ABSTRACT
Limited safety data are available on inadvertent exposure to quadrivalent human papillomavirus vaccine (4vHPV) during pregnancy. We conducted a descriptive observational postlicensure safety surveillance study in Kaiser Permanente Southern California and Northern California to assess congenital anomaly and miscarriage among pregnancies exposed to 4vHPV. Using electronic medical records, we identified women who received a dose of 4vHPV between August 2006 and March 2008 within 30 days preconception or any time during a possible pregnancy. A broad algorithm was developed using diagnostic and procedure codes and laboratory tests to identify pregnancy, congenital anomalies, and miscarriages. Medical records of all potential congenital anomaly cases and a random sample of 100 potential miscarriage cases were reviewed to confirm pregnancy exposure and diagnosis. Results were reviewed by an independent Safety Review Committee (SRC). Among the population of 189,629 females who received at least one dose of 4vHPV during the study period, 2,678 females were identified as possibly having a 4vHPV-exposed pregnancy. Among 170 potential congenital anomalies identified, 44 (26%) were found to be both 4vHPV-exposed and confirmed congenital anomaly cases. Among the 633 potential miscarriages identified, the records of a random sample of 100 cases were reviewed, and 9 cases (9%) were confirmed as 4vHPV-exposed miscarriages. The SRC noted no safety signal for congenital anomaly or miscarriage associated with 4vHPV exposure during pregnancy. The rate of major congenital anomaly (3.6%) was in the range of background estimates from the literature. There was no apparent pattern of timing of 4vHPV exposure among 4vHPV-exposed miscarriages.
KEYWORDS: papillomavirus vaccines, pharmacovigilance, postmarketing product surveillance, pregnancy outcome, safety
Introduction
The quadrivalent human papillomavirus vaccine (4vHPV) is indicated in females age 9–26 years for the prevention of cervical, vulvar, vaginal, and anal cancer; genital warts; and precancerous or dysplastic lesions.1 The Advisory Committee on Immunization Practices (ACIP) recommends routine HPV vaccination at age 11–12 years, starting as early as 9 years. HPV vaccination is also recommended for females age 13–26 years if not previously vaccinated.2 While 4vHPV is not recommended for use in pregnant women, the eligibility age for vaccination poses a risk for inadvertent administration in women who do not realize they are pregnant.
Limited safety data regarding 4vHPV in pregnant women are available. While the 4vHPV clinical trials excluded pregnant women, 3,819 females aged 16–45 years in the trials reported at least one pregnancy.1 Adverse outcomes, defined as the combined number of spontaneous abortions, late fetal deaths, and congenital anomaly cases, were observed in 22.6% of the 4vHPV group vs. 23.1% of the placebo group. Additionally, a voluntary registry for females inadvertently vaccinated during pregnancy was established by the manufacturer as part of its postlicensure commitment to the US Food and Drug Administration (FDA).3 The registry enrolled women exposed to 4vHPV within 1 month before the last menstrual period or any time during pregnancy. Among the 1,752 reports with a known pregnancy outcome, the overall rate of spontaneous abortion was 6.7 per 100 outcomes, and the prevalence of major birth defects was 2.4 per 100 live-born neonates. Furthermore, among 147 non-manufacturer reports to the Vaccine Adverse Events Reporting System (VAERS) of 4vHPV administered to pregnant women, the most frequent pregnancy-specific outcome was spontaneous abortion in 15 (10.2%) reports, and only two reports of major birth defects were received.4 Finally, in a nationwide study conducted in Denmark, 4vHPV during pregnancy was not associated with significantly greater risks of adverse pregnancy outcomes, including major birth defects and spontaneous abortion.5
Overall, the existing safety data do not suggest an increased risk of spontaneous abortion, fetal malformations, or adverse pregnancy outcomes beyond that found in the general population.6 These prior studies have some potential limitations, however. The clinical trials populations tend to be healthier and more homogeneous than the general public that receives vaccines postlicensure. Furthermore, in clinical trials, women were tested for pregnancy prior to vaccination, and were not vaccinated if they tested positive. As such, the majority of estimated dates of conceptions occurred at least 6 months after vaccination. In contrast, during real world use, women may be pregnant without their knowledge at the time of 4vHPV initiation. Voluntary, spontaneous reports lack a clear denominator, are prone to biased reporting, and have limited data for medical adjudication. The Danish study relied on diagnoses recorded in the national registry without record review, and did not consider women vaccinated within 30 days preconception as being potentially exposed. Using a large, geographically diverse population of women enrolled in two large integrated health care organizations, we undertook a descriptive observational postlicensure safety surveillance study to assess pregnancy outcomes (congenital anomaly and miscarriage) among pregnancies with inadvertent exposure to 4vHPV.
Results
Potential 4vHPV-exposed pregnancies
Among the population of 189,629 females who received at least one dose of 4vHPV during the study period, 187,905 (99%) were between 9–26 years of age at their first dose. There were 2,678 females identified as possibly having a 4vHPV-exposed pregnancy, of whom 1,740 (65%) had a pregnancy outcome recorded in the electronic medical record (EMR). These outcomes included 665 live births (38%), 633 potential miscarriages (36%), and 442 potential elective abortions (25%).
Congenital Anomaly
There were 170 potential congenital anomalies that were identified among 4vHPV-exposed pregnancies (Fig. 1): 157 from live births (92%) and 13 from medical genetics visits (not live births) (8%). However, after initial review by the Principal Investigators (PIs) and subsequent review by the Pregnancy Case Review Committee (PCRC) of the 170 potential congenital anomalies in 4vHPV-exposed pregnancies identified electronically, 44 (26%) of the potential cases were either not pregnancy cases or not 4vHPV-exposed pregnancies (28 excluded by PIs; 16 excluded by PCRC). For the remaining potential cases, 77 (45%) were refuted (e.g., low fetal weight but not a congenital anomaly), 5 (3%) were not confirmed due to insufficient information or a tie among the PCRC reviewers, and 44 (26%) were confirmed congenital anomaly cases.
Figure 1.
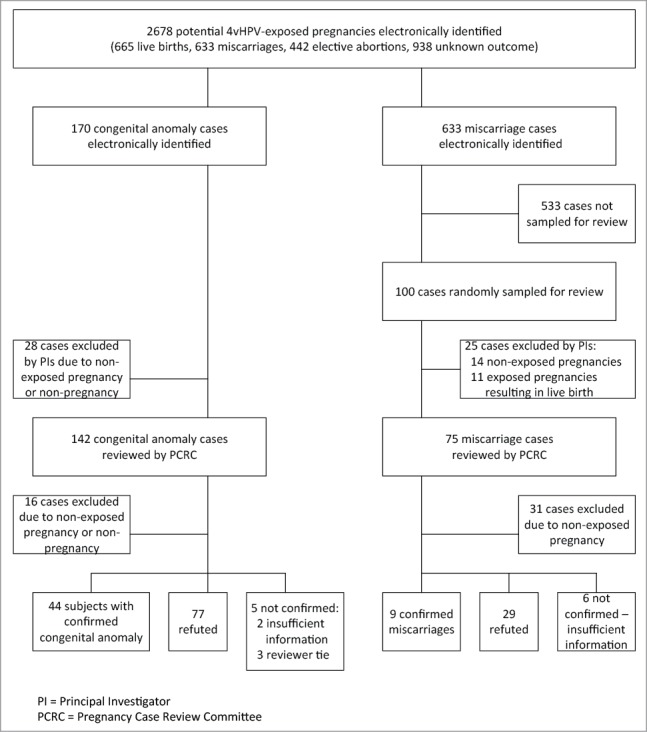
Congenital anomaly and miscarriage case review among potential 4vHPV‐exposed pregnancies, vaccinated August 2006 – March 2008.
The confirmed congenital anomalies spanned a wide range of body systems, as described in Table 1. Of the 44 babies with confirmed anomalies, 43 were from live births and 1 was identified from a medical genetics visit. Two babies each had 2 anomalies. There were 42 cases among females who received 1 dose of 4vHPV during pregnancy; 18 received 4vHPV within 30 days preconception, 22 within the first trimester, and 2 within the second trimester. There were 2 cases among females who received 2 doses of 4vHPV during pregnancy; both females received one dose in the first trimester and one dose in the second trimester. Maternal age at birth ranged from 15 to 34 years. There were 16 mothers who received other vaccinations during the same visit they received 4vHPV. Among the 665 live births followed for up to 180 days after birth in the pregnancy population, 43 babies (6.5% of live births) had a confirmed anomaly, and of these, 24 babies (3.6% [95% confidence interval: 2.3-5.3%] of live births) had a major anomaly according to Metropolitan Atlanta Congenital Defects Program (MACDP) criteria.
Table 1.
Confirmed congenital anomaly cases among women with 4vHPV-exposed pregnancies, vaccinated August 2006 – March 2008.
| Case Number | Case Review Result | Timing of 4vHPV exposure | Number of 4vHPV doses during pregnancy | MACDP categorization |
|---|---|---|---|---|
| 1 | 1. Gastroschisis 2. Patent Ductus Arteriosus |
Second trimester | 1 | 1. Major 2. Minor |
| 2 | Anencephaly Note: Identified with genetics visit code (not live birth) |
Preconception | 1 | Major |
| 3 | Atrial septal defect | Preconception | 1 | Major |
| 4 | Atrial septal defect, ventricular septal defect | First trimester | 1 | Major |
| 5 | Branch pulmonary artery stenosis | Preconception | 1 | Major |
| 6 | Cerebral atrophy | Preconception | 1 | Major |
| 7 | Cleft lip / palate | First trimester | 1 | Major |
| 8 | Congenital hypoplasia depressor anguli oris muscle | Preconception | 1 | Major |
| 9 | Congenital knee dislocation | First trimester | 1 | Major |
| 10 | Congenital torticollis | First trimester | 1 | Major |
| 11 | Congenital torticollis | First trimester | 1 | Major |
| 12 | Down's syndrome | First trimester | 1 | Major |
| 13 | Glanular hypospadias | First trimester | 1 | Major |
| 14 | Hydronephrosis | Preconception | 1 | Major |
| 15 | Hydronephrosis | First trimester | 1 | Major |
| 16 | Hypospadias | First trimester | 1 | Major |
| 17 | Hypospadias / epispadias | Preconception | 1 | Major |
| 18 | Imperforate anus | Preconception | 1 | Major |
| 19 | Imperforate anus with rectovaginal fistula | First trimester | 1 | Major |
| 20 | Jejunal atresia | Preconception | 1 | Major |
| 21 | Plagiocephaly | First trimester | 1 | Major |
| 22 | Ptosis | First trimester | 1 | Major |
| 23 | Sacrococcygeal teratoma | First trimester | 1 | Major |
| 24 | Ventricular septal defect | First trimester; second trimester | 2 | Major |
| 25 | Ventricular septal defect | Preconception | 1 | Major |
| 26 | Accessory nipple | Second trimester | 1 | Minor |
| 27 | Hemangioma | First trimester | 1 | Minor |
| 28 | Lacrimal dacryostenosis | First trimester | 1 | Minor |
| 29 | Lacrimal dacryostenosis | First trimester | 1 | Minor |
| 30 | Lacrimal dacryostenosis | First trimester | 1 | Minor |
| 31 | Laryngomalacia | First trimester | 1 | Minor |
| 32 | Left preauricular skin tag | Preconception | 1 | Minor |
| 33 | Mongolian spots | Preconception | 1 | Minor |
| 34 | Patent ductus arteriosus | Preconception | 1 | Minor |
| 35 | Tongue tie | Preconception | 1 | Minor |
| 36 | Tongue tie | First trimester | 1 | Minor |
| 37 | Tongue tie | Preconception | 1 | Minor |
| 38 | Tongue tie | First trimester | 1 | Minor |
| 39 | Tongue tie | Preconception | 1 | Minor |
| 40 | Undescended testis | First trimester | 1 | Minor |
| 41 | Undescended testis | First trimester; second trimester | 2 | Minor |
| 42 | Undescended testis | Preconception | 1 | Minor |
| 43 | Undescended testis | Preconception | 1 | Minor |
| 44 | Undescended testis | First trimester | 1 | Minor |
MACDP = Metropolitan Atlanta Congenital Defects Program
Case 12 was vaccinated at 13th week of pregnancy and is classified as first trimester exposure (originally in the study, it was classified as second trimester exposure). For cases 24 and 41, both trimesters of vaccine exposure are shown here (originally in the study, only most recent trimester was counted).
There was no apparent pattern or distribution of anomalies by concomitant vaccinations (given at same health care visit as 4vHPV), nor was there any apparent pattern or distribution of anomalies by maternal age. The SRC determined that the distribution of anomaly cases was consistent with that of the general population.
Miscarriage
Among the 633 potential miscarriages identified electronically, a random sample of 100 (16%) cases was identified for medical record review (Fig. 1). After initial review by the PIs and subsequent review by the PCRC, 45 of the potential cases were not 4vHPV-exposed pregnancies, 11 were exposed pregnancies resulting in live birth, 29 were refuted (e.g., due to evidence of elective abortion), 6 had insufficient information, and 9 exposed pregnancies were confirmed as miscarriage.
Among the 9 confirmed miscarriage cases, the mean maternal age was 21 years (standard deviation = 8, median = 18, range = 15-41). All of the cases had only 1 dose of 4vHPV during pregnancy. Four of the cases received concomitant vaccines during the visit when 4vHPV was administered. There was no apparent pattern or clustering to the estimated gestational age at miscarriage (mean = 10 weeks, standard deviation = 7 weeks), nor in timing of exposure to 4vHPV, which ranged from 4 weeks prior to beginning of pregnancy to 11 weeks of pregnancy (i.e., 4 preconception and 5 first trimester exposures). The SRC reviewed these data pertaining to miscarriages and noted no safety signal of miscarriage associated with exposure to 4vHPV.
Discussion
In this postlicensure study, we assessed outcomes following inadvertent 4vHPV exposure during pregnancy. After evaluation of 2,678 potential 4vHPV exposed pregnancies, we found that 3.6% (95% CI: 2.3-5.3%) of live births had a major anomaly according to MACDP criteria. This was in the range of background estimates from the literature. The annual rate of major birth defects at birth among babies born in the United States overall and in California is 3.0%.7,8 From 4vHPV clinical trials, there were 31 congenital or other anomalies reported at birth among 1,447 live births (2.1%).9 From the manufacturer's pregnancy registry for 4vHPV, the prevalence of major congenital anomalies at birth was 2.4% per live born infant, according to MACPD criteria.3 In a recent study of major structural birth defects in large healthcare datasets from 2004 to 2013, the period prevalence was 1.7 per 100 live births based on algorithms involving criteria for number, timing, and setting of diagnoses, vs. 4.4 per 100 live births based on a single ICD-9 code in the first year of life.10
We conducted detailed medical record review on a random sample of suspected miscarriages, and characterized confirmed 4vHPV-exposed miscarriage cases. Detailed information on miscarriages among 4vHPV-exposed pregnancies was not available in the published literature. However, in an observational cohort study on the risk of miscarriage among women 15–25 years of age exposed to 2vHPV in the United Kingdom,11 the mean gestational age at miscarriage was approximately 11 weeks and the mean maternal age at pregnancy was 18 years, while in our study the mean gestational age at miscarriage was 10 weeks and the mean maternal age at pregnancy was 21 years.
There are several potential limitations to this study. First, our ability to identify 4vHPV-exposed pregnancies, congenital anomalies, and miscarriages was limited by available data. Among 2,678 potential 4vHPV exposed pregnancies, 938 lacked pregnancy outcomes in the EMR. These women may not have actually been pregnant (given the highly sensitive algorithm that was applied to identify potential pregnancies), may have left the Kaiser Permanente system, may have had a miscarriage or elective abortion outside the system, and may not have presented for medical care particularly with early miscarriages. Second, in order to ensure we captured all subjects with possible exposure during pregnancy, we applied a wide window for exposure. While this likely resulted in overestimation of the number of 4vHPV-exposed pregnancies, it was considered appropriate because it likely identified all potential 4vHPV-exposed pregnancies of interest. We found that approximately 26% of potential congenital anomalies and 45% of potential miscarriages were found not to be 4vHPV-exposed pregnancies or not pregnant. Having limited pregnancy dating information was a challenge not unique to our study; in other EMR database studies of vaccine safety during pregnancy, gestational age data were incomplete for pregnancies that did not end in live births.12 In 4vHPV clinical trials, only 6.3% of pregnancies had an estimated date of conception within 30 days of vaccination.9 Third, another limitation is that a limited random sample (n = 100, 16%) of potential miscarriages was selected for case review. However, there is no reason to expect a systematic difference in outcomes among potential cases randomly sampled for review and those that were not selected. Together, these limitations made it difficult to calculate rates of miscarriage among 4vHPV-exposed pregnancies. Nevertheless, we were able to estimate the prevalence of major anomalies among live births in our pregnancy population. Fourth, the lack of a direct internal comparison group is a limitation of this descriptive study, necessitating a more cautious interpretation of the results. Finally, it is unclear how these findings for 4vHPV translate to 2vHPV or 9vHPV.
A strength of this study included the use of broadly inclusive algorithms for electronic identification of potential miscarriages and congenital anomalies diagnosed within 180 days after birth. Another strength was the thorough case review process of the medical records by a committee of neonatal-perinatal physicians to confirm or refute the diagnoses identified electronically and to estimate the date of conception. Finally, the study was conducted in a large, racially and socioeconomically diverse US population of women who received the vaccine in the course of routine clinical care.
In conclusion, the rate of major congenital anomaly was comparable to that of the general population, and there was no apparent pattern of timing of 4vHPV exposure among 4vHPV-exposed miscarriages. While 4vHPV is not recommended during pregnancy, this study provides some reassurance to those women who may have inadvertently been exposed during pregnancy, along with their providers. This study adds to a modest but important literature on the safety of inadvertent 4vHPV exposure during pregnancy.
Methods
Setting
We conducted a safety surveillance study of 4vHPV in routine use among women as part of a postlicensure commitment to Food and Drug Administration and the European Medicines Agency. The surveillance study was registered at www.ClinicalTrials.gov (NCT01078220). Findings on general safety and autoimmune conditions have previously been reported;13,14 here we report on pregnancy outcomes. The study duration was based on the time needed to accrue the required sample size for the general safety aim of the postlicensure commitment. This was a descriptive study; no formal comparison was planned due to the anticipated low frequency of inadvertent exposure during pregnancy in this young population.
We conducted this descriptive pregnancy safety surveillance study in Kaiser Permanente Southern California (KPSC) and Kaiser Permanente Northern California (KPNC), each with over 3 million members generally representative of the California and US populations on several key demographic and socioeconomic variables.15,16 Care delivery is facilitated by the integrated network and use of extensive electronic medical records (EMR), which capture data on sociodemographics, utilization (outpatient, emergency department, and inpatient encounters), diagnoses, vaccinations, laboratory tests, pharmacy utilization, and membership. In these pre-paid integrated systems, members are strongly encouraged to stay within the network for all care including routine preventive services. This study was approved by the Institutional Review Boards of KPSC and KPNC.
Subjects
We identified eligible study subjects through the EMR. Women were eligible for inclusion if they received a dose of 4vHPV between August 2006 (when 4vHPV was first available within Kaiser Permanente) and March 2008. We then identified the subset of women who were vaccinated with 4vHPV within 30 days preconception or any time during a possible pregnancy. A broad algorithm was developed using pregnancy tests and diagnostic and procedure codes (International Classification of Diseases, 9th Revision [ICD-9], and Current Procedural Terminology, 4th Edition [CPT-4]) to identify pregnancy (Appendix A).
Information necessary to date pregnancies (e.g., date of last menstrual period or date of delivery) was available in the EMR for approximately half of the females in the pregnancy population. We used these data whenever possible. For the remaining pregnancies, we broadly captured females vaccinated with 4vHPV any time up to 10 months before or 9 months after the event indicating the pregnancy (i.e., diagnostic or procedure code) in the EMR, in order to include all months of a potential pregnancy and 30 days preconception. If there was more than one event in the EMR suggestive of pregnancy, but no definite pregnancy start or end date, the window for defining potential pregnancy exposure to 4vHPV was shortened using the specific data available for each potential case. For example, if a woman had 4 encounters with pregnancy-related codes in her EMR over a 4 month period, but no pregnancy start or end date, the window would start 10 months before the last encounter and end 9 months after the first encounter, for a total span of 15 months instead of 19 months.
Outcomes
As with the approach for identifying exposed pregnancies, identification of pregnancy outcomes was designed to be as inclusive as possible, to achieve high sensitivity for detecting outcomes. Subjects were passively followed through the EMR to subsequently identify congenital anomalies in the infants and miscarriages. The EMRs of infants were linked to study subjects and we searched for congenital anomalies up to 180 days after birth. We included the full range of ICD-9 codes related to congenital anomalies (Appendix B). To capture potential congenital anomalies not yet diagnosed within the 180-day window, a search of expanded codes (Appendix B) was also performed in the infant's record if available; if not available, the mother's record was searched. These included records for suspected or unspecified fetal abnormality, fetal demise, poor or excess fetal growth, unspecified fetal and placental problem, “light-for-dates”, fetal growth retardation, and lack of expected physiological development. The mother's EMR was reviewed for coding indicative of a medical genetics visit, and birth certificates were screened for indication of congenital anomaly. Miscarriages were identified using codes for spontaneous abortion, fetal demise, or surgical treatment for spontaneous abortion (Appendix C).
Medical record review
By casting a wide net to detect outcomes, our approach had high sensitivity but low specificity. Medical record review was needed to sort out the true positives (i.e., confirmed diagnosis of congenital anomaly or miscarriage and pregnancy exposure to 4vHPV) from the false positives. Medical records associated with all potential congenital anomalies and a random sample of 100 potential miscarriages were obtained and redacted as to mother's vaccination status. Due to the large number of potential miscarriages identified by the comprehensive detection algorithm, only a random sample of potential miscarriages was reviewed to keep the study within resource and time constraints. In cases of potential congenital anomalies, both the infant's and mother's records were reviewed. Records were reviewed from the estimated date of conception or date of last menstrual period through pregnancy resolution or 180 days following a live birth. If staff noted details within the record that would obviously exclude a case (e.g., not a pregnancy), the Principal Investigator (PI) at each site reviewed and confirmed whether the case should be excluded.
To assess the validity of congenital anomaly and miscarriage diagnoses, an independent Pregnancy Case Review Committee (PCRC) was formed, consisting of three neonatal-perinatal physicians with expertise in teratology, obstetrics, or developmental biology. The PCRC reviewed the redacted medical records of all congenital anomalies and the sample of miscarriage cases, and classified each as “confirmed”, “refuted”, or “insufficient information”. For confirmed cases, the PCRC also determined the timing (both date of outcome and estimated date of conception). Cases were ultimately classified as “confirmed” or “refuted” based upon the majority determination of PCRC members. Cases with a 3-way tie or with insufficient information were considered “not confirmed”.
Analysis
We tabulated the number of potential 4vHPV exposed pregnancies, along with the frequencies and proportions of live births, congenital anomalies, and miscarriages among those pregnancies. Using information from PCRC review on estimated date of conception, along with date of 4vHPV vaccination from the EMR, some potential cases were excluded because they were ultimately determined as not being 4vHPV-exposed during pregnancy. We tabulated congenital anomaly and miscarriage cases confirmed and refuted by the PCRC. For confirmed events, we characterized the gestational age at vaccination, maternal age at outcome, and number of 4vHPV doses received during pregnancy. We further classified congenital anomalies as major or minor according to the criteria in the Metropolitan Atlanta Congenital Defects Program (MACDP).17 MACDP only includes major anomalies when estimating birth defect rates.
Safety Review Committee
An external Safety Review Committee (SRC) reviewed the pregnancy safety surveillance results for any evidence of a safety signal. The SRC comprised five independent experts in pediatrics, clinical epidemiology, vaccinology, perinatology/teratology, pediatric rheumatology, and pharmacoepidemiology. The SRC was unmasked to 4vHPV exposure status, and assessed the results for any association between 4vHPV exposure during pregnancy and congenital anomaly and miscarriage.
Appendix A.
Criteria for Identifying Pregnancy
The presence of any of the following codes or criteria was used to identify pregnancy:
1. A positive urine or serum pregnancy test
2. Receipt of prenatal care Current Procedural Terminology, 4th Edition (CPT-4) codes
Abortion
59840, 59841, 59850–59852, 59855–59857
Miscarriage/stillbirth
59812, 59820, 59821, 59830
Live birth
59400, 59409, 59410, 59510, 59514, 59515, 59610, 59612, 59614, 59618, 59620, 59622
3. Pregnancy International Classification of Diseases, 9th Revision (ICD-9) codes
630–633 Ectopic and molar pregnancy
640–649 Complications mainly related to pregnancy
650–659 Normal delivery and other indication for care in pregnancy, labor and delivery
660–669 Complications occurring mainly in the course of labor and delivery
670–677 Complications of the puerperium
V22, V22.x Normal pregnancy
V23, V23.x Supervision of high-risk pregnancy
V27 outcomes of delivery
V28 Antenatal screening
4. Notation of therapeutic abortion, or miscarriage/spontaneous abortion ICD-9 codes
69.01 Dilation and curettage for termination of pregnancy
69.02 Dilation and curettage following delivery or abortion
69.51 Aspiration curettage of uterus for termination of pregnancy
69.52 Aspiration curettage following delivery or abortion
69.93 Insertion of laminaria
73.1 Other surgical induction of labor
74.91 Hysterotomy to terminate pregnancy
75.0 Intra-amniotic injection for abortion
634 Spontaneous abortion
635 Legally induced abortion
636 Illegally induced abortion
637 Unspecified abortion
638 Fail attempted abortion
639 Complication following abortion or ectopic and molar pregnancies
5. Stillbirth ICD-9 codes
656.4 Fetal demise
V27.1 Single Stillborn
V27.3 Twins, one liveborn and one stillborn
V27.4 Twin, both stillborn
V27.6 Other multiple birth, some liveborn
V27.7 Other multiple birth, all stillborn
V27.9 Unspecified outcome of delivery
6. Live birth
KPSC:
Record in KPSC Perinatal Services System.
ICD-9 codes for live birth:
V27.0 Single liveborn
V27.2 Twins, both liveborn
V27.3 Twins, one liveborn and one stillborn
V27.5 Other multiple birth, all liveborn
V27.6 Other multiple birth, some liveborn
V30-39 Live born infants
KPNC:
KPNC pulled live births from the hospital files for children with a date of birth between October 1, 2006 and January 31, 2009. These records had live birth ICD-9 codes.
Note: ICD-9 codes included all additional fourth- or fifth- level digits where applicable. For example, code 651 listed above (in 650–659) included any 651.xx code (e.g., 651.00, 651.01, 651.03, 651.10, etc.).
Appendix B.
Criteria for Identifying Congenital Anomaly
The presence of any of the following codes or criteria was used to identify suspected cases of congenital anomaly:
Original ICD-9 diagnosis codes (screened baby's chart)
Nervous system
740 Anencephalus and similar anomalies
741 Spina bifida
742 Other congenital anomalies of nervous system
Eye, ear, face and neck
743 Congenital anomalies of eye
744 Congenital anomalies of ear, face, and neck
Circulatory system
745 Bulbus cordis anomalies and anomalies of cardiac septal closure
746 Other congenital anomalies of heart
747 Other congenital anomalies of circulatory system
Respiratory system
748 Congenital anomalies of respiratory system
Digestive system
749 Cleft palate
750 Other congenital anomalies of upper alimentary tract
750.5 Pyloric stenosis
751 Other congenital anomalies of digestive system
751.3 Congenital megacolon
Genital organs
752 Congenital anomalies of genital organs
Urinary system
753 Congenital anomalies of urinary system
753.2 Congenital hydronephrosis
Musculoskeletal system
754 Certain congenital musculoskeletal deformities
754.3 Hip dysplasia
754.7 Club foot
755 Other congenital anomalies of limbs
756 Other congenital musculoskeletal anomalies
Other
757 Congenital anomalies of the integument
758 Chromosomal anomalies
759 Other and unspecified congenital anomalies
Expanded ICD-9 codes for identifying suspected congenital anomalies (screened baby's chart; if baby's chart was not available, screened mother's chart)
655.8 Other known or suspected fetal abnormality, not elsewhere classified
655.9 Unspecified, fetal abnormality
656.4 Fetal demise
656.5 Poor fetal growth
656.6 Excess fetal growth
656.9 Unspecified fetal and placental problem
764.0 “Light-for-dates” without mention of fetal malnutrition
764.9 Fetal growth retardation, unspecified
783.4 Lack of expected normal physiological development in childhood
Medical genetics visit (screened mother's chart)
V26.33 Genetic counseling visit
Birth certificate
For live births, birth certificates in administrative database were screened for indication of congenital anomaly.
Note: ICD-9 codes included all additional fourth- or fifth- level digits where applicable.
Appendix C.
Criteria for Identifying Miscarriage
The presence of any of the following codes was used to identify suspected cases of miscarriage:
Miscarriage/ spontaneous abortion
630, 631,632, 633, 634, 637, 639, 640, 646.3
Fetal demise
656.4 Fetal demise
V27.1 Single Stillborn
V27.3 Twins, one liveborn and one stillborn
V27.4 Twin, both stillborn
V27.6 Other multiple birth, some liveborn
V27.7 Other multiple birth, all stillborn
V27.9 Unspecified outcome of delivery
Surgical treatment of spontaneous abortion
59812 Treatment of incomplete abortion, any trimester, complete surgically
59820 Treatment of missed abortion, complete surgically, first trimester
59821 Treatment of missed abortion, complete surgically, second trimester
59830 Treatment septic abortion, complete surgically
Note: ICD-9 codes included all additional fourth- or fifth- level digits where applicable.
Disclosure of potential conflicts of interest
The study sponsor, Merck & Co., provided substantial input into the study design and analytic plan, and took part in reviewing analyses and drafting and revising the manuscript. All data were collected and analyzed at Kaiser Permanente. Kaiser Permanente authors made final decisions regarding manuscript edits.
Dr. Chao, Dr. Jacobsen, and Mr. Slezak received research funding from Merck & Co. for another study related to 4vHPV. Dr. Chao also received research funding from Merck & Co., Pfizer, Amgen, and Seattle Genetics for other unrelated studies. Dr. Jacobsen served as an unpaid consultant to Merck & Co. Dr. Klein received research support from Merck & Co., GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Cheetham received research funding from Bristol-Myers Squibb for an unrelated study. Drs. Velicer and Liaw are employees of Merck & Co.
Acknowledgments
We thank the Safety Review Committee members for their valuable input throughout this study (affiliations at the time of the study are listed): Dr. Robert Davis, Center for Health Research, Southeast, Kaiser Permanente of Georgia; Dr. Neal Halsey, Bloomberg School of Public Health, Johns Hopkins University; Dr. Robert Jacobson, Chair, Department of Pediatric and Adolescent Medicine, Mayo Clinic; Dr. Edward Lammer, Children's Hospital Oakland Research Institute; and Dr. Ann Reed, Chair, Department of Pediatric Rheumatology, Mayo Clinic. We thank the Pregnancy Case Review Committee Members for their valuable contribution to the case review process: Dr. Harold Bass, Dr. Leslie Casper, and Dr. George Tiller. We also thank the following individuals for their assistance in preparing medical records for the Case Review Committee: Ms. Sandy Bauska, Ms. Felicia Bixler, Ms. Nancy Canul, Mr. Alexander Carruth, Ms. Marilyn Foley, Ms. Mamie Ford, Ms. Joy Gelfond, Ms. Michelle McGuire, Ms. Catalina Magallon, Ms. Margarita Magallon, Mr. Barry Nichols, Ms. Virginia Robinson, Ms. Pat Ross, and Ms. Ellenie Tuazon.
Funding
This study was funded by Merck & Co.
Author contributions
The authors are responsible for the work described in this paper. All authors were involved in at least one of the following: conception and design of the study, acquisition of data, and/or analysis and interpretation of data. All authors drafted and/or revised the manuscript for important intellectual content, and all authors provided final approval of the submitted manuscript.
References
- 1.Food and Drug Administration Product approval-prescribing information [Package insert]. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant], Merck & Co, Inc. US Department of Health and Human Services, Food and Drug Administration, 2015. [Accessed 22-Nov-2016.] http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111263.pdf. [Google Scholar]
- 2.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA Jr, Unger ER, Centers for Disease Control and Prevention (CDC) . Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 3.Goss MA, Lievano F, Buchanan KM, Seminack MM, Cunningham ML, Dana A. Final report on exposure during pregnancy from a pregnancy registry for quadrivalent human papillomavirus vaccine. Vaccine. 2015;33:3422–8. doi: 10.1016/j.vaccine.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Moro PL, Zheteyeva Y, Lewis P, Shi J, Yue X, Museru OI, Broder K. Safety of quadrivalent human papillomavirus vaccine (Gardasil) in pregnancy: adverse events among non-manufacturer reports in the Vaccine Adverse Event Reporting System, 2006–2013. Vaccine. 2015;33:519–22. doi: 10.1016/j.vaccine.2014.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheller NM, Pasternak B, Molgaard-Nielsen D, Svanstrom H, Hviid A. Quadrivalent HPV Vaccination and the Risk of Adverse Pregnancy Outcomes. New England journal of medicine. 2017;376:1223–33. doi: 10.1056/NEJMoa1612296. [DOI] [PubMed] [Google Scholar]
- 6.Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV Bivalent and Quadrivalent Vaccines During Pregnancy. Annals of pharmacotherapy. 2011;45:258–62. doi: 10.1345/aph.1P396. [DOI] [PubMed] [Google Scholar]
- 7.California Birth Defects Monitoring Program Maternal, Child, & Adolescent Health Division of the California Department of Public Health. Preventing birth defects through research. 2010. [Accessed 07-Apr-2010.] https://www.cdph.ca.gov/Programs/CFH/DGDS/Pages/cbdmp/default.aspx. [Google Scholar]
- 8.Centers for Disease C, Prevention Update on overall prevalence of major birth defects–Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 9.Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, et al. . Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: A combined analysis of five randomized controlled trials. Obstetrics and gynecology. 2009;114:1179–88. doi: 10.1097/AOG.0b013e3181c2ca21. [DOI] [PubMed] [Google Scholar]
- 10.Kharbanda EO, Vazquez-Benitez G, Romitti PA, Naleway AL, Cheetham TC, Lipkind HS, Sivanandam S, Klein NP, Lee GM, Jackson ML, et al. . Identifying birth defects in automated data sources in the Vaccine Safety Datalink. Pharmacoepidemiol Drug Saf. 2017;26:412–20. doi: 10.1002/pds.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baril L, Rosillon D, Willame C, Angelo MG, Zima J, van den Bosch JH, Van Staa T, Boggon R, Bunge EM, Hernandez-Diaz S, et al. . Risk of spontaneous abortion and other pregnancy outcomes in 15–25 year old women exposed to human papillomavirus-16/18 AS04-adjuvanted vaccine in the United Kingdom. Vaccine. 2015;33:6884–91. doi: 10.1016/j.vaccine.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, Kharbanda EO, Irving S, Cheetham TC, McCarthy NL, et al. . Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013;31:2898–903. doi: 10.1016/j.vaccine.2013.03.069. [DOI] [PubMed] [Google Scholar]
- 13.Chao C, Klein NP, Velicer CM, Sy LS, Slezak JM, Takhar H, Ackerson B, Cheetham TC, Hansen J, Deosaransingh K, et al. . Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. Journal of internal medicine. 2012;271:193–203. doi: 10.1111/j.1365-2796.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- 14.Klein NP, Hansen J, Chao C, Velicer C, Emery M, Slezak J, Lewis N, Deosaransingh K, Sy L, Ackerson B, et al. . Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Archives of pediatrics & adolescent medicine. 2012;166:1140–8. doi: 10.1001/archpediatrics.2012.1451. [DOI] [PubMed] [Google Scholar]
- 15.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Permanente journal. 2012;16:37–41. doi: 10.7812/TPP/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sukumaran L, McCarthy NL, Li R, Weintraub ES, Jacobsen SJ, Hambidge SJ, Jackson LA, Naleway AL, Chan B, Tao B, et al. . Demographic characteristics of members of the Vaccine Safety Datalink (VSD): A comparison with the United States population. Vaccine. 2015;33:4446–50. doi: 10.1016/j.vaccine.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Correa A, Cragan JD, Kucik JE, Alverson CJ, Gilboa SM, Balakrishnan R, Strickland MJ, Duke CW, O'Leary LA, Riehle-Colarusso T, et al. . Reporting birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol. 2007;79:65–186. [DOI] [PubMed] [Google Scholar]


