ABSTRACT
Tick-borne encephalitis and West Nile fever are endemic flavivirus diseases in Europe. Climate change, virus evolution, and social factors may increase the risk of these flavivirus infections and may lead to the emergence of other flaviviruses in Europe that are endemic in (sub)tropical regions of the world. Control of the spread of flaviviruses is very difficult considering the cycling of flaviviruses between arthropod vectors and animal reservoir hosts. The increasing threat of flavivirus infections emphasizes the necessity of a sustainable vector surveillance system, an active animal health surveillance system and an adequate human surveillance system for early detection of flavivirus infections. Vaccination is the most important approach to prevent flavivirus infections. Effective inactivated whole virus vaccines against tick-borne encephalitis (TBE) infection are available. Implementation of TBE vaccination based on favorable cost-effectiveness estimates per region and per target group can reduce the disease burden of TBE infection. At present, several West Nile virus (WNV) vaccine candidates are in various stages of clinical development. A major challenge for WNV vaccine candidates is to demonstrate efficacy, because of the sporadic nature of unpredictable WNV outbreaks. Universal WNV vaccination is unlikely to be cost-effective, vaccination of high-risk groups will be most appropriate to protect against WNV infections.
KEYWORDS: epidemiology, flavivirus, surveillance, TBE vaccines, tick-borne encephalitis, vaccination strategy, West Nile fever, WNV vaccine development
Introduction
Flaviviruses
The Flaviviridae family comprises more than 70 different viruses, many of which are arthropod-borne and transmitted by either mosquitoes or ticks. With respect to occurrence and disease impact, the most important flaviviruses are yellow fever virus (YFV), dengue virus (DENV), Japanese encephalitis virus (JEV), West Nile virus (WNV), tick-borne encephalitis virus (TBEV), and Zika virus. With the exception of members of TBEV that are transmitted by ticks, mosquitoes transmit the major human pathogenic flaviviruses. In Europe, tick-borne encephalitis and West Nile fever are endemic flavivirus diseases.1,2
It is extremely difficult to control the spread of flaviviruses, because most flavivirus life cycles are maintained between arthropod vectors and reservoir hosts in the absence of humans. Moreover, no specific antivirals are available. Vaccination is considered the most important intervention to prevent flavivirus infections. Effective inactivated or live attenuated whole virus vaccines against yellow fever, Japanese encephalitis, and tick-borne encephalitis infections are available, but vaccines against West Nile virus, dengue virus, and Zika virus are still in development.1,2
Clinical diagnosis of flavivirus infection is often not reliable because the manifestations of the disease are often not specific. Therefore, laboratory diagnosis, based on the presence of serum antibodies against flaviviruses, is needed to confirm the etiology of the disease. Usually specific IgM- and IgG-serum antibodies are determined by enzyme-linked immuno-sorbent assay (ELISA), since these antibodies are detectable in practically every case at the time of hospitalization.3,4 Positive ELISA results are confirmed by flavivirus neutralization tests. However, serologic testing is challenging due to cross-reactivity between the flaviviruses.3–5 Virus isolation from blood, or the detection of specific nucleic acids in blood or cerebrospinal fluid by reverse-transcriptase polymerase chain reaction (RT-PCR) overcomes the problem of serological cross-reactivity, but is only successful during the first viremic phase of the disease, before seroconversion.3 With the onset of the second phase of disease, the virus can only be detected from the cerebrospinal fluid.
Neutralizing antibodies have a critical role in the long-term protection from disease and at present their measurement provides the best correlate of flavivirus immunity.2,6 The flavivirus envelope (E) protein, involved in host cell attachment and membrane fusion, is the major target of virus neutralizing antibodies.2,5,6 The amino acid sequence identity in the E protein ranges from 40–44% for unrelated flaviviruses to 60–70% within closely related flaviviruses. The extent and duration of cross-neutralization and even cross-protection is strongly dependent on the degree of amino acid similarity in the E protein. Infection with any one of the four DENV serotypes induces life-long protection against the same serotype but only for few months against the other serotypes.2 A human cohort study with sera from persons with a history of vaccination against TBEV, JEV and YFV showed that some individuals vaccinated against TBEV and JEV were able to neutralize WNV.7 The presence of non-neutralizing antibodies or neutralizing antibodies at suboptimal concentrations, however, may cause antibody-dependent enhancement of infection (ADE). These antibodies can facilitate virus entry through Fc receptor binding leading to increased infection instead of protection. The most clearly established role for ADE in vivo exists for DENV.2,8 For the development of flavivirus vaccines, it is therefore important to rule out ADE induction by the vaccine. On the other hand, flaviviral cross-reactivity could help to develop wide spectrum vaccines against flaviviruses.
In this article the challenges for vaccination and preparations that should be considered to protect for the endemic flavivirus diseases in Europe, i.e. TBE and West Nile fever, are addressed.
Tick borne encephalitis virus
Virus and transmission
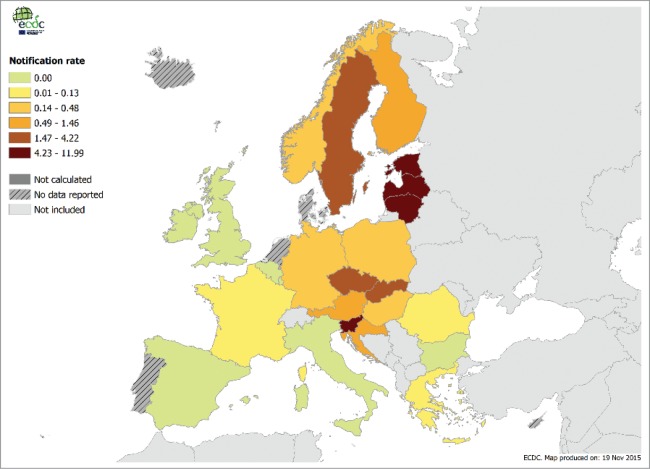
TBE is an infectious disease involving the central nervous system caused by the tick-borne encephalitis virus (TBEV). There are three distinct subtypes of TBEV, i.e. the European subtype (TBEV-Eu), widely distributed in Western, Central, Northern, and Eastern Europe; the Far Eastern subtype (TBEV-Fe), circulating in China, Japan, and eastern Russia; and the Siberian subtype (TBEV-Si), present in Siberia and some parts of Russia.9 TBEV is maintained in nature by numerous species of reservoir hosts, particularly rodents, and various vector tick species. Rodents act as maintenance, amplifying and reservoir hosts. Humans and horses are dead-end hosts. For human infections, viruses are transmitted by tick bites (adult and nymph) or intake of non-pasteurized dairy products.3,9 Ixodes ricinus is the main tick vector for TBEV-Eu, whereas Ixodes persulcatus is the main vector for TBEV-Fe and TBEV-Si, although TBEV can be transmitted by as many as 11 different tick species.9 Once infected, the tick is infective for its lifespan that can be up to 3 years.10 TBE virus has been shown to spread from infected ticks to humans within hours. TBE cases occur in humans most frequently during the highest period of tick activity (between April and November) (Fig. 1).11
Figure 1.
Confirmed TBE cases per 100,000 inhabitants in Europe per country, 2014.11
Clinical disease and incidence
Clinical manifestations following infection with TBE virus can vary between TBEV subtypes. About 10–30% of human infections are symptomatic.1 Typical TBEV infection is biphasic in approximately 75% of patients.12,13 The first symptoms occur on average 7 days after a tick bite, although incubation of up to 28 days has been described.12–14 The median duration of the first stage of illness is 5 days followed by an asymptomatic period of approximately one week before the second phase. In the first stage, the dominant symptoms are fever, headache, fatigue, myalgia, nausea, and/or vomiting.1,12,14 Significant morbidity and mortality is associated with the second phase of disease. The clinical spectrum ranges from mild to severe meningitis (50%), severe meningoencephalitis (40%) and meningoencephalomyelitis (10%).12,13 Following encephalitis, up to 40% of cases result in long-term neurological sequelae, which include diverse manifestations such as spinal nerve paralysis, neuropsychiatric complaints, dysphasia, ataxia and paresis.1,15 TBEV-Fe infection is more frequently associated with severe neurologic disease, relatively high case fatality rate and an increased propensity for neurological sequelae in survivors. Fatality rates are reported to be ≥ 20%, 6–8%, and 1–2% in TBEV-Fe, TBEV-Si, and TBEV-Eu infections, respectively.1 According to the European Surveillance System, the number of confirmed TBE cases in 2014 was 1,986. The proportion of confirmed TBE cases was higher in men (59.2%). The majority of cases belonged to the age group 45–65 years (40.4%), i.e. 0.62 cases per 100,000 population, followed by the age group ≥ 65 years (0.42 cases per 100,000 population). The lowest rates were observed in children.11 The incidence of clinical cases is reported to be between 10,000 and 15,000 per year worldwide, though it is probably underestimated, because notification of the disease is not mandatory in all countries.9 Numbers of reported TBE cases have increased in recent decades, because of climate change, increased outdoor activities and improved surveillance systems.1
Vaccines
At present, there is no drug with demonstrated efficacy available against TBEV. Other than the avoidance of exposure to the bite of an infected tick, vaccination is the most effective means of disease prevention. Different inactivated whole virus vaccines produced in Europe and Russia can prevent TBE.2,10 The effectiveness of both the European and Russian vaccine is very high: it reaches 98% when the proper vaccination schedule is applied, and has led to a dramatic decline of disease incidence in the vaccinated population.2 The primary immunization schedule includes 3 doses; booster doses are recommended at varying intervals in different countries (see Table 1 for WHO recommended immunization schedules against TBE). The first TBEV vaccine, FSME-IMMUN®, an inactivated whole virus vaccine (TBEV-Eu serotype) produced on primary chicken embryo cells was approved and used in Austria since 1976. For pediatric use, FSME-IMMUN®(Junior) was launched in 2003.1 Encepur®, also an inactivated whole virus vaccine (TBEV-Eu subtype), was licensed in 1991. For pediatric use, Encepur®K was approved in 1994. Two additional vaccines manufactured in Russia, TBE-Moscow® and EnceVir®, based on strains of TBEV-Fe subtype, are only available in Russia and some neighboring countries. All TBEV vaccines showed to give high seroconversion rates (88–100%) following three immunizations. Cross-neutralizing antibodies against TBEV-Fe and TBEV-Si were detected in humans immunized with FSME-IMMUN® containing TBEV-Eu antigens. In general, it is assumed that cross-protective immunity against all three serotypes can be induced by any TBEV vaccine.1 TBEV vaccination was successfully implemented into routine immunization programs; the incidence rate of TBEV infection in Austria has declined from 5.7/100,000 population (average from 1972–1981) to 0.9/100,000 (average of 2002–2011) (Table 1).16
Table 1.
Immunization schedules for tick-borne encephalitis vaccines according to WHO recommendations.
| Basic immunization: conventional schedule (dose 1 on day 0) |
Basic immunization: rapid schedule (dose 1 on day 0) |
||||||
| Vaccine |
2nd dose (mo) |
3rd dose (mo) |
2nd dose |
3rd dose |
4th dose (mo) |
1st booster |
Subsequent boosters (yrs) |
| FSME-Immun® | 1–3 mo | 5–12 mo | 14 d* | 5–12 mo* | — | 3 yrs | 5† |
| Encepur® | 1–3 mo (14 d) | 9–12 mo | 7 d | 21 d | — | 12–18 mo | 5† |
| TBE-Moscow vaccine® | 1–7 mo | 12 mo | — | — | — | 3 yrs | 3 |
| EnceVir® | 5–7 mo | 12 mo | 21–35 d‡ | 42–70 d‡ | 6–12 | 3 yrs | 3 |
Intervals given in months (mo) unless indicated as years (yrs) or days (d).
Interval of 3 yrs in persons ≥ 50 years of age (in Austria an interval of 3 y for persons ≥60 years of age.
Double dose of total 1.0 ml.
For FSME-immun, the licensed rapid scheme is only licensed for adults. For FSME-Immun and Encepur, after the first booster dose, intervals of 5 years are now recommended by the manufacturers for persons below 50 and 60 years of age, respectively.
Adapted from Kollaritsch.10
West Nile virus
Virus and transmission
West Nile virus (WNV) is a flavivirus attracting worldwide attention because it has spread rapidly across the US since its first appearance in New York City in 1999. Presently, WNV is categorized into five genetic distinct lineages, though most isolates fall into lineage 1, clade 1a or lineage 2.17 The New York 1999 WNV strain belongs to lineage 1, clade 1a. Viruses of clade 1a are found worldwide. Lineage 2 comprises virus isolates from Sub-Saharan Africa and Madagascar and emerged in 2004 in central Europe and southern European countries.18,19 Humans are dead-end hosts.18 Human infection is most often the result of bites from infected mosquitoes, usually in the summer season or in early autumn. WNV is maintained in mosquito populations through vertical transmission (adults to eggs.20,21 Mosquitoes become infected when they feed on infected birds, the prime reservoir host. The virus will accumulate and replicate in the salivary glands of mosquitoes, which will result in high viremia in the saliva. During feeding after a mosquito bite, the virus can be transmitted to mammalian hosts, where it can multiply and cause illness. Mosquitoes of the genus Culex are the principal vectors of WNV, in particular Cx. pipiens.20 Introduction of WNV into new areas is generally considered to be initiated by migratory birds.
Clinical disease and incidence
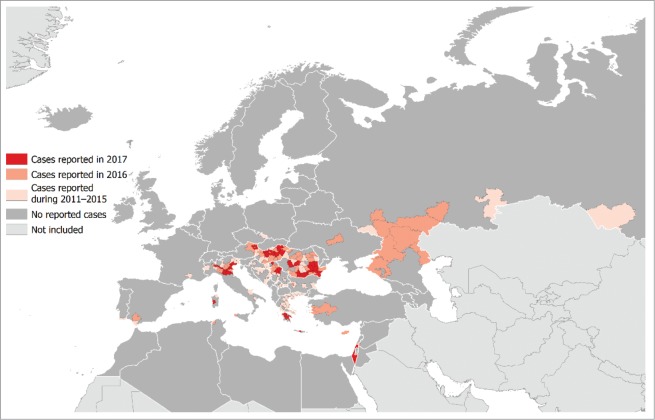
Most cases of WNV infection are subclinical or asymptomatic, approximately 20–30% will present as West Nile fever (WNF).17,22 WNF cases are considerably underreported, since routine diagnostic testing is not recommended and many patients do not seek medical care.23 Most symptomatic patients experience an acute systemic febrile illness that often includes headache, weakness, myalgia, or arthralgia; gastrointestinal symptoms and a transient maculopapular rash are also commonly reported. Less than 1% of the infected persons develop more severe West Nile neuroinvasive disease (WNND).17,23,24 which manifests as encephalitis, meningitis, or acute flaccid paralysis that may result in respiratory failure. Some patients with WNND experience long-term neurological dysfunction requiring assistance with daily activities.17 Persons of all ages are susceptible to WNV infection, but the incidence of neuroinvasive disease and death increases with age. The incidence of WNND is also higher in immunocompromised patients, and it is slightly higher in male patients.25 Until the mid-1990s, human WNV cases were sporadic with mild manifestations. During the 1990's, more severe outbreaks with increased neuroinvasive disease were seen in North Africa, the US and southern Europe. Following the introduction of WNV into the US in 1999, the number of human infections there rose dramatically, peaking in 2002–2003 (2003: 9,862 cases).26 The case-fatality rate was reported to be 4.2% in the US, whereas the case-fatality rate among patients with WNND was 9.6%.17 The case fatality rates of <40-year-old, 40–59-year-old, and≥60-year-old displaying neurological diseases were 0.8%, 3%, and 17%, respectively.1 Following the WNV outbreak in Greece with 262 reported cases in 2010, the European Union has begun efforts to improve surveillance. In 2010–2012, southern European and neighboring countries demonstrated a total of 2,414 WNV cases with 127 associated deaths resulting in a case-fatality rate of 5.3%, similar to the rate observed in the United States.17 The highest notification rate was reported in the ≥65-year-old age group (0.04 cases per 100,000,). Only one case was reported among children under the age of 15 years (Fig. 2).27
Figure 2.
Distribution of West Nile fever cases by affected areas in Europe and Mediterranean basis in 2017 (current season) and previous seasons; updated 7 September 2017.28
Immunity against WNV
Antibodies against WNV, measured with immunoassays using WNV recombinant proteins (premembrane/envelope), start to appear about three to seven days following infection.18,29 WNV-specific IgM antibodies, but also IgA, have been shown to be detectable on day 3 after being tested positive for WNV and persist for at least 6 months after infection.29 The diagnosis of WNV infection generally relies on the demonstration of specific antibodies against WNV in serum or cerebrospinal fluid, although cross-reactivity with infections caused by other flaviviruses is a problem in serological diagnostic tests.18 Animal experiments indicate that specific antibodies are responsible for terminating viremia, while CD8+ T cells have an important function in clearing infection from tissues and preventing viral persistence.30 However, it cannot be excluded that T cells, apart from recovery of WNV encephalitis, may also cause immunopathology based on experiments in CD8+ deficient mice.18,31 A number of animal studies using passive immunization have shown that transfer of neutralizing antibodies to naïve animals is sufficient for protection against lethal WNV infection.17
Vaccines in clinical development
The increasing incidence of West Nile neuro-invasive disease (WNND), new outbreaks, the endemic virus circulation in temperate areas, and lack of specific therapeutic treatments for humans have promoted research and development activities on vaccines against WNV. Although there are several veterinary vaccines for horses licensed.32 there is no human WNV vaccine available yet.
Various vaccine concepts against WNV are in development, including DNA-vectored vaccines, live chimeric/recombinant vaccines expressing WNV protein(s), live attenuated vaccines, subunit (i.e. protein-, peptide-, or virus-like particle based) vaccines or inactivated whole virus vaccines. Ideally, WNV vaccine needs to protect against all WNV genotypes that can cause WNND in humans. This supports the development of a whole virus vaccine, either inactivated or live-attenuated, in order to achieve a broad coverage of the vaccine.
So far, some vaccine concepts have reached the early clinical phase of development, but none of them has progressed any further. The WNV vaccine concepts, including antigen targets that have reached early clinical stage are listed in Table 2. All tested vaccines appeared to have a good safety profile, and seroconversion rates showed to be high. Geometric mean antibody titers, although not always published, seem to vary considerably between subjects.33–39
Table 2.
WNV vaccine concepts that have reached the clinical stage.
| Vaccine name (sponsor) |
Vaccine type |
Antigen |
Phase (year) |
Target group |
Study design |
Summarized results or Trial ID |
| Chimerivax-WN02 (Sanofi Pasteur) | Live attenuated chimeric | Prm and E of WNV NY99 into YEF 7D virus | Phase II | 18–40 y (n = 95) | 1-dose (dose-ranging) | PRNT seroconversion: >96% all (dose and age) groups |
| 2005–9 33 | 41–64 yrs (n = 33) | 4.103, 4.104 and 4.105 pfu | PRNT seroconversion: 92–95% all (dose) groups | |||
| 2008/9 34 | ≥ 65 yrs (n = 31) | 4.105 pfu | ||||
| ≥50 years (n = 359) | 4.105 pfu | |||||
| 1-dose (dose-ranging) | ||||||
| 4.103, 4.104 and 4.105 pfu | ||||||
| VRC-WNVDNA020-00-VP 35 | Plasmid DNA | Prm, E of WNV NY99 with CMV/R promotor | Phase I 2006 35 | healthy adults (n = 30) | 3-dose regimen | Vaccine-induced antibody (ELISA) response in both age groups in 93% |
| (replaces VRC-WNVDNA017-00 VP 36) (NIAID) | split in: 18–50 y (n = 15) | Vaccine-induced neutralizing antibody response in both age groups in 97% | ||||
| 51–65 y (n = 15) | 24–45% showed CD4+ or CD8+ T cell responses to E and Prm peptide pools | |||||
| WN/DEN4-3′Δ30 (NIAID) | Live attenuated chimeric | Prm, E of WNV NY99 into dengue type 4 virus | Phase I 2004 37 | 18–50 y | 1 dose (103, 104 or 105 pfu) | Seroconversion was observed in 74% (103 PFU), 75% (104 pfu), and 55% (105 PFU) |
| 2007 37 | 18–50 y | 2-dose regimen (104 or 105 pfu) | A 2nd (105 pfu) dose 6 months after first dose increased the seroconversion rate to 89%. | |||
| 2014 38 | 50–65 y (n = 28) | 2-dose regimen (104 pfu) | Seroconversion rate 95% | |||
| WN-80E (other name HBV-002) (Hawaii Biotech) | Rec. E subunit to aluminium hydroxide | E | Phase I 2008–9 39 | Low level neutralizing antibodies | ||
| HydroVax-001 (NIAID) | Inactive whole WNV virion | Phase I 2015 | NCT02337868 |
CMV, cytomegalovirus; E, envelope protein; pfu, plaque forming units; Prm, premembrane protein; PRNT; plaque reduction neutralization titers; n, numbers of participants that received the WNV vaccine; NIAID, National Institute of Allergy and Infectious Diseases; NY99, New York 1999 WNV strain; YEF, yellow Fever.
A hurdle for human WNV vaccine development is the limited feasibility to perform phase III efficacy trials, because of the relatively low incidence and unpredictable sporadic nature of WNV outbreaks. This poses not only a challenge for clinical study design, but also for implementation of a new WNV vaccine.40 This may be partly overcome by maintaining and/or strengthening the surveillance efforts, and thereby planning trials in regions of high WNV incidence, and monitoring for vaccine efficacy over a prolonged period of time (possibly 1 to 3 years).17
Discussion
Increasing risk for flaviviruses in Europe and surveillance
Infected mosquito vectors might be introduced in EU countries via international travel and commerce with other continents where flaviviruses are endemic. Moreover, rising temperatures will expand geographic areas and widen Europe's seasonal window for the potential spread of vector-borne viral diseases. For instance, Culex pipiens mosquitoes that were collected in 2010–2011 for screening in Northern Italy appeared to be infected with JEV. This finding may indicate a wider range of distribution of the vector and virus and a potential public health threat in Europe.3 In 2012–2013, a dengue outbreak occurred in Madeira, Portugal with 1080 confirmed cases. The main vector for dengue, Aedes aegypti, was detected for the first time in Madeira in 2005.4 Other human pathogenic flaviviruses exist that rarely cause human disease, such as usutu virus. The usutu virus have been detected in Austria, Italy, Germany, Spain, Hungary, Switzerland, Poland, England, Czech Republic, Greece, and Belgium, where it caused unusual mortality in birds.41 Reports on clinically apparent human usutu virus infections, however, are scarce and only four cases are described so far in the literature.42 Furthermore, it has been shown that other flaviviruses can cause human disease in Europe by tick bite transmission, i.e. Omsk haemorrhagic fever virus, louping ill virus, and Powassan virus. Omsk haemorrhagic fever virus infections seemed to be confined to some regions of western Siberia. In more recent years, most human cases have been related to direct contact with musk-rats. Only few cases of disease caused by louping ill virus and Powassan virus have been reported in the literature.15
The surveillance of TBE in the European countries is not uniform and not always mandatory. Efforts to reach a final diagnosis, especially in less severe cases, vary at present as well as the awareness of the disease in low endemic regions. An adequate national surveillance of TBE cases is important and should be recommended in all European countries. The unpredictable nature of WNV outbreaks necessitates the establishment and maintenance of surveillance systems capable of detecting increases in WNV transmission activity. In addition, an active animal health surveillance system to detect new WNV cases in birds and horses is essential.
Vaccination strategy
Tick-borne encephalitis (TBE) is a substantial public health problem in many parts of Europe. With various safe and effective vaccines currently available, vaccination is the most effective protection against TBE. However, in most endemic countries vaccination coverage is too low to reduce the TBE burden significantly. Among all European countries, vaccination coverage is highest in Austria, where ≈85% of the total population have received >1 doses of the vaccine.16 This high vaccination coverage has led to a dramatic decline in the overall incidence of TBE in Austria. The field effectiveness of the vaccine for preventing disease appeared to be high, i.e. 96%–99% after regular vaccination and best-case assumptions. Even among persons with a history of irregular vaccinations, the average protection rate was still >90%. In Austria during 2000–2011, it was calculated that vaccination prevented >4,000 cases of TBE.16 Moreover, vaccine effectiveness is excellent among elderly persons, for whom risk for severe forms of TBE and neuropathological sequelae is highest.16 This indicates that TBE vaccination is an excellent way to prevent disease in all age groups. However, important factors to consider before implementation of vaccination, are: incidence threshold, which WHO recommends to be 5/100,000 and cost-effectiveness. Yet, incidence could be relevant on a regional, rather than a national scale, and cost-effectiveness could be considered based on age groups, rather than the whole population, as shown by an Estonian study where vaccination of the ≥50 year olds was more cost-effective from the health care perspective than vaccination of the whole population.43
Severe neuroinvasive disease caused by WNV occurs in less than 1% of infected persons and mostly affects elderly and immunocompromised individuals.25 Universal WNV vaccination is therefore unlikely to be cost-effective. Especially considering that almost 3 million Americans have likely been infected with WNV, but most patients are not seeking medical care because of asymptomatic or mildly symptomatic disease.17 Therefore, further studies are needed to determine if targeted vaccine campaigns focused on at-risk groups or geographical regions will provide a favorable cost-effectiveness. These studies should preferably include not only direct health care costs but also costs associated with productivity loss, WNV surveillance, prevention, and outbreak response.
Summarizing, climate change, virus evolution, and social factors may lead to further spread of vector-borne infectious diseases in the future. It remains to be seen whether (sub)tropical diseases such as yellow fever, dengue and Zika will emerge in Europe, but considering the fact that the vectors are already present and the expected increase in global temperatures there is a theoretical risk. Therefore, an adequate vector-, animal health- and human-surveillance system for rising and emerging endemic flaviviruses within Europe is essential. Vaccination is considered the most important approach to prevent flavivirus infections and vaccine development should be supported. Implementation of vaccination against endemic flaviviruses should be based on favorable cost-effectiveness estimates per region and per target group.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ishikawa T, Yamanaka A, Konishi E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine. 2014;32(12):1326–37. doi: 10.1016/j.vaccine.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30(29):4301–6. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 3.Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21 Suppl 1:S36–40. doi: 10.1016/S0264-410X(02)00819-8. [DOI] [PubMed] [Google Scholar]
- 4.Hogrefe WR, Moore R, Lape-Nixon M, Wagner M, Prince HE. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J Clin Microbiol. 2004;42(10):4641–8. doi: 10.1128/JCM.42.10.4641-4648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck C, Jimenez-Clavero MA, Leblond A, Durand B, Nowotny N, Leparc-Goffart I, Zientara S, Jourdain E, Lecollinet S. Flaviviruses in Europe: Complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int J Environ Res Public Health. 2013;10(11):6049–83. doi: 10.3390/ijerph10116049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe. 2008;4(3):229–38. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansfield KL, Horton DL, Johnson N, Li L, Barrett AD, Smith DJ, et al.. Flavivirus-induced antibody cross-reactivity. J Gen Virol. 2011;92(Pt 12):2821–9. doi: 10.1099/vir.0.031641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, et al.. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328(5979):745–8. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amicizia D, Domnich A, Panatto D, Lai PL, Cristina ML, Avio U, et al.. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum Vaccin Immunother. 2013;9(5):1163–71. doi: 10.4161/hv.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollaritsch H, Paulke-Korinek M, Holzmann H, Hombach J, Bjorvatn B, Barrett A. Vaccines and vaccination against tick-borne encephalitis. Expert Rev Vaccines. 2012;11(9):1103–19. doi: 10.1586/erv.12.86. [DOI] [PubMed] [Google Scholar]
- 11.http://ecdc.europa.eu/en/healthtopics/emerging_and_vector-borne_diseases/tick_borne_diseases/tick_borne_encephalitis/Pages/Annual-epidemiological-report-2016.aspx. Annual epidemiological report 2015; Tick-borne encephalitis (reporting on 2014 data) European Centre for Disease Prevention and Control (ECDC, Stockholm). 2016.
- 12.Zambito Marsala S, Pistacchi M, Gioulis M, Mel R, Marchini C, Francavilla E. Neurological complications of tick borne encephalitis: the experience of 89 patients studied and literature review. Neurol Sci. 2014;35(1):15–21. doi: 10.1007/s10072-013-1565-8. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser R. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994–98: A prospective study of 656 patients. Brain. 1999;122 (Pt 11):2067–78. doi: 10.1093/brain/122.11.2067. [DOI] [PubMed] [Google Scholar]
- 14.Ruzek D, Dobler G, Donoso Mantke O. Tick-borne encephalitis: Pathogenesis and clinical implications. Travel Med Infect Dis. 2010;8(4):223–32. doi: 10.1016/j.tmaid.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Charrel RN, Attoui H, Butenko AM, Clegg JC, Deubel V, Frolova TV, Gould EA, Gritsun TS, Heinz FX, Labuda M, et al.. Tick-borne virus diseases of human interest in Europe. Clin Microbiol Infect. 2004;10(12):1040–55. doi: 10.1111/j.1469-0691.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- 16.Heinz FX, Stiasny K, Holzmann H, Grgic-Vitek M, Kriz B, Essl A, et al.. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis. 2013;19(1):69–76. doi: 10.3201/eid1901.120458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amanna IJ, Slifka MK. Current trends in West Nile virus vaccine development. Expert Rev Vaccines. 2014;13(5):589–608. doi: 10.1586/14760584.2014.906309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzoli A, Jimenez-Clavero MA, Barzon L, Cordioli P, Figuerola J, Koraka P, Martina B, Moreno A, Nowotny N, Pardigon N, et al.. The challenge of West Nile virus in Europe: knowledge gaps and research priorities. Euro Surveill. 2015;20(20):pii=21135. doi: 10.2807/1560-7917.ES2015.20.20.21135. [DOI] [PubMed] [Google Scholar]
- 19.Linke S, Ellerbrok H, Niedrig M, Nitsche A, Pauli G. Detection of West Nile virus lineages 1 and 2 by real-time PCR. Journal of virological methods. 2007;146(1–2):355–8. doi: 10.1016/j.jviromet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 20.http://www.who.int/mediacentre/factsheets/fs354/en/. West Nile virus. WHO Fact sheet No 354. July 2011. [Google Scholar]
- 21.Fechter-Leggett E, Nelms BM, Barker CM, Reisen WK. West Nile virus cluster analysis and vertical transmission in Culex pipiens complex mosquitoes in Sacramento and Yolo Counties, California, 2011. J Vector Ecol. 2012;37(2):442–9. doi: 10.1111/j.1948-7134.2012.00248.x. [DOI] [PubMed] [Google Scholar]
- 22.Murray KO, Walker C, Gould E. The virology, epidemiology, and clinical impact of West Nile virus: A decade of advancements in research since its introduction into the Western Hemisphere. Epidemiol Infect. 2011;139(6):807–17. doi: 10.1017/S0950268811000185. [DOI] [PubMed] [Google Scholar]
- 23.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect. 2013;141(3):591–5. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, et al.. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet. 2001;358(9278):261–4. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 25.Gyure KA. West Nile virus infections. J Neuropathol Exp Neurol. 2009;68(10):1053–60. doi: 10.1097/NEN.0b013e3181b88114. [DOI] [PubMed] [Google Scholar]
- 26.https://www.cdc.gov/westnile/resources/pdfs/data/2-west-nile-virus-disease-cases-reported-to-cdc-by-state_1999-2015_07072016.pdf. West Nile virus disease cases reported to CDC by state of residence, 1999–2015. Centers for Disease Control and Prevention (CDC). 2015. [Google Scholar]
- 27.http://ecdc.europa.eu/en/healthtopics/west_nile_fever/Pages/Annual-epidemiological-report-2016.aspx. Annual epidemiological report; West Nile fever (reporting on 2014 data) European Centre for Disease Prevention and Control (ECDC, Stockholm). 2016.
- 28.https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc. Disease data from ECDC Surveillance Atlas – West Nile fever. ECDC (Stockholm), West Nile fever maps. 2017. [Google Scholar]
- 29.Prince HE, Tobler LH, Lape-Nixon M, Foster GA, Stramer SL, Busch MP. Development and persistence of West Nile virus-specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43(9):4316–20. doi: 10.1128/JCM.43.9.4316-4320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78(15):8312–21. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Lobigs M, Lee E, Mullbacher A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol. 2003;77(24):13323–34. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Filette M Ulbert S, Diamond M, Sanders NN. Recent progress in West Nile virus diagnosis and vaccination. Vet Res. 2012;43:16. doi: 10.1186/1297-9716-43-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biedenbender R, Bevilacqua J, Gregg AM, Watson M, Dayan G. Phase II, randomized, double-blind, placebo-controlled, multicenter study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J Infect Dis. 2011;203(1):75–84. doi: 10.1093/infdis/jiq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dayan GH, Bevilacqua J, Coleman D, Buldo A, Risi G. Phase II, dose ranging study of the safety and immunogenicity of single dose West Nile vaccine in healthy adults >/ = 50 years of age. Vaccine. 2012;30(47):6656–64. doi: 10.1016/j.vaccine.2012.08.063. [DOI] [PubMed] [Google Scholar]
- 35.Ledgerwood JE, Pierson TC, Hubka SA, Desai N, Rucker S, Gordon IJ, Enama ME, Nelson S, Nason M, Gu W, et al.. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203(10):1396–404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, Andrews CA, Xu Q, Davis BS, Nason M, et al.. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196(12):1732–40. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durbin AP, Wright PF, Cox A, Kagucia W, Elwood D, Henderson S, Wanionek K, Speicher J, Whitehead SS, Pletnev AG. The live attenuated chimeric vaccine rWN/DEN4Delta30 is well-tolerated and immunogenic in healthy flavivirus-naive adult volunteers. Vaccine. 2013;31(48):5772–7. doi: 10.1016/j.vaccine.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce KK, Whitehead SS, Kirkpatrick BD, Grier PL, Jarvis A, Kenney H, Carmolli MP, Reynolds C, Tibery CM, Lovchik J, et al.. A Live Attenuated Chimeric West Nile Virus Vaccine, rWN/DEN4Delta30, Is Well Tolerated and Immunogenic in Flavivirus-Naive Older Adult Volunteers. J Infect Dis. 2017;215(1):52–5. doi: 10.1093/infdis/jiw501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Hoeven N, Joshi SW, Nana GI, Bosco-Lauth A, Fox C, Bowen RA, Clements DE, Martyak T, Parks DE, Baldwin S, et al.. A Novel Synthetic TLR-4 Agonist Adjuvant Increases the Protective Response to a Clinical-Stage West Nile Virus Vaccine Antigen in Multiple Formulations. PLoS One. 2016;11(2):e0149610. doi: 10.1371/journal.pone.0149610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandler S, Tangy F. Vaccines in development against West Nile virus. Viruses. 2013;5(10):2384–409. doi: 10.3390/v5102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashraf U, Ye J, Ruan X, Wan S, Zhu B, Cao S. Usutu virus: an emerging flavivirus in Europe. Viruses. 2015;7(1):219–38. doi: 10.3390/v7010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allering L, Jost H, Emmerich P, Gunther S, Lattwein E, Schmidt M, Seifried E, Sambri V, Hourfar K, Schmidt-Chanasit J, et al.. Detection of Usutu virus infection in a healthy blood donor from south-west Germany, 2012. Euro Surveill. 2012;17(50):pii=20341. [PubMed] [Google Scholar]
- 43.Smit R, Postma MJ. Vaccines for tick-borne diseases and cost-effectiveness of vaccination: A public health challenge to reduce the diseases' burden. Expert Rev Vaccines. 2016;15(1):5–7. doi: 10.1586/14760584.2016.1111142. [DOI] [PubMed] [Google Scholar]