ABSTRACT
This study set out to evaluate influenza- and respiratory-related illnesses recorded during primary care physician consultations in England following the H1N1 pandemic in 2009 and to enable the development of a dynamic disease model. Data were obtained from the Clinical Practice Research Datalink of primary care records over four influenza seasons (2010–2014). The primary outcome of the study was incidence of influenza- and respiratory-related diagnoses, calculated per practice and by season and age group. Upper respiratory tract infection diagnoses were most frequently recorded (mean seasonal practice level incidence; 3,762 consultations per 100,000 [SD = 1,989]), and influenza-related diagnoses were least frequently recorded across all seasons, except one. Incidence rates for the under 18 population were higher than those for the general population, in particular for upper respiratory tract infection (range of 8,024–9,950 versus 3,228–4,120, respectively) and otitis media diagnoses (2,668–3,652 versus 782–1,057, respectively). For influenza-related diagnoses, the 65+ age group, the 0 to <2 and 2 to <4 groups had a higher risk (risk ratio = 1.33, 1.12 and 1.16, respectively) than other age groups. This study provides valuable insight into the incidence of influenza- and respiratory-related diagnoses in the primary care setting in England, and suggests a higher burden of disease in young children and the elderly. The study also indicates that some influenza illness is likely to be reported under respiratory-related diagnoses, given the low incidence of influenza-related diagnoses in the study.
KEYWORDS: Influenza, Respiratory disease, United Kingdom, Vaccination, CPRD
Introduction
Seasonal influenza is associated with a significant clinical and economic burden.1 The World Health Organization estimates that annual influenza epidemics result in around 3–5 million cases of severe illness, and approximately 250,000–500,000 deaths globally.2 In Europe, influenza is estimated to cause 40,000 deaths each year.3
Influenza also causes secondary infections and can exacerbate pre-existing chronic conditions, further contributing to its clinical and economic burden.4,5 In particular, adults aged 65 years and over, pregnant women, those with certain chronic medical conditions and young children are at high risk of developing influenza-associated illnesses such as acute lower respiratory infections (ALRI), including pneumonia and bronchitis.6 A large-scale study of the global burden of influenza and associated illnesses estimated that 90 million new cases of influenza, 20 million cases of influenza-associated ALRI, and 1 million cases of influenza-associated severe ALRI occur globally in children under 5 years old.7 Globally, influenza is associated with 10% of respiratory hospitalizations in children younger than 18 years old.8 In an average influenza season in the United Kingdom (UK), 2.4% of children under 5 years old visited primary care providers (PCPs) for respiratory illness attributed to influenza.9
Influenza vaccination policies have traditionally targeted the elderly and those in other clinical risk groups, including pregnant women.10 More recently, recommendations on vaccination policies have also been extended to otherwise healthy children (those aged <18 years).10 Children are more likely to be infected with influenza than adults, with attack rates reaching up to 30%.11 Additionally, young children have particularly high rates of complications from influenza infection, with acute otitis media developing in nearly 40% of children under 3 years.12 Children are also considered key transmitters of influenza infection because they shed higher viral titers for longer periods of time than adults, are in close contact with one another in schools and other settings, and may have limited pre-existing immunity and poor hygiene.13–16 In 2013, based on such findings and a thorough evaluation of the different influenza prevention strategies, the UK health authority implemented a childhood influenza vaccination program with live attenuated influenza vaccine on the recommendation of the Joint Committee on Vaccination and Immunisation.17–19 In the first year of the program during the 2013–2014 influenza season, all children aged 2 and 3 years were offered the vaccine.20 In addition, 7 local regions offered vaccinations to children aged between 4 and 1117 in school and community settings. The program has been extended to older age groups in subsequent seasons with the aim to increase vaccine uptake rates.
The UK's childhood influenza vaccination program provides an opportunity to investigate the impact of vaccinating children against influenza on the burden of disease in the general population. Our overall aim is to develop a dynamic disease model to measure the impact of the childhood vaccination program on respiratory disease burden in England. In preparation for this, there was a need to describe the distribution and patterns of respiratory disease burden in the general population and across age groups. To achieve this, we undertook a descriptive analysis of the seasonal rate and variability of influenza- and respiratory-related PCP consultations in England for the years following the 2009 H1N1 pandemic. While studies have been published on the burden of influenza-attributable respiratory illness prior to 2009,21,22 there is limited information about the burden after the 2009 H1N1 pandemic. One study by Hardelid et al. measured the consultation rate of influenza-like-illness PCP consultations23 after the pandemic; however, no studies to date have reported the rate of overall respiratory-related PCP consultations and patterns across respiratory diagnoses. It is important to obtain contemporary estimates of influenza- and respiratory-related PCP-consultations as it is likely that physician diagnosing behaviors were altered following the 2009 H1N1 pandemic. The results of this study will be used to inform the design and development of a dynamic disease model, which will be utilized to estimate the impact of the childhood influenza vaccination program in England.
Results
Table 1 provides a description of the included population for the study in relation to gender and age for each season. The study population was similar across seasons, with over 2 million participants in each season.
Table 1.
Description of practice-level population characteristics, by season.
| Season |
||||||
|---|---|---|---|---|---|---|
| 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 | All seasons combined | ||
| Population characteristics | (n = 2,625,838)a | (n = 2,527,191)a | (n = 2,495,908)a | (n = 2,206,712)a | (n = 9,855,649) | |
| Male gender | Practice mean % (SD) | 44.2 (1.9) | 44.2 (1.7) | 44.2 (1.5) | 44.2 (1.4) | 44.20 (1.64) |
| Practice median % (Q1–Q3) | 44.0 (43.2–45.0) | 44.1 (43.2–45.1) | 44.1 (43.2–44.9) | 44.1 (43.4–44.9) | 44.07 (43.21–44.99) | |
| Age 0 to <2 | Practice mean % (SD) | 3.5 (2.0) | 3.8 (1.4) | 3.4 (1.3) | 2.6 (1.0) | 3.36 (1.56) |
| Practice median % (Q1–Q3) | 3.4 (2.7–4.1) | 3.7 (2.9–4.7) | 3.2 (2.5–4.0) | 2.4 (1.9–3.2) | 3.21 (2.42–4.05) | |
| Age 2 to <4 | Practice mean % (SD) | 2.9 (1.1) | 3.5 (1.2) | 4.4 (1.4) | 4.8 (1.5) | 3.84 (1.47) |
| Practice median % (Q1–Q3) | 2.8 (2.2–3.6) | 3.4 (2.7–4.2) | 4.2 (3.4–5.1) | 4.6 (3.8–5.6) | 3.66 (2.80–4.72) | |
| Age 4 to <5 | Practice mean % (SD) | 1.2 (0.5) | 1.4 (0.56) | 1.6 (0.6) | 2.0 (0.6) | 1.54 (0.63) |
| Practice median % (Q1–Q3) | 1.2 (0.9–1.4) | 1.4 (1.1–1.8) | 1.6 (1.3–2.0) | 1.9 (1.6–2.3) | 1.46 (1.10–1.90) | |
| Age 5 to <11 | Practice mean % (SD) | 4.7 (1.8) | 5.3 (1.9) | 6.2 (2.2) | 7.1 (2.5) | 5.77 (2.25) |
| Practice median % (Q1–Q3) | 4.5 (3.5–5.5) | 5.1 (4.1–6.3) | 5.9 (4.7–7.3) | 6.8 (5.7–8.4) | 5.41 (4.24–6.89) | |
| Age 11 to <18 | Practice mean % (SD) | 4.1 (1.3) | 4.2 (1.2) | 4.4 (1.3) | 4.6 (1.3) | 4.30 (1.28) |
| Practice median % (Q1–Q3) | 4.0 (3.3–4.8) | 4.1 (3.4–4.9) | 4.2 (3.6–5.1) | 4.4 (3.7–5.3) | 4.15 (3.49–5.06) | |
| Age 18 to <65 | Practice mean % (SD) | 43.3 (6.8) | 41.6 (5.6) | 40.1 (5.5) | 38.9 (5.5) | 41.07 (6.09) |
| Practice median % (Q1–Q3) | 43.6 (39.7–47.1) | 41.50 (38.0–45.1) | 39.9 (36.6–43.4) | 38.5 (35.1–42.1) | 41.05 (37.27–44.67) | |
| Age 65+ | Practice mean % (SD) | 40.4 (9.9) | 40.1 (9.3) | 40.0 (9.4) | 40.1 (9.3) | 40.13 (9.48) |
| Practice median % (Q1–Q3) | 40.6 (34.4–46.1) | 40.6 (33.7–46.1) | 40.5 (33.6–46.3) | 40.2 (33.8–46.3) | 40.46 (33.77–46.20) | |
SD, standard deviation.
n = total number of registered patients meeting the study inclusion criteria at the beginning of the season.
Table 2 details the practice-level incidence rates for each of the diagnosis groups collected during the study across the general population (children and adults). Upper respiratory tract infections (URTIs) were the most frequently recorded diagnoses across all seasons, with mean seasonal practice-level incidence of 3,762 consultations per 100,000 population (standard deviation [SD] = 1,989). The next most frequent diagnoses were lower respiratory tract infections (LRTIs) (2,214 per 100,000 population [SD = 1,362]) and other acute respiratory tract infections (ARTIs) (1,457 per 100,000 population [SD = 736]). Influenza-related diagnoses were the least frequently recorded diagnoses across all seasons, except for the 2010–2011 season. Inter-practice variability (measured in terms of coefficients of variation [CV]) was <1 for all diagnosis groups with the exception of influenza-related diagnoses. Incidence rates for diagnosis groups across seasons had limited variability with the exception of influenza-related diagnoses.
Table 2.
Practice-level IR for general population (adults and children).
| Season |
||||||
|---|---|---|---|---|---|---|
| 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 | All seasons combined | ||
| Diagnosis | (n = 2,625,838)a | (n = 2,527,191)a | (n = 2,495,908)a | (n = 2,206,712)a,* | (n = 9,855,649) | |
| Influenza | Practice mean IR (SD) | 129.3 (207.4) | 48.5 (93.3) | 74.6 (186.2) | 41.3 (82.0) | 75.4 (158.9) |
| Practice median IR (Q1–Q3) | 75.2 (24.3–145.4) | 22.8 (0.0–56.1) | 28.7 (0.0–79.4) | 18.4 (0.0–42.1) | 30.4 (0.0–81.6) | |
| Co-efficient of variation | 1.604 | 1.924 | 2.496 | 1.985 | 2.107 | |
| Upper respiratory tract infection | Practice mean IR (SD) | 4,119.7 (2,140.4) | 3,710.0 (1,930.6) | 3,896.5 (2,026.2) | 3,228.1 (1,688.6) | 3,761.9 (1,988.6) |
| Practice median IR (Q1–Q3) | 3,832.2 | 3,517.0 | 3,691.8 | 2,992.4 | 3,545.2 | |
| (2,704.5–5,238.5) | (2,386.4–4,747.4) | (2,570.4–5,044.4) | (2,113.0–4,217.8) | (2,389.7–4,926.8) | ||
| Co-efficient of variation | 0.52 | 0.52 | 0.52 | 0.523 | 0.529 | |
| Lower respiratory tract infection | Practice mean IR (SD) | 2,407.2 (1,455.6) | 2,256.6 (1,292.0) | 2,248.0 (1,409.9) | 1,884.5 (1,207.1) | 2,213.5 (1,361.9) |
| Practice median IR (Q1–Q3) | 2,180.4 | 2,068.7 | 1,985.8 | 1,704.9 | 1,987.9 | |
| (1,348.3–3,233.8) | (1,309.7–3,030.4) | (1,168.3–2,974.8) | (1,022.3–2,572.0) | (1,197.6–2,977.7) | ||
| Co-efficient of variation | 0.605 | 0.573 | 0.627 | 0.641 | 0.615 | |
| Other acute respiratory tract infection | Practice mean IR (SD) | 1,477.4 (741.3) | 1,494.3 (746.4) | 1,478.5 (764.4) | 1,363.1 (679.3) | 1,456.9 (736.2) |
| Practice median IR (Q1–Q3) | 1,327.6 | 1,368.1 | 1,379.1 | 1,280.6 | 1,338.3 | |
| (976.6–1,848.3) | (1,008.0–1,875.6) | (980.7–1,873.5) | (928.3–1,765.8) | (969.8–1,838.5) | ||
| Co-efficient of variation | 0.502 | 0.499 | 0.517 | 0.498 | 0.505 | |
| Pneumonia | Practice mean IR (SD) | 108.4 (84.1) | 109.2 (88.7) | 101.3 (85.9) | 83.2 (81.6) | 101.3 (85.7) |
| Practice median IR (Q1–Q3) | 99.0 (50.0–146.3) | 95.4 (44.4–162.9) | 86.8 (38.6–146.0) | 61.2 (28.3–123.1) | 86.9 (38.9–145.1) | |
| Co-efficient of variation | 0.776 | 0.812 | 0.848 | 0.981 | 0.846 | |
| Otitis media | Practice mean IR (SD) | 1057.4 (557.1) | 933.6 (511.0) | 930.4 (498.2) | 782.4 (428.0) | 933.3 (512.6) |
| Practice median IR (Q1–Q3) | 977.9 | 885.6 | 912.7 | 756.7 | 885.6 | |
| (678.0–1,356.3) | (604.4–1,242.7) | (588.8–1,243.0) | (492.4–1,033.5) | (582.5–1,221.3) | ||
| Co-efficient of variation | 0.527 | 0.547 | 0.535 | 0.547 | 0.549 | |
| Asthma exacerbation | Practice mean IR (SD) | 654.3 (499.1) | 628.8 (499.8) | 627.3 (493.2) | 536.5 (408.4) | 615.0 (480.6) |
| Practice median IR (Q1–Q3) | 555.9 (356.8–778.9) | 492.9 (306.2–751.0) | 487.6 (310.2–818.4) | 460.8 (276.4–671.0) | 494.3 (314.8–751.4) | |
| Co-efficient of variation | 0.763 | 0.795 | 0.786 | 0.761 | 0.781 | |
| Total | Practice mean IR (SD) | 8,503.2 (3,080.7) | 7,894.8 (2,835.0) | 8,086.9 (3,004.0) | 6,877.2 (2,579.5) | 7,884.3 (2,948.2) |
| Practice median IR (Q1–Q3) | 8,509.2 | 7,927.6 | 8,142.7 | 6,904.7 | 7,890.2 | |
| (6,539.0–1,0240) | (6,375.1–9,818.6) | (6,395.6–9,995.4) | (5,439.2–8,483.5) | (6,078.4–9,753.3) | ||
| Co-efficient of variation | 0.362 | 0.359 | 0.371 | 0.375 | 0.374 | |
IR, incidence rates; SD, standard deviation.
n = total number of registered patients meeting the study inclusion criteria at the beginning of the season.
Childhood influenza vaccination program introduced 2013–2014.
Practice-level incidence rates for each diagnosis group for the under 18 years population are provided in Table 3. Incidence rates for the under 18 years population were higher than those for the general population, in particular for URTI and otitis media (incidence rate per 100,000 population within the range of 8,024–9,950 vs 3,228–4,120 for URTI and 2,668–3,652 vs 782–1,057 for otitis media diagnoses in the under 18 years and general population, respectively). This general trend was not observed for certain diagnoses such as influenza-related illness and LRTI, for which lower incidence rates were observed for patients within the 11–18-year-old age group. Inter-practice variability (measured by CV) was similar to the general population, with some evidence that there were higher levels of variability between practices for influenza-related and pneumonia diagnoses (CV range 2.1–5.9 and 1.3–2.0, respectively). For the majority of diagnosis outcomes, as well as for total outcomes combined, similar incidence rates were observed across the first three seasons, with a decrease in incidence rates observed during the 2013–2014 season.
Table 3.
Practice-level IR for <18 years population.
| Season |
||||||
|---|---|---|---|---|---|---|
| 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 | All seasons combined | ||
| Diagnosis | (n = 2,625,838)a | (n = 2,527,191)a | (n = 2,495,908)a | (n = 2,206,712)a,* | (n = 9,855,649) | |
| Influenza | Practice mean IR (SD) | 105.2 (225.7) | 30.2 (100.2) | 52.7 (286.0) | 35.4 (209.2) | 57.4 (217.5) |
| Practice median IR (Q1–Q3) | 32.9 (0.0–124.2) | 0.0 (0.0–0.0) | 0.0 (0.0–39.5) | 0.0 (0.0–0.0) | 0.0 (0.0–53.4) | |
| Co-efficient of variation | 2.145 | 3.318 | 5.427 | 5.91 | 3.789 | |
| Upper respiratory tract infection | Practice mean IR (SD) | 9,949.6 (4,627.6) | 9,128.8 (4,187.6) | 9,728.9 (4,483.6) | 8,023.8 (3,791.2) | 9,258.5 (4,360.7) |
| Practice median IR (Q1–Q3) | 9,850.9 | 9,113.6 | 9,528.1 | 7,818.1 | 9,113.6 | |
| (6,978.4–12559) | (6,174.5–11,820) | (6,882.4–12,839) | (5,363.2–10,461) | (6221.9–11,891) | ||
| Co-efficient of variation | 0.465 | 0.459 | 0.461 | 0.472 | 0.471 | |
| Lower respiratory tract infection | Practice mean IR (SD) | 1,959.3 (1,486.0) | 1,928.0 (1,435.6) | 1,904.0 (1,457.2) | 1,579.3 (1,301.5) | 1,853.8 (1,432.7) |
| Practice median IR (Q1–Q3) | 1,653.8 | 1,662.9 | 1,521.3 | 1,274.6 | 1,521.8 | |
| (911.9–2,570.1) | (872.1–2,454.9) | (908.4–2,516.6) | (657.3–2,064.2) | (841.8–2,439.6) | ||
| Co-efficient of variation | 0.758 | 0.745 | 0.765 | 0.824 | 0.773 | |
| Other acute respiratory tract infections | Practice mean IR (SD) | 2,521.9 | 2,714.1 | 2,728.7 | 2,761.5 | 2,675.8 |
| (1,515.6) | (1,530.6) | (1,560.7) | (1,510.5) | (1,530.8) | ||
| Practice median IR (Q1–Q3) | 2,271.5 | 2,539.7 | 2,554.7 | 2,588.9 | 2,501.5 | |
| (1,445.8–3,297.5) | (1,654.5–3,434.9) | (1,760.6–3,479.7) | (1,756.1–3,596.3) | (1,643.6–3,450.7) | ||
| Co-efficient of variation | 0.601 | 0.564 | 0.572 | 0.547 | 0.572 | |
| Pneumonia | Practice mean IR (SD) | 59.7 (75.1) | 46.1 (77.9) | 33.4 (66.4) | 25.9 (45.4) | 42.2 (69.3) |
| Practice median IR (Q1–Q3) | 39.2 (0.0–99.7) | 0.0 (0.0–68.8) | 0.0 (0.0–51.5) | 0.0 (0.0–42.7) | 0.0 (0.0–67.3) | |
| Co-efficient of variation | 1.258 | 1.69 | 1.988 | 1.753 | 1.642 | |
| Otitis media | Practice mean IR (SD) | 3,651.6 (1,865.2) | 3,214.4 (1,764.9) | 3,253.3 (1,750.1) | 2,667.8 (1,438.8) | 3,222.8 (1,755.6) |
| Practice median IR (Q1–Q3) | 3,583.8 | 3,066.5 | 3,164.6 | 2,589.9 | 3,084.8 | |
| (2,393.6–4,763.5) | (1,932.8–4,311.9) | (2,072.8–4,321.9) | (1,704.5–3,515.8) | (2,020.9–4,301.1) | ||
| Co-efficient of variation | 0.511 | 0.549 | 0.538 | 0.539 | 0.545 | |
| Asthma exacerbation | Practice mean IR (SD) | 855.2 (605.7) | 820.8 (592.1) | 745.5 (526.4) | 690.7 (533.1) | 782.8 (570.4) |
| Practice median IR (Q1–Q3) | 758.0 | 646.2 | 630.8 | 570.4 | 652.1 | |
| (479.6–1,057.6) | (423.8–1,106.3) | (385.1–1,023.9) | (336.7–909.1) | (405.0–,1018.4) | ||
| Co-efficient of variation | 0.708 | 0.721 | 0.706 | 0.772 | 0.729 | |
| Total | Practice mean IR (SD) | 15,373 (5,415.3) | 14,533 (5,106.1) | 15,123 (5,483.0) | 12,934 (4,776.6) | 14,556 (5,292.4) |
| Practice median IR (Q1–Q3) | 15,488 | 14,871 | 15,068 | 13,099 | 14,708 | |
| (11,819–18,520) | (11,614–17,682) | (11,782–18,756) | (10,241–16,071) | (11,339–17,832) | ||
| Co-efficient of variation | 0.352 | 0.351 | 0.363 | 0.369 | 0.364 | |
IR, incidence rates; SD, standard deviation.
n = total number of registered patients meeting the study inclusion criteria at the beginning of the season.
Childhood influenza vaccination program introduced 2013–2014.
A mixed-effect model was used to estimate the effect of age and gender (fixed effect) on the risk of each diagnosis group, with season and practice as random effects, as illustrated in Fig. 1. The 18 to <65 age group and males were used as the reference groups for age and gender, respectively. The findings for the different diagnostic categories are as follows:
-
•
Influenza-related illness (Fig. 1a): There was a higher risk associated with the 65+ age group (risk ratio [RR] = 1.33), with some evidence that there was also a higher risk for the 0 to <2 and 2 to <4 age groups (RR = 1.12 and 1.16, respectively). The 5 to <11 and 11 to <18 age groups both showed a decreased risk (RR = 0.63 and 0.59, respectively).
-
•
Other ARTI (Fig. 1b): All age groups under 18 and the 65+ age group showed an increased risk of other ARTI.
-
•
LRTI (Fig. 1c) and pneumonia (Fig. 1d): Age groups below 5 and the 65+ age group were at higher risk of LRTI or pneumonia diagnosis, with the 5 to <11 and 11 to <18 age groups at lower risk.
-
•
URTI (Fig. 1e) and otitis media (Fig. 1f): All age groups under 18 years were at higher risk of URTI and otitis media, with a lower risk associated with the 65+ age group.
-
•
Asthma exacerbation (Fig. 1g): The 0 to <2 age group had a lower risk for asthma exacerbations, with higher risk associated with all other age groups under 18 years.
-
•
Across all outcomes (Fig. 1h), female subjects were associated with a higher risk of an influenza or respiratory diagnosis.
Figure 1.
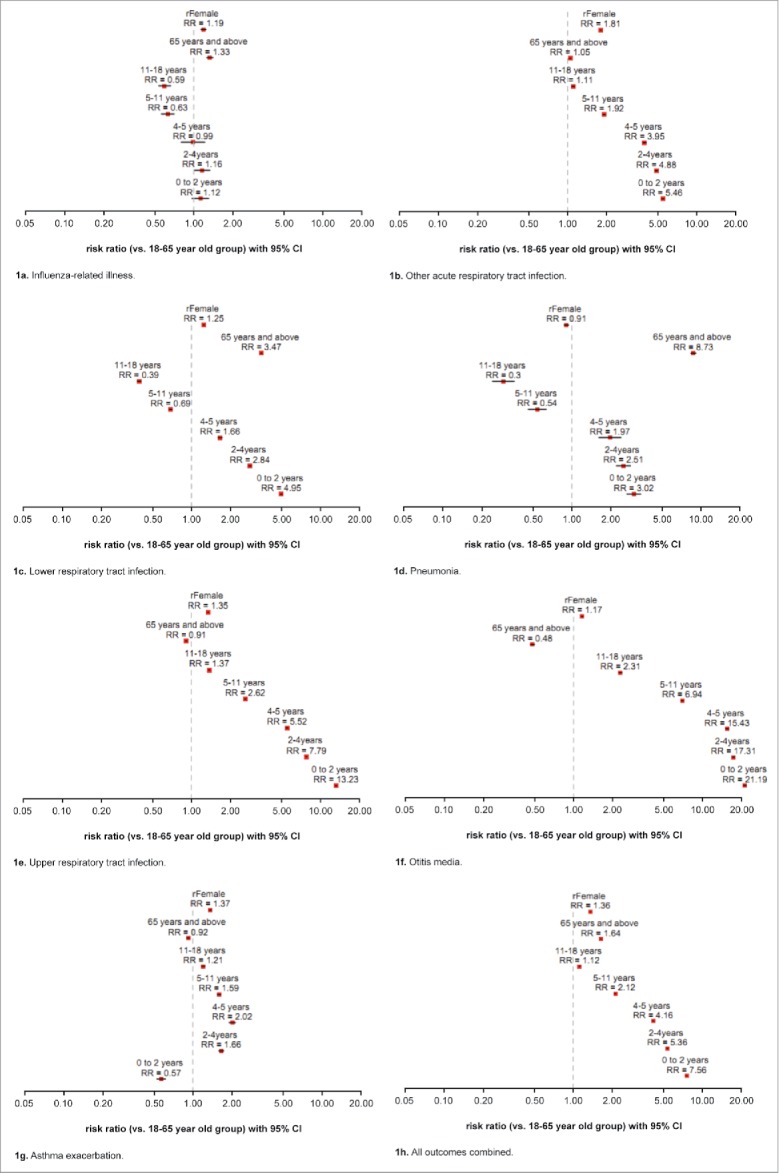
(a) Influenza-related illness. (b) Other acute respiratory tract infection. (c) Lower respiratory tract infection. (d) Pneumonia. (e) Upper respiratory tract infection. (f) Otitis media. (g) Asthma exacerbation. (h) All outcomes combined.
Figure 2 shows the seasonal and practice effects associated with each of the diagnosis groups. Seasonal effects were similar in direction across all diagnosis groups, with the 2010–2011 season associated with an increased risk and the 2013–2014 season associated with a decreased risk of influenza- or respiratory-related diagnoses. The magnitude of the seasonal effect was visibly larger for influenza-related illness, with all other diagnosis groups exhibiting smaller variations across season. Practice effects (increased variability in observed incidence rate) were also much higher for influenza-related illness, as is illustrated within the lower graph of Figure 2a. Practice effects were also comparatively higher for pneumonia in comparison with all other diagnosis groups.
Figure 2.
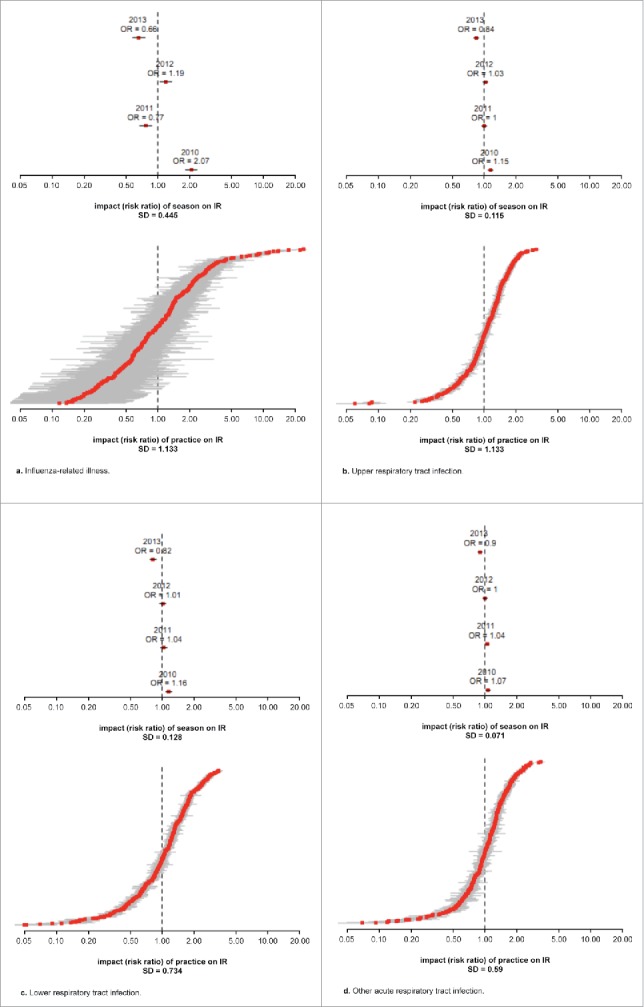
(a) Influenza-related illness. (b) Upper respiratory tract infection. (c) Lower respiratory tract infection. (d) Other acute respiratory tract infection. (e) Pneumonia. (f) Otitis media. (g) Asthma exacerbation. (h) All outcomes combined.
Figure 2.
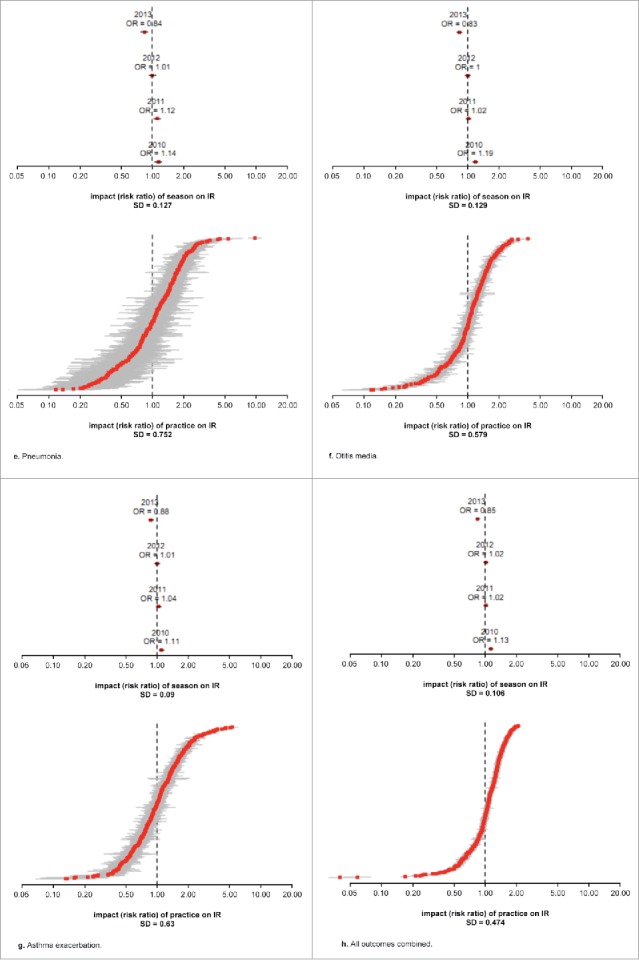
(Continued).
Discussion
This analysis provides valuable insight into the population-level incidence of influenza- and respiratory-related disease in England during the winter months across four consecutive influenza seasons after the 2009 H1N1 pandemic, and the diagnostic labeling of the conditions in UK primary care. While previous studies have reported on the incidence of respiratory disease ascertained through PCP consultations and hospitalizations,9,24 no studies have reported incidence rates for distinct groups of respiratory conditions. Furthermore, no studies at the time of publication have reported on PCP diagnosis rates for seasons following the 2009 H1N1 pandemic, after which PCP diagnosing behavior may have changed and new surveillance processes were initiated.
This study provides specific insights into how respiratory illnesses are classified by PCPs (e.g. ARTI, LRTI, URTI, etc.), and allows us to decide which of these measures would be most appropriate for use in a dynamic disease model, which will enable us to estimate the impact of vaccinating children of different ages on the overall burden of influenza in England. It is recognized that PCPs may not always employ precise criteria when diagnosing patients with respiratory-related conditions, and this descriptive analysis allowed for the characterization of diagnosing behavior using diagnosis categories defined by an expert in influenza and experienced PCP. As the study was conducted over four influenza seasons after the 2009 H1N1 pandemic, the analysis also captures any variability in diagnosing behavior that might have occurred as a result of the pandemic. The most frequent diagnoses during respiratory-related consultations were found to be URTI, followed by LRTI and other ARTI. Comparatively, the frequency of influenza-related illness was low. As influenza is one of the most predominant circulating pathogens during the winter period, our findings suggest that a substantial proportion of primary care consultations presenting with respiratory symptoms and with an underlying influenza infection are being diagnosed as URTI, LRTI, or other ARTI, similar to what has been seen in other studies of influenza illness in primary care practice in the UK.9,24 Further evidence of this is provided when the rates of influenza-related diagnoses from this study are compared with the rates reported by Public Health England (PHE) within the routine influenza surveillance reports,25 which mimic the same pattern as this study, though with significantly lower rates. This is also illustrated by the high level of inter-practice variability associated with the influenza-related illness outcome in comparison with the other outcomes collected in the present study. The findings from this novel categorization of respiratory diagnosis codes after the 2009 pandemic indicate that care should be taken when selecting diagnoses for observational studies of influenza and measuring the impact of influenza vaccination programs, as influenza diagnoses may be recorded under respiratory diagnoses.
The findings from evaluating consultation rates by age in this study are in agreement with previous studies that have highlighted the high burden of influenza and risk of complications in young children and the elderly.1,6 The results observed in this study may be due to the true higher disease burden in the young and elderly, but could be confounded by the fact that young and elderly patients are more likely to visit a PCP for consultation. Only children under the age of 5 were found to be at a higher risk of the more severe diagnoses (pneumonia, LRTI). One potential explanation of this finding is the immaturity of the immune system in children, in particular, the mechanisms that usually recognize influenza viruses and activate immune responses to reduce viral replication.26
The use of the CPRD means that the findings here should be broadly representative of the UK population.27 However, the CPRD does not capture those with influenza who did not seek medical attention, or those who received medical care outside of PCPs. In addition, it is possible that some influenza- and respiratory-related diagnoses could have been attributed to other respiratory viruses, such as respiratory syntactical virus (RSV). One of the major limitations of this study is the lack of virology data, which has been used to perform regression modeling by others to validate the disease burden.9,28,29 However, even when virology data has been used in other studies, it has been limited to influenza A, B and RSV in the past. Hence, presenting the complete information as is, without modeling for virology, lends itself to a more conservative interpretation of the data. Finally, the high levels of inter-practice variability associated with the influenza-related illness outcome in comparison with the other outcomes collected in the present study may represent a weakness in our data; however, future studies will help establish whether this finding persists.
The variability in observed incidence rates across seasons may be attributed to differences in the virulence of the seasonal strains, differences in the level of vaccine match, or differences in the diagnosing practices of PCPs, or of the general population in relation to propensity to consult a PCP. It is possible, for example, that the diagnosing behavior of PCPs may change following a particularly severe influenza season. However, what can be seen is that the overall inter-seasonal trends observed with CPRD data are similar to those observed within PHE virology data.
It is noted that there is a small year-on-year decrease in the total practice-level population across the seasons included within our study (see Table 1). This is likely due to a corresponding decrease in the number of practices registered with CPRD. The number of practices included in the study varied over time depending on how long practices contributed to the CPRD, and was not documented for the purposes of this analysis. However, as population characteristics in terms of age and gender were similar across the seasons considered (as shown in Table 1), it is likely that the underlying population is comparable across seasons, and the decrease in the total population does not bias the results of the analysis. A potential limitation of the study is that multiple diagnoses can be recorded for each PCP respiratory consultation, meaning that a single consultation can be counted in different diagnosis groups. As there is no way of discerning between primary and secondary diagnoses for a consultation, it is not possible to account for this limitation. However, this does not impact the primary objective of this study, which is to evaluate the distribution of practice-level incidence rates of respiratory diagnoses.
The findings of this descriptive study will be used to inform future observational studies and modeling work evaluating the burden of influenza and the impact of vaccination against influenza.
Methods
The study was undertaken as a retrospective observational analysis. Data were obtained from the Clinical Practice Research Datalink (CPRD), which were eligible for linkage to the Hospital Episode Statistics and Office of National Statistics databases. CPRD is a research database made up of a network of PCP practices across the UK and holds anonymized longitudinal primary care records from more than 11 million patients, and is considered broadly representative of the UK population.27 Laboratory data were not used in the analysis as the objective of this study was to investigate overall rates and patterns of respiratory diagnoses and not to attribute burden to influenza or other circulating pathogens.
Data were collected for four consecutive influenza seasons: 2010–2011, 2011–2012, 2012–2013 (before the introduction of the UK's childhood influenza vaccination program), and 2013–2014, the first year of the childhood influenza vaccination program. The start date for each season was 1 September (index date) and the season end date was defined as 13 April (the end of the notable period of community transmission of influenza30). Previous research has used the CPRD to estimate the burden of influenza-attributable illnesses.9
Subjects were included in the analysis for each influenza season if they were registered with an eligible PCP practice within the CPRD network on the start date of that season and had at least 12 months of registered history within the database. Subjects aged <1 year on the start date were included for each season if they were registered with an eligible practice on the index date (1 September) of the respective season.
Subjects were analyzed by age using the following age groups: 0 to <2 years, 2 years to <4 years, 4 years to <5 years, 5 years to <11 years, 11 years to <18 years, 18 years to <65 years, and 65 years and above. The age groups were split in this way to facilitate analysis of the impact of the childhood influenza program, which is being rolled out in a gradual manner across the aforementioned age bands.
Study outcomes
The primary outcome of the study was the incidence of influenza- or respiratory-related PCP diagnoses, chosen based on expert opinion and previous modeling work on age-specific influenza-related hospitalization and mortality in the UK.9,31 Diagnoses were categorized according to the following seven groups based on medical read codes recorded at the time of the PCP consultation.32
-
•
Influenza-related illnesses
-
•
URTI
-
•
LRTI
-
•
Other ARTI
-
•
Pneumonia
-
•
Otitis media
-
•
Asthma exacerbations.
Categories were defined based on input from an influenza clinical expert. To avoid double counting the same underlying infection, only the first PCP consultation during which a relevant code was recorded was counted for each diagnosis group. Any subsequent consultations within the same season in which a code for the same diagnosis group was recorded would not be counted as a separate consultation for that particular diagnosis group.
Statistical analysis
The patient population for each season was described in terms of gender and age group. The distribution of practice-level incidence rates of influenza- and respiratory-related PCP diagnosis outcomes and all outcomes combined were reported by season for the general population (adults and children) and childhood population (<18 years old). Both measures of central values (e.g., means, medians) and dispersion/variations (e.g., interquartile ranges, SD, CV; calculated as a ratio of empirical SD and mean) across practices were calculated. A mixed-effect model was applied to the four seasons to simultaneously capture and disentangle the various sources of variability; namely season and practice. Specifically, a Poisson model was used to describe age and gender effects as a fixed effect, as well as seasonal and practice variability as a random effect for each of the seven influenza- and respiratory-related PCP diagnoses outcomes and all outcomes combined.33–36 No patient-level covariate adjustment was performed. Missing data was not imputed due to the descriptive nature of the study.
This study was approved by the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency (Protocol Number 14_169R). No patient consent was required given the retrospective, non-interventional design of the study.
Abbreviations
- ALRI
acute lower respiratory infections
- ARTI
acute respiratory tract infections
- CPRD
Clinical Practice Research Datalink
- CV
coefficients of variation
- IR
Incidence rate
- LRTI
lower respiratory tract infections
- PCP
primary care provider
- PHE
Public Health England
- RSV
respiratory syntactical virus
- SD
standard deviation
- UK
United Kingdom
- URTI
upper respiratory tract infection
Disclosure of potential conflicts of interest
Richard Lawson, Betina Blak, and Robert Brody are employees of AstraZeneca. Sankarasubramanian Rajaram and Tehseen Salimi were employees of AstraZeneca during the study. Judith Hackett and Yanli Zhao are employees of MedImmune, the biologics arm of AstraZeneca. Witold Wiecek and Billy Amzal are employees of LASER Analytica, who have received funding for the current study from AstraZeneca. Vishal Patel is a former employee of LASER Analytica.
Acknowledgments
Medical writing support was provided by Talya Underwood, MPhil (Cantab) of Prime Global, Knutsford, UK, in accordance with Good Publication Practice guidelines (Link) and funded by AstraZeneca. AstraZeneca was involved in the study design and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors. Clinical guidance and expertise on the diagnosis categories was provided by Dr. Douglas Fleming.
Funding
This study was supported by AstraZeneca.
Declarations
Ethics approval and consent to participate
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available in the CPRD repository, https://www.cprd.com/intro.asp.
Authors' contributions
Sankarasubramanian Rajaram, Tehseen Salimi, Judith Hackett, and Betina Blak were involved in the study. Billy Amzal, Vishal Patel, and Witold Wiecek undertook the analyses. All authors were involved in developing the manuscript and approved the final version for submission.
References
- 1.Antonova EN, Rycroft CE, Ambrose CS, Heikkinen T, Principi N. Burden of paediatric influenza in Western Europe: a systematic review. BMC Public Health. 2012; 12:968. doi: 10.1186/1471-2458-12-968. PMID:23146107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Influenza (Seasonal) Fact sheet No 211. 2014. [accessed 2016 Dec 01]. http://www.who.int/mediacentre/factsheets/fs211/en/ [Google Scholar]
- 3.European Centre for Disease Prevention and Control. Seasonal influenza key messages. 2017 [accessed 2017 Nov 16]. https://ecdc.europa.eu/en/seasonal-influenza/facts/key-messages [Google Scholar]
- 4.Hardelid P, Pebody R, Andrews N. Mortality caused by influenza and respiratory syncytial virus by age group in England and Wales 1999–2010. Influenza Other Respir Viruses. 2013; 7:35–45. doi: 10.1111/j.1750-2659.2012.00345.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox NJ, Subbarao K. Influenza. Lancet. 1999; 354:1277–82. doi: 10.1016/S0140-6736(99)01241-6 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Influenza symptoms. 2016 [Google Scholar]
- 7.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, et al.. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011; 378:1917–30. doi: 10.1016/S0140-6736(11)61051-9 [DOI] [PubMed] [Google Scholar]
- 8.Lafond KE, Nair H, Rasooly MH, Valente F, Booy R, Rahman M, Kitsutani P, Yu H, Guzman G, Coulibaly D, et al.. Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis. PLoS Med. 2016; 13:e1001977. doi: 10.1371/journal.pmed.1001977. PMID:27011229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming DM, Taylor RJ, Haguinet F, Schuck-Paim C, Logie J, Webb DJ, Lustig RL, Matias G. Influenza-attributable burden in United Kingdom primary care. Epidemiol Infect. 2016; 144:537–47. doi: 10.1017/S0950268815001119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Vaccines against influenza. WHO position paper. 2012. [accessed 2016 Dec 01]. http://www.who.int/immunization/position_papers/PP_influenza_november2012_summary.pdf [Google Scholar]
- 11.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993; 110:145–60. doi: 10.1017/S0950268800050779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heikkinen T, Silvennoinen H, Peltola V, Ziegler T, Vainionpaa R, Vuorinen T, Kainulainen L, Puhakka T, Jartti T, Toikka P, et al.. Burden of influenza in children in the community. J Infect Dis. 2004; 190:1369–73. doi: 10.1086/424527 [DOI] [PubMed] [Google Scholar]
- 13.Chin TD, Mosley WH, Poland JD, Rush D, Belden EA, Johnson O. Epidemiologic studies of type B influenza in 1961–1962. Am J Public Health Nations Health. 1963; 53:1068–74. doi: 10.2105/AJPH.53.7.1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glezen WP. Herd protection against influenza. J Clin Virol. 2006; 37:237–43. doi: 10.1016/j.jcv.2006.08.020 [DOI] [PubMed] [Google Scholar]
- 15.Savidan E, Chevat C, Marsh G. Economic evidence of influenza vaccination in children. Health Policy. 2008; 86:142–52. doi: 10.1016/j.healthpol.2007.09.009 [DOI] [PubMed] [Google Scholar]
- 16.Frank AL, Taber LH, Wells CR, Wells JM, Glezen WP, Paredes A. Patterns of shedding of myxoviruses and paramyxoviruses in children. J Infect Dis. 1981; 144:433–41. doi: 10.1093/infdis/144.5.433 [DOI] [PubMed] [Google Scholar]
- 17.Kassianos G, White S, Reynolds AJ, Rajaram S. Review of the experiences from the first childhood influenza vaccination programme with a live attenuated influenza vaccine in England and Scotland. Drugs Context. 2015; 4:212280. doi: 10.7573/dic.212280. PMID:25972905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013; 10:e1001527. doi: 10.1371/journal.pmed.1001527. PMID:24115913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joint Committee on Vaccination and Immunisation Minutes of the meeting held on Friday 13 April 2012. 2012 [Google Scholar]
- 20.Department of Health PHE, and NHS England The flu immunisation programme 2013/14 – extension to children. 2013 [Google Scholar]
- 21.Pitman RJ, Melegaro A, Gelb D, Siddiqui MR, Gay NJ, Edmunds WJ. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect. 2007; 54:530–8. doi: 10.1016/j.jinf.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 22.Cromer D, van Hoek AJ, Jit M, Edmunds WJ, Fleming D, Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect. 2014; 68:363–71. doi: 10.1016/j.jinf.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 23.Hardelid P, Rait G, Gilbert R, Petersen I. Recording of Influenza-Like Illness in UK Primary Care 1995–2013: Cohort Study. PLOS ONE. 2015; 10:e0138659. doi: 10.1371/journal.pone.0138659. PMID:26390295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming DM. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun Dis Public Health. 2000; 3:32–8 [PubMed] [Google Scholar]
- 25.Public Health England Surveillance of influenza and other respiratory viruses in the United Kingdom: Winter 2013/14. 2014 [Google Scholar]
- 26.Principi N, Esposito S. Severe influenza in children: incidence and risk factors. Expert Rev Anti Infect Ther. 2016; 14:961–8. doi: 10.1080/14787210.2016.1227701 [DOI] [PubMed] [Google Scholar]
- 27.Walley T, Mantgani A. The UK General Practice Research Database. Lancet 1997; 350:1097–9. doi: 10.1016/S0140-6736(97)04248-7 [DOI] [PubMed] [Google Scholar]
- 28.Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ, GLaMOR Collaborating Teams . Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: a modeling study. PLoS Med. 2013; 10:e1001558. doi: 10.1371/journal.pmed.1001558. PMID:24302890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paget WJ, Balderston C, Casas I, Donker G, Edelman L, Fleming D, Larrauri A, Meijer A, Puzelli S, Rizzo C, et al.. Assessing the burden of paediatric influenza in Europe: the European Paediatric Influenza Analysis (EPIA) project. Eur J Pediatr. 2010; 169:997–1008. doi: 10.1007/s00431-010-1164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Public Health England PHE weekly national influenza report. Summary of UK surveillance of influenza and other seasonal respiratory illneses. 24 April 2014 – Week 17 report (up to week 16 data). 2014. [accessed 2016 Dec 01]. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/325236/Flu_report_24_April_2014.pdf
- 31.Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016; 16:481. doi: 10.1186/s12889-016-3128-4. PMID:27278794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jick H, Hagberg KW. Effectiveness of influenza vaccination in the United kingdom, 1996–2007. Pharmacotherapy. 2010; 30:1199–206. doi: 10.1592/phco.30.12.1199 [DOI] [PubMed] [Google Scholar]
- 33.Poljak Z, Carman S, McEwen B. Assessment of seasonality of influenza in swine using field submissions to a diagnostic laboratory in Ontario between 2007 and 2012. Influenza Other Respir Viruses. 2014; 8:482–92. doi: 10.1111/irv.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmier J, Li S, King JC Jr., Nichol K, Mahadevia PJ. Benefits and costs of immunizing children against influenza at school: an economic analysis based on a large-cluster controlled clinical trial. Health Aff (Millwood). 2008; 27:w96–104. doi: 10.1377/hlthaff.27.2.w96 [DOI] [PubMed] [Google Scholar]
- 35.Smits AJ, Hak E, Stalman WA, van Essen GA, Hoes AW, Verheij TJ. Clinical effectiveness of conventional influenza vaccination in asthmatic children. Epidemiol Infect. 2002; 128:205–11. doi: 10.1017/S0950268801006574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poljak Z, Dewey CE, Martin SW, Christensen J, Friendship RM. Field efficacy of an inactivated bivalent influenza vaccine in a multi-site swine production system during an outbreak of systemic porcine circovirus associated disease. Can J Vet Res. 2010; 74:108–17 [PMC free article] [PubMed] [Google Scholar]


