ABSTRACT
Botulinum neurotoxins (BoNTs) are the most potent toxins to mammals. A toxoid vaccine was previously used for prevention of botulinum intoxication; however, this vaccine is no longer available. Currently, no approved botulinum vaccines are available from the Food and Drug Administration (FDA). Recently, a recombinant host cell receptor-binding subunit created for use as a potential vaccine completed phase 2 clinical trials. The current study designed a vaccine candidate against BoNT type A (BoNT/A) using a structural design. Our vaccine candidate was the BoNT/A heavy chain C-terminal region (HCR) that contained the point mutation BA15 (R1269A) within the ganglioside-binding site. A Biacore affinity test showed that the affinity of BA15 for ganglioside GT1b was 100 times lower than that of the HCR. A SNAP25 cleavage assay revealed that immunized sera blocked SNAP25 cleavage of the BoNT/A toxin via BA15. In an in vivo experiment, mice and guinea pigs immunized with BA15 produced neutralizing antibodies that protected against 3,000 LD50 of BoNT/A. In conclusion, the results of both in vitro and in vivo assays showed that our BA15 vaccine candidate was similar to the recombinant host cell receptor-binding subunit vaccine. The inability of BA15to bind ganglioside shows that BA15 is a potential safe vaccine candidate.
KEYWORDS: Botulinum, guinea pig, mouse, mutation, recombinant vaccine
Introduction
Botulism, a fatal paralytic disease, is caused by potent neurotoxins that are produced by the anaerobic Gram-positive spore-forming bacterium Clostridium botulinum.1 Botulinum neurotoxins (BoNTs) are categorized into eight serotypes: A, B, C, D, E, F, G and H. Serotypes A, B, E, F, and H can infect humans, whereas serotypes C and D can infect other animals, such as cattle and birds.2,3 Because of their potent toxicity, BoNTs were one of the first agents considered a biological warfare agent.4 BoNTs are composed of a 50 kDa light chain and a 100 kDa heavy chain linked by a single disulfide bond. The N-terminal catalytic light chain functions as a zinc metalloprotease, whereas the heavy chain contains two functional domains: a translocation domain and a receptor-binding domain.5 BoNTs can enter the body through the respiratory system (inhalational botulism), the gastrointestinal tract (foodborne and infant botulism), and infected wounds (wound botulism).4,6,7 Upon infection, the circulatory system transports the toxin to a peripheral nerve, primarily targeting neuromuscular junctions. In nerve cells, BoNT inhibits the exocytosis of acetylcholine, thereby inducing neuromuscular paralysis.8–10 Vaccination is the most effective medical countermeasure to prevent botulism.11 Current commercial vaccines are toxoids (i.e., inactivated whole toxins). Toxoid vaccines have side effects such as local and systemic reactions that are increased in individuals who receive a second shot.12 To overcome the drawback of toxoid vaccines, researchers have studied a recombinant protein of the heavy chain to develop a vaccine.13–15 The most advanced recombinant-based vaccine is the recombinant botulinum toxin A/B (rBV A/B) vaccine, which is currently undergoing clinical trials.16 However, no current Food and Drug Administration (FDA)-approved recombinant subunit vaccines exist for the prevention of botulism. Therefore, a recombinant subunit vaccine with improved efficacy and safety must be developed. Przedpelski et al. developed a heavy chain C-terminal region (HCR) subunit vaccine that has the point mutation W1266A within the ganglioside-binding site.17 These authors demonstrated the efficacy of the vaccine. The current study designed a vaccine candidate using BoNT/A HCR/A that contained the point mutation BA15 (R1269A) within the ganglioside-binding site. For the first time, we investigated the efficacy of BA15 using in vivo and in vitro assays. We demonstrated that our vaccine candidate protects mice and guinea pigs against a lethal botulinum A toxin challenge, and our in vitro results should contribute to the development of this vaccine.
Results
Homology modeling of HCR/A mutants
The analysis of the HCR domain of the BoNT/A complex using the ganglioside GT1b structure was defined by the amino acids involved in the binding of the HCR domain and GT1b. The Y1117, E1203, F1252, H1253, S1264, W1266, S1275, R1276 and R1269 amino acid residues were shown to be present in the ganglioside-binding pocket (GBP).18 Y1117 interacts with the terminal 5′-sialic acid (Sia5) via hydrophobic stacking and hydrogen bonding. E1203 and H1253 hydrogen bond to galactose (Gal4) and N-acetyl galactosamine (GalNAc3), and the carbonyl oxygen of F1252 interacts by hydrogen bonding to Gal4S1264 and S1275 hydrogen bond to Gal4 and Sia5, respectively. W1266 interacts with Gal4 via hydrophobic stacking and hydrogen bonds to Sia6. R1269 indirectly interacts with GalNAc3 via water-mediated hydrogen bonding (Fig. 1). We selected five of the amino acids located at the GT1b binding site composed of three HCR/A mutants substituted with alanine. The template structure for homology modeling was used for the binding domain of the BoNT/A complex with GT1b (PDB:2VU9). A 3D HCR/A model was generated using the MODELER module in DS 3.5. For each of the HCR/A mutants generated, ten models were selected for the lowest probability density function (PDF) energy and discrete optimized protein energy (DOPE) score, showing only the top five models. The final models were optimized using the Verified protein MODELER tool. After verification, a slight decrease in the normalized DOPE score (−1.448294, −1.429964, and −1.41263) was found, which indicated the structural stability of the models (Table 1).19 We obtained the backbone root mean square deviation (RMSD) for the model after super imposing 2VU9 onto the most-homologous template for BA15. The PDF, DOPE score and RMSD are shown in Table 1. All models showed low RMSDs of < 0.25 Å. In addition, more than 96% of the Ramachandran plot distribution was contained in the favored regions. None of the residues were outliers, except glycine (data not shown).
Figure 1.
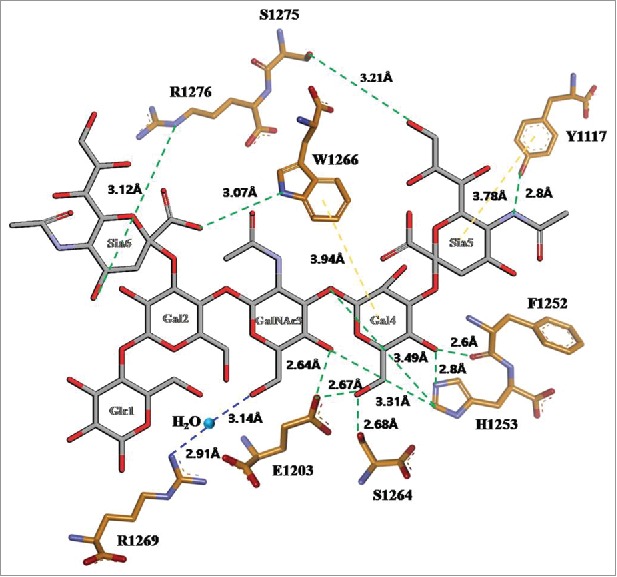
GT1b binding site of the HCR/A in 2D structure. GT1b polysaccharide represented as sticks with graycarbons. HCR/A represented as sticks with orange carbons. Water molecule represented as sphere in light blue. The hydrogen bonds between the HCR/A and GT1b are shown as dotted green lines, the water mediating hydrogen bonds as dotted blue lines, the hydrophobic stacking interaction as dotted purple lines, oxygen atoms in red, and nitrogen atoms in blue. Numbered monosaccharide names are shown; Glc: glucose, GalNac3: N-acetylgalactosamine, Gal: galactose, Sia: sialic acid.
Table 1.
HCR/A mutant Model score and verified model score in the D.S 3.5 program.
| BA15 Model Scores |
BA15 Verified Model Score |
|||||
|---|---|---|---|---|---|---|
| Model number | PDF Total Energy | PDF Physical Energy | DOPE Score | DOPE Score | Normalized DOPE Score | RMSD(Å) |
| BA15.M0010 | 2370.948 | 1305.723062 | −51868.07422 | −51867.96094 | −1.448294 | 0.228 |
| BA15.M0008 | 2378.1191 | 1306.420585 | −51706.68359 | − | ||
| BA15.M0002 | 2450.8145 | 1306.257814 | −51503.38672 | − | ||
| BA15.M0001 | 2459.8896 | 1304.817089 | −51762.25781 | − | ||
| BA15.M0009 | 2463.1392 | 1294.060374 | −51623.08984 | − | ||
We compared the GT1b binding site of the three HCR/A mutants after completing homology modeling (Fig. 2A). When arginine 1269 was substituted with alanine (BA15), the water-mediated hydrogen bonding between R1269A and GalNac3 disappeared (Fig. 2B). In addition, the Y1117 and E1203 interaction was not observed at the binding site. This bonding appeared to change the angle of the residues in the modeling process; therefore, we concluded that it would not affect the actual interaction. The interaction between H1253 and W1266 that plays an important role in the GT1b binding affinity was maintained without any change. Therefore, we considered our optimized model satisfactory and reliable for the vaccine study.
Figure 2.
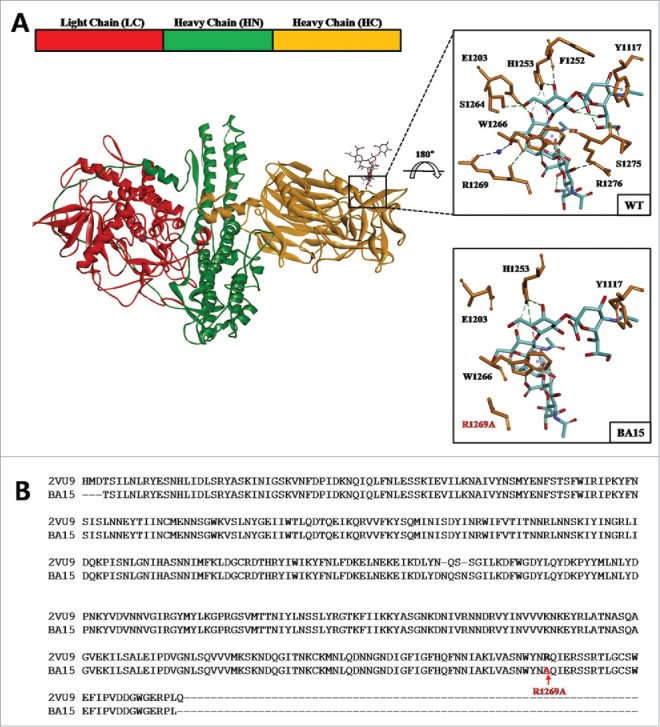
HCR/A mutant modeling structure. (A) Linear schematic of the BoNT/A structure and color-coded crystal structure with ganglioside GT1b modeled into the binding site (PDBID:3BTA). Close up of the GT1b binding site shown as a square. HCR/A amino acids as orange sticks, GT1b in cyan sticks, and interactions between HCR/A and GT1b are shown as dotted lines. (B) GT1b binding site of the BA15 (R1269A) model, with alanine 1269 colored red.
BA15 protects mice and guinea pigs during a BoNT/A challenge
Mice were immunized with 30 µg of BA15 and HCR/A in 2% aluminum hydroxide and challenged with 100 LD50 to 5,000 LD50 of BoNT/A. BoNT/A LD50 (a lethal dose that kills 50% of the mice) was evaluated at 0.125 ng/head using a probit analysis for the intraperitoneal challenge of BoNT/A (Table 2). During the immunization period, all mice showed normal behavior, and side effects were not observed. In the first experiment, immunized mice were protected completely against the challenge with 100 LD50, 500 LD50 and 1,000 LD50 of BoNT/A. In the second experiment, immunized mice survived (10 of 10) against the challenge with 3,000 LD50of BoNT/A (Table 3); however, immunized mice died after challenge with 5,000 LD50 of BoNT/A. These results indicate that 30 µg of these vaccine candidates protects mice against 3,000 LD50. In the third experiment, mice were immunized with 3 µg or 0.3 µg of BA15 and HCR/A in 2% aluminum hydroxide and challenged with 3,000 LD50 and 1,000 LD50. All of the mice survived and showed normal behavior. These results demonstrated that 0.3 µg of BA15 and HCR/A produce protective IgG antibodies against BoNT/A.
Table 2.
LD50 of mouse and guinea pigs against BoNT/A toxin.
| 95% confidence limit |
|||
|---|---|---|---|
| Animal | LD50 (ng/head) | lower | upper |
| Mouse | 0.129 | 0.08 | 0.524 |
| Guinea pig | 4.624 | 3.296 | 8.109 |
Table 3.
Vaccine candidate protection to BoNT/A challenge in the mice.
| No. of mouse survivors/ Total no. |
||||||
|---|---|---|---|---|---|---|
| Total dose (ug) | Antigen | 100LD50a | 500 LD50b | 1000 LD50c | 3000 LD50d | 5000LD50e |
| 30 | Adjuvant only | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| BA15 | 10/10 | 10/10 | 10/10 | 10/10 | 0/10 | |
| HCR/A | 10/10 | 10/10 | 10/10 | 10/10 | 0/10 | |
| 3 | BA15 | 10/10 | 10/10 | 10/10 | 10/10 | 0/10 |
| HCR/A | 10/10 | 10/10 | 10/10 | 10/10 | 0/10 | |
| 0.3 | BA15 | 10/10 | 10/10 | 10/10 | 0/10 | Not done |
| HCR/A | 10/10 | 10/10 | 10/10 | 0/10 | Not done | |
Mouse 100LD50: 12.5 ng/head.
Mouse 500 LD50: 62.5 ng/head.
Mouse 1,000 LD50: 125 ng/head.
Mouse 3,000 LD50: 375 ng/head.
Mouse 5,000 LD50: 625 ng/head.
Guinea pigs were immunized with 75 µg of BA15 and HCR/A in 2% aluminum hydroxide and challenged with 500 LD50 to 5,000 LD50 of BoNT/A. The BoNT/A LD50 of the guinea pigs was evaluated at 4.2 ng/kg using a probit analysis for the intra-muscular challenge of BoNT/A (Table 2). As shown in Table 4, the vaccination of BA15 and HCR/A led to the full protection against a dose of up to 3,000 LD50 of BoNT/A, and all animals showed normal activity.
Table 4.
Vaccine candidate protection to BoNT/A challenge in the guinea pig.
| No. of guinea pig survivors/ Total no. |
|||||
|---|---|---|---|---|---|
| Total dose (ug) | Antigen | 500LD50a | 1,000 LD50b | 3,000 LD50c | 5,000 LD50d |
| 75 | Adjuvant only | 0/10 | 0/10 | 0/10 | 0/10 |
| BA15 | 10/10 | 10/10 | 10/10 | 7/10 | |
| HCR/A | 10/10 | 10/10 | 10/10 | 7/10 | |
Guinea pig 500 LD50: 21 μg/kg.
Guinea pig 1,000 LD50: 42 μg/kg.
Guinea pig 3,000 LD50: 126 μg/kg.
Guinea pig 5,000 LD50: 210 μg/kg.
Immunization with BA15 antisera protects naïve mice against BoNT/A toxin
We investigated whether immunized sera prevented intoxication of naïve mice exposed to BoNT/A toxin. Three- and 5-week sera of mice immunized withBA15, HCR/A and adjuvant only were separately mixed 1:1 with 10 LD50 of BoNT/A toxin. Immunized sera with adjuvant only did not protect naïve mice against BoNT/A 10 LD50. Mice administered 3- and 5-week immunized sera with the BA15 and BoNT/A toxin mixture were 100% protected. However, mice that received 3-week immunized sera with HCR/A died following challenge with10 LD50 BoNT/A (Table 5).
Table 5.
Passive protection against 10LD50 BoNT/A.
| Total Dose (ug) | Antigen | Immunized sera + 10LD50 BoNT/A | No. of mouse survivors/ Total no. |
|---|---|---|---|
| 0.2 | Adjuvant only | 3 week sera + BoNT/A | 0/5 |
| HCR/A | 3 week sera + BoNT/A | 1/5 | |
| BA15 | 3 week sera + BoNT/A | 5/5 | |
| 0.3 | Adjuvant only | 5 week sera + BoNT/A | 0/5 |
| HCR/A | 5 week sera + BoNT/A | 5/5 | |
| BA15 | 5 week sera + BoNT/A | 5/5 |
Mouse serum with vaccination blocked SNAP25 cleavage from BoNT/A
BoNT/A catalyzes the cleavage of the SNAP25 protein at the neuromuscular junction. The cleavage of SNAP25 blocks synaptic vesicle exocytosis and leads to paralysis. The ability of the immunized mouse serum to inhibit SNAP25 cleavage was analyzed using the Neuro 2A cell line. Immunized mouse serum was added to cell cultures of the Neuro 2A cells in the presence of BoNT/A, and the cleavage of intracellular SNAP25 was measured via immunoblot. To confirm the SNAP25 cleavage patterns, we assessed the appropriate concentrations of BoNT/A and serum in dose-dependent treatments (data not shown). The SNAP 25 cleavage patterns were confirmed with 20 μg/ml BoNT/A toxin treatment (Fig. 3A, lane 2). Eight units of NIBSC anti-BoNT/A equine serum with BoNT/A toxins were used as a positive control for the comparison of the neutralizing efficacy (Fig. 3A, lane 3). In the absence of immunized serum, BoNT/A toxins cleaved cellular SNAP25 (Fig. 3A and B, lane 2). SNAP25 was also cleaved in pre-immunized mouse serum treatment with BoNT/A toxins (Fig. 3A, lane 4). Neuro 2A cells were treated with only mouse serum to test the effects of serum against SNAP 25 cleavage. SNAP25 was not affected by mouse serum treatments in the absence of toxins (Fig. 3B, lane 3). The cleavages of SNAP25 were blocked with HCR/A or BA15 immunized mouse serum at week 10 (Fig. 3B, lanes 4 – 9). However, SNAP25 cleavage showed greater inhibition in the presence of 5% BA15 immunized mouse serum (Fig. 3B, lanes 8 – 9) compared to HCR/A immunized serum (Fig. 3B, lanes 4 and 6, lower bands). These results showed that BA15 immunized serum fully prevents the BoNT/A-induced cleavage of SNAP25.
Figure 3.
SNAP25 cleavage assay for in vitro neutralization using the immunized mouse serum. (A) Inhibition of BoNT/A-mediated SNAP-25 cleavage during co-incubation with BoNT/A toxins and control samples. Lane 1, SNAP25 of untreated Neuro 2A cells; lane 2, SNAP25 of Neuro 2A cells treated with 20 μg/ml BoNT/A toxins; lane3, SNAP25 of Neuro 2A cells treated with 20 μg/ml BoNT/A toxins and 8 units of NIBSC; lane 4, SNAP25 of Neuro 2A cells treated with BoNT/A toxins and 5% pre-immune mouse serum (B) Inhibition of BoNT/A-mediated SNAP-25 cleavage during co-incubation with BoNT/A toxins and mouse serum immunized with vaccine candidates. Lane 1, SNAP25 of untreated Neuro 2A cells; lane 2, SNAP25 of Neuro 2A cells treated with 20 μg/ml BoNT/A toxins; lane3, SNAP25 of Neuro 2A cells treated with 5% pre-immune mouse serum; lane 4 – 6, SNAP25 of Neuro 2A cells treated with BoNT/A toxins and 5% HCR/A immunized mouse serum (n = 3, three different mice serum); lane 7 – 9, SNAP25 of Neuro 2A cells treated with BoNT/A toxins and 5% BA15 immunized mouse serum (n = 3, three different mice serum).
BA15 protein elicits an immune response in mice and guinea pigs
The sera of mice and guinea pigs were analyzed to determine IgG titers. One week after the second immunization, IgG titers were approximately 100–1,000 in the sera of both mice and guinea pigs. One week after the third immunization, the IgG titers increased 10- to 50-fold compared with the second immunization (Fig. 4A). The ELISA results indicated that the IgG of the sera neutralized BoNT/A, and protective immunity was generated in the animals. In the additional experiment, we analyzed the IgG titers of low-dose immunization (3 μg or 0.3 μg) in mice immunized with BA15 or HCR/A. The IgG titers of 3 μg immunization were greater than those of 0.3 μg in both the BA15 and HCR/A immunizations. The IgG titers of 0.3 µg of BA15 immunization were significantly greater than those of the HCR/A immunization in sera at 3 weeks and 5 weeks (Fig. 4A). A third experiment analyzed IgG titers over a long-term period (15 weeks) in the mice immunized with BA15 or HCR/A. The IgG titer of BA15 was approximately maintained until 11 weeks, whereas the IgG titer of HCR/A markedly decreased at 9 weeks after immunization (Fig. 4B).
Figure 4.
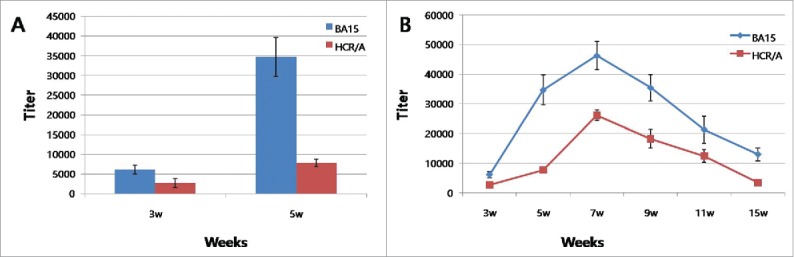
IgG titer to HCR/A and BA15. (A) The HCR/A or BA15 was administered to mice (0.1μg/head) in an initial intramuscular injection (0 days) and in two subsequent boosts (14 and 28 days). The results showed that IgG titer of BA15 was markedly higher than that of HCR/A. (B)The HCR/A or BA15 was administered to mice (10 μg/head) in an initial intramuscular injection (0 days) and in two subsequent boosts (14 and 28 days).IgG titer was analyzed until 15 weeks. IgG titer of BA15 was higher than that of HCR/A during 15 weeks.
The ganglioside GT1b binding affinity of BA15 and HCR/A
Affinities of ganglioside GT1b against HCR/A and BA15 were observed with surface plasmon resonance. The dissociation constant of BA15 was approximately 100 times lower than that of the HCR/A (Table 6), signifying that the candidate lost some of its binding affinity due to the designed mutations. By losing binding ability against GT1b, BA15 was unable to recognize and bind to the designated cell surface or HCR/A; thus, it might have a lower cellular translocation rate.
Table 6.
Dissociation constant of antigens against ganglioside GT1b.
| Antigen | Dissociation constant |
|---|---|
| HCR/A | 4.23 × 10−5M |
| BA15 | 1.33 × 10−3M |
Discussion
Botulism is a fatal neuroparalytic disease. Human botulism cases are primarily caused by botulinum toxins type A, B and E.6 A pentavalent (ABCDE) botulinum toxoid vaccine has been used for individuals at risk for exposure (e.g., those in the research, veterinary and military fields). However, the use of this pentavalent toxoid vaccine was recently stopped, and only an ABEF toxoid vaccine is used in Japan. In 1998, the crystal structure of BoNT/A was determined, and the HCR domain was found to be composed of amino acids 872 to 1296.20 The HCR domain of BoNT/A has become a major target for developing a new vaccine. Recombinant HCR subunit vaccines are currently being developed and evaluated. One such vaccine, rBV A/B, is composed of HCR domains from botulinum type A and B and is currently undergoing clinical trials.16 In 2013, Przedpelski et al. investigated an HCR subunit vaccine with the point mutation W1266A within the ganglioside.17 BoNT/A acts by binding to receptors on the nerve cell surface, and the HCR region of a single ganglioside-binding site mediates this action.21 In fact, several investigators have shown that the BoNT/A HCR W1266 mutant plays a critical role in ganglioside binding. In addition, BoNT/A HCR W1266A lacked detectable binding to neurons without changing their structure.17 Moreover, this mutant showed a more protective immune response to the wild-type BoNT/A challenge than the HCR/A without a mutation in a mouse model.17 The current study approached the design of recombinant HCR/A vaccines by focusing on the mutation of amino acids related to the ganglioside-binding site. We modeled the elimination of receptor-binding affinity using amino acid substitution (BA15:R1269A). Because Arg, Tyr, His, Glu and Trp are conserved within the GBP, these mutations might be introduced into the HCR to induce a greater immune response and decrease binding to the nerve cells. A substitution or combination of the five amino acids might improve vaccine efficacy and block potential reactivity with the cell. We then predicted a 3D structure of the three vaccine candidates using homology modeling. Structurally, W1266A has a relatively strong interaction with Gal4 and Sia6 in GT1b. Thus, the mutation W1266A inhibits the binding between HCR/A and GT1b. In this study, the mutated amino acid R1269A indirectly interacted with the GalNac3 of GT1b via water. Therefore, the interactions involved in the binding between HCR/A and GT1b are relatively weak compared to other amino acids. However, when R1269A was substituted with alanine, the binding capacity to GT1b was approximately 100 times lower, which could be indirect evidence of inhibition of intracellular entry. In other words, both W1266A and R1269A have high potential as a HCR/A vaccine capable of minimizing structural changes and inhibiting intracellular inflow by a single mutation. A vaccine candidate was confirmed via the immune response and the protective efficacy against the BoNT/A toxin as a recombinant protein. The vaccination results of mice and guinea pigs in the current study demonstrated that BA15 did not cause adverse side effects or toxicity and was capable of eliciting neutralizing antibodies. These results showed that recombinant mutant proteins have epitopes that can produce neutralizing antibodies against BoNT/A. Previous studies, including animal and human vaccine studies, have reported that the epitopes inducing an immune response are located within the HCR subunit. David et al. showed that the recombinant HCR of BoNT/A protected mice from BoNT/A intoxication.22 In fact, the most advanced vaccine candidate for botulinum is a recombinant HCR subunit that is currently undergoing clinical trials. Although recombinant HCRs can protect animals, they can also enter neurons that are similar to BoNT/A via synaptic vesicle protein 2.23–25 The toxicity of the recombinant HCRs entering cells remains unknown. However, because the receptor-binding site was mutated, our vaccine candidate has a weak binding ability to the GBP. An affinity assay demonstrated that HCR/A showed 100 times higher affinity for GT1b than affinity for BA15. Therefore, BA15 in the body has a low binding ability to cells and increased circulation. As a result, the immunogenicity of BA15 was increased, and the IgG titer of BA15 was higher. Our ELISA results demonstrated that the IgG titer of BA15 was markedly higher than that of HCR/A. The IgG titer of BA15 was maintained until 11 weeks, but the IgG titer of HCR/A decreased at 9 weeks. In addition, a low dose immunization of BA15 induceda faster and stronger immune response than HCR/A. Przedpelski et al.17 also showed that a W1266A mutant of HCR hadgreater protective efficacy than HCR/A. These authors reasoned that the greater protective efficacy of the mutant HCR might be because of the increased circulation of the immunogen due to the elimination of receptor binding.
It is important to produce a neutralizing antibody titer when administering a vaccine to an animal. Antibody titers are not normally correlated with neutralization capacity. Takeda et al. revealed that the serum ELISA titer was not correlated with the neutralization titer.26 The present study identified a point mutation region of HCR capable of generating a neutralizing antibody. To confirm whether the sera from immunized mice neutralize the toxin, we conducted a SNAP25 cleavage assay. A SNAP25 cleavage assay can be used to screen for neutralizing antibodies. BoNT/A cleaves SNAP25, a plasma membrane protein region of the SNARE protein. The cleavage of SNAP25 blocks synaptic vesicle exocytosis and leads to paralysis in mammals.27 The ability of BoNT/A to cleave SNAP25 blocks vesicle exocytosis. Fig. 3 shows that the vaccinated sera played an important role in blocking the cleavage of SNAP25, whereas the normal sera failed to block the SNAP25 cleavage. The positive control, NIBSC polyclonal botulinum A antibody, completely blocked the cleavage of the SNAP25 protein. Our SNAP25 cleavage assay demonstrated that BA15 elicited an immune response to produce neutralizing antibodies against BoNT/A. These data strongly demonstrate that BA15 is a crucial region for generating neutralizing antibodies, and it should be considered in future vaccine designs.
In conclusion, BA15 has a weak binding affinity to the ganglioside GT1b, and it is capable of producing protective antibodies against BoNT/A. In addition, BA15 induces more neutralizing antibodies than HCR/A at 3 weeks after immunization. Antibodies against BA15 can block SNAP25 cleavage on Neuro 2A cells and inhibit the toxicity of intact BoNT/A in mice and guinea pigs.
The present study did not assess different adjuvants or attempt to optimize the neutralizing antibody response. Future studies should analyze whether the vaccine candidate is quantitatively or qualitatively altered when administered with different adjuvants and only two immunizations.
Materials and methods
Modeling of HCR/A mutants
We downloaded the PDB file of the HCR/A complex with the ganglioside GT1b (PDB: 2VU9) from the RCSB Protein Data Bank and corrected its incomplete structure using Discovery Studio's clean protein software (DS version 3.5, BIOVIA, San Diego, CA). This software defined the amino acids involved in the ganglioside-binding site between HCR/A and GT1b. The result calculated based on the binding energy of the point mutation was then selected as the vaccine candidate. Homology modeling, mutation energy (stability) calculation and affinity with GT1b were conducted using the DS 3.5 program. A CHARMM force field was used to simulate and refine the model.
Construction and purification
The downloaded HCR domain of BoNT/A (residues T876 to L1296) was synthesized (CosmoGentech, Seoul, South Korea) with codon optimization for E.coli expression. The HCR template was amplified and subcloned into BamHI and NotI restriction sites of a pGEX4T3 vector (GE Healthcare Life Sciences, Chicago, IL) and tagged with N-terminal glutathione S-transferase. HCR/A mutants were created via overlap PCR mutagenesis. BA15 was transformed into BL21 DE3 cells (Invitrogen, Carlsbad, CA) for protein expression.
Transformed cells were grown in LB media (BD Biosciences, San Jose, CA) containing ampicillin. Expression was induced by adding isopropyl β-D-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM with a cell density of 0.4–0.5 OD600 in an overnight culture at 18°C. Cell pellets were resuspended in ice-cold 20 mMTris pH 8.0, 200 mMNaCl lysis buffer and then disrupted via sonication (Qsonica, Newtown, CT). Supernatants were loaded onto a GST column (GE Healthcare Life Sciences). BA15 was eluted and cleaved to remove the purification tag via thrombin (Sigma-Aldrich, St. Louis, MO). The final purification was loaded onto a Superdex 200 column (GE Healthcare Life Sciences, Chicago, IL).
Toxin production
The BoNT/A complexed toxin was purified from Clostridium botulinum type A, as previously described,28 althougha slight modification was applied. A BoNT/A LD50 dose in the mouse and guinea pig was evaluated at 0.125 ng and 4.2 ng each (Table 1). The LD50 dose is the dose that kills 50% of the animals
Affinity assay of ganglioside GT1b binding
The purified HCR of BoNT/A and BA15 were analyzed using surface plasmon resonance (Biacore X-100, GE Healthcare Life Sciences, Chicago, IL). The proteins of interest were immobilized on a CM5 chip with a ligand level of 3,000. Then, 1 to 100 µM of ganglioside GT1b (Santa Cruz Biotech, Dallas, TX) was sequentially added to analyze the affinity against the proteins. The association and dissociation times for each condition were 120 and 600 seconds, respectively.
Vaccination of mice and guinea pigs
Ten female 6-week-old ICR mice (Orient Bio, Sung-nam, South Korea) were immunized intramuscularly three times with 2 week interval with 10 µg, 1 µg or 0.1 µg of BA15 or HCR/A with 2% alhydrogel (InvivoGen, San Diego, CA) as an adjuvant. A control group of mice was composed of animals vaccinated with phosphate buffered saline (PBS) with 2% alhydrogel. Blood samples were collected on days 21 and 35 from the retro-orbital plexus, and serum was collected via centrifugation. Mice were challenged on day 37 with 100, 500, 1,000, 3,000 and 5,000 LD50 of the BoNT/A. Survival was monitored until 14 days after toxin administration.
Seven-week-old female Hartley guinea pigs (Orient Bio, Sung-nam, South Korea) were randomly segregated into 5 groups. The animals in groups 1 through 4 were vaccinated intramuscularly on days 0, 14 and 28 with 25 µg of BA15 and 2% alhydrogel as an adjuvant. Group 5 was composed of animals vaccinated with PBS with 2% alhydrogel. Blood samples were collected from the cephalic vein on days 21 and 35, and the serum preparation was the same as described above for the mice. Guinea pigs were challenged on day 37 with 500, 1000, 3,000 and 5,000 LD50 of the BoNT/A. The Animal Care and Use Committee at the Agency for Defense Development approved the mouse and guinea pig experiments.
Long-term antibody titer analysis following BA15 and HCR/A immunization
Ten female 6-week-old ICR mice (Orient Bio, Sung-nam, South Korea) were immunized intramuscularly with 10 µg of BA15 or HCR/A with 2% alhydrogel (InvivoGen, San Diego, CA) as an adjuvant. A control group of mice was composed of animals vaccinated with PBS with 2% alhydrogel. BA15 or HCR/A was administered on days 0, 14 and 28. Blood samples were collected on days 21, 35, 49, 63, 77 and 105 from the retro-orbital plexus, and serum was collected via centrifugation.
Passive protection assay
For confirmation of neutralizing antibodies induced by vaccination, each BA15 and HCR/A immunized sera (21 and 35 days sera) and naïve sera were mixed with 10 LD50 of BoNT/A toxin. Sera and toxin were incubated at room temperature for1 hour. Naïve animals were administered these mixtures via intraperitoneal challenge. Survival was monitored for 10 days.
ELISA assay
Antibody titers for BA15 and HCR/A in mice were determined following standard procedures. Briefly, flat-bottom 96-well plates (Nunc, Rochester, NY) were coated with BA15 or HCR/A (100 ng/well) and incubated at 4°C overnight, followed by washing with PBST (pH 7.4, Sigma-Aldrich, St. Louis, MO). The plates were blocked with 5% skim milk (Sigma-Aldrich, St. Louis, MO) in PBS for 1 hour and washed 3 times. Twofold serial dilutions of serum samples were added to the plates and incubated at 37°C for 60 min. Immunoglobulin G titers were determined using horseradish peroxidase-conjugated rabbit anti-mouse IgG (Sigma-Aldrich, St. Louis, MO). Anti-mouse IgG was diluted 1:8,000 in 5% skim milk in PBS, incubated for 60 min at 37°C and washed 3 times. DAB (Sigma-Aldrich, St. Louis, MO) was added as a substrate, and the plates were incubated for 20 min in darkness. The OD value was evaluated at an absorbance of 492 nm.
SNAP25 cleavage assay
Neuro 2A cells (ATCC, Manassas, VA) were grown on 100-mm dishes and maintained inMEM medium (Life Technologies, Carlsbad, CA) with 10% fetal bovine serum (FBS;Life Technologies, Carlsbad, CA) and 1% antibiotics (penicillin/streptomycin;Life Technologies, Carlsbad, CA). Cells were plated 1 × 105 cells per well into 6-well plates (Nunc, Rochester, NY) for 24 hours, and the cells were treated with 50 μg/ml of ganglioside GT1b (Santa Cruz Biotech, Dallas, TX). After 24 hours, 20 µg/ml of the BoNT/A solutions were incubated with mouse sera from immunized HCR/A and BA15 (10 µg three times, 10 weeks) for 1 hour. Then, the cells were treated with solutions for 48 hours, harvested and lysed with RIPA buffer (Sigma-Aldrich, St. Louis, MO) containing a protease (Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitor cocktail (Roche Diagnostics, Basel, Switzerland). The concentrations of cellular proteins were determined using a BCA protein assay reagent kit (Thermo Scientific Inc.Waltham, MA). Cellular proteins were separated via NuPAGE 4–12% Bis-Tris gel (Thermo Scientific Inc.Waltham, MA) and transferred onto a 0.45-µm polyvinylidene fluoride (PVDF) membrane (GE Healthcare Life Sciences, Chicago, IL) using a transfer Pierce G2 Fast Blotter (Thermo Scientific Inc.Waltham, MA). Membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 1 hour at room temperature. Following blocking, the blots were probed with a 1:5,000 dilution of anti-SNAP25 primary antibody (R&D system, Minneapolis, MN) in 1% skim milk-TBS-T at 4°C overnight. Then, the blots were washed three times with TBS-T for 10 min. After washing, they were incubated with a 1:10,000 dilution of HRP-conjugated donkey anti-sheep (R&D system, Minneapolis, MN) in 1% skim milk containing TBS-T on a shaker for 2 hours at room temperature. The results were developed via an enhanced chemiluminescence (ECL) solution (Amersham, Buckinghamshire, UK).
Statistical analysis
The values were assessed as the mean and standard error of the mean. Statistical analysis between sets of data was performed by Student's t-test. P < 0.05 was considered statistically significant.
Funding Statement
This work was supported by a grant from the Agency for Defense Development, Republic of Korea (611665-912400201).
Disclosure of potential conflicts of interest
The authors have no conflict of interest to declare.
References
- 1.Johnson E. Clostridium botulinum and Clostridium tetani In: Borriello S, Murra P, Funke G, editors. Topley and Wilson's microbiology and microbial infections. London: Hodder Arnold; 2005. p. 1035–88. [Google Scholar]
- 2.Dover N, Barash JR, Hill KK, Xie G, Arnon SS. Molecular characterization of a novel botulinum neurotoxin Type H gene. J Infect Dis. 2014;209(2):192–202. doi: 10.1093/infdis/jit450. PMID:24106295 [DOI] [PubMed] [Google Scholar]
- 3.Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol. 2014;12(8):535–49. doi: 10.1038/nrmicro3295. PMID:24975322 [DOI] [PubMed] [Google Scholar]
- 4.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, et al.. Botulinum toxin as a biological weapon:medical and public health management. JAMA. 2001;285(8):1059–70. doi: 10.1001/jama.285.8.1059. PMID:11209178 [DOI] [PubMed] [Google Scholar]
- 5.Montecucco C, Schiavo G. Structure and function of tetanus and botulinum neurotoxins. Q RevBiophys. 1995;28(4):423–72. doi: 10.1017/S0033583500003292 [DOI] [PubMed] [Google Scholar]
- 6.Hill KK, Smith TJ, Helma CH, Ticknor LO, Foley BT, Svensson RT, Brown JL, Johnson EA, Smith LA, Okinaka RT, et al.. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J Bacteriol. 2007;189(3):818–32. doi: 10.1128/JB.01180-06. PMID:17114256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–93. doi: 10.1146/annurev.pharmtox.44.101802.121554. PMID:14744243 [DOI] [PubMed] [Google Scholar]
- 8.Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. PMID:20233039 [DOI] [PubMed] [Google Scholar]
- 9.Rusnak JM, Smith LA. Botulinum neurotoxin vaccines: past history and recent developments. Hum Vaccin. 2009;5(12):794–805. doi: 10.4161/hv.9420. PMID:19684478 [DOI] [PubMed] [Google Scholar]
- 10.Smith LA, Rusnak JM. Botulinum neurotoxin vaccines: past, present, and future. Crit Rev Immunol. 2007;27(4):303–18. doi: 10.1615/CritRevImmunol.v27.i4.20. PMID:18197811 [DOI] [PubMed] [Google Scholar]
- 11.Keller JE. Characterization of new formalin-detoxified botulinum neurotoxin toxoids. Clin VaccineImmunol. 2008;15(9):1374–79. doi: 10.1128/CVI.00117-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith LA. Botulism and vaccines for its prevention. Vaccine. 2009;27:D33–D39. doi: 10.1016/j.vaccine.2009.08.059. PMID:19837283 [DOI] [PubMed] [Google Scholar]
- 13.Baldwin MR, Tepp WH, Przedpelski A, Pier CL, Bradshaw M, Johnson EA, Barbieri JT. Subunit vaccine against the seven serotypes of botulism. Infect Immun. 2008;76(3):1314–18. doi: 10.1128/IAI.01025-07. PMID:18070903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henkel JS, Tepp WH, Przedpelski A, Fritz RB, Johnson EA, Barbieri JT. Subunit vaccine efficacy against botulinum neurotoxin subtypes. Vaccine. 2011;29(44):7688–95. doi: 10.1016/j.vaccine.2011.07.134. PMID:21839134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb RP, Smith TJ, Wright P, Brown J, Smith LA. Production of catalytically inactive BoNT/A1 holoprotein and comparison with BoNT/A1 subunit vaccines against toxin subtypes A1, A2, and A3. Vaccine. 2009;27(33):4490–97. doi: 10.1016/j.vaccine.2009.05.030. PMID:19450643 [DOI] [PubMed] [Google Scholar]
- 16.Shearer JD, Manetz TS, House RV. Preclinical safety assessment of recombinant botulinum vaccine A/B (rBV A/B). Vaccine. 2012;30(11):1917–26. doi: 10.1016/j.vaccine.2012.01.035 [DOI] [PubMed] [Google Scholar]
- 17.Przedpelski A, Tepp WH, Kroken AR, Fu Z, Kim JJ, Johnson EA, Barbieri JT. Enhancing the protective immune response against botulism. Infect Immun. 2013;81(7):2638–44. doi: 10.1128/IAI.00382-13. PMID:23670557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenmark P, Dupuy J, Imamura A, Kiso M, Stevens RC. Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b-insight into the toxin-neuron interaction. PLoSPathog. 2008;4(8):e1000129. doi: 10.1371/journal.ppat.1000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15(11):2507–24. doi: 10.1110/ps.062416606. PMID:17075131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. Crystal structure of botulinum neurotoxin type A and implications for toxicity. NatStruct Biol. 1998;5(10):898–902. doi: 10.1038/2338. doi: 10.1038/2338 [DOI] [PubMed] [Google Scholar]
- 21.Rummel A, Mahrhold S, Bigalke H, Binz T. The HCC -domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol Microbiol. 2004;51(3):631–43. doi: 10.1046/j.1365-2958.2003.03872.x. PMID:14731268 [DOI] [PubMed] [Google Scholar]
- 22.David AB, Diamant E, Barnea A, Rosen O, Torgeman A, Zichel R. The receptor binding domain of botulinum neurotoxin serotype A (BoNT/A) inhibits BoNT/A andBoNT/E intoxications in vivo. Clin Vaccine Immunol. 2013;20:1266–73. doi: 10.1128/CVI.00268-13. PMID:23761665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin MR, Barbieri JT. Association of botulinum neurotoxin serotypes A and B with synaptic vesicle protein complexes. Biochemistry. 2007;46(11):3200–10. doi: 10.1021/bi602396x. PMID:17311420 [DOI] [PubMed] [Google Scholar]
- 24.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312(5773):592–6. doi: 10.1126/science.1123654. PMID:16543415 [DOI] [PubMed] [Google Scholar]
- 25.Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580(8):2011–14. doi: 10.1016/j.febslet.2006.02.074. PMID:16545378 [DOI] [PubMed] [Google Scholar]
- 26.Takeda M, Kasai H, Torii Y, Mukamoto M, Kohda T, Tsukamoto K, Kozaki S. Protective effect of botulinum C / D mosaic toxoid against avian botulism. J Vet Med Sci. 2006;68(4):325–30. doi: 10.1292/jvms.68.325. PMID:16679722 [DOI] [PubMed] [Google Scholar]
- 27.Schiavo G, Rossetto O, Catsicas S, De Laureto PP, DasGupta B, Benfenati F, Montecucco C. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J. Biol Chem. 1993;268:23784–87. PMID:8226912 [PubMed] [Google Scholar]
- 28.Malizio C, Goodnough M, Johnson E. Purification of Clostridium botulinum type A neurotoxin. Purification of Clostridium botulinum type A neurotoxin In: Holst O, editor, Bacterial toxins: methods and protocols. Totowa, NJ: Humana Press; 2000. p. 27–39. [DOI] [PubMed] [Google Scholar]


