ABSTRACT
A 9-valent HPV (9vHPV) vaccine has been developed to protect against HPV type 6/11/16/18/31/33/45/52/58-related infection and disease. Previous safety analyses from 7 clinical trials conducted in 9vHPV vaccine recipients 9–26 years of age, including comparisons of 9vHPV and quadrivalent HPV (qHPV) vaccines in girls and women 16–26 years of age, showed that the 9vHPV vaccine was generally well tolerated. Additional safety analyses were conducted to include the results of new clinical studies. The safety profile of the 9vHPV vaccine in prior qHPV vaccine recipients (n = 3756 from 1 randomized controlled trial and 2 open-label extension studies) and young men (n = 248 9vHPV and n = 248 qHPV vaccine recipients from 1 randomized controlled trial) was evaluated. Vaccine was administered as a 3-dose regimen (at Day 1 and Months 2 and 6), and adverse events (AEs) were monitored. The most common AEs were injection-site events (91.1% and 79.0% in prior qHPV vaccine recipients and young men, respectively), the majority of which were mild. Discontinuations due to an AE were rare (0.2% and 0.0% among prior qHPV vaccine recipients and young men, respectively). In young men, the AE profile of the 9vHPV vaccine was generally similar to that of the qHPV vaccine. Overall, the 9vHPV vaccine was generally well tolerated in prior qHPV vaccine recipients and in young men, with an AE profile generally consistent with that previously reported with the broader clinical program.
KEYWORDS: 9vHPV, cervical canJEL CODE
Introduction
A 9-valent human papillomavirus (9vHPV) vaccine (Gardasil 9™, Merck & Co., Inc., Kenilworth, NJ, USA) has been developed to protect against the 4 HPV types covered by the quadrivalent (qHPV) vaccine, HPV6/11/16/18, as well as the 5 oncogenic types next most commonly associated with cervical cancer (HPV31/33/45/52/58).1,2 The 9vHPV vaccine has the potential to prevent approximately 90% of cervical cancers; HPV-related vulvar, vaginal, and anal cancers; and cases of anogenital warts, as well as 70%–85% of cervical precancerous lesions.3–6 The 9vHPV vaccine was licensed in the United States in 2014; Canada, the European Union, and Australia in 2015; and several other countries between 2015 and 2017.
In a recent combined analysis of safety from 7 Phase 3 studies, the 9vHPV vaccine was generally well tolerated in males and females 9–26 years of age.7 The adverse event (AE) profiles were generally similar for the 9vHPV vaccine and qHPV vaccine in girls and women, although injection-site AEs were more common with the 9vHPV versus qHPV vaccine.7 Safety results from additional Phase 3 studies have now become available, including 2 extension studies offering 9vHPV vaccine to prior qHPV vaccine recipients from the control arms of their respective core studies, and 1 study comparing 9vHPV and qHPV vaccines in men. To supplement the safety analyses previously reported,7 we describe safety analyses from these additional studies on 9vHPV vaccine safety in prior qHPV vaccine recipients and in male participants.
Methods
Study design
Three studies contributed to the safety assessment in prior qHPV vaccine recipients.
-
·
Study 006 (Merck Protocol V503-006; NCT01047345) was a randomized, double-blinded, placebo-controlled study assessing the safety and immunogenicity of 9vHPV vaccine in girls and women 12–26 years of age (N=924) who had previously received qHPV vaccine.8 To be eligible for the study, participants had to be prior recipients of a 3-dose regimen of qHPV vaccine. Participants were randomized in a 2:1 ratio to receive 3 doses of 9vHPV vaccine or saline placebo (on Day 1, Month 2, and Month 6 of the study) and followed for 7 months for safety and immunogenicity. Details of the study design and primary results have been reported previously.8
-
·
Study 006-02 (Merck Protocol V503-006-02; NCT01047345) was an optional open-label extension of Study 006, in which participants randomized to placebo in the base study were offered a 3-dose regimen of 9vHPV vaccine (on Day 1 and Months 2 and 6 of the extension) and followed for safety for 7 months. No serum samples were collected. Study 006-02 was conducted between May 8, 2014 and November 28, 2015 at 18 sites across 6 participating countries (Canada, Colombia, Hong Kong, Mexico, Sweden and the United States).
-
·
Study 001-04 (Merck Protocol V503-001-04; NCT00543543) was an optional open-label extension of Study 001 (Merck Protocol V503-001; NCT00543543), a randomized, double-blinded study of the safety, efficacy, and immunogenicity of the 9vHPV vaccine compared with qHPV vaccine in young women (age 16–26 years).9 Study 001-04 was conducted between July 21, 2014 and July 7, 2016. The study extension enrolled 2 cohorts. Cohort 1 enrolled 150 participants who received 3 doses of 9vHPV vaccine in the core study and the fourth dose in the study extension to assess immune memory; results from Cohort 1 have been summarized separately.10 Cohort 2 was a sub-study to offer 9vHPV vaccine to subjects in the control arm (qHPV vaccine) of the core study. Participants who were randomized to and received at least 1 dose of qHPV vaccine in the base study at sites participating in the extension (total of 95 sites across 18 countries [i.e., the same countries as in the core study9]) were offered a 3-dose regimen of the 9vHPV vaccine in the extension, administered at extension study Day 1, Month 2, and Month 6 and followed for safety for 7 months. No serum samples were collected.
One study provided safety assessment in young men.
-
·
Study 020 (Merck Protocol V503-020; NCT02114385) was a double-blind, randomized, qHPV vaccine-controlled, immunogenicity, and safety study of 9vHPV vaccine in young men 16–26 years of age (N = 500) in 3 European countries.11 Participants were equally randomized to receive 3 doses of 9vHPV or qHPV vaccine (on Day 1, Month 2, and Month 6 of the study) and followed for 7 months for safety and immunogenicity. Details of the study design and primary results have been published previously.11
Safety assessment
In Study 006 and Study 020, vaccination-report-card–aided surveillance was utilized to collect information about injection-site and systemic AEs, as previously described.7 In all studies, serious AEs (SAEs) were collected (regardless of causality) throughout the study at each of the 3 vaccination visits and 1 month after the last vaccine dose. Female subjects underwent pregnancy testing before each vaccination, as described previously7; those who were pregnant at the first vaccination visit were not vaccinated, and those who became pregnant after receiving 1 or more doses of vaccine were discontinued from further vaccination until after resolution of the pregnancy. Pregnancies detected during the study were followed to outcome.
Data analysis
All participants who received at least 1 vaccination and had follow-up data are included in the analyses. AEs were summarized using frequencies and percentages according to study group and the type of AE reported. AE rates were compared between prior qHPV vaccine recipients in Study 006 and individuals without prior HPV vaccination; since all participants in Study 006 were prior qHPV vaccine recipients, the 9vHPV vaccine cohort of the pivotal efficacy study of the 9vHPV vaccine (Study 001; NCT005435439) was used as a comparator. For this comparison, the 95% CI for the difference in rates was estimated based on a 2-sample test for binomial proportions under normal-theory method. Since participants in Studies 006, 006-02, and 001-04 were all prior qHPV vaccine recipients, a combined analysis of the safety endpoints collected in the 3 studies was conducted. This combined analysis provides a cross-study summary of SAEs and AEs resulting in discontinuation and pregnancy outcomes.
Results
Study disposition
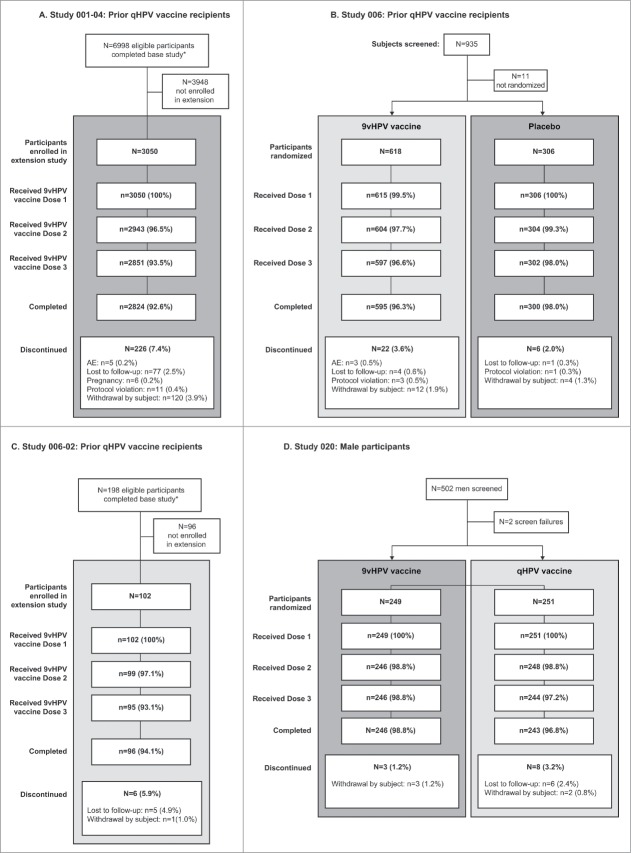
Participant disposition is shown in Fig. 1. Of the 3050, 618, and 102 participants enrolled in Studies 001-04, 006, and 006-02, respectively, to receive the 9vHPV vaccine, 2824 (92.6%), 595 (96.3%), and 96 (94.1%), respectively, completed the study. Of the 249 participants enrolled in Study 020 to receive the 9vHPV vaccine, 246 (98.8%) completed the study.
Figure 1.
Participant flow in prior qHPV vaccine recipients from Study 001-04 (A), Study 006 (B) and Study 006-02 (C) and among male participants from Study 020 (D) AE: adverse event.
*Includes eligible participants from those study sites that participated in the study extension. Participant disposition in Study 0068 and Study 02011 have been reported previously
Safety assessment in prior qHPV vaccine recipients
AEs (any grade) reported within 15 days following a vaccination visit in female prior qHPV vaccine recipients 12–26 years of age from Study 006 are summarized in Table 1. Injection-site AEs, which have been previously reported,8 are summarized in Table 1 for completeness. Participants receiving 9vHPV vaccine were more likely to experience injection-site events (91.1% overall) compared with placebo recipients (43.9% overall), most commonly mild-to-moderate pain, swelling, erythema, pruritus, and hematoma (Table 1). It is noteworthy that injection-site swelling and injection-site erythema were more common in prior qHPV vaccine recipients in Study 006 (49.0% and 42.3%, respectively; Table 2) than in individuals who did not receive prior HPV vaccination (in Study 001, frequencies of injection-site swelling and injection-site erythema were 40.0% and 34.0%, respectively).7,9 Even though most AEs of injection-site swelling and injection-site erythema were mild-to-moderate in intensity, the frequencies of injection-site swelling and injection-site erythema of severe intensity (i.e., maximum size >5 cm) were higher in prior qHPV vaccine recipients (7.6% and 3.3%, respectively, in Study 006) than in individuals without prior HPV vaccination (3.8% and 1.6%, as previously reported in Study 0017) (Table 2). The incidence of injection-site AEs increased across subsequent doses, similar to the findings in prior studies involving participants who were not previously vaccinated with qHPV vaccine.7 In prior qHPV vaccine recipients (Table 1), injection-site swelling and injection-site erythema increased from 27.1% or 20.1% after Dose 1 to 35.5% or 26.4% after Dose 3, respectively. In individuals without prior qHPV vaccination, injection-site swelling and injection-site erythema increased from 12.5% or 10.6% after Dose 1 to 28.3% or 22.6% after Dose 3, respectively, as previously reported.7
Table 1.
AEs reported from Day 1 to Day 15 following a vaccination visit in Study 006 in females 12–26 years of age who previously received qHPV vaccine.
| 9vHPV vaccine |
Saline placebo |
|||||||
|---|---|---|---|---|---|---|---|---|
| After Dose 1 | After Dose 2 | After Dose 3 | After any dose | After Dose 1 | After Dose 2 | After Dose 3 | After any dose | |
| Number of subjects with follow-up | 608 | 600 | 594 | 608 | 305 | 303 | 299 | 305 |
| Number (%) of subjects with the following AEs | ||||||||
| Injection-site event* | 486 (79.9) | 449 (74.8) | 445 (74.9) | 554 (91.1) | 89 (29.2) | 62 (20.5) | 58 (19.4) | 134 (43.9) |
| Pain | 473 (77.8) | 430 (71.7) | 429 (72.2) | 549 (90.3) | 74 (24.3) | 55 (18.2) | 48 (16.1) | 116 (38.0) |
| Mild | 368 (60.5) | 318 (53.0) | 294 (49.5) | 316 (52.0) | 71 (23.3) | 55 (18.2) | 40 (13.4) | 105 (34.4) |
| Moderate | 102 (16.8) | 102 (17.0) | 119 (20.0) | 209 (34.4) | 3 (1.0) | 0 (0.0) | 7 (2.3) | 10 (3.3) |
| Severe | 3 (0.5) | 9 (1.5) | 14 (2.4) | 24 (3.9) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (0.3) |
| Unknown | 0 (0.0) | 1 (0.2) | 2 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Swelling | 165 (27.1) | 164 (27.3) | 211 (35.5) | 298 (49.0) | 11 (3.6) | 8 (2.6) | 7 (2.3) | 18 (5.9) |
| Mild (0 to ≤2.5 cm) | 117 (19.2) | 116 (19.3) | 132 (22.2) | 182 (29.9) | 8 (2.6) | 4 (1.3) | 6 (2.0) | 13 (4.3) |
| Moderate (>2.5 cm to ≤5.0 cm) | 28 (4.6) | 32 (5.3) | 45 (7.6) | 65 (10.7) | 2 (0.7) | 2 (0.7) | 1 (0.3) | 3 (1.0) |
| Severe (>5.0 cm) | 19 (3.1) | 14 (2.3) | 26 (4.4) | 46 (7.6) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| Unknown | 1 (0.2) | 2 (0.3) | 8 (1.3) | 5 (0.8) | 0 (0.0) | 2 (0.7) | 0 (0.0) | 1 (0.3) |
| Erythema | 122 (20.1) | 135 (22.5) | 157 (26.4) | 257 (42.3) | 15 (4.9) | 8 (2.6) | 10 (3.3) | 26 (8.5) |
| Mild (0 to ≤2.5 cm) | 102 (16.8) | 108 (18.0) | 114 (19.2) | 190 (31.3) | 14 (4.6) | 6 (2.0) | 9 (3.0) | 22 (7.2) |
| Moderate (>2.5 cm to ≤5.0 cm) | 13 (2.1) | 14 (2.3) | 26 (4.4) | 39 (6.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe (>5.0 cm) | 5 (0.8) | 8 (1.3) | 11 (1.9) | 20 (3.3) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (0.3) |
| Unknown | 2 (0.3) | 5 (0.8) | 6 (1.0) | 8 (1.3) | 1 (0.3) | 2 (0.7) | 0 (0.0) | 3 (1.0) |
| Pruritus | 29 (4.8) | 14 (2.3) | 21 (3.5) | 47 (7.7) | 1 (0.3) | 1 (0.3) | 3 (1.0) | 4 (1.3) |
| Mild | 23 (3.8) | 10 (1.7) | 13 (2.2) | 31 (5.1) | 1 (0.3) | 1 (0.3) | 3 (1.0) | 4 (1.3) |
| Moderate | 6 (1.0) | 4 (0.7) | 6 (1.0) | 14 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 2 (0.3) | 2 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hematoma | 14 (2.3) | 9 (1.5) | 7 (1.2) | 29 (4.8) | 3 (1.0) | 0 (0.0) | 4 (1.3) | 7 (2.3) |
| Mild | 12 (2.0) | 8 (1.3) | 5 (0.8) | 24 (3.9) | 2 (0.7) | 0 (0.0) | 4 (1.3) | 6 (2.0) |
| Moderate | 2 (0.3) | 1 (0.2) | 2 (0.3) | 5 (0.8) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| Systemic event† | 249 (41.0) | 166 (27.7) | 150 (25.3) | 363 (59.7) | 121 (39.7) | 75 (24.8) | 58 (19.4) | 170 (55.7) |
| Vaccine-related‡systemic event | 107 (17.6) | 80 (13.3) | 62 (10.4) | 186 (30.6) | 53 (17.4) | 30 (9.9) | 22 (7.4) | 79 (25.9) |
| Headache | 64 (10.5) | 46 (7.7) | 35 (5.9) | 119 (19.6) | 40 (13.1) | 21 (6.9) | 8 (2.7) | 55 (18.0) |
| Pyrexia | 7 (1.2) | 13 (2.2) | 14 (2.4) | 31 (5.1) | 0 (0.0) | 0 (0.0) | 5 (1.7) | 5 (1.6) |
| Nausea | 15 (2.5) | 10 (1.7) | 4 (0.7) | 24 (3.9) | 4 (1.3) | 1 (0.3) | 1 (0.3) | 6 (2.0) |
| Dizziness | 6 (1.0) | 9 (1.5) | 5 (0.8) | 18 (3.0) | 1 (0.3) | 2 (0.7) | 3 (1.0) | 5 (1.6) |
| Fatigue | 9 (1.5) | 3 (0.5) | 2 (0.3) | 11 (1.8) | 4 (1.3) | 3 (1.0) | 1 (0.3) | 7 (2.3) |
| Number of subjects with temperature data | 600 | 585 | 587 | 604 | 304 | 294 | 292 | 304 |
| Number (%) of subjects with the followingmaximum temperatures* | ||||||||
| ≥37.8°C | 8 (1.3) | 17 (2.9) | 18 (3.1) | 39 (6.5) | 0 (0.0) | 1 (0.3) | 8 (2.7) | 9 (3.0) |
| ≥38.9°C | 0 (0.0) | 3 (0.5) | 5 (0.9) | 8 (1.3) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (0.3) |
9vHPV: 9-valent human papilloma virus; AE: adverse event; qHPV: quadrivalent human papilloma virus
Days 1–5 following a vaccination visit
Days 1–15 following a vaccination visit
As determined by the reporting investigator
Injection-site and systemic AEs shown are those with incidence ≥2% in any vaccination group during the study
Table 2.
Percentage of study participants with injection-site and systemic AEs after any 9vHPV vaccine dose among prior qHPV vaccine recipients (Study 006) and individuals with no prior HPV vaccination (Study 001).
| 9vHPV vaccine |
|||
|---|---|---|---|
| Prior qHPV vaccine recipients (N = 608) | Individuals with no prior HPV vaccination (N = 7071) | Difference % (95% CI)§ | |
| Number (%) of subjects with the following AEs | |||
| Injection-site event* | 554 (91.1) | 6414 (90.7) | 0.4 (−2.0, 2.8) |
| Pain | 549 (90.3) | 6356 (89.9) | 0.4 (−2.0, 2.9) |
| Severe | 24 (3.9) | 302 (4.3) | −0.3 (−1.9, 1.3) |
| Swelling | 298 (49.0) | 2830 (40.0) | 9.0 (4.9, 13.1) |
| Severe (>5.0 cm) | 46 (7.6) | 272 (3.8) | 3.7 (1.6, 5.9) |
| Erythema | 257 (42.3) | 2407 (34.0) | 8.2 (4.2, 12.3) |
| Severe (>5.0 cm) | 20 (3.3) | 114 (1.6) | 1.7 (0.2, 3.1) |
| Pruritus | 47 (7.7) | 388 (5.5) | 2.2 (0.1, 4.4) |
| Severe | 2 (0.3) | 7 (0.1) | — |
| Systemic event† | 363 (59.7) | 3948 (55.8) | 3.9 (−0.2, 7.9) |
| Vaccine-related‡systemic event | 186 (30.6) | 2086 (29.5) | 1.1 (−2.7, 4.9) |
| Headache | 119 (19.6) | 1031 (14.6) | 5.0 (1.7, 8.3) |
| Pyrexia | 31 (5.1) | 357 (5.0) | 0.0 (−1.8, 1.9) |
9vHPV: 9-valent human papilloma virus; AE: adverse event; CI: confidence interval; qHPV: quadrivalent human papilloma virus
Days 1–5 following a vaccination visit
Days 1–15 following a vaccination visit
As determined by the reporting investigator
95% CI is based on a 2-sample test for binomial proportions under normal-theory method
Injection-site and systemic AEs shown are those with incidence ≥5% in any vaccination group during the study
Data for individuals with no prior HPV vaccination (Study 001; NCT00543543) have been previously reported7,9
Vaccine-related systemic events were reported at similar frequencies in the active and placebo groups, with incidence generally decreasing across subsequent doses, a result that was not previously reported (Table 1). The frequencies of vaccine-related systemic AEs in prior qHPV vaccine recipients (30.6%) were similar to those previously reported in individuals without prior HPV vaccination (29.5%) (Table 2). The frequencies of vaccine-related headache were higher in prior qHPV vaccine recipients (19.6%) than in individuals without prior HPV vaccination (14.6%).
Taken together, Study 006, Study 006-02, and Study 001-04 enrolled 3770 participants to receive 9vHPV vaccine, 3756 (99.6%) of whom had safety follow-up. New, combined analyses of SAEs, AEs resulting in discontinuations, and pregnancy outcomes reported in these 3 studies were conducted. Among the total participants from Study 006, Study 006-02 and Study 001-04, a total of 30 subjects (0.8%) reported SAEs at any time, including 6 (0.2%) who reported SAEs within 15 days of any 9vHPV vaccine dose (Table 3). SAEs most commonly involved the system organ class of pregnancy, puerperium, and perinatal conditions, followed by infections and infestations (Table 3). No participants died during the studies. Overall, in prior qHPV vaccine recipients, the frequencies of SAEs were generally similar to those previously reported in individuals without HPV vaccination (in Study 001, frequencies of SAEs in 9vHPV vaccine recipients were 0.4% for SAEs occurring within 15 days after a vaccination and 3.3% for SAEs during the entire study7,9).
Table 3.
Subjects with SAEs* (by system organ class) among subjects who received 9vHPV vaccine in Study 006, Study 006-02 and Study 001-04 after previously receiving qHPV vaccine.
| Within 15 days following any vaccination (N = 3756) |
At any time (N = 3756) |
|||
|---|---|---|---|---|
| Count | (%) | Count | (%) | |
| With ≥1 SAE | 6 | (0.2) | 30 | (0.8) |
| Cardiac disorders | 0 | (0.0) | 1 | (0.0) |
| Gastrointestinal disorders | 2 | (0.1) | 2 | (0.1) |
| Hepatobiliary disorders | 0 | (0.0) | 1 | (0.0) |
| Infections and infestations† | 1 | (0.0) | 6 | (0.2) |
| Injury, poisoning, and procedural complications | 0 | (0.0) | 2 | (0.1) |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 0 | (0.0) | 3 | (0.1) |
| Adenocarcinoma of the cervix‡ | 0 | (0.0) | 1 | (0.0) |
| Breast cancer | 0 | (0.0) | 1 | (0.0) |
| Glioma | 0 | (0.0) | 1 | (0.0) |
| Nervous system disorders | 2 | (0.1) | 3 | (0.1) |
| Pregnancy, puerperium, and perinatal conditions | 0 | (0.0) | 9 | (0.2) |
| Renal and urinary disorders | 0 | (0.0) | 1 | (0.0) |
| Skin and subcutaneous tissue disorders | 1 | (0.0) | 1 | (0.0) |
| Surgical and medical procedures | 0 | (0.0) | 3 | (0.1) |
9vHPV: 9-valent human papilloma virus; N: number of subjects as-treated who received at least 1 dose of the 9vHPV vaccine and had at least 1 follow-up visit for adverse event; qHPV: quadrivalent human papilloma virus; SAE: serious adverse event
SAEs were predefined as any AE that resulted in death, were deemed by the investigator to be life threatening, resulted in a persistent or significant disability or incapacity, resulted in or prolonged an existing in-patient hospitalization, or was a congenital anomaly, a cancer, or an “other important medical event”. Per protocol, SAEs were reportable regardless of causality for the entire study period; events of fetal loss were to be reported as SAEs for any pregnancy with last menstrual period at any time during the study
One SAE was considered by the reporting investigator to be vaccine-related (tonsillitis in Study 006; described previously8). The sponsor does not downgrade the investigator's causality assessment
Cervical adenocarcinoma tissue sample was not available for analysis; this subject tested positive by PCR at Day 1 of the base study (pre-vaccination) for HPV16 and continued to test positive by PCR for this HPV type for the entire duration of the base study
The summaries provided are counts of subjects and the percentages were calculated relative to the number of subjects as-treated. System organ class categories reported are those with incidence >0% during the study. A subject is counted once within a category and may be counted in more than 1 category
One participant in Study 001-04 developed an SAE of cervical adenocarcinoma, based on a biopsy of an endocervical polyp and histopathological diagnosis of endocervical carcinoma in situ 20 days after the second vaccination in the study extension. The details and outcome of the SAE are unknown because the participant decided to withdraw consent for personal reasons; the biopsy sample was not available. During the base study, this participant was seropositive at Day 1 for HPV16, and gynecological swabs were positive for HPV16 at Day 1 and Months 7, 12, 18, 24, 30, and 36 visits; and for HPV56 at the Month 24 visit. Cervical cytology showed inflammation (with changes consistent with bacterial vaginosis) at Day 1 and Months 7 and 12, and was negative at Months 18, 24, 30, and 36. Although the causal HPV type could not be determined, it is likely that this lesion may have been caused by persistent infection with HPV16, as this participant was positive by serology and PCR prior to enrollment in the base study.
Across the 3 studies, 8 participants (0.2%) discontinued vaccination due to an AE, including 7 who experienced vaccine-related AEs that were not considered serious and 1 who experienced an SAE that was not considered vaccine-related (see Supplementary Information for details). Overall, in prior qHPV vaccine recipients, the frequencies of AEs resulting in discontinuation of vaccination were low (0.2%) and generally similar to those previously reported in individuals who did not receive prior HPV vaccination (in Study 001, frequencies of AEs resulting in discontinuation of vaccination were 0.1%7).
There were a total of 72 pregnancies (69 with known outcomes) in female prior qHPV vaccine recipients from Studies 006 (n = 2 pregnancies in the 9vHPV vaccine group), 006-02 (n = 2), and 001-04 (n = 68 [65 with known outcomes]). The majority (n = 58; 84.1%) resulted in live births; there were 8 spontaneous abortions (11.6%), and 3 elective abortions (4.3%), all among participants from Study 001-04. There were no reports of congenital anomalies. Overall, the frequencies of adverse pregnancy outcomes were within the ranges reported in the general population or previous studies of the 9vHPV vaccine.7 It is noteworthy that the frequency of spontaneous abortions observed in pregnancies occurring in prior qHPV vaccine recipients who received 9vHPV vaccine (11.6%) is almost identical to the frequencies of spontaneous abortions reported in pregnancies occurring in individuals who were not previously vaccinated for HPV and received the 9vHPV, qHPV, or bivalent HPV vaccine in clinical studies (9.1% and 11.1% for the 9vHPV and qHPV vaccine groups of Study 001, respectively7; 13.3% for bivalent HPV vaccine recipients in the Costa Rica HPV Vaccine Trial12).
Safety assessment in young men
In male participants 16–26 years of age from Study 020, the most common injection-site AEs (any grade) were pain, swelling, and erythema (Table 4). Most events were mild or moderate in intensity. Injection-site and systemic AEs over the entire course of 9vHPV or qHPV vaccine injection have been previously reported11 and are summarized in Table 4 for completeness. However, AEs occurring after each vaccination have not been reported before. When AEs occurring after each vaccine dose were analyzed separately, the frequencies and intensities of injection-site AEs were generally similar across the 3 subsequent doses (Table 4). Injection-site AEs were reported in 60.5%, 62.4%, and 58.1% of subjects after each 9vHPV vaccination, and 48.8%, 51.6%, and 46.5% of subjects after each qHPV vaccination, respectively, over the 3-dose series of injections. Although the frequency of injection-site swelling increased with dose, the frequency of severe injection-site swelling was similar across the 3 doses. The frequencies of vaccine-related systemic events were generally highest following the first vaccine dose and decreased with subsequent doses (Table 4). Vaccine-related systemic AEs were reported in 17.3%, 7.8%, and 5.3% of subjects after the first, second, and third 9vHPV vaccinations, and 12.1%, 6.5%, and 7.0% of subjects after qHPV vaccinations, respectively, over the entire series of injections. As described previously, 6 SAEs were reported (all in the qHPV vaccine group), none of which were considered vaccine-related.11 No subjects discontinued due to AEs, and no participants died during the study.11
Table 4.
AEs reported from Day 1 to Day 15 after a vaccination visit in males 16–26 years of age in Study 020.
| 9vHPV Vaccine |
qHPV Vaccine |
|||||||
|---|---|---|---|---|---|---|---|---|
| After Dose 1 | After Dose 2 | After Dose 3 | After any dose | After Dose 1 | After Dose 2 | After Dose 3 | After any dose | |
| Number of subjects with follow-up | 248 | 245 | 246 | 248 | 248 | 246 | 243 | 248 |
| Number (%) of subjects with the following AEs | ||||||||
| With ≥1 AEs* | 165 (66.5) | 160 (65.3) | 154 (62.6) | 204 (82.3) | 154 (62.1) | 141 (57.3) | 132 (54.3) | 203 (81.9) |
| Injection-site event† | 150 (60.5) | 153 (62.4) | 143 (58.1) | 196 (79.0) | 121 (48.8) | 127 (51.6) | 113 (46.5) | 179 (72.2) |
| Pain | 140 (56.5) | 147 (60.0) | 140 (56.9) | 193 (77.8) | 111 (44.8) | 120 (48.8) | 107 (44.0) | 174 (70.2) |
| Mild | 129 (52.0) | 127 (51.8) | 123 (50.0) | 157 (63.3) | 98 (39.5) | 107 (43.5) | 91 (37.4) | 138 (55.6) |
| Moderate | 11 (4.4) | 20 (8.2) | 17 (6.9) | 36 (14.5) | 13 (5.2) | 13 (5.3) | 16 (6.6) | 36 (14.5) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Swelling | 10 (4.0) | 17 (6.9) | 20 (8.1) | 36 (14.5) | 7 (2.8) | 12 (4.9) | 15 (6.2) | 23 (9.3) |
| Mild (0 to ≤2.5 cm) | 8 (3.2) | 16 (6.5) | 17 (6.9) | 31 (12.5) | 4 (1.6) | 8 (3.3) | 11 (4.5) | 15 (6.0) |
| Moderate (>2.5 cm to ≤5.0 cm) | 0 (0.0) | 1 (0.4) | 2 (0.8) | 2 (0.8) | 1 (0.4) | 3 (1.2) | 2 (0.8) | 4 (1.6) |
| Severe (>5.0 cm) | 2 (0.8) | 0 (0.0) | 1 (0.4) | 3 (1.2) | 2 (0.8) | 1 (0.4) | 2 (0.8) | 4 (1.6) |
| Erythema | 17 (6.9) | 25 (10.2) | 20 (8.1) | 38 (15.3) | 15 (6.0) | 26 (10.6) | 21 (8.6) | 43 (17.3) |
| Mild (0 to ≤2.5 cm) | 16 (6.5) | 25 (10.2) | 19 (7.7) | 36 (14.5) | 14 (5.6) | 24 (9.8) | 20 (8.2) | 40 (16.1) |
| Moderate (>2.5 cm to ≤5.0 cm) | 1 (0.4) | 0 (0.0) | 1 (0.4) | 2 (0.8) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 3 (1.2) |
| Severe (>5 cm) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| Movement impairment | 5 (2.0) | 2 (0.8) | 0 (0.0) | 5 (2.0) | 7 (2.8) | 2 (0.8) | 2 (0.8) | 9 (3.6) |
| Induration | 2 (0.8) | 2 (0.8) | 2 (0.8) | 4 (1.6) | 4 (1.6) | 2 (0.8) | 0 (0.0) | 6 (2.4) |
| Pruritus | 0 (0.0) | 1 (0.4) | 3 (1.2) | 4 (1.6) | 3 (1.2) | 1 (0.4) | 1 (0.4) | 5 (2.0) |
| Systemic event* | 69 (27.8) | 29 (11.8) | 42 (17.1) | 101 (40.7) | 66 (26.6) | 33 (13.4) | 45 (18.5) | 100 (40.3) |
| Vaccine-related‡ systemic event | 43 (17.3) | 19 (7.8) | 13 (5.3) | 57 (23.0) | 30 (12.1) | 16 (6.5) | 17 (7.0) | 54 (21.8) |
| Headache | 13 (5.2) | 9 (3.7) | 4 (1.6) | 20 (8.1) | 13 (5.2) | 5 (2.0) | 7 (2.9) | 22 (8.9) |
| Pyrexia | 2 (0.8) | 2 (0.8) | 2 (0.8) | 5 (2.0) | 1 (0.4) | 4 (1.6) | 3 (1.2) | 7 (2.8) |
| Nausea | 3 (1.2) | 2 (0.8) | 1 (0.4) | 5 (2.0) | 2 (0.8) | 1 (0.4) | 1 (0.4) | 3 (1.2) |
| Diarrhea | 2 (0.8) | 2 (0.8) | 2 (0.8) | 5 (2.0) | 5 (2.0) | 0 (0.0) | 1 (0.4) | 6 (2.4) |
| Fatigue | 3 (1.2) | 1 (0.4) | 1 (0.4) | 5 (2.0) | 3 (1.2) | 0 (0.0) | 5 (2.1) | 8 (3.2) |
| Number of subjects with temperature data | 245 | 237 | 242 | 248 | 247 | 237 | 238 | 248 |
| Number (%) of subjects with the following maximum temperatures† | ||||||||
| ≥37.8°C | 1 (0.4) | 3 (1.3) | 4 (1.7) | 7 (2.8) | 0 (0.0) | 4 (1.7) | 3 (1.3) | 6 (2.4) |
| ≥38.9°C | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.4) |
9vHPV: 9-valent human papilloma virus; AE: adverse event; qHPV: quadrivalent human papilloma virus
Days 1–15 following a vaccination visit
Days 1–5 following a vaccination visit
As determined by the reporting investigator
Injection-site and systemic AEs shown are those with incidence ≥2% in any vaccination group during the study
Discussion
Safety analyses of 9vHPV vaccination in prior qHPV vaccine recipients have been conducted, including previously reported results for ∼600 9vHPV vaccine recipients (Study 006 base study8), as well as new data from qHPV vaccine recipients who received 9vHPV vaccine during 2 extension studies, which increases the clinical trial safety database to ∼3700 prior qHPVvaccine recipients. The 9vHPV vaccine was generally well tolerated. SAEs occurring within 15 days of vaccination and AEs resulting in discontinuation were rare (0.4% and 0.2%, respectively); no participant died during the studies. The 9vHPV vaccine safety profile is similar in prior qHPV-vaccine recipients versus individuals who did not receive prior HPV vaccination. Injection-site swelling and injection-site erythema are more likely to occur in prior qHPV-vaccine recipients than individuals without prior HPV vaccination. As the 9vHPV vaccine becomes more widely available, observations in prior qHPV vaccine recipients are of increasing interest, as individuals who previously received qHPV vaccine may seek additional HPV type coverage provided by the 9vHPV vaccine. Although professional societies have not issued a formal recommendation regarding 9vHPV vaccination of prior qHPV vaccine recipients, proposed guidelines have been made available.13,14
Safety analyses in young men have been conducted previously.11,15 The safety profile of the 9vHPV vaccine in men is further defined with the additional analyses presented herein (i.e., by vaccination visit) comparing the 9vHPV and qHPV vaccines. The format of these analyses mirrors that of the analyses previously reported in women.7 Safety assessments previously reported from 1 study in young women9 and 2 studies in young men11,15 have indicated that AEs occurred less frequently in men than in women. The results presented herein lend further support for this conclusion. A lower proportion of male participants reported AEs at each vaccination visit compared with the previously described frequency at each visit among female participants.7 In addition, there was no increase in the frequency of severe injection-site AEs with subsequent vaccinations in men, while previous observations in women have indicated an increase in the frequency of severe injection-site AEs at the later vaccination visits.7 Interest in gender-neutral vaccination (males in addition to females) has increased, with the aim of protecting males against HPV-related anogenital disease and maximizing herd effects of HPV vaccination.16–19 Therefore, these observations are expected to be relevant to medical practice.
Limitations of these analyses include the relatively small sample size, which only allows analysis of the most common AEs. Rarer AEs are expected to be assessed in pharmacovigilance and post-licensure safety analyses. The prior qHPV vaccine recipients and individuals with no prior HPV vaccination were from different studies; as in any cross-study comparison, differences in study populations may affect the results of the comparison of AEs between these 2 groups.
In conclusion, these analyses supplement the combined safety analyses previously reported for 7 Phase 3 clinical studies,7 as well as the recently reported results from 2 additional studies.20,21 Overall, 9vHPV vaccine safety has been assessed in over 20,000 clinical study participants. Additional safety evaluations of the 9vHPV vaccine are planned. Post-approval regulatory commitments22,23 include the continued assessment of 9vHPV vaccine safety in long-term follow-up clinical studies, a post-authorization safety study, and a pregnancy registry (similar to that recently completed for the qHPV vaccine24,25); these studies are ongoing. Post-licensure pharmacovigilance monitoring is also ongoing, and the US Centers for Disease Control and Prevention (Atlanta, GA) have announced plans to continue to monitor the safety of the 9vHPV vaccine post-licensure.26
Supplementary Material
Disclosure of potential conflicts of interest
EDM reports grants and personal fees from Merck & Co., Inc., Kenilworth, NJ, USA. ARG's institution has received grants from Merck & Co., Inc., Kenilworth, NJ, USA on her behalf for her research; she is a member of the Scientific Advisory Board for Merck & Co., Inc., Kenilworth, NJ, USA. JdH reports research grants from Abide, Amgen, Galderma, Genentech, GlaxoSmithKline, Janssen Research & Development, Lilly Chorus, MSD, a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, Novartis, Sanofi Pasteur, UCB and Vertex; and consultancy for Ablynx, Amgen, Eli Lilly, Genentech, and UCB. OEI has received personal fees from Merck & Co., Inc., Kenilworth, NJ, USA for conducting clinical HPV vaccine trials and for Scientific Advisory Committee meetings and has received lecture fees from Sanofi Pasteur MSD. EAJ has received funding and personal fees from Merck & Co., Inc., Kenilworth, NJ, USA, SPMSD, and GSK. JR has nothing to disclose. PVD acts as principal investigator for vaccine trials conducted on behalf of the University of Antwerp, for which the University obtains research grants from vaccine manufacturers; speaker's fees for presentations on vaccines are paid directly to an educational fund held by the University of Antwerp; PVD receives no personal remuneration for this work. CV's university has received grants from GSK, AdImmune and Sanofi-Pasteur for clinical studies for which she was principal investigator. MCE, AK, CS, BH, and AL are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ USA, and may own stock or stock options.
Acknowledgments
Medical writing assistance was provided by Erin Bekes, PhD, of Complete Medical Communications, Hackensack, NJ.
NCT numbers: NCT01047345, NCT00543543, NCT02114385.
Funding
Funding for this research and writing assistance was provided by Merck & Co., Inc., Kenilworth, NJ USA.
References
- 1.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR et al.. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. PMID:20952254 [DOI] [PubMed] [Google Scholar]
- 2.Pitisuttithum P, Velicer C, Luxembourg A. 9-Valent HPV vaccine for cancers, pre-cancers and genital warts related to HPV. Expert Rev Vaccines. 2015;14(11):1405–1419. doi: 10.1586/14760584.2015.1089174. PMID:26366475 [DOI] [PubMed] [Google Scholar]
- 3.Alemany L, Saunier M, Alvarado-Cabrero I, Quirós B, Salmeron J, Shin HR, Pirog E, Guimerá N, Hernández-Suarez GA, Felix A et al.. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136(1):98–107. doi: 10.1002/ijc.28963. PMID:24817381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, Joura EA. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. doi: 10.1086/597071. PMID:19199546 [DOI] [PubMed] [Google Scholar]
- 5.Joura EA, Ault A, Bosch FX, Brown D, Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh W et al.. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1997–2008. doi: 10.1158/1055-9965.EPI-14-0410. PMID:25274978 [DOI] [PubMed] [Google Scholar]
- 6.Serrano B, de Sanjosé S, Tous S, Quiros B, Muñoz N, Bosch X, Alemany L. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer. 2015;51(13):1732–1741. doi: 10.1016/j.ejca.2015.06.001. PMID:26121913 [DOI] [PubMed] [Google Scholar]
- 7.Moreira ED Jr, Block SL, Ferris D, Giuliano AR, Iversen OE, Joura EA, Kosalaraksa P, Schilling A, Van Damme P, Bornstein J, et al.. Safety profile of the 9-valent HPV vaccine: a combined analysis of 7 Phase III clinical trials. Pediatrics. 2016;138(2):e20154387. doi: 10.1542/peds.2015-4387. PMID:27422279 [DOI] [PubMed] [Google Scholar]
- 8.Garland SM, Cheung TH, McNeill S, Petersen LK, Romaguera J, Vazquez-Narvaez J, Bautista O, Shields C, Vuocolo S, Luxembourg A. Safety and immunogenicity of a 9-valent HPV vaccine in females 12–26 years of age who previously received the quadrivalent HPV vaccine. Vaccine. 2015;33(48):6855–6864. doi: 10.1016/j.vaccine.2015.08.059. PMID:26411885 [DOI] [PubMed] [Google Scholar]
- 9.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, et al.. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. PMID:25693011 [DOI] [PubMed] [Google Scholar]
- 10.Guevara A, Cabello R, Woelber L, Moreira ED Jr, Joura E, Reich O, Shields C, Ellison MC, Joshi A, Luxembourg A. Antibody persistence and evidence of immune memory at 5 years following administration of the 9-valent HPV vaccine. Vaccine. 2017;35:5050–5057. doi: 10.1016/j.vaccine.2017.07.017. PMID:28789851 [DOI] [PubMed] [Google Scholar]
- 11.Van Damme P, Meijer CJ, Kieninger D, Schuyleman A, Thomas S, Luxembourg A, Baudin M. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine. 2016;34(35):4205–4212. doi: 10.1016/j.vaccine.2016.06.056. PMID:27354258 [DOI] [PubMed] [Google Scholar]
- 12.Panagiotou OA, Befano BL, Gonzalez P, Rodriguez AC, Herrero R, Schiller JT, Kreimer AR, Schiffman M, Hildesheim A, Wilcox AJ et al.. Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: long term observational follow-up in the Costa Rica HPV Vaccine Trial. BMJ. 2015;351:h4358. doi: 10.1136/bmj.h4358. PMID:26346155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Damme P, Bonanni P, Bosch FX, Joura E, Kjaer SK, Meijer CJ, Petry KU, Soubeyrand B, Verstraeten T, Stanley M. Use of the nonavalent HPV vaccine in individuals previously fully or partially vaccinated with bivalent or quadrivalent HPV vaccines. Vaccine. 2016;34(6):757–761. doi: 10.1016/j.vaccine.2015.12.063. PMID:26772631 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Supplemental information and guidance for vaccination providers regarding use of 9-valent HPV. 2016. November 29 [accessed 2017March28] https://www.cdc.gov/hpv/downloads/9vhpv-guidance.pdf.
- 15.Castellsague X, Giuliano AR, Goldstone S, Guevara A, Mogensen O, Palefsky JM, Group T, Shields C, Liu K, Maansson R et al.. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine. 2015;33(48):6892–6901. doi: 10.1016/j.vaccine.2015.06.088. PMID:26144901 [DOI] [PubMed] [Google Scholar]
- 16.Audisio RA, Icardi G, Isidori AM, Liverani CA, Lombardi A, Mariani L, Mennini FS, Mitchell DA, Peracino A, Pecorelli S et al.. Public health value of universal HPV vaccination. Crit Rev Oncol Hematol. 2016;97:157–167. doi: 10.1016/j.critrevonc.2015.07.015. PMID:26346895 [DOI] [PubMed] [Google Scholar]
- 17.Lehtinen M, Apter D. Gender-neutrality, herd effect and resilient immune response for sustainable impact of HPV vaccination. Curr Opin Obstet Gynecol. 2015;27(5):326–332. doi: 10.1097/GCO.0000000000000208. PMID:26308204 [DOI] [PubMed] [Google Scholar]
- 18.Schmeler KM, Sturgis EM. Expanding the benefits of HPV vaccination to boys and men. Lancet. 2016;387(10030):1798–1799. doi: 10.1016/S0140-6736(16)30314-2. PMID:27203488 [DOI] [PubMed] [Google Scholar]
- 19.Stanley M. HPV vaccination in boys and men. Hum Vaccin Immunother. 2014;10(7):2109–2111. doi: 10.4161/hv.29137. PMID:25424825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Nur Azurah AG, Fong SM et al.. Immunogenicity of the 9-valent HPV vaccine using 2-Dose regimens in girls and boys vs a 3-Dose Regimen in women. JAMA. 2016;316(22):2411–2421. doi: 10.1001/jama.2016.17615. PMID:27893068 [DOI] [PubMed] [Google Scholar]
- 21.Iwata S, Murata S, Han S, Wakana A, Sawata M, Tanaka Y. Safety and immunogenicity study of a 9-valent human papillomavirus vaccine administered to 9- to 15-year old Japanese Girls. Jpn J Infect Dis. 2017;70:368–373. doi: 10.7883/yoken.JJID.2016.299. PMID:28003597 [DOI] [PubMed] [Google Scholar]
- 22.European Medicines Agency (EMA) Summary of the risk management plan (RMP) for Gardasil 9 (human papillomavirus 9-valent vaccine (recombinant, adsorbed)) - EMA/226536. 2015. [accessed 2017February16]. www.ema.europa.eu/docs/en_GB/document_library/EPAR-Risk-management-plan_summary/human/003852/WC500184709.pdf.
- 23.United States Food and Drug Administration Approval Letter - Gardasil 9. 2014. December 10 [accessed 2017February16] http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426520.htm.
- 24.Dana A, Buchanan KM, Goss MA, Seminack MM, Shields KE, Korn S, Cunningham ML, Haupt RM. Pregnancy outcomes from the pregnancy registry of a human papillomavirus type 6/11/16/18 vaccine. Obstet Gynecol. 2009;114(6):1170–1178. doi: 10.1097/AOG.0b013e3181c2a122. PMID:19935016 [DOI] [PubMed] [Google Scholar]
- 25.Goss MA, Lievano F, Buchanan KM, Seminack MM, Cunningham ML, Dana A. Final report on exposure during pregnancy from a pregnancy registry for quadrivalent human papillomavirus vaccine. Vaccine. 2015;33(29):3422–3428. doi: 10.1016/j.vaccine.2015.04.014. PMID:25869893 [DOI] [PubMed] [Google Scholar]
- 26.Gee J, Weinbaum C, Sukumaran L, Markowitz LE. Quadrivalent HPV vaccine safety review and safety monitoring plans for nine-valent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12(6):1406–1417. doi: 10.1080/21645515.2016.1168952. PMID:27029786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.