SUMMARY
Unequal transmission of nutritive signaling during cell division establishes fate disparity between sibling lymphocytes, but how asymmetric signaling becomes organized is not understood. We show that receptor-associated class I phosphatidylinositol 3-kinase (PI3K) signaling activity, indexed by phosphatidylinositol (3,4,5)-trisphosphate (PIP3) staining, is spatially restricted to the microtubule-organizing center and subsequently to one pole of the mitotic spindle in activated T and B lymphocytes. Asymmetric PI3K activity co-localizes with polarization of antigen receptor components implicated in class I PI3K signaling and with facultative glucose transporters whose trafficking is PI3K dependent and whose abundance marks cells destined for differentiation. Perturbation of class I PI3K activity disrupts asymmetry of upstream antigen receptors and downstream glucose transporter traffic. The roles of PI3K signaling in nutrient utilization, proliferation, and gene expression may have converged with the conserved role of PI3K signaling in cellular symmetry breaking to form a logic for regenerative lymphocyte divisions.
In Brief
Activated lymphocytes self-renew while producing differentiated progeny, a process linked to unequal transmission of anabolic PI3K signaling during cell division. Chen et al. find that a PI3K-mediated polarity pathway causes the lopsided arrangement of the very receptors and transporters that mediate PI3K-induced cell fate changes.

INTRODUCTION
Adaptive immunity requires selected T and B lymphocytes to produce differentiated cellular descendants (effector cells and plasma cells) while self-renewing the less differentiated fate. The initial cell divisions of a selected CD8+ T cell appear to yield a more activated, proliferative, and differentiation-prone daughter cell alongside a less activated and more quiescent sibling cell (Chang et al., 2007; Lin et al., 2015, 2016; Pollizzi et al., 2016; Verbist et al., 2016). After 3 or 4 divisions, the more activated progenitor cell appears to give rise to an irreversibly determined effector cell with de novo silencing of TCF1 expression, alongside a self-renewing progenitor cell, which is more anabolic and activated than a quiescent memory cell, but maintains TCF1 expression and the bipotency to yield daughter cells with discordant silencing of TCF1 (Adams et al., 2016; Lin et al., 2015, 2016; Nish et al., 2017b).
Dividing B cell progenitors likewise appears to give rise to an irreversibly determined plasmablast with de novo silencing of Pax5 expression, alongside a self-renewing progenitor cell that retains the bipotency to yield daughter cells with discordant silencing of Pax5 (Adams et al., 2016; Lin et al., 2015). A hallmark of the cell divisions giving rise to sibling cells with discordant silencing of Pax5 and TCF1 is the discordant transmission of phosphatidylinositol 3-kinase (PI3K) signaling, as indexed by inactivation of FoxO1 through its nuclear displacement (Lin et al., 2015). Herein, examination of the subcellular localization of antigen receptor-associated class I PI3K activity revealed its asymmetric positioning prior to cytokinesis. A PI3K-mediated polarity mechanism, operative before and during cell division, is apparently responsible for unequal PI3K-driven nutrient uptake and differentiation between sibling cells.
RESULTS
Asymmetric Division of Functional PI3K Activity in CD8+ T Cells
PI3K activation of lymphocytes results from antigen and costimulatory receptor signaling (Frauwirth et al., 2002; Fruman et al., 2017; Garçon et al., 2008; Keppler et al., 2015; Lucas et al., 2016). Additionally, endocytosis of antigen receptors has emerged as a critical mechanism for maximal activation of T and B lymphocytes, including adequate anabolic induction and proliferation (Chaturvedi et al., 2011; Willinger et al., 2015; Yudushkin and Vale, 2010). We, therefore, used confocal microscopy to image P14 CD8+ T cells that were activated in vitro for 3 to 4 days with gp33 peptide and antigen-presenting cells (APCs). At this point, activated T cells had undergone several cell divisions and begun to repress TCF1 (Figure S1A). In contrast to unstimulated CD8+ T cells, which contained symmetrical, concentric, plasma membrane-localized CD3, activated T cells exhibited CD3 staining that was polarized to the microtubule organizing center (MTOC) and more internalized than in resting cells (Figure 1A). Using a monoclonal antibody that detects phosphatidylinositol (3,4,5)-trisphosphat (PIP3), a lipid signaling intermediate generated by receptor-associated class I PI3K enzymatic activity, we found that class I PI3K activity (PIP3) co-localizes with CD3 at the MTOC of dividing cells and that it remains polarized at one end of the mitotic spindle in metaphase cells (Figure 1B).
Figure 1. Asymmetric Division of Functional PI3K Activity in CD8+ T Cells.
(A) Confocal immunofluorescence (IF) microscopy of CD3 localization in unstimulated (N = 16) or gp33 peptide-stimulated interphase P14 CD8+ T cells activated for 3 days (N = 23).
(B) Top: CD3 and PIP3 localization in interphase and metaphase P14 blasts at day 3 post-activation. Bottom left: quantification of CD3 and PIP3 asymmetry in metaphase cells. ****p < 0.001. Chi-square test with Bonferroni correction. Bottom right: among PIP3 asymmetric cells, proportion with CD3 asymmetric concordant (same MTOC), CD3 asymmetric discordant (opposite MTOC), or CD3 symmetric localization.
(C) Left: Glut1 localization in interphase and metaphase P14 blasts. Right: quantification of Glut1 asymmetry in metaphase cells. ****p < 0.001. Fisher’s exact test.
(D) CD8+ T cells expressing myc-tagged Glut1 were stimulated with anti-CD3/CD28 + IL-2 for 3.5 days. Left: surface Glut1 versus cell division, gated for high and low populations. Middle: TCF1 expression versus cell division within surface Glut1-high and -low populations. Right: quantification of TCF1-low population frequency. *p < 0.05. Paired t test.
For all panels, dashed blue line denotes an asymmetry cutoff value of 0.2, and scale bars are 5 μm.
See also Figures S1 and S2.
To determine whether the asymmetry of PI3K activity might have functional consequences, we examined the localization of Glut1, the major inducible glucose transporter of lymphocytes (Caro-Maldonado et al., 2014; Macintyre et al., 2014). Glut1 is up-regulated upon T cell activation, and PI3K activity is essential for trafficking Glut1 from recycling endosomes to the plasma membrane (Jacobs et al., 2008; Wieman et al., 2007). In activated interphase cells, Glut1 was primarily found in discrete intracellular puncta, largely polarized to the MTOC, with some transporter also detectable at the plasma membrane (Figure 1C). In metaphase, Glut1 remained asymmetric at one end of the mitotic spindle (Figure 1C). Consistent with the role of aerobic glycolysis in effector determination (Adams et al., 2016; Caro-Maldonado et al., 2014; and references therein), cells expressing higher levels of surface Glut1 were also enriched in TCF1-silenced effector cells (Figure 1D). Asymmetric polarization of PI3K activity and glucose transporter abundance at metaphase could therefore represent a means to bias cell metabolism and cell fate between incipient sibling cells during and after cytokinesis.
Asymmetric Division of Functional PI3K Activity in B Cells
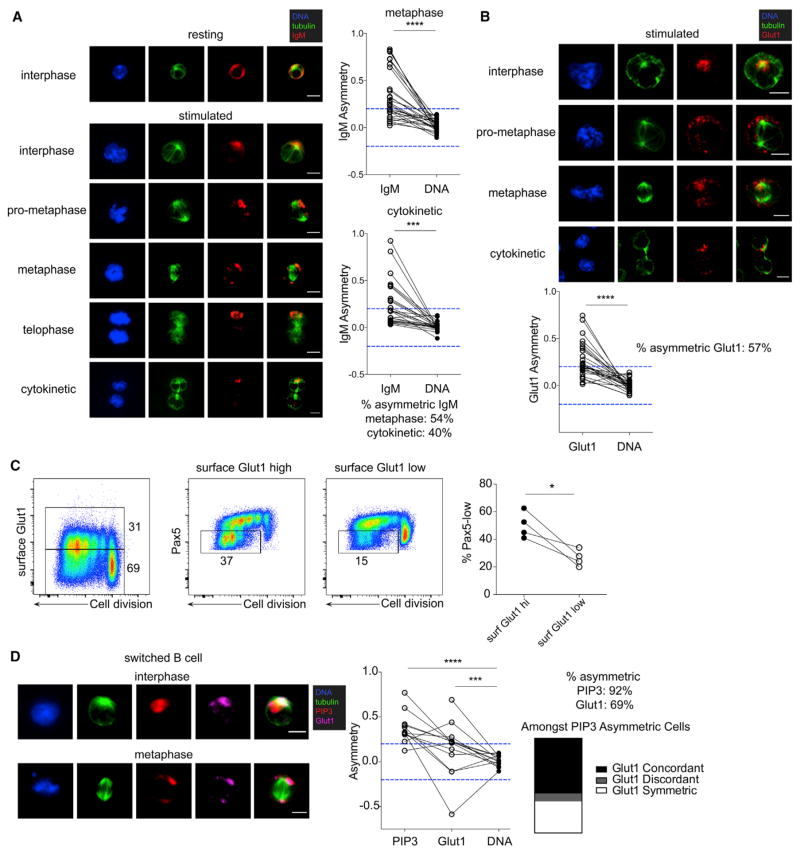
Modeled differentiation of B cells using lipopolysaccharide (LPS) stimulation in vitro recapitulates in vivo responses to immunization, including PI3K-dependent, intra-divisional bifurcation of self-renewing Pax5-expressing B cells and differentiated Pax5-silenced plasmablasts (Figure S1B) (Adams et al., 2016; Lin et al., 2015). LPS-stimulated B cells transmit their activating signals, including PI3K, through immunoglobulin M (IgM) and CD19 (Keppler et al., 2015; Schweighoffer et al., 2017). Compared to resting B cells, which contained concentric, plasma membrane-localized IgM, dividing B cells activated with LPS for 3 days contained predominantly intracellular IgM, which was polarized to the MTOC (Figure 2A). Asymmetry of internalized IgM was maintained in dividing B cells throughout the cell cycle.
Figure 2. Asymmetric Division of Functional PI3K Activity in B Cells.
(A) Confocal IF microscopy analysis of IgM localization in LPS-stimulated B cells at day 3 post-activation. Left: IgM localization in unstimulated (resting) interphase (N = 16) or LPS-stimulated interphase and mitotic B cells (N = 19). Right: quantification of IgM asymmetry in metaphase and cytokinetic cells. ****p < 0.0001; ***p < 0.001. Fisher’s exact test.
(B) Left: Glut1 localization in LPS-stimulated mitotic B cells at indicated stages of the cell cycle. Right: quantification of Glut1 asymmetry in metaphase cells. ****p < 0.0001. Fisher’s exact test.
(C) B cells expressing myc-tagged Glut1 stimulated with LPS for 3.5 days. Left: surface Glut1 versus cell division, gated for high and low populations. Middle: Pax5 expression versus cell division within the surface Glut1-high and -low populations. Right: quantification of Pax5-low population frequency. *p < 0.05. Paired t test.
(D) Class-switched (IgM negative), resting memory B cells stimulated with LPS and analyzed by IF microscopy on day 3 post-activation. Left: subcellular localization of PIP3 and Glut1 in interphase and metaphase blasts. Middle: quantification of PIP3 and Glut1 asymmetry in metaphase cells. ****p < 0.0001; ***p < 0.001. Chi-square test with Bonferroni correction. Right: among cells with asymmetric PIP3, proportion of asymmetric concordant, asymmetric discordant, and symmetric Glut1 localization.
See also Figures S1 and S2.
We found that LPS-stimulated B cells, like antigen-activated T cells (Figure 1C), exhibited asymmetric localization of Glut1 throughout the cell cycle (Figure 2B), and that cells expressing higher levels of surface Glut1 were enriched in Pax5-silenced plasmablasts (Figure 2C). The reagent that detects PIP3, a mouse IgM monoclonal antibody, was used to stain class-switched, IgM-negative memory B cells that had been activated with LPS, a condition that recapitulates bifurcation into Pax5-expressing and Pax5-silenced cells after 3 to 4 divisions (Figure S1B). Co-staining of Glut1 and PIP3 in activated, class-switched memory B cells revealed concordant asymmetry of PI3K activity and Glut1. Sites of PI3K-transmitting receptor polarization (CD19) were often denser in membrane staining (Figure S2A), prompting us to consider whether receptor polarity would be a universal finding under these conditions. Examination of CD98, a receptor implicated in asymmetric mammalian target of rapamycin (mTOR) induction in T cells (Pollizzi et al., 2016; Verbist et al., 2016), revealed that asymmetric localization of CD3 was not accompanied by coordinate asymmetry of CD98 (Figure S2B). Co-localization of signaling receptors, PI3K activity, and glucose transporter trafficking might thus be a conserved mechanism to generate clonal diversification of lymphocytes.
Asymmetry of PI3K Signaling Governed by a PI3K-Mediated Polarity Pathway
In addition to roles in signaling to metabolic adaptation, gene expression, and membrane trafficking, PI3K pathways play a conserved role in several forms of cellular symmetry breaking, often in conjunction with actin and microtubule cytoskeletal rearrangements (Berzat and Hall, 2010; Engelman et al., 2006; Marat and Haucke, 2016; Nish et al., 2017a; and references therein). To test whether PI3K-dependent polarity pathways control asymmetry of antigen receptors and Glut1, we performed transient pharmacological inhibition experiments. For interphase cells, a decentralization score was calculated based on the proximity of the molecule of interest to the MTOC (see Experimental Procedures). Compared to cells activated in the absence of an inhibitor, CD3 became substantially decentralized away from the MTOC in interphase cells as well as becoming more symmetrical in metaphase cells when the PI3K inhibitor LY294002 was present (Figures 3A and 3B). Consistent with observations that lipid-based (including PI3K-mediated) polarity pathways typically interact with regulated cytoskeletal re-arrangements, we found that inhibition of actin-based myosin II motors with Blebbistatin resulted in decentralization of both CD3 and PIP3 staining away from the MTOC (Figures 3A and 3C).
Figure 3. PI3K-Dependent Polarity Control of Asymmetric PI3K Signaling.
(A) P14 CD8+ T cells activated as in Figure 1 and treated with indicated drugs to perturb polarity. Left: CD3 localization in cells treated with no drug, LY294002, or myosin II inhibitor (Blebbistatin). Lower right: CD3 decentralization score. **p < 0.01; *p < 0.05. Kruskal-Wallis non-parametric test.
(B) CD3 localization in metaphase cells treated with no drug or LY294002. Lower panel: quantification of CD3 asymmetry. **p < 0.01. Fisher’s exact test.
(C) PIP3 localization in interphase CD8+ T cells treated with no drug or Blebbistatin. Lower panel: PIP3 decentralization score. ***p < 0.001. Mann-Whitney U test.
(D) Class I PI3K-driven TCF1 repression and facultative glucose transporter function. Upper row: cell division and TCF1 expression at day 4 in the absence or presence of idelalisib (PI3Kδ inhibitor), pictilisib (pan-class I PI3K inhibitor), or LY294002 (pan-PI3K inhibitor). Lower left: quantification of surface Glut1 in P14 CD8+ T cells 3 days post-activation in the absence or presence of indicated inhibitors. Lower right: uptake of fluorescent glucose analog 2-NBDG in the absence or presence of the indicated inhibitors. *p < 0.05; **p < 0.01; ****p < 0.0001. One-way ANOVA.
(E) Left: CD3 localization in interphase CD8+ T cells activated in the absence or presence of idelalisib or pictilisib. Middle: CD3 decentralization score. **p < 0.01; ***p < 0.001. Kruskal-Wallis non-parametric test. Far right: quantification of CD3 asymmetry in metaphase CD8+ T cells activated in the absence or presence of idelalisib. p = 0.05. Mann-Whitney U test.
(F and G) Representative IF images and quantification of IgM asymmetry in LPS-activated B cells in the absence or presence of LY294002 at indicated stages of cell division. *p < 0.05 by Fisher’s exact test.
(H) Representative images of Glut1 localization in interphase LPS-stimulated B cells in the absence or presence of LY294002. Decentralization score of indicated groups quantified beneath microscopy. **p < 0.01. Mann-Whitney U test.
All error bars within this figure represent mean ± SEM. See also Figures S3 and S4.
In view of the importance of class I PI3K molecules, particularly PI3Kδ, in antigen receptor signaling (Fruman et al., 2017; Garçon and Okkenhaug, 2016; Garçon et al., 2008; Lucas et al., 2016), we also tested the effect of idelalisib, a PI3Kδ-specific inhibitor, and pictilisib, a pan-class I PI3K inhibitor on T cell fate, nutrient utilization, and polarity. Like LY294002, the more specific inhibition resulted in defective silencing of TCF1, reduced cell surface trafficking of Glut1, diminished glucose uptake (Figure 3D), and defective CD3 polarity, the latter appearing more severe in interphase cells than metaphase cells (Figure 3E). Pictilisib also resulted in loss of PIP3 staining, as assessed by microscopy (Figures S3A) and flow cytometry (Figure S3B), which, along with the dissimilar pattern of PIP2 staining (Figure S3C), underscores the specificity of the observed polarity in class I PI3K activity. In activated dividing B cells, addition of the inhibitor LY294002 also disrupted polarity and asymmetry of internalized IgM, Glut1, and CD19 (Figures 3F, 3G, 3H, and S4). A PI3K-associated polarity mechanism may thus be responsible for organizing asymmetric distribution of receptors that transduce PI3K signaling to nutrient utilization, anabolism, gene expression, and membrane trafficking.
Asymmetric PI3K Signaling and Glut1 Inheritance In Vivo
To determine whether subcellular compartmentalization and asymmetry of PI3K signaling is a physiological occurrence in vivo, we examined CD8+ T cells from mice infected with Listeria monocytogenes (L. monocytogenes). TCR transgenic P14 CD8+ T cells expressing a Tcf7GFP/+ reporter (Choi et al., 2015) were transferred intravenously to naive recipient mice, followed by infection of recipients with L. monocytogenes expressing the LCMV peptide gp33 (LM-gp33). Spleens were harvested at day 4 to 5 post-infection, when the majority of activated P14 cells had undergone 4 or more rounds of cell division (Lin et al., 2016). Tcf7-GFPhi cells were sorted for analysis by confocal immunofluorescence microscopy. Similar to cells activated in vitro (Figures 1 and 3), we found that CD3, PIP3, and Glut1 were concordantly co-localized at the MTOC and at one end of the spindle pole during interphase and metaphase, respectively (Figure 4). These results support a model wherein CD8+ T cells undergoing asymmetric fate determination during later cell divisions in vivo possess coordinated asymmetry of endocytosed antigen receptors, class I PI3K activity, and facultative glucose transporter trafficking.
Figure 4. Asymmetric PI3K Activity Organizing Unequal Inheritance of Antigen Receptor and Glucose Transporter In Vivo.
(A) Polarization and asymmetric inheritance of CD3 and PIP3 in interphase and metaphase P14 CD8+ T cells in vivo during L. monocytogenes infection. FACS-sorted TCF7-GFP reporter P14 CD8+ T cells at day 4 to 5 post-infection analyzed by IF microscopy. Left: PIP3 and CD3 localization in interphase and metaphase blasts. Right: quantification of PIP3 and CD3 asymmetry in metaphase cells. ****p < 0.0001. Chi-square test with Bonferroni correction.
(B) Polarization and asymmetric inheritance of Glut1 in vivo. Left: Glut1 localization in interphase and metaphase P14 CD8+ T cells processed as in (A). Right: quantification of Glut1 asymmetry in metaphase cells. ****p < 0.0001. Fisher’s exact test.
(C) Concordant polarity of PIP3 and Glut1 in vivo. Left: PIP3 and Glut1 localization in metaphase P14 CD8+ T cells. Middle: quantification of PIP3 and Glut1 asymmetry in metaphase cells. ****p < 0.0001. Chi-square test with Bonferroni correction. Right: proportion of PIP3 asymmetric cells with Glut1 asymmetric concordant (same MTOC), Glut1 asymmetric discordant (opposite MTOC), or Glut1 symmetric localization.
DISCUSSION
Genetic studies yielding incomplete phenotypes have led to uncertainty about the role of the evolutionarily conserved PAR polarity network in lymphocyte asymmetric division (reviewed by Nish et al., 2017a). In dividing lymphocytes, class I PI3K activity, triggered by antigen and costimulatory signals and indexed by PIP3 production, appears to be asymmetrically positioned alongside both the receptors that initiate class I PI3K activity and downstream protein targets, such as Glut1, that are trafficked by class I PI3K activity. The asymmetric localization of receptors that trigger PI3K activity appears to be at least partly dependent on class IA PI3Kδ activity, which also couples glucose uptake with silencing of TCF1, the hallmark of irreversible effector cell differentiation (Lin et al., 2015, 2016; Nish et al., 2017b). Although PI3K activity may be dispensable for glucose uptake in differentiated killer T cells (Macintyre et al., 2011), the present results are consistent with prior evidence that PI3K signaling is required for glucose uptake and Glut1 trafficking in newly differentiating T cells (Frauwirth et al., 2002; Jacobs et al., 2008). The convergence of PI3K-dependent metabolic, transcriptional, and cell-polarity functions during cell division appears to form a mechanism that ensures self-renewal accompanies differentiation of lymphocytes.
Spatially segregated class I PI3K activity and accompanying cytoskeletal rearrangement is an evolutionarily conserved response to guidance cues for directed migration, axon outgrowth of neurons, immunological synapsis, apico-basal polarity, and asymmetric epithelial progenitor division (Berzat and Hall, 2010; Dainichi et al., 2016; Engelman et al., 2006; Garçon and Okkenhaug, 2016; Garçon et al., 2008; Le Floc’h et al., 2013, 2015; Marat and Haucke, 2016; Nish et al., 2017a). Following T lymphocyte activation, focal PI3K activity and actin remodeling co-localize at the immune synapse (Garçon and Okkenhaug, 2016; Garçon et al., 2008; Le Floc’h et al., 2013; Singleton et al., 2009). Receptor-induced actin remodeling, PI3K activity, and their associated GTPases and exchange factors can act in a self-reinforcing feedback loop to create a domain of cytoskeletal-driven polarity (Berzat and Hall, 2010; Le Floc’h and Huse, 2015). Although some features of immune synapsis may be PI3K-independent (Garçon et al., 2008), focal actin remodeling at the synapse is both PI3Kδ-dependent (Garçon and Okkenhaug, 2016; Le Floc’h et al., 2013) and critical in establishing polarity of synaptic antigen receptors (reviewed by Le Floc’h and Huse, 2015).
Insofar as endocytosed antigen receptors propagate anabolic activation of lymphocytes (Chaturvedi et al., 2011; Willinger et al., 2015; Yudushkin and Vale, 2010), membrane-trafficking events mediated by class II and III PI3K signaling could potentially cooperate with class-I-initiated polarity to maintain asymmetry through cell division. Consistent with this hypothesis, inhibition of class IA PI3Kδ perturbed CD3 polarity more severely in cells in interphase than in metaphase (Figure 3E). Some asymmetric divisions in flies result from polarized endocytic trafficking of receptor components independently of the PAR polarity network (Emery et al., 2005). Whether other PI3K isoforms and classes, mTOR activity, and PAR polarity proteins play complementary or downstream roles to class I PI3K in asymmetric lymphocyte division will require further investigation (Engelman et al., 2006; Goldstein and Macara, 2007; Marat and Haucke, 2016; Pollizzi et al., 2016; Verbist et al., 2016).
EXPERIMENTAL PROCEDURES
Mice
All animal work was conducted in accordance with the Institutional Animal Care and Use Guidelines of Columbia University in specific pathogen-free conditions. Male and female mice were used, all between 6 and 8 weeks of age. Strains used include C57BL/6 (wild-type), P14 TCR transgenic (P14) recognizing LCMV peptide gp33-41/Db, TCF7GFP/+ reporter (Choi et al., 2015), and homozygous myc-Glut1 mice containing an exofacial (surface) myc tag in the gene encoding the Glut1 transporter (Macintyre et al., 2014).
Lymphocyte Activation
Wild-type B cells, myc-tagged Glut1 B cells, or myc-tagged Glut1 CD8+ T cells were enriched from splenocytes using magnetic bead negative selection kits (Miltenyi Biotec). B cells were stimulated with 20 μg/mL LPS, and polyclonal CD8+ T cells were stimulated with plate-bound anti-CD3/CD28 (1 μg/mL) supplemented with 100 IU/mL interleukin-2 (IL-2) (Adams et al., 2016). For P14 CD8+ T cells, bulk splenocytes were activated with gp33 peptide (1 μg/mL) as previously described (Lin et al., 2016). For switched memory B cells, CD23+, IgD−, IgM−, and lineage-negative (CD4, CD8, and Gr1) cells were sorted from bulk splenocytes and then stimulated with LPS (20 μg/mL). Lymphocytes were labeled with CellTrace Violet (CTV) prior to stimulation. Where indicated, cells were treated with the following drugs: idelalisib (GS-1101, Selleckchem, 1 μM), pictilisib (GDC-0941, Selleckchem, 1 μM), LY294002 (Cell Signaling Technology, 2.5–5 μM), and Blebbistatin (Abcam, 20 μM).
Infectious Challenge
For in vivo experiments, 1 ×106 to 3 ×106 Tcf7-GFP reporter P14 CD8+ T cells were adoptively transferred intravenously (i.v.) into allotype-disparate C57BL/6 wild-type mice, which were subsequently infected with 5 × 103 colony-forming units (CFUs) of L. monocytogenes expressing gp33 (LM-gp33) via i.v. injection. Spleens were harvested at day 4 to 5 of infection, and CD8+ T cells were then MACS-enriched for cell sorting of transferred, Tcf7-GFP+ P14 cells.
Flow Cytometry
Single-cell suspensions were prepared and stained as previously described (Lin et al., 2016). Antibodies used include rabbit anti-myc tag (71D10, Cell Signaling Technology), mouse anti-myc tag (4A6, Millipore), TCF1 (C63D9, Cell Signaling Technology), Pax5 (1H9, eBioscience), IgM (Il/41, eBioscience), IgD (11-26c.2a, BioLegend), CD23 (B3B4, eBioscience), CD4 (RM405, BioLegend), CD8α (53-6.7, eBioscience), and Gr1 (RB6-8C5, BD PharMingen). Glucose uptake was determined by staining with the fluorescent glucose analog 2-NBDG (Cayman, 100 μM) in glucose-free RPMI.
Confocal Microscopy
Immunofluorescence microscopy was performed as described (Lin et al., 2015). Antibodies included α-tubulin (YOL1/34, Abcam), β-tubulin (AA2, Sigma), CD19 (1D3, BD Pharmingen), CD98 (RL388, BioLegend), IgM (II/41, eBioscience), PIP3 (RC6F8, Thermo Fisher Scientific), PIP2 (2C11, Abcam), Glut1 (EPR3915, Abcam), CD3ε (ebio500A2, eBioscience), anti-mouse-IgM (Thermo Fisher Scientific), and Alexa-Fluor-conjugated secondary antibodies (Thermo Fisher Scientific). Metaphase cells were identified by the presence of two MTOCs with opposed spindle poles. Cytokinetic cells were identified by the presence of a tubulin-containing bridge. To quantify polarity in inter-phase cells, a decentralization score was calculated based on the proximity of fluorescent puncta to the MTOC. Cells were divided into 6 equal sectors, and each sector that contained at least 1 fluorescent punctum was counted toward the decentralization score. The sector containing the MTOC is worth 0 points; the sector directly opposite the MTOC is 3 points; the two sectors adjacent to the MTOC sector are 1 point each; the two sectors adjacent to the sector opposite the MTOC are 2 points each. Cells with fluorescent puncta entirely localized to the MTOC received a score of 0, whereas cells with decentralized puncta had higher scores. For quantification of asymmetry, fluorescent signal was thresholded to remove background, and integrated fluorescence density in each nascent daughter cell or half the metaphase cell was calculated. Asymmetry was calculated as (fluorescence daughter 1 + fluorescence daughter 2)/(fluorescence daughter 1 + fluorescence daughter 2), with values over 0.2 considered asymmetric.
Statistical Analyses
Statistical significance between experimental groups was assessed using GraphPad Prism software (version 7). Unless indicated, data were reported as mean ± SEM. All t tests were two-tailed. Parametric and non-parametric tests were used, as specified in each figure legend. Significance cutoffs and post-test corrections are stated in each figure legend. Not significant (n.s.); *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
Supplementary Material
Highlights.
Class I PI3K activity is asymmetrically polarized in activated, mitotic lymphocytes.
Receptors upstream of PI3K activity polarize via a PI3K-dependent polarity mechanism.
Focal PI3K activity correlates with unequal traffic of inducible glucose transporters.
PI3K-driven differentiation and asymmetry merge to enable divergent sister cell fate.
Acknowledgments
We are grateful to anonymous reviewers for suggestions regarding PI3K perturbation. This study was supported by NIH grants AI113365, AI076458 (S.L.R.), and T32 GM007367 and the Charles H. Revson Foundation.
Footnotes
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2017.12.087.
AUTHOR CONTRIBUTIONS
Y.-H.C., R.K., W.-H.W.L. and S.L.R. conceived and designed the study and wrote the manuscript. Y.-H.C., R.K., W.-H.W.L., N.J.R., B.Y., W.C.A., and S.A.N. performed experiments. J.C.R. provided reagents and expertise.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Adams WC, Chen YH, Kratchmarov R, Yen B, Nish SA, Lin WW, Rothman NJ, Luchsinger LL, Klein U, Busslinger M, et al. Anabolism-associated mitochondrial stasis driving lymphocyte differentiation over self-renewal. Cell Rep. 2016;17:3142–3152. doi: 10.1016/j.celrep.2016.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzat A, Hall A. Cellular responses to extracellular guidance cues. EMBO J. 2010;29:2734–2745. doi: 10.1038/emboj.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol. 2014;192:3626–3636. doi: 10.4049/jimmunol.1302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A, Martz R, Dorward D, Waisberg M, Pierce SK. Endocytosed BCRs sequentially regulate MAPK and Akt signaling pathways from intracellular compartments. Nat Immunol. 2011;12:1119–1126. doi: 10.1038/ni.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, Love PE, Peng W, Xue HH, Crotty S. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol. 2015;16:980–990. doi: 10.1038/ni.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainichi T, Hayden MS, Park SG, Oh H, Seeley JJ, Grinberg-Bleyer Y, Beck KM, Miyachi Y, Kabashima K, Hashimoto T, et al. PDK1 is a regulator of epidermal differentiation that activates and organizes asymmetric cell division. Cell Rep. 2016;15:1615–1623. doi: 10.1016/j.celrep.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garçon F, Okkenhaug K. PI3Kδ promotes CD4(+) T-cell interactions with antigen-presenting cells by increasing LFA-1 binding to ICAM-1. Immunol Cell Biol. 2016;94:486–495. doi: 10.1038/icb.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garçon F, Patton DT, Emery JL, Hirsch E, Rottapel R, Sasaki T, Okkenhaug K. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler SJ, Gasparrini F, Burbage M, Aggarwal S, Frederico B, Geha RS, Way M, Bruckbauer A, Batista FD. Wiskott-Aldrich syndrome interacting protein deficiency uncovers the role of the co-receptor CD19 as a generic hub for PI3 kinase signaling in B cells. Immunity. 2015;43:660–673. doi: 10.1016/j.immuni.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc’h A, Huse M. Molecular mechanisms and functional implications of polarized actin remodeling at the T cell immunological synapse. Cell Mol Life Sci. 2015;72:537–556. doi: 10.1007/s00018-014-1760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc’h A, Tanaka Y, Bantilan NS, Voisinne G, Altan-Bonnet G, Fukui Y, Huse M. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J Exp Med. 2013;210:2721–2737. doi: 10.1084/jem.20131324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WH, Adams WC, Nish SA, Chen YH, Yen B, Rothman NJ, Kratchmarov R, Okada T, Klein U, Reiner SL. Asymmetric PI3K signaling driving developmental and regenerative cell fate bifurcation. Cell Rep. 2015;13:2203–2218. doi: 10.1016/j.celrep.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, Nish SA, Yen B, Chen YH, Adams WC, Kratchmarov R, Rothman NJ, Bhandoola A, Xue HH, Reiner SL. CD8+ T lymphocyte self-renewal during effector cell determination. Cell Rep. 2016;17:1773–1782. doi: 10.1016/j.celrep.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CL, Chandra A, Nejentsev S, Condliffe AM, Okkenhaug K. PI3Kδ and primary immunodeficiencies. Nat Rev Immunol. 2016;16:702–714. doi: 10.1038/nri.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, Feijoo C, Okkenhaug K, Cantrell DA. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marat AL, Haucke V. Phosphatidylinositol 3-phosphates-at the interface between cell signalling and membrane traffic. EMBO J. 2016;35:561–579. doi: 10.15252/embj.201593564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nish SA, Lin WW, Reiner SL. Lymphocyte fate and metabolism: a clonal balancing act. Trends Cell Biol. 2017a;27:946–954. doi: 10.1016/j.tcb.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nish SA, Zens KD, Kratchmarov R, Lin WW, Adams WC, Chen YH, Yen B, Rothman NJ, Bhandoola A, Xue HH, et al. CD4+ T cell effector commitment coupled to self-renewal by asymmetric cell divisions. J Exp Med. 2017b;214:39–47. doi: 10.1084/jem.20161046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Sun IH, Patel CH, Lo YC, Oh MH, Waickman AT, Tam AJ, Blosser RL, Wen J, Delgoffe GM, et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol. 2016;17:704–711. doi: 10.1038/ni.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighoffer E, Nys J, Vanes L, Smithers N, Tybulewicz VLJ. TLR4 signals in B lymphocytes are transduced via the B cell antigen receptor and SYK. J Exp Med. 2017;214:1269–1280. doi: 10.1084/jem.20161117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KL, Roybal KT, Sun Y, Fu G, Gascoigne NR, van Oers NS, Wülfing C. Spatiotemporal patterning during T cell activation is highly diverse. Sci Signal. 2009;2:ra15. doi: 10.1126/scisignal.2000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist KC, Guy CS, Milasta S, Liedmann S, Kamiński MM, Wang R, Green DR. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532:389–393. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Staron M, Ferguson SM, De Camilli P, Flavell RA. Dynamin 2-dependent endocytosis sustains T-cell receptor signaling and drives metabolic reprogramming in T lymphocytes. Proc Natl Acad Sci USA. 2015;112:4423–4428. doi: 10.1073/pnas.1504279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudushkin IA, Vale RD. Imaging T-cell receptor activation reveals accumulation of tyrosine-phosphorylated CD3ζ in the endosomal compartment. Proc Natl Acad Sci USA. 2010;107:22128–22133. doi: 10.1073/pnas.1016388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.