ABSTRACT
Purpose: Two new Staphylococcus aureus vaccines, S. aureus four-antigen (SA4Ag) and three-antigen (SA3Ag) vaccines, have good immunogenicity and tolerance. However, the safety of these vaccines is worth exploring. Here, we performed a meta-analysis to investigate the safety of SA3Ag and SA4Ag by evaluating systemic and local adverse events.
Methods: The Medline, EMBASE, and Cochrane databases were searched for randomized clinical trials confirming the safety of SA4Ag and SA3Ag. Two investigators independently selected suitable trials, assessed trial quality, and extracted data.
Results: Three studies comprising a total of 1,148 participants were included in this review. The two S. aureus vaccines did not increase systemic adverse events (relative ratio 1.1 [95% confidence interval 0.98, 1.24]), but increased the incidence of local adverse events (2.89 [2.15, 3.90]). However, the incidence of severe local adverse events (4.06 [0.78, 21.24]) did not rise significantly.
Conclusions: SA4Ag and SA3Ag have acceptable safety in adults.
KEYWORDS: local adverse events, Staphylococcus aureus, Staphylococcus aureus four-antigen vaccine, Staphylococcus aureus three-antigen vaccine, systemic adverse events
Introduction
Staphylococcus aureus is a leading cause of infection-related morbidity and mortality1–4 and the most common cause of postoperative infection.5–7 Erythromycin, penicillin, gentamicin, and vancomycin are typically used for the treatment of S. aureus infection. However, due to the abuse of antibiotics, new multidrug-resistant strains have emerged, thus hindering the management of S. aureus. Therefore, the study of S. aureus vaccines has once again become a pressing issue.
In 1981, Watson et al. created inactivated and live attenuated S. aureus vaccines; however, these S. aureus vaccines were whole-cell bacterial vaccines that contain many unrelated and toxic components besides the effective antigen.8 Thus, vaccine toxicity is high and clinical application is limited. The complexity of the virulence mechanism of S. aureus poses challenges for the development of prophylactic vaccines. From the experience of previous failures, a successful prophylactic S. aureus vaccine must address a combination of well-conserved virulence factors expressed by most strains and generate antibodies that effectively kill the organism.9–14 Recently, researchers have successfully developed two kinds of multi-antigen vaccine candidates, namely S. aureus three-antigen (SA3Ag) and S. aureus four-antigen (SA4Ag) vaccines. The former includes bacterial capsular polysaccharide type 5 (CP5), CP8, and clumping factor A (ClfA), and the latter comprises CP5, CP8, ClfA, and recombinant P305A (rP305A).15–20 SA3Ag and SA4Ag exhibit superior immunogenicity than previous vaccines. Former candidate vaccines have only demonstrated the ability to generate anti-staphylococcal binding antibodies using enzyme-linked immunosorbent assays and bacterial uptake by phagocytic cells, while SA3Ag and SA4Ag can induce high levels of antibodies to kill S. aureus in opsonophagocytosis assays.9,12 However, Frenck et al. observed systematic adverse events (SAE) in participants who had been vaccinated with SA4Ag, including remarkable diarrhoea.16 Related studies16–18,20 also showed that participants had significant local adverse events (LAE) after two SA4Ag or SA3Ag inoculations, such as redness, swelling, and injection site pain and some LAE were severe. Therefore, the purpose of the present study was to identify and combine all relevant published clinical randomized controlled trials (RCTs) to investigate the safety of SA4Ag and SA3Ag in healthy adults.
Results
Literature search
The literature search identified 8 records of clinical trials in Medline, 6 records in EMBASE, and 7 records in the Cochrane databases (Fig. 1). After checking for duplicates, 8 unique references remained; 2 were excluded for reasons such as irrelevance to S. aureus vaccine or describing a nonclinical trial21,22 and the remaining 6 full texts underwent further evaluation. Among these, 3 articles15,18,19 were excluded as they did not provide the necessary data for our meta-analysis.
Figure 1.
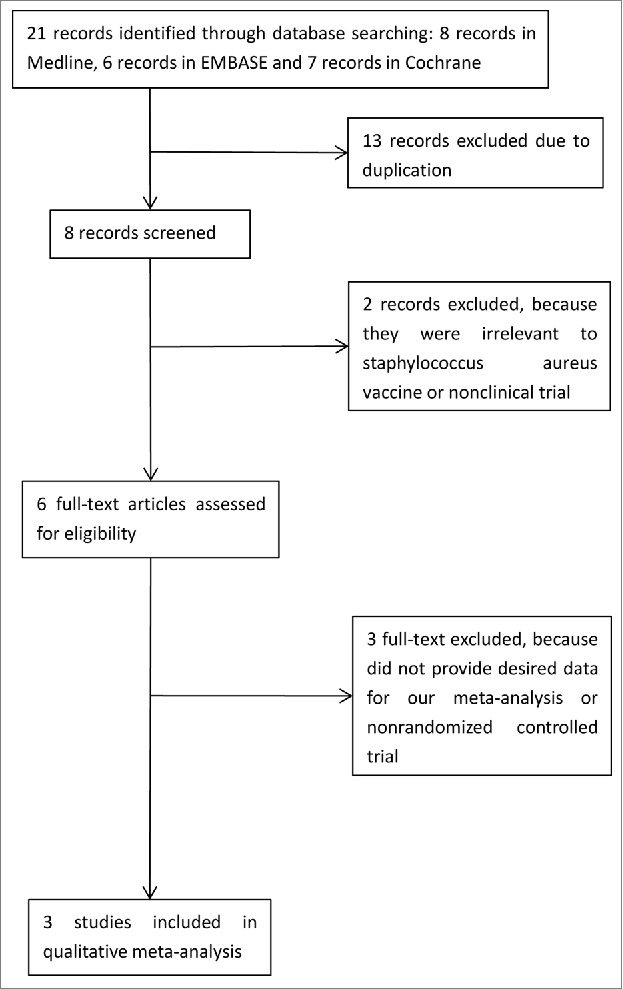
Flow diagram of study search and selection
Types of vaccine injected
Of the three included studies, one [17] involved participants receiving one of three dose levels (low, mid, high) of SA4Ag or one dose level of SA3Ag or placebo. In this study, the same intervention group with multiple doses was recommended for data consolidation; however, to observe potential differences between the two vaccines, the data were equally divided into two subgroups, according to the Cochrane Handbook.23 One16 involved participants receiving one of three dose levels (low, mid, high) of SA4Ag or placebo. Data from the same intervention group with multiple doses were consolidated. Another study20 established two age strata, each corresponding to one of three dose levels of SA3Ag or placebo. Thus, this study was considered as two results because of the independence of the two participant groups (Table 1).
Table 1.
Study design characteristics.
| Study | Year | Design | Participants | Administrating method | Dosing groups |
|---|---|---|---|---|---|
| Creech17 | 2017 | Prospective,multicentre,randomized, controlled, double-blind | Participants receiving SA3Ag or SA4Ag or placebo | a single 0.5 mL intramuscular injection into the deltoid muscle | SA3Ag, low-dose SA4Ag, mid-dose SA4Ag, high-dose SA4Ag, placebo |
| Frenck16 | 2017 | Prospective,multicentre,randomized, controlled, double-blind | Participants receiving SA4Ag or placebo | a single 0.5 mL intramuscular injection into the deltoid muscle | Low-dose SA4Ag, mid-dose SA4Ag, high-dose SA4Ag, placebo |
| Nissen20 | 2015 | Prospective,multicentre,randomized, controlled, double-blind | Participants in the two age groups receiving SA3Ag or placebo, independently | Not Reported | Low-dose SA3Ag, mid-dose SA3Ag, high-dose SA3Ag, placebo |
SA3Ag: Staphylococcus aureus three-antigen vaccine; SA4Ag: Staphylococcus aureus four-antigen vaccine.
S.aureus vaccine protocols
In one study17, three dose levels (low, mid, high) of SA4Ag were evaluated; each dose level included 30 µg CP5-CRM197, 30 µg CP8-CRM197, and 60 µg recombinant surface protein clumping factor A (rmClfA) and one of three dose levels of rP305A (low, 20 µg; mid, 60 µg; or high, 200 µg). The SA3Ag comparator vaccine consisted of the same fixed-dose levels of 30 µg CP5-CRM197, 30 µg CP8-CRM197, and 60 µg rmClfA without rP305A. Study vaccine or placebo was administered as a single 0.5-mL intramuscular injection into the deltoid muscle. In another study16, three dose levels of SA4Ag were evaluated, each containing the same fixed-dose levels of 30 µg CP5-CRM197, 30 µg CP8-CRM197, and 60 µg rmClfA plus one of three dose levels of rP305A (low, 20 µg; mid, 60 µg; or high, 200 µg) per 0.5-mL dose. SA4Ag was administered as a non-adjuvanted, lyophilized vaccine reconstituted with 60 mM sodium chloride prior to administration. Study vaccine or placebo was administered as a single 0.5-mL intramuscular injection into the deltoid muscle of the non-dominant arm. In another study20, SA3Ag was administered as a non-adjuvanted, lyophilized vaccine containing 10 µg each of CP5- and CP8-CRM197 and 20 µg of rmClfA (low-dose level), 30 µg of CP5- and CP8-CRM197 and 60 µg of rmClfA (mid-dose level), or 100 µg of CP5- and CP8-CRM197 and 200 µg of rmClfA (high-dose level), reconstituted with 60 mM sodium chloride.
Patient characteristics
The mean ages of included participants ranged from 21.3 to 70.9 years (Table 2), and the percentages of males varied from 30.2% to 51.9%. Whites, including non-Hispanic or non-Latino subjects, accounted for the majority of subjects, with proportions ranging from 73.2% to 94.9% and 64.4% to 99.7%, respectively. The average body mass index ranged from 25.4% to 29.5%, while smoking ranged from 9.0% to 19.1%.
Table 2.
Patient characteristics.
| Study | Year | Number of subjects | Age | Male | Race(White) | Ethnicity(Non-Hispanic and non-Latino) | BMI (kg/m2) | Smoking |
|---|---|---|---|---|---|---|---|---|
| Creech17 | 2017 | 284 | 70.9 ± 4.62 | 49.2% | 91.5% | 64.4% | 29.4 ± 5.67 | 10.2% |
| Frenck16 | 2017 | 456 | 45.0 ± 13.13 | 43.2% | 73.2% | 73.7% | 29.5 ± 6.42 | 19.1% |
| Nissen20 18-24-year age stratum | 2015 | 96 | 21.3 ± 1.65 | 30.2% | 91.7% | 99.0% | 25.4 ± 5.26 | 17.7% |
| Nissen20 50-85-year age stratum | 2015 | 312 | 63.6 ± 8.15 | 51.9% | 94.9% | 99.7% | 28.8 ± 5.82 | 9.0% |
Primary outcome
The primary outcome was SAE and sSAE. The vaccine group did not exhibit increased incidence of diarrhoea (RR 1.33 [95% CI 0.87, 2.02]) (Fig. 2) compared with the placebo group, and there was no evidence for statistical heterogeneity (χ2 = 8.06, I2 = 48.7%, and Pheterogeneity = 0.099). Joint pain, muscle pain, vomiting, headache, and fatigue had RRs [95% CIs] of 1.10 [0.76, 1.59], 1.25 [0.91, 1.71], 1.11 [0.48, 2.53], 0.96 [0.79, 1.16], and 1.19 [0.95, 1.50], respectively. According to these results, the incidence of SAE was not increased in the vaccine group compared with the placebo group. Overall effects seemed to increase the incidence of SAE (1.1 [0.98, 1.24]) compared to placebo; however, this difference was not significant and no statistical heterogeneity was observed (χ2 = 18.86, I2 = 0%, and Pheterogeneity = 0.896). Additionally, the vaccine group also did not demonstrate increased incidence of sSAE (1.57 [0.74, 3.30]) (Fig. 3) compared to the placebo group, and no statistical heterogeneity was found (χ2 = 1.25, I2 = 0%, and Pheterogeneity = 0.742).
Figure. 2.
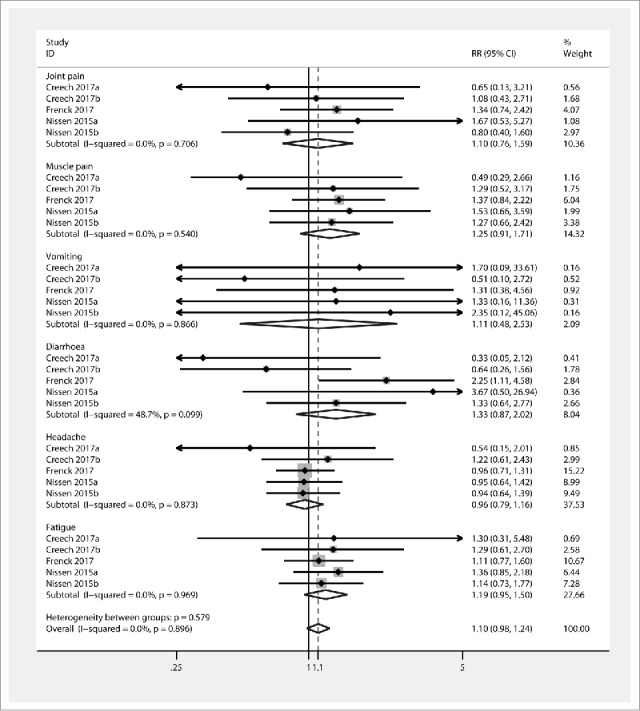
Forest plot showing RRs for the incidence of systemic events following vaccination with Staphylococcus aureus four- and three-antigen vaccines. A significant effect of S. aureus vaccines was assumed if the 95% CI did not include the value 1 for RR. CI confidence interval, RR relative ratio
Figure 3.
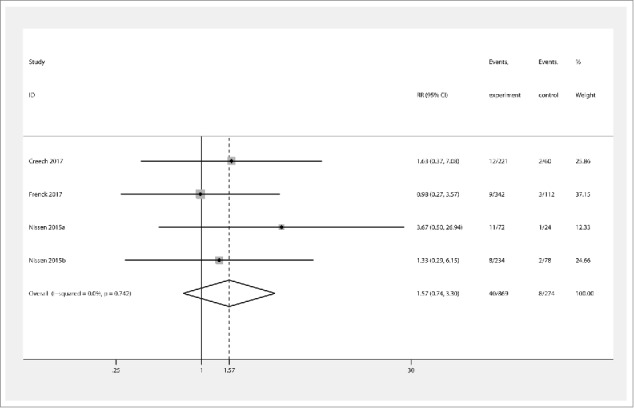
Forest plot showing RRs for the incidence of severe systemic events following vaccination with Staphylococcus aureus four- and three-antigen vaccines. A significant effect of S. aureus vaccines was assumed if the 95% CI did not include the value 1 for RR. CI confidence interval, RR relative ratio
Secondary outcome
The incidence of redness was significantly increased by vaccine injection (RR 2.97 [95% CI 1.57, 5.62]) (Fig. 4) compared to the placebo group, and no statistical heterogeneity was found (χ2 = 5.08, I2 = 11.9%, and Pheterogeneity = 0.338). The levels of swelling reported in the three studies also showed significant differences between the intervention and placebo groups (3.34 [1.45, 7.67]), with no statistical heterogeneity (χ2 = 2.69, I2 = 0%, and Pheterogeneity = 0.685). The intervention group exhibited increased incidence of injection site pain (2.79 [1.92, 4.04]) compared to the placebo group, with no statistical heterogeneity (χ2 = 5.23, I2 = 0%, and Pheterogeneity = 0.464). Overall effects also showed the intervention group demonstrated increased secondary outcomes (2.89 [2.15, 3.90]), and no statistical heterogeneity was found (χ2 = 11.78, I2 = 0%, and Pheterogeneity = 0.720). However, the vaccine group did not show increased incidence of sLAE (4.06 [0.78, 21.24]) (Fig. 5) compared to the placebo group, and there was no heterogeneity (χ2 = 0.03, I2 = 0%, and Pheterogeneity = 0.985).
Figure 4.

Forest plot showing RRs for the incidence of local reactions following vaccination with Staphylococcus aureus four- and three-antigen vaccines. A significant effect of S. aureus vaccines was assumed if the 95% CI did not include the value 1 for RR. CI confidence interval, RR relative ratio
Figure 5.
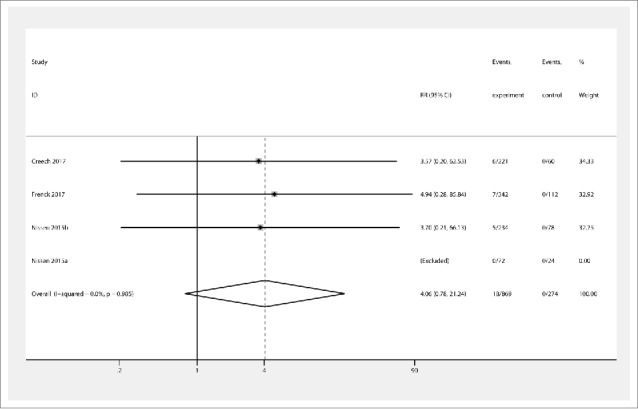
Forest plot showing RRs for the incidence of severe local reactions following vaccination with Staphylococcus aureus four- and three-antigen vaccines. A significant effect of S. aureus vaccines was assumed if the 95% CI did not include the value 1 for RR. CI confidence interval, RR relative ratio
Quality of studies
One study performed randomization by computer20 and was considered to be low risk (Fig. 6). However, the other two studies16,17 did not specify the randomization method and thus were considered to have unclear risk. In one study20, allocation concealment was achieved by a computer, which was defined as low risk of bias, whereas the two remaining studies16,17 did not give explicit concealment methods. Although all studies were double-blinded (participant- and investigator-blind), there was insufficient information on the effectiveness of the blinding, so performance bias was defined as unclear risk in all studies. Additionally, insufficient information was available to determine whether effective outcome assessments were carried out. All studies exhibited withdrawal bias, but missing data did not affect the analysis; thus these studies were defined as low risk. No selectivity bias or other bias was found in all studies.
Figure 6.
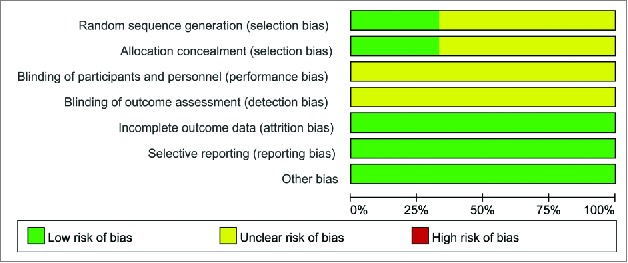
Risk of bias summary
Publication bias
Funnel plots of the study were visually symmetric and a statistical analysis of funnel plots also suggested that no publication bias was present (Harbord's test, P = 0.779) (Fig. 7).
Figure 7.
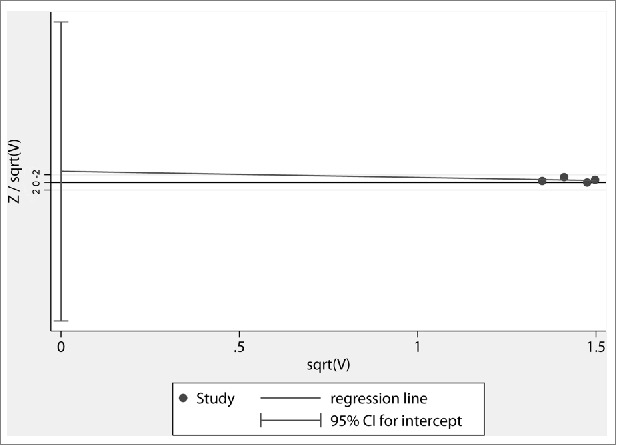
Harbord's funnel plot of all studies included in the meta-analysis
Discussion
The complexity of the virulence mechanism of S. aureus poses challenges for the development of prophylactic vaccines. Previous candidate vaccines, despite generating immune responses, have not proved successful in preventing S. aureus infections12,24,25, and increasing vaccine dose is bound to cause increased side effects. The reason for the lack of efficacy with previous vaccine candidates is not clear, but may be related to the target of a single virulence pathway.24,25 Therefore, a successful prophylactic S. aureus vaccine must address a combination of well-conserved virulence factors expressed by most strains and generate antibodies that effectively kill the organism. A multi-antigen vaccine candidate targeting S. aureus CP5, CP8, ClfA, and rP305A, developed from a lipoprotein manganese transporter C, is under development. S. aureus produces capsular polysaccharides to evade the host's immune system26, and all S. aureus strains have the genetic pathway for synthesis of either CP5 or CP8.27 ClfA is a highly conserved surface antigen that binds fibrinogen, complement proteins, and platelets, which promotes S. aureus infection and mediates adhesion to host tissue.28 RP305A, the recombinant, nonlipidated form of the manganese transporter C protein, is not expressed in vitro by S. aureus, but it is rapidly upregulated and expressed in vivo early in the infectious process.29 Moreover, deletion of manganese transporter C results in loss of bacterial viability in assays that mimic the neutrophil's intracellular environment30, confirming that it is a virulence factor. While both SA3Ag and SA4Ag have good immunogenicity and tolerability, the safety of these vaccines is worth exploring.
In this meta-analysis, we conducted a review of the side effects of SA3Ag and SA4Ag in healthy adults using data from three RCTs comprising a total of 1,148 patients. The objective of our study was to confirm whether these new S. aureus vaccines have good safety. The main finding of this study was that SA3Ag and SA4Ag did not significantly increase SAE. In addition, although LAE were increased in the vaccine group, the incidence of sLAE was not statistically different between the vaccine and placebo groups.
SAE is a key index for evaluating vaccine safety. In this meta-analysis, we included some of the common adverse reactions, such as joint pain, muscle pain, vomiting, diarrhoea, headache, and fatigue. Fever was not included because of the lack of data. Frenck et al.16 found that SA4Ag can increase the incidence of diarrhoea. Our results showed that the vaccine group seemed to exhibit an increased incidence of diarrhoea compared to the placebo group; however, the difference was not significant. In addition, the incidence of joint pain, muscle pain, vomiting, and fatigue events tended to increase in the vaccine group, but there was no statistical difference. It is worth mentioning that if the subgroup of headache was removed, the combined effects of the primary end point were statistically significant, suggesting that vaccination may have an impact on SAE. However, the incidence of sSAE in the vaccine group did not increase, suggesting that vaccine safety was acceptable.
LAE are very common after vaccination. In this meta-analysis, we incorporated three of the most common adverse reactions: redness, swelling, and pain. Predictably, our results showed a marked increase in LAE in the vaccine group, but sLAE were not statistically significant, which also showed that the two vaccines had good safety. Moreover, there was no statistically significant difference in sSAE or sLAE according to vaccine subgroup analysis.
There are some limitations associated with the present study. First, the RCTs included in the meta-analysis did not evaluate all SAE, such as fever, which could change the outcome of the combined effect. Although our results showed that the vaccine group did not exhibit increased sSAE and sLAE, we used the definition applied by the investigators of the respective studies when examining the endpoint events. This means that varying severities of events could have been classified as identical, and we may have erroneously pooled these events in the meta-analysis. Second, most of these studies had an inadequate blinding design for performance and outcome assessment, which is seemingly more difficult to achieve in RCTs than in trials investigating a pharmacological agent. In this meta-analysis, all included studies had bias in the performance and outcome evaluation design.
Overall, our meta-analysis showed that these two novel S. aureus vaccines, SA3Ag and SA4Ag, had acceptable safety according to the incidence of SAE and LAE. However, the study was based on a small number of subjects and participants were predominantly white. Moreover, the inadequate blinding design may also have led to false positives in the data. Therefore, additional, large-scale RCTs must be carried out to further determine the immunogenicity, tolerance, and safety of SA3Ag and SA4Ag.
Methods
Data sources
The Medline, EMBASE, and Cochrane databases and the reference lists found in original and review articles were searched independently by two reviewers (XX, HZ) using medical subject heading terms, key words, titles, and abstracts. The search terms were “Staphylococcus aureus four-antigen vaccine,” “SA4Ag,” “Staphylococcus aureus three-antigen vaccine,” and “SA3Ag.” All historical literature was searched up until July 2017.
Study selection
An initial eligibility screen of all retrieved titles and abstracts was conducted, and original studies were included in our meta-analysis if they met the following criteria: (1) human subjects; (2) participants in general good health, including those with stable pre-existing chronic medical conditions; (3) participants were randomly assigned to receive either prophylactic S. aureus vaccine or placebo; and (4) sufficient data on SAE and LAE were available. Full manuscripts were obtained for all selected articles based on the initial assessment of abstracts. Only fully published trials were included (abstracts and congress presentations were not included). The primary outcome was the combined endpoint, SAE, including joint pain, muscle pain, vomiting, diarrhoea, headache, and fatigue. The secondary outcome was LAE, including redness, swelling, and injection site pain. Trials not reporting any of these parameters were excluded from the review. Two investigators (XX, HZ) independently reviewed all full-text articles that potentially met the inclusion criteria according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement31 and the Cochrane Handbook guidelines.23 In cases of disagreement, a consensus was obtained by discussion with the third author (HL).
Data extraction
All selected papers were reviewed by two reviewers (XX, HZ), who independently extracted data to a data sheet. Data extraction included year of publication, study design, sample size, patient characteristics, inclusion and exclusion criteria, control and intervention protocols, randomization, blinding, and follow-up, as well as the outcome parameters described above. SAE and severe systemic adverse events (sSAE) were the primary endpoints; the former included all systemic adverse events, whereas the latter was only included severe side effects. The secondary outcome was also classified into LAE and severe local adverse events (sLAE), defined similarly. For a study involving multiple intervention groups and one control group, data for different doses of intervention groups were combined, while data for intervention groups with different vaccine types were equally segmented, according to the Cochrane Handbook guidelines.23 Where data were presented in a graph but not in the text, we requested the data from the corresponding author of the paper. If the data were not provided, we extrapolated them from the graph using a charting digital tool (GetData Graph Digitizer, http://getdata-graph-digitizer.com). Following the extraction of relevant data by two of the authors, data were examined for possible inconsistencies, which were then resolved by discussion. If a consensus could not be reached, then the third author was consulted (HL). Studies were not directly conducted on humans and ethical approval was therefore unnecessary.
Quality assessment
Two authors (XX, HZ) used the seven domains of the Cochrane risk of bias tool to evaluate the quality of the included studies, using the following criteria: randomization sequence generation, concealment of randomization sequence, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Studies were classified as having low risk, high risk, or unclear risk of bias for each item, as suggested in the Cochrane Handbook.23
Statistical analysis
The verified data were analysed using Stata software (version 13.0; Stata Corporation, College Station, TX, USA) and REVMAN software (version 5.2; Cochrane Collaboration, Oxford, UK). One author (XX) entered the data and a second author (HZ) verified data entry. The relative ratio (RR) and corresponding 95% confidence intervals (CIs) were calculated for dichotomous data. A fixed effects model was used to analyse data with values higher than 0.05 by heterogeneity testing (χ2-based Q-test), while a random effects model was used when values were less than 0.05. The magnitude of heterogeneity was assessed by I2 test (I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 = 75–100%, extreme heterogeneity). An intervention was assumed to have had a significant effect if the 95% CIs did not include the value 1 for RR. In the analysis for small-study effects, publication bias was assessed using funnel plot techniques and Harbord's test.32
Abbreviations
- CI
confidence interval
- ClfA clumping factor A
CP5 capsular polysaccharide type 5
- CP8
capsular polysaccharide type 8
- LAE
local adverse events
- RCT
randomized controlled trial
- rP305A
recombinant P305A
- RR
relative ratio
- SA3Ag
Staphylococcus aureus three-antigen vaccine
- SA4Ag
Staphylococcus aureus four-antigen vaccine
- SAE
systematic adverse events
- sLAE
severe local adverse events
- sSAE
severe systematic adverse events
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Acknowledgments
This study was supported by the National Research Foundation for Health and Family Planning Commission of China (WKJ-ZJ-1604) and Key Research and Development Project of Zhejiang Provincial Science and Technology Department (2017C03005). We thank Christina Croney, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing a draft of this manuscript.
Funding
This study was supported by the National Research Foundation for Health and Family Planning Commission of China (WKJ-ZJ-1604) and Key Research and Development Project of Zhejiang Provincial Science and Technology Department (2017C03005).
Author contributions
Designed the study: Huoyang Lv and Xiaoqun Xu. Performed the study: Xiaoqun Xu and Houyong Zhu. Analysed the data: Xiaoqun Xu, Houyong Zhu, and Huoyang Lv. Wrote the paper: Xiaoqun Xu and Houyong Zhu. Revised the manuscript: Huoyang Lv.
Ethical approval
Studies were not directly conducted on humans and ethical approval was therefore unnecessary.
Informed consent
Studies were not directly conducted on humans and informed consent was therefore unnecessary.
References
- 1.Shorr AF, Tabak YP, Gupta V, Johannes RS, Liu LZ, Kollef MH. Morbidity and cost burden of methicillin-resistant Staphylococcus aureus in early onset ventilator-associated pneumonia. Crit Care. 2006;10(3):R97. doi: 10.1186/cc4934. PMID:16808853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troidle L, Eisen T, Pacelli L, Finkelstein F. Complications associated with the development of bacteremia with Staphylococcus aureus. Hemodial Int. 2007;11(1):72–75. doi: 10.1111/j.1542-4758.2007.00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Amissah NA, van Dam L, Ablordey A, Ampomah OW, Prah I, Tetteh CS, van der Werf TS, Friedrich AW, Rossen JW, van Dijl JM et al.. Epidemiology of Staphylococcus aureus in a burn unit of a tertiary care center in Ghana. Plos One. 2017;12(7):e181072. doi: 10.1371/journal.pone.0181072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosimi RA, Beik N, Kubiak DW, Johnson JA. Ceftaroline for Severe Methicillin-Resistant Staphylococcus aureus Infections: A Systematic Review. Open Forum Infect Dis. 2017;4(2):x84. doi: 10.1093/ofid/ofx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adogwa O, Elsamadicy AA, Sergesketter A, Vuong VD, Mehta AI, Vasquez RA, Cheng J, Bagley CA, Karikari IO. Prophylactic use of intraoperative vancomycin powder and postoperative infection: an analysis of microbiological patterns in 1200 consecutive surgical cases. J Neurosurg Spine. 2017;27(3):328-334. doi: 10.3171/2017.2.SPINE161310. [DOI] [PubMed] [Google Scholar]
- 6.Fu T, Liu YM. Antibacterial Effect of Bacteriocin Isolated From Lactobacillus Plantarum ATCC 8014 on Postoperative Infection of Mandibular Fracture In Vivo. J Craniofac Surg. 2017;28(3):679–682. doi: 10.1097/SCS.0000000000003469. [DOI] [PubMed] [Google Scholar]
- 7.Oksuz E, Deniz FE, Gunal O, Demir O, Barut S, Markoc F, Erkorkmaz U. Which method is the most effective for preventing postoperative infection in spinal surgery? Eur Spine J. 2016;25(4):1006–1011. doi: 10.1007/s00586-015-3941-y. [DOI] [PubMed] [Google Scholar]
- 8.Watson DL, Kennedy JW. Immunisation against experimental staphylococcal mastitis in sheep – effect of challenge with a heterologous strain of Staphylococcus aureus. Aust Vet J. 1981;57(7):309–313 doi: 10.1111/j.1751-0813.1981.tb05834.x. [DOI] [PubMed] [Google Scholar]
- 9.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R et al.. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346(7):491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 10.Shinefield HR, Black S. Prospects for active and passive immunization against Staphylococcus aureus. Pediatr Infect Dis J. 2006;25(2):167–168. doi: 10.1097/01.inf.0000199887.18267.9a. [DOI] [PubMed] [Google Scholar]
- 11.Shinefield HR. Use of a conjugate polysaccharide vaccine in the prevention of invasive staphylococcal disease: is an additional vaccine needed or possible? Vaccine. 2006;24 Suppl 2:S2–S65 [DOI] [PubMed] [Google Scholar]
- 12.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS et al.. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. Jama. 2013;309(13):1368–1378. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 13.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine. 2013;31(25):2723–2730. doi: 10.1016/j.vaccine.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Zorman JK, Esser M, Raedler M, Kreiswirth BN, Ala'Aldeen DA, Kartsonis N, Smugar SS, Anderson AS, McNeely T, Arduino JM. Naturally occurring IgG antibody levels to the Staphylococcus aureus protein IsdB in humans. Hum Vaccin Immunother. 2013;9(9):1857–1864. doi: 10.4161/hv.25253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begier E, Seiden DJ, Patton M, Zito E, Severs J, Cooper D, Eiden J, Gruber WC, Jansen KU, Anderson AS et al.. SA4Ag, a 4-antigen Staphylococcus aureus vaccine, rapidly induces high levels of bacteria-killing antibodies. Vaccine. 2017;35(8):1132–1139. doi: 10.1016/j.vaccine.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Frenck RJ, Creech CB, Sheldon EA, Seiden DJ, Kankam MK, Baber J, Zito E, Hubler R, Eiden J, Severs JM, et al.. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): Results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine. 2017;35(2):375–384. doi: 10.1016/j.vaccine.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Creech CB, Frenck RJ, Sheldon EA, Seiden DJ, Kankam MK, Zito ET, Girgenti D, Severs JM, Immermann FW, McNeil LK, et al.. Safety, tolerability, and immunogenicity of a single dose 4-antigen or 3-antigen Staphylococcus aureus vaccine in healthy older adults: Results of a randomised trial. Vaccine. 2017;35(2):385–394. doi: 10.1016/j.vaccine.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Marshall H, Nissen M, Richmond P, Shakib S, Jiang Q, Cooper D, Rill D, Baber J, Eiden J, Gruber WC et al.. Safety and immunogenicity of a booster dose of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults: A randomized phase 1 study. J Infect. 2016;73(5):437–454. doi: 10.1016/j.jinf.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Rozemeijer W, Fink P, Rojas E, Jones CH, Pavliakova D, Giardina P, Murphy E, Liberator P, Jiang Q, Girgenti D et al.. Evaluation of approaches to monitor Staphylococcus aureus virulence factor expression during human disease. Plos One. 2015;10(2):e116945. doi: 10.1371/journal.pone.0116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nissen M, Marshall H, Richmond P, Shakib S, Jiang Q, Cooper D, Rill D, Baber J, Eiden J, Gruber W, et al.. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine. 2015;33(15):1846–1854. doi: 10.1016/j.vaccine.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Park HM, Yoo HS, Oh TH, Kim D, Han HR. Immunogenicity of alpha-toxin, capsular polysaccharide (CPS) and recombinant fibronectin-binding protein (r-FnBP) of Staphylococcus aureus in rabbit. J Vet Med Sci. 1999;61(9):995–1000. doi: 10.1292/jvms.61.995. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad I, Kleven SH, Avakian AP, Glisson JR. Sensitivity and specificity of Mycoplasma gallisepticum agglutination antigens prepared from medium with artificial liposomes substituting for serum. Avian Dis. 1988;32(3):519–526. doi: 10.2307/1590922. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J, Altman D, Gotzsche P. Cochrane Handbook for Systematic Reviews of Interventions: BMJ; 2011. [Google Scholar]
- 24.Fattom A, Fuller S, Propst M, Winston S, Muenz L, He D, Naso R, Horwith G. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine. 2004;23(5):656–663. doi: 10.1016/j.vaccine.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Fattom AI, Horwith G, Fuller S, Propst M, Naso R. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine. 2004;22(7):880–887. doi: 10.1016/j.vaccine.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 26.O'Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17(1):218–234 doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy E, Lin SL, Nunez L, Andrew L, Fink PS, Dilts DA, Hoiseth SK, Jansen KU, Anderson AS. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: monitoring antigenic diversity. Hum Vaccin. 2011;7 Suppl:51–59 [DOI] [PubMed] [Google Scholar]
- 28.O'Brien L, Kerrigan SW, Kaw G, Hogan M, Penades J, Litt D, Fitzgerald DJ, Foster TJ, Cox D. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol Microbiol. 2002;44(4):1033–1044 doi: 10.1046/j.1365-2958.2002.02935.x. PMID:12010496 [DOI] [PubMed] [Google Scholar]
- 29.Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, Nunez L, Carriere M, Singer C, Dilts DA et al.. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis. 2012;205(11):1688–1696. doi: 10.1093/infdis/jis272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handke LD, Hawkins JC, Miller AA, Jansen KU, Anderson AS. Regulation of Staphylococcus aureus MntC expression and its role in response to oxidative stress. PLOS ONE. 2013;8(10):e77874. doi: 10.1371/journal.pone.0077874. PMID:24205007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535 doi: 10.1136/bmj.b2535. PMID:19622551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]


