ABSTRACT
Universal vaccination programmes against Hepatitis B Virus (HBV) have significantly reduced the burden of the disease; nevertheless, HBV infection remains a relevant issue for high-risk subjects, such as healthcare workers (HCWs), who may potentially be exposed to blood or body fluids. Our study evaluates the long-term duration of the immunological memory of HBV vaccination 11–23 years after primary immunization by examining the response to booster doses in HCWs and students of health disciplines at Careggi Teaching Hospital in Florence (Italy). All participants (n = 2,203) had received a complete HBV immunization course in infancy or adolescence. Blood samples were collected to measure antibody levels against the HBV surface antigen (anti-HBs); an anti-HBs titre <10 mIU/mL was considered as negative. The administration of the vaccination course during infancy induced lower long-term anti-HBs titres compared to those in case of vaccination performed during adolescence (titre <10 mIU/mL: 51.1% and 12.2% respectively; p < 0.001), also considering that an equal number of years has elapsed since vaccination. A booster dose administered to subjects vaccinated in infancy is able to induce anamnestic immunological response in a higher percentage of vaccinated people (p < 0.001). Few subjects (n. = 4) accepted a fifth dose of vaccine in the case of persistent anti-HBs negative titres; this aspect requires further investigation.
The total absence of acute hepatitis B among vaccinated subjects suggests that the long incubation period of the disease allows the activation of immunologic memory mechanisms, which is also true in case of low anti-HBs level. In conclusion HCWs still represent a high-risk category; it is therefore, necessary to increase efforts to protect and vaccinate these subjects.
KEYWORDS: Hepatitis B, Vaccination, Coverage, Protection, Boosters, Health Care Workers
Introduction
All over the world, 2 billion people have evidence of past or present infection of Hepatitis B Virus (HBV), 240 million are chronic carriers of HBV surface antigen (HBsAg) and around 680,000 people die each year from hepatitis B complications.1
Italy was one of the first countries to introduce a routine simultaneous double-cohort vaccination program against HBV in 1991, even before the World Health Organization (WHO) recommended universal immunization.2,3
In particular, the Italian vaccination plan against HBV included universal immunization of new-borns in the first year of life and 12-year-old adolescents with the aim to reduce and in the long term eliminate the transmission of HBV by creating 24 generations of immune subjects within the first 12 years of vaccination implementation.
As expected, 20 years after the introduction of universal vaccination, a significant decrease in the incidence of acute hepatitis B cases was observed.4
Although universal vaccination of new-borns and adolescents has reduced the burden of disease, HBV infection remains an issue for high-risk subjects, such as healthcare workers (HCWs), who may potentially be exposed to blood or body fluids.5
The risk for HCWs of being exposed to a virus is partly proportional to the prevalence of that infection among patients6; therefore, the risk of HBV infection has certainly decreased in Italy due to the implementation of universal vaccination for the last 25 years. However, the risk for HCWs is still relevant.
Vaccination of HCWs in addition to the universal precautions adopted during occupational activity represents the main strategy of protection highlighted by the WHO and adopted in Italy for a long time.2,7–11
The Italian policy for the protection of HCWs against HBV infection also includes a vigilant screening through the serological test for antibodies against HBsAg (anti-HBs) before starting the occupational activity.12 Scientific evidences, show that subjects with a negative anti-HBs result (<10 mIU/mL) should receive up to three additional doses of vaccine in order to achieve immunological response.7,13,14 The Italian Health Ministry recommends this protocol in case the subject is identified as a non-responder to the basic immunization course.15
We analysed the data obtained from HCWs and students of health disciplines attending an Italian teaching hospital, who have undergone occupational medicine visits. The aims of the study are: to assess the antibody levels against HBV after 11–23 years from administration of the primary vaccination course; analyse whether vaccination administered in the first years of life can guarantee protection in adulthood, when the risk of infection increases, and evaluate the effectiveness of booster doses in increasing the immunological response.
Results
A total of 2,203 subjects (1.408 females and 795 males) were included in the study. All of them had received vaccination against HBV (three doses) during infancy or adolescence. The main descriptive results are shown in Table 1.
Table 1.
Descriptive data of the subjects evaluated in the study.
| Anti-HBs titre (mIU/mL), n. of subjects (%) |
||||||
|---|---|---|---|---|---|---|
| Group | Year of birth | N. of subjects | <10 | 10-100 | ≥ 101 | ≥ 10 |
| 1 | 1980 | 74 | 8 (10.8) | 27 (36.5) | 39 (52.7) | 66 (89.2) |
| 1981 | 82 | 6 (7.3) | 29 (35.4) | 47 (57.3) | 76 (92.7) | |
| 1982 | 85 | 4 (4.7) | 26 (30.6) | 55 (64.7) | 81 (95.3) | |
| 1983 | 109 | 4 (3.7) | 44 (40.4) | 61 (55.9) | 105 (96.3) | |
| 1984 | 118 | 11 (9.3) | 31 (26.3) | 76 (64.4) | 107 (90.7) | |
| 1985 | 132 | 18 (13.6) | 46 (34.8) | 68 (51.6) | 114 (86.4) | |
| 1986 | 114 | 23 (20.2) | 31 (27.2) | 60 (52.6) | 91 (79.8) | |
| 1987 | 108 | 18 (16.7) | 45 (41.7) | 45 (41.6) | 90 (83.3) | |
| 1988 | 97 | 14 (14.4) | 28 (28.9) | 55 (56.7) | 83 (85.6) | |
| 1989 | 97 | 15 (15.5) | 37 (38.1) | 45 (46.4) | 82 (84.5) | |
| 1990 | 136 | 20 (14.7) | 54 (39.7) | 62 (45.6) | 116 (85.3) | |
| Total | 1980-1990 | 1152 | 141 (12.2) | 398 (34.5) | 613 (53.3) | 1.011 (87.8) |
| 2 | 1991 | 152 | 47 (30.9) | 47 (30.9) | 58 (38.2) | 105 (69.1) |
| 1992 | 182 | 99 (54.4) | 60 (33.0) | 23 (12.6) | 83 (45.6) | |
| 1993 | 253 | 135 (53.4) | 85 (33.4) | 33 (13.2) | 118 (46.6) | |
| 1994 | 232 | 131 (56.5) | 83 (35.8) | 18 (7.7) | 101 (43.5) | |
| 1995 | 155 | 83 (53.5) | 55 (35.5) | 17 (11.0) | 72 (46.4) | |
| 1996 | 77 | 42 (54.5) | 29 (37.7) | 6 (7.8) | 35 (45.5) | |
| Total | 1991-1996 | 1051 | 537 (51.1) | 359 (34.2) | 155 (14.7) | 514 (48.9) |
| TOTAL | 1980-1996 | 2203 | 678 (30.8) | 757 (34.4) | 768 (34.8) | 1525 (69.2) |
The exact intervals between primary vaccination and anti-HBs titres check were: 17.2 years (minimum 12, maximum 23 years) in Group 1 (individuals born between 1980 and 1990 and vaccinated at 12 years of age) and 19.3 years (minimum 17, maximum 22 years) in subject born between 1992 and 1996 (belonging to Group 2 and vaccinated in the first year of life). Group 2 also included subjects born in 1991 (n 152), most of whom (79.6%) were vaccinated in adolescence; the interval between primary vaccination and anti-HBs levels check was 11.4 years. Conversely, 20.4% received vaccine in infancy, for them the interval was 22.8 years.
From the analysis of serum samples that were collected during the occupational health visits, 678 subjects (30.8%) had anti-HBs titre <10 mIU/mL, 757 (34.4%) between 10 and 100 mIU/mL and 768 (34.8%) had ≥ 101 mIU/mL (Table 1).
Figure 1 shows the proportion of subjects with non-protective titre of anti-HBs (<10mIU/mL) broken down by year of birth.
Figure 1.
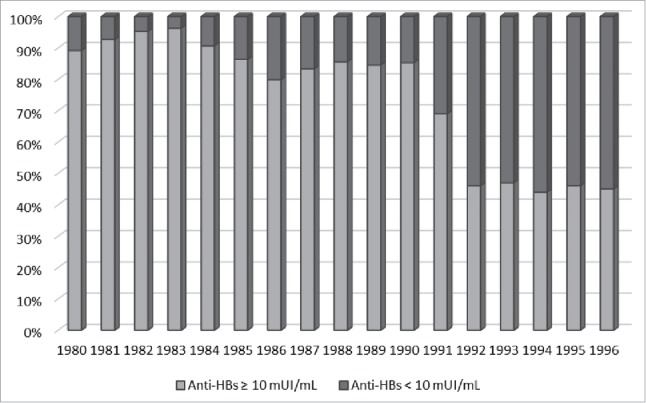
Proportion of subjects with anti-HBs < 10 mUI/mL and ≥ 10 mUI/mL broken down by year of birth.
Group 1 was composed of 1,152 people, of which 141 (12.2%) had anti-HBs titre < 10 mIU/mL, 398 (34.5%) had between 10 and 100 mIU/mL, and 613 (53.3%) had ≥ 101 mIU/mL. Group 2 was composed of 1,051 people, of which 537 (51.1%) had anti-HBs titre < 10 mIU/mL, 359 (34.2%) between 10 and 100 mIU/mL and 155 (14.7%) had ≥ 101 mIU/mL (Fig. 2).
Figure 2.
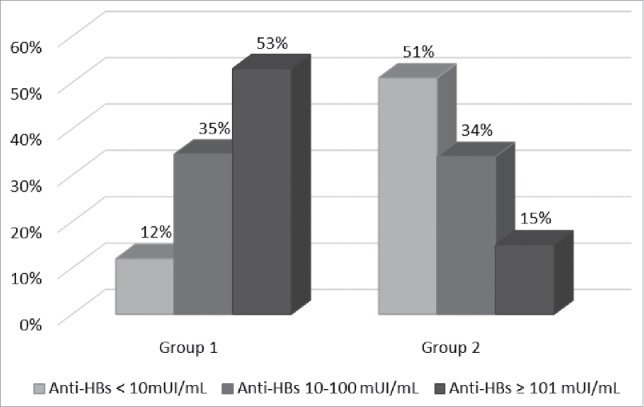
Proportion of subjects with anti-HBs < 10 mUI/mL, between 10 and 100 mUI/mL and ≥ 101 mUI/mL broken down by group (Group 1 = vaccinated at 12 years of age; Group 2 = vaccinated in the first year of life).
We analysed the relation between the titre of anti-HBs < 10 mIU/mL and the timing of vaccination (at 12 years of age = Group 1, or in the first year of life = Group 2). The proportion of subjects with anti-HBs < 10 mIU/mL was found to be significantly higher in Group 2 (OR = 7.49, 95% CI 6.05-9.27, p < 0.001).
We also examined the possible differences among the proportion of negative subjects within each group: no associations were found in Group 1 (p > 0.05). Conversely, in Group 2, a statistically significant difference was found between years of birth 1992–1996 and year of birth 1991. The latter showed a higher proportion of individuals with negative anti-HBs (p < 0.05).
In order to compare the antibody response of people vaccinated at 12 years of age with those vaccinated in the first year of life on an equal number of years since vaccination, we evaluated the proportion of subjects with anti-HBs < 10 mIU/mL within each ‘vaccination year cohort’. As shown in Table 2, in all six cohorts, individuals vaccinated in the first year of life had non-protective antibody titres more frequently than those vaccinated at 12 years of age.
Table 2.
Proportion of subjects with anti-HBs < 10 mUI/mL by year of birth in each ‘vaccination year cohort’. Logistic regression analysis. Odds Ratio (OR); 95% Confidence Interval (95% CI); p values.
| Vaccination year cohort | Years of birth (% of subjects with anti-HBs < 10 mIU/mL) | OR | 95% CI | p |
|---|---|---|---|---|
| 1991 | 1991 (30.9) vs 1980 (10.8) | 3.69 | 1.64-8.30 | <0.005 |
| 1992 | 1992 (54.4) vs 1981 (7.3) | 15.10 | 6.26-36.45 | < 0.0001 |
| 1993 | 1993 (53.4) vs 1982 (4.7) | 23.16 | 8.23-65.40 | < 0.0001 |
| 1994 | 1994 (56.5) vs 1983 (3.7) | 34.04 | 12.13-95.53 | < 0.0001 |
| 1995 | 1995 (53.5) vs 1984 (9.3) | 11.21 | 5.58-22.49 | < 0.0001 |
| 1996 | 1996 (54.5) vs 1985 (13.6) | 7.60 | 3.89-14.84 | < 0.0001 |
Among the 678 subjects with anti-HBs titre < 10 mIU/mL, 330 (48.7%) received a fourth dose of vaccine, while the others refused. The measurement of the antibody response one month after this further dose shows that 37 subjects (11.2%) still had anti-HBs titre < 10 mIU/mL; conversely, 293 (88.8%) subjects achieved a protective antibody titre. Figure 3 shows the proportion of subjects with different levels of anti-HBs after the fourth dose of vaccine, broken down by group. Subjects of Group 2 showed a lower failure to boost anti-HBs titre than those of Group 1 (OR = 0.23, 95% CI 0.11-0.47, p < 0.001).
Figure 3.
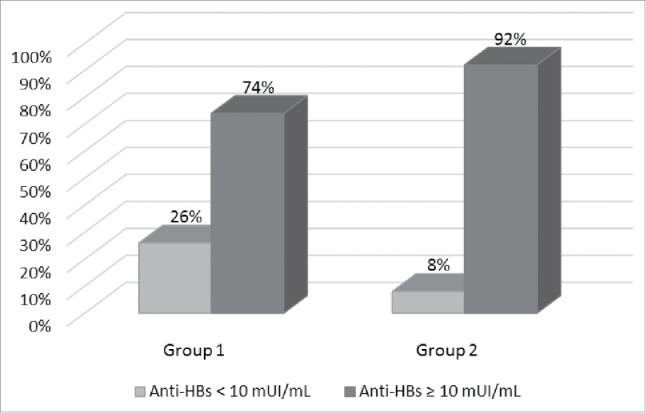
Proportion of subjects with anti-HBs < 10 mUI/mL and ≥ 10 mUI/mL, after the fourth dose of vaccine, broken down by group (Group 1 = vaccinated at 12 years of age; Group 2 = vaccinated in the first year of life).
Finally, we proposed a fifth vaccine dose to the 37 non- protected subjects. This protocol was started in October 2015 and only four people (half belonging to Group 1 and half to Group 2) were enrolled. All of them developed a protective anti-HBs titre one month after the fifth dose.
Discussion
Our study confirms a high adherence to the Italian universal routine vaccination program against HBV in the age group 1–36 years. All enrolled subjects had received three doses of vaccine during infancy or adolescence.
After three intramuscular doses of hepatitis B vaccine, more than 90% of healthy adults and more than 95% of infants, children, and adolescents were found to develop adequate antibody responses.13
Conventionally, a level of anti-HBs ≥ 10 mIU/mL is considered protective as a proxy of the acquisition of immunological memory against HBsAg.7,16
From literature data, it is evident that antibody levels decline with time: the achievement of the maximum antibody titre is usually about one month after the last vaccine dose. A sharp decay in the titre occurs over the months, but the declining trend of the antibody concentration slows down in the following years.13,17
However, immunologic memory persists for more than 20 years following immunization, and healthy subjects with declining antibody levels are still protected against HBV infection.18
In our study, the 30.8% of the total sample showed non-protective antibody titres. The proportion of subjects with anti-HBs titre < 10 mIU/mL in subjects immunized in the first year of life was significantly higher that those immunized at 12 years of age (51.1% and 12.2% respectively; p < 0.001); this difference was also confirmed when the analysis was performed considering an equal number of years since vaccination. These results are in accordance with a recent Italian study conducted on 717 healthcare students immunized against HBV in infancy or adolescence.19 Similar to our results, 29.3% of the subjects showed non-protective antibody titres. Moreover, a higher prevalence of subjects with anti-HBsAg titre < 10 mIU/mL was found among people vaccinated as infants compared to those vaccinated as adolescents (p <0.001).
Furthermore, among people vaccinated at 12 years of age, no difference was found in the percentage of negative results for anti-HBs between subjects born in different years. Conversely, among people vaccinated in the first year of life, a significant difference emerged between subjects vaccinated in 1991 (about 24 years before the study) and those vaccinated in the following years (1992-1996) (p < 0.05).
In particular, contrary to expectation, people born in 1991 showed a higher proportion of anti-HBs protective level. This can be explained by the fact that the compulsory vaccination against HBV, was issued in Italy on 27 May 1991, but was implemented in the whole country only from October 1991.Therefore, several subjects born in 1991 were in reality vaccinated at 12 years of age, thus making the 1991 birth cohort a mixed population in regard to the age of administration of vaccination.
In public health programmes of universal HBV immunization, the control of seroconversion after vaccination is not considered a cost-effective practice because of the high level of protection obtained in the community. On the other hand, it is important to verify the existence of protection in subjects exposed to a high risk of infection, such as HCWs. For this reason, workers and students in the healthcare sector need to be checked for anti-HBs. If they are found to be positive, no further action is needed. In subjects who received hepatitis B vaccine in the distant past and have anti-HBs titre <10 mIU/mL, it is not possible to distinguish between failure to respond to the initial vaccination (lack of protection) and response to the initial vaccination followed by reduction of antibody titre (protected). In these cases, the subjects should receive a ‘challenge’ dose of vaccine, and should be checked again one month later. If a booster response is evident, this means that immunological memory exists, and no further action is needed. On the contrary, a persistently negative result requires the completion of the second vaccination course with two doses (one immediate and one after four to six months) in order to obtain the acquisition of immunological memory (40–70% of initial non-protected subjects show seroconversion to the new series).7,13,14 Only people with titre <10 mIU/mL after two full series of the hepatitis B vaccine (six doses) would be considered non-responders.
In our study, after a fourth dose of vaccine was administered to subjects with low anti-HBs titre, only 11.2% did not develop anti-HBs protective levels at one month; most of them were vaccinated at 12 years of age (p < 0.001). On the other hand, those vaccinated in infancy were more frequently anti-HBs negative at the first check, but showed a booster effect after the ‘challenge’ dose more often.
Therefore, although anti-HBs titres achieved in case of vaccination in infancy are lower than in case of immunization at adolescent age and the likelihood of a negative anti-HBs result is much higher many years later, our results seem to confirm that immunological memory in case of infant immunization is almost invariably acquired and conserved 11–23 years later.
Similar data about the long-term protection of HBV vaccine after primary immunization are demonstrated in the study by Dini et al., where the protection was shown to last more than 20 years; but a lower failure to respond to booster dose was reported compared to our results (only in 5% of subjects).19 Furthermore, they did not find a difference between subjects vaccinated in infancy and those vaccinated in adolescence in terms of the anamnestic response to a booster dose of vaccine. Other studies confirm the persistence of a strong immune memory after primary immunization: in the study by Zanetti et al., an anamnestic response is present in more than 95% of cases 10 years after a primary immunization performed in infancy or adolescence.20 Moreover, in this study an anti-HBs titre <10 mIU/mL was prevalent in subjects vaccinated in infancy (36%) compared to those vaccinated in adolescence (11%). Spada et al. extended the study of Zanetti et al. in order to evaluate the immune memory achieved by primary immunization in infancy; they concluded that vaccination in the first year of life lasts for at least 17 years and additional booster doses are not needed at this time to maintain long-term protection.21 The dramatic decrease of acute hepatitis B occurrence in the Italian vaccinated cohorts suggests that the long incubation period of the disease allows the activation of immunologic memory mechanisms, even when anti-HBs are no longer detectable.22,23
We found a poor adherence to revaccination among HCWs and students (only 48.7% accepted a fourth dose of vaccine). This is a critical aspect that will need further work, particularly in terms of communication about benefits and drawbacks of both vaccination and non-vaccination.24
One limitation of the study is that in the absence of documentation regarding vaccination, we checked the vaccination status for HBV based on self-reporting; therefore, this might be subject to recall bias. This issue only affects 14.4% of the subjects; however, in the literature, self-reporting of influenza, pneumococcal polysaccharide, hepatitis A, hepatitis B, and HPV vaccination status among adults has proved to be sensitive and specific.25
Others limitations are related to the fact that no data about peak antibody titre are available and that information about specific conditions and behaviours of participants (e.g., obesity, alcohol consumption and smoking habits), which are considered to be variables involved in the decrease of the immunogenicity of HBV vaccine over time, was not collected.26
Conclusion
Preventive occupational medicine visits provided the occasion to study a sample of Italian HCWs and students of health disciplines, in order to analyse HBV vaccination coverage, the kinetics of anti-HBs levels and the immunological response to booster doses of vaccine. The administration of the vaccination course during infancy elicits lower anti-HBs titres compared to the vaccination performed during adolescence, but a booster dose induces an anamnestic response more frequently.
Although population preventive programmes, such as the universal vaccination introduced in 1991, are making HBV transmission to HCWs an increasingly rare event, the occupational risk is still considerable. It is, therefore, necessary to continue and increase efforts to protect and vaccinate all these subjects. Administration of up to three further doses of vaccine in non-responder subjects should further decrease the number of non-immune HCWs and contribute to the progress towards the goal of a zero-risk for acquisition of new HBV infections in Italy.
Materials and methods
The study was conducted from January 2014 to December 2015 in the Occupational Medicine Service of Careggi Teaching Hospital, a tertiary adult acute care center in Tuscany in the city of Florence (Italy), with nearly 1,300 beds. The subjects involved were all students of health disciplines and HCWs, born in Italy between 1 January 1980 and 31 December 1996, attending or employed at the Careggi Hospital during the study period.
All involved subjects were verified as being fully vaccinated. The verification was done by checking their vaccination certificate or booklet generated by vaccination registry, which was available in 85.6% of cases. When this was not available, we asked the person about his/her own vaccination status (self-reporting). During preventive visits and periodic controls, as required by law,11 the subjects underwent blood sampling to measure anti-HBs by ADVIA Centaur® assay, an antibody-capture microparticle direct chemiluminometric immunoassay used to measure the amount of anti-HBs in human serum and plasma. The criteria provided by the producer were applied for the qualitative evaluation of antibodies and antigen detection, according to specific instruction manual. Anti-HBs titres were classified into three levels: < 10 mIU/mL, from 10 to 100 mIU/mL and ≥ 101 mIU/mL. According to current International Standards, subjects with anti-HBs concentrations of < 10 mIU/ml following the primary series of vaccination are considered non-protected against HBV infection, while an anti-HBs titre ≥10 mIU/mL indicates a protective level. Subjects with anti-HBs ≥ 101 mIU/ml after the primary series can be regarded as good-responders to the vaccine.27,28
Age, sex and primary vaccination course were also recorded.
In order to analyse the possible differences in the titre of anti-HBs, we classified students and HCWs into two groups on the basis of their year of birth: in Group 1 (subjects born between 1980 and 1990) the three doses were administered at 12 years of age, while in Group 2 (born between 1991 and 1996) the first series of vaccine was administered in the first year of life. However, subjects born in 1991 might have received immunization as either infants or adolescents. We also divided subjects into six ‘vaccination year cohorts’ based on the year of vaccination, with the aim of comparing the antibody response of people vaccinated at 12 years of age with those vaccinated in the first year of life, after an equal number of years since the vaccination. The vaccination cohort of 1991 included subjects born in 1980 and 1991; the vaccination cohort of 1992 included subjects born in 1981 and 1992; the vaccination cohort of 1993 included subjects born in 1982 and 1993; the vaccination cohort of 1994 included subjects born in 1983 and 1994; the vaccination cohort of 1995 included subjects born in 1984 and 1995 and the vaccination cohort of 1996 included subjects born in 1985 and 1996. The descriptive analysis of results was performed using the Microsoft Excel 2010 software, while the statistical analysis was carried out with the software SAS 9.3. The association between binary variables was evaluated using the simple logistic regression model. The research was carried out in compliance with the Helsinki Declaration.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.World Health Organization (WHO) Hepatitis B. Fact sheet N. 204; 2016. WHO web-site Available at: http://www. who.int/mediacentre/factsheets/fs204/en/. Updated July 2016.
- 2.Law n. 165 , 27 May 1991. Obbligatorietà della vaccinazione contro l'epatite virale B. Gazz. Uff., n. 127, 1° giugno 1991. [Google Scholar]
- 3.World Health Organization (WHO) Hepatitis B. Immunization surveillance, assessment and monitoring. WHO web-site Available at: http://www.who.int/immunization_monitoring/diseases/hepatitis/en/index. html. Last accessed: October 2012.
- 4.Boccalini S, Pellegrino E, Tiscione E, Pesavento G, Bechini A, Levi M, Rapi S, Mercurio S, Mannelli F, Peruzzi M, et al. Sero-epidemiology of hepatitis B markers in the population of Tuscany, Central Italy, 20 years after the implementation of universal vaccination. Hum Vaccin Immunother. 2013;9(3):636–41. doi: 10.4161/hv.23259. PMID:23354158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice BD, Tomkins SE, Ncube FM. Sharp truth: health care workers remain at risk of bloodborne infection. Occup Med (Lond). 2015;65(3):210–4.. doi: 10.1093/occmed/kqu206. PMID:25663385. [DOI] [PubMed] [Google Scholar]
- 6.Expert Advisory Group on AIDS and the Advisory Group on Hepatitis Guidance for clinical healthcare workers: Protection against infection with bloodborne viruses. London: UK Health Departments; 1998. Available at: http://webarchive.nationalarchives.gov.uk/20121104233313/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4014474.pdf. [Google Scholar]
- 7.Bonanni P, Bonaccorsi G. Vaccination against hepatitis B in health care workers. Vaccine. 2001;19(17–19):2389–94. https://doi.org/10.1016/S0264-410X(00)00460-6. doi: 10.1016/S0264-410X(00)00460-6. PMID:11257366. [DOI] [PubMed] [Google Scholar]
- 8.Kretzschmar M, de Wit A. Universal hepatitis B vaccination. Lancet Infect Dis. 2008;8(2):85–7; author reply 90. doi: 10.1016/S1473-3099(08)70003-3. PMID:18222156. [DOI] [PubMed] [Google Scholar]
- 9.The Council of the European Union Council Directive 2010/32/EU of 10 May 2010 implementing the Framework Agreement on prevention from sharp injuries in the hospital and healthcare sector concluded by HOSPEEM and EPSU. Off J Eur Union. 2010;53:66–72. [Google Scholar]
- 10.World Health Organization (WHO) WHO global health sector strategy on viral hepatitis 2016–2021. Publication date: June 2016. WHO web-site Available at: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua = 1.
- 11.Law n. 81 , 9 April 2008. Testo Unico sulla salute e sicurezza sul lavoro. Gazz. Uff. n. 101, 30 aprile 2008. [Google Scholar]
- 12.Italian Ministry of Health Ministerial Circular n.20, 4 October 1991. Disposizioni relative all'applicazione della legge 27 maggio 1991, n. 165. Gazz. Uff. n. 251, 25 ottobre 1991. [Google Scholar]
- 13.Centers for Disease Control and Prevention Epidemiology and prevention of vaccine-preventable diseases. Hamborsky J, Kroger A, Wolfe S, 13th ed Washington D.C.: Public Health Foundation; 2015. Chapter 10, Hepatitis B Available at: https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html. [Google Scholar]
- 14.Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15:1–8. https://doi.org/10.1016/S0749-3797(98)00003-8. doi: 10.1016/S0749-3797(98)00003-8. PMID:9651632. [DOI] [PubMed] [Google Scholar]
- 15.Italian Ministry of Health Piano Nazionale Prevenzione Vaccinale (PNPV) 2017–2019. Gazz. Uff. n. 41,l 18 febbraio 2017. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf
- 16.Westmoreland D, Player V, Heap DC, Hammond A. Immunization against hepatitis B — what can we expect? Epidemiol Infect. 1990;104:499–509. doi: 10.1017/S0950268800047506. PMID:2140795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jilg W, Schmidt M, Deinhardt F, Zachoval R. Hepatitis B vaccination: how long does protection last? Lancet. 1984;2:458. doi: 10.1016/S0140-6736(84)92926-X. PMID:6147519. [DOI] [PubMed] [Google Scholar]
- 18.West DJ, Calandra GB. Vaccine induced immunologic memory for hepatitis B surface antigen: implications for policy on booster vaccination. Vaccine. 1996;14:1019–27. doi: 10.1016/0264-410X(96)00062-X. PMID:8879096. [DOI] [PubMed] [Google Scholar]
- 19.Dini G, Toletone A, Barberis I, Debarbieri N, Massa E, Paganino C, Bersi F, Montecucco A, Alicino C, Durando P. Persistence of protective anti-HBs antibody levels and anamnestic response to HBV booster vaccination: A cross-sectional study among healthcare students 20 years following the universal immunization campaign in Italy. Hum Vaccin Immunother. 2017;13(2):440–4. doi: 10.1080/21645515.2017.1264788. PMID:27925503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanetti AR, Mariano A, Romanò L, D'Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366(9494):1379–84. doi: 10.1016/S0140-6736(05)67568-X. PMID:16226616. [DOI] [PubMed] [Google Scholar]
- 21.Spada E, Romanò L, Tosti ME, Zuccaro O, Paladini S, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, et al. Hepatitis B immunity in teenagers vaccinated as infants: an Italian 17-year follow-up study. Clin Microbiol Infect. 2014;20(10):O680–6. doi: 10.1111/1469-0691.12591. PMID:24528380. [DOI] [PubMed] [Google Scholar]
- 22.Epicentro, Istituto Superiore di Sanità Aspetti epidemiologici in Italia. 2011. Available at: http//www. epicentro.iss.it/problem/epatite/EpidemiologiaItalia. asp. Last accessed: October 2012.
- 23.SEIEVA. (Sistema epidemiologico integrato dell'epatite virale acuta) Istituto Superiore di Sanità. SEIEVA website: www.iss.it/seieva/docu/cont. Last accessed: October 2012.
- 24.Topuridze M, Butsashvili M, Kamkamidze G, Kajaia M, Morse D, McNutt LA. Barriers to hepatitis B vaccine coverage among healthcare workers in the Republic of Georgia: An international perspective. Infect Control Hosp Epidemiol. 2010;31:158–64. doi: 10.1086/649795. PMID:20038247. [DOI] [PubMed] [Google Scholar]
- 25.Rolnick SJ, Parker ED, Nordin JD, Hedblom BD, Wei F, Kerby T, Jackson JM, Crain AL, Euler G. Self-report compared to electronic medical record across eight adult vaccines: do results vary by demographic factors? Vaccine. 2013;31:3928–35. doi: 10.1016/j.vaccine.2013.06.041. PMID:23806243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg C, Bovin NV, Bram LV, Flyvbjerg E, Erlandsen M, Vorup-Jensen T, Petersen E. Age is an important determinant in humoral and T cell responses to immunization with hepatitis B surface antigen. Hum Vaccin Immunother. 2013;9(7):1466–76. doi: 10.4161/hv.24480. PMID:23571167. [DOI] [PubMed] [Google Scholar]
- 27.Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention (CDC) Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(RR–7):1–45. [PubMed] [Google Scholar]
- 28.Westmoreland D, Player V, Heap DC, Hammond A. Immunization against hepatitis B–what can we expect? Results of a survey of antibody response to immunization in persons ‘at risk’ of occupational exposure to hepatitis B. Epidemiol Infect. 1990;104(3):499–509. doi: 10.1017/S0950268800047506. PMID:2140795. [DOI] [PMC free article] [PubMed] [Google Scholar]


