributed to writing the report.
Declaration of interests
JC worked on this research while an FDA employee, and no longer contributed after leaving the agency; at the time of manuscript submission, JC is an employee of AstraZeneca. No potential conflicts of interest were disclosed by the remaining authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of Immune-Related Response Criteria and RECIST v1. 1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34(13):1510–7. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 4.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936–43. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 21(8):1712–7. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 6.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Regan KN, Jagannathan JP, Ramaiya N, Hodi FS. Radiologic aspects of immune-related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapy. AJR Am J Roentgenol. 2011;197(2):W241–6. doi: 10.2214/AJR.10.6032. [DOI] [PubMed] [Google Scholar]
- 8.Long GV, Weber JS, Larkin J, et al. Nivolumab for Patients With Advanced Melanoma Treated Beyond Progression: Analysis of 2 Phase 3 Clinical Trials. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George S, Motzer RJ, Hammers HJ, et al. Safety and Efficacy of Nivolumab in Patients With Metastatic Renal Cell Carcinoma Treated Beyond Progression: A Subgroup Analysis of a Randomized Clinical Trial. JAMA Oncol. 2016;2(9):1179–86. doi: 10.1001/jamaoncol.2016.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escudier B, Motzer RJ, Sharma P, et al. Treatment Beyond Progression in Patients with Advanced Renal Cell Carcinoma Treated with Nivolumab in CheckMate 025. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1. 1-defined disease progression in clinical trials. Semin Oncol. 2017;44(1):3–7. doi: 10.1053/j.seminoncol.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 13.Beaver JA, Theoret MR, Mushti S, et al. FDA Approval of Nivolumab for the First-Line Treatment of Patients with BRAFV600 Wild-Type Unresectable or Metastatic Melanoma. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-0714. [DOI] [PubMed] [Google Scholar]
- 14.Hazarika M, Chuk MK, Theoret MR, et al. U.S. FDA Approval Summary: Nivolumab for Treatment of Unresectable or Metastatic Melanoma Following Progression on Ipilimumab. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-0712. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1558–68. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 17.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 18.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53(282):457–81. [Google Scholar]
- 23.Blumenthal GM, Theoret MR, Pazdur R. Treatment Beyond Progression With Immune Checkpoint Inhibitors–Known Unknowns. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.1819. [DOI] [PubMed] [Google Scholar]
- 24.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e52. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.