The molecular mechanisms underlying the inhibition of the cardiac Na+–Ca2+ exchanger by cytoplasmic protons are poorly defined. Using mutagenesis and electrophysiology, John et al. reveal specific residues within this plasma membrane transporter that are important for pH regulation.
Abstract
The cardiac Na+–Ca2+ exchanger (NCX) plays a critical role in the heart by extruding Ca2+ after each contraction and thus regulates cardiac contractility. The activity of NCX is strongly inhibited by cytosolic protons, which suggests that intracellular acidification will have important effects on heart contractility. However, the mechanisms underlying this inhibition remain elusive. It has been suggested that pH regulation originates from the competitive binding of protons to two Ca2+-binding domains within the large cytoplasmic loop of NCX and requires inactivation by intracellular Na+ to fully develop. By combining mutagenesis and electrophysiology, we demonstrate that NCX pH modulation is an allosteric mechanism distinct from Na+ and Ca2+ regulation, and we show that cytoplasmic Na+ can affect the sensitivity of NCX to protons. We further identify two histidines (His 124 and His 165) that are important for NCX proton sensitivity and show that His 165 plays the dominant role. Our results reveal a complex interplay between the different allosteric mechanisms that regulate the activity of NCX. Because of the central role of NCX in cardiac function, these findings are important for our understanding of heart pathophysiology.
Introduction
The plasma membrane Na+–Ca2+ exchanger (NCX) utilizes the electrochemical gradient of Na+ to move Ca2+ against its concentration gradient (Nicoll et al., 2013), making it an important regulator of Ca2+ homeostasis (Ottolia et al., 2013; Shattock et al., 2015). To control intracellular Ca2+ levels, the cell tightly regulates NCX activity via a variety of allosteric mechanisms. High intracellular Na+ evokes a time-dependent decay of NCX exchange current. This slow inactivation arises from the occupancy of the transport sites by intracellular Na+ (Hilgemann et al., 1992b). However, mutagenesis has demonstrated that a stretch of hydrophobic and positive amino acids (219–238) just distal to the fifth transmembrane span (TMS) in the large intracellular loop is implicated in this process (Matsuoka et al., 1997). This region is called XIP (exchanger inhibitory peptide), as the application of a 20-amino-acid peptide with an identical sequence inhibits transport activity (Li et al., 1991). In contrast, cytoplasmic Ca2+ strongly activates NCX and rescues it from Na+-dependent inactivation (Hilgemann et al., 1992a,b). This calcium is not transported but binds to two Ca2+-binding domains (CBDs; CBD1, amino acids 371–501; and CBD2, amino acids 508–683) whose structure has been determined by nuclear magnetic resonance and x-ray crystallography (Hilge et al., 2006, 2009; Besserer et al., 2007; Wu et al., 2009, 2010; Giladi et al., 2012).
Cytoplasmic protons are also powerful modulators of NCX, and small increases in proton concentration strongly inhibit NCX activity (Baker and Blaustein, 1968; Philipson et al., 1982; Hilgemann et al., 1992b; Doering and Lederer, 1993, 1994; Boyman et al., 2011). The inhibitory effects of protons on NCX activity likely play an important role during hypoxia and ischemia, when the cytoplasm becomes acidic (Vaughan-Jones et al., 2009). Despite the potential role of this regulation under pathophysiological conditions, the underlying mechanisms of NCX pH regulation are not completely understood and remain controversial. Previous studies showed that digestion with chymotrypsin abrogates NCX sensitivity to cytoplasmic protons (Doering and Lederer, 1993, 1994). Because chymotrypsin also removes Na+ and Ca2+ regulation (Hilgemann, 1990), it has been hypothesized that these regulations may share a common molecular mechanism (Doering and Lederer, 1993, 1994; Blaustein and Lederer, 1999; Boyman et al., 2011). Indeed, the inhibition of NCX by cytoplasmic protons appears to require both the Na+-dependent inactivation (Doering and Lederer, 1994) and Ca2+-binding domains, which have been indicated as potential binding sites for protons (Boyman et al., 2011).
The goal of this work was to gain insights into the molecular mechanisms by which protons regulate NCX. We demonstrate that protons can inhibit NCX independently of both Na+ and Ca2+ regulation, indicating that NCX pH modulation relies on a distinct mechanism. Nevertheless, the sensitivity of NCX to cytoplasmic protons can be fine-tuned by intracellular Na+ ions. Moreover, we constructed a series of mutants to reveal which specific amino acids of NCX are necessary for this allosteric regulation. We found that replacement of two histidines (His 124 and His 165) drastically decreases the response of NCX to intracellular protons, indicating that these histidines are part of the underlying mechanism linked to NCX pH modulation. Our data show that between these two residues, His 165 is strategically important in conferring proton sensitivity to NCX, suggesting that this residue is a key element of the NCX pH sensor.
Materials and methods
Molecular biology
Mutations were generated using QuikChange Mutagenesis (Agilent) and sequenced, as previously described (Ottolia et al., 2005). RNA was synthesized using mMESSAGE mMACHINE (Ambion) and injected into Xenopus laevis oocytes as described previously (Ottolia et al., 2005, 2009).
Electrophysiology
Outward NCX currents were recorded using the inside-out giant patch technique (Hilgemann and Lu, 1998). Borosilicate glass pipettes were pulled and fire-polished to a final inner diameter of ∼20 to 30 µm and coated with a mixture of parafilm and mineral oil. Before experiments, the vitelline membrane of the oocyte was removed manually in the bath solution. After membrane patch excision, solutions were rapidly changed using a computer controlled 20-channel solution switcher. Recordings of the outward exchanger current (reverse mode) were obtained using the following solutions: pipette solution (mM): 100 N-methylglucamine, 10 HEPES, 20 TEAOH, 0.2 niflumic acid, 0.2 ouabain, and 8 Ca(OH)2, pH 7 (using MES); and bath solution (mM): 100 CsOH or 100 NaOH, 20 TEAOH, and 10 HEPES or MOPS, adjusted to various intracellular pHs (using MES). We omitted calcium buffers in our cytoplasmic solution, as they are pH sensitive. The contaminant Ca2+ was measured as ∼4 µM using a Ca2+ electrode.
To characterize the Ca2+ sensitivity of the mutant exchangers, calcium buffers were added to the cytoplasmic solutions composed as follows (mM): 100 CsOH or 100 NaOH, 20 TEAOH, 10 HEPES, 10 EGTA or HEDTA, and different Ca(OH)2 concentrations to obtain the desired final free Ca2+ concentrations, pH 7.0 (using MES). Free Ca2+ concentrations were calculated according to the WEBMAXC program (Patton et al., 2004) and confirmed with a Ca2+ electrode.
Outward currents were evoked by the rapid replacement of 100 mM Cs+ with 100 mM Na+. Because NCX does not transport Cs+, there is no current and only upon application of Na+ does the exchange cycle initiate.
To screen our large library of mutants, we first used a protocol that simultaneously applies Na+ and pH while maintaining constant intracellular Ca2+ (contaminant ∼4 µM Ca2+; Fig. S1 A, simultaneous protocol). Before the coapplication of Na+ and pH, the cytoplasmic side of the membrane patch is continuously perfused with a solution of Cs+ at pH 7.0 (physiological pH of oocytes). Application of intracellular Na+ initiates NCX transport, generating a current. This current activates rapidly, peaks, and then decays to a steady state. The peak current mainly depends on the number of exchangers available at pH 7 before Na+ application. The decay in current is caused by the Na+-dependent inactivation process and any response to the applied pH. Alternatively, we modified the intracellular pH in steps while maintaining constant Na+ and Ca2+ regulation (Fig. S1 B, step protocol). Often, both protocols were used within the same patch. These protocols are advantageous because they minimize the exposure of the patch to stressful pHs, favoring a higher success rate. Therefore, they allow us to investigate efficiently and rapidly how pH affects the steady-state current.
Residues showing altered pH sensitivity at steady state were then further investigated by preexposing the intracellular side of the patch for 20–25 s with various pHs before activation of transport by Na+ (Fig. S1 C, preexposure protocol). This protocol allows investigation of the pH sensitivity both at peak and steady state. As this protocol is stressful to the patch because of prolonged exposure to acidic pHs, we limited its use to only mutant exchangers that showed significant differences from WT in our initial screening protocol. Of particular note is that the pH dependency measured at steady state is the same independently of the protocols adopted (Fig. S1 D).
Data were acquired on-line at a sampling rate of 250 Hz and filtered at 50 Hz using an 8-pole Bessel filter. PCLAMP (Molecular Devices) was used for acquisition and analysis. Experiments were performed at 35°C and at a holding potential of 0 mV.
Statistical analysis
Data were normalized to maximum values (pH 9) and fitted to a Hill function to extrapolate the apparent H+ affinity and the Hill coefficient. Each value is from 3–12 individual experiments. Statistical analysis was performed using Real Statistics Excel plug in for Excel. One-way ANOVA was used to compare multiple groups. A two-sample t test was used for comparison between two groups. Bonferroni’s multiple comparisons test was used post hoc; α was adjusted to P < 0.0025 for multiple comparisons.
Online supplemental material
Fig. S1 shows the specific protocols used to determine the pH sensitivity of NCX. Fig. S2 shows the biophysical properties of the mutant exchangers K229Q-E516L, E516L, and D466-499 compared with those of the WT exchanger. Fig. S3 depicts ionic currents from mutant H165A recorded at different cytoplasmic Ca2+ concentrations while maintaining the pH constant (pH 7), and it compares the sensitivity of the currents recorded from mutants with different amino acid substitutions at position 165.
Results
Cytoplasmic pH modulates NCX activity
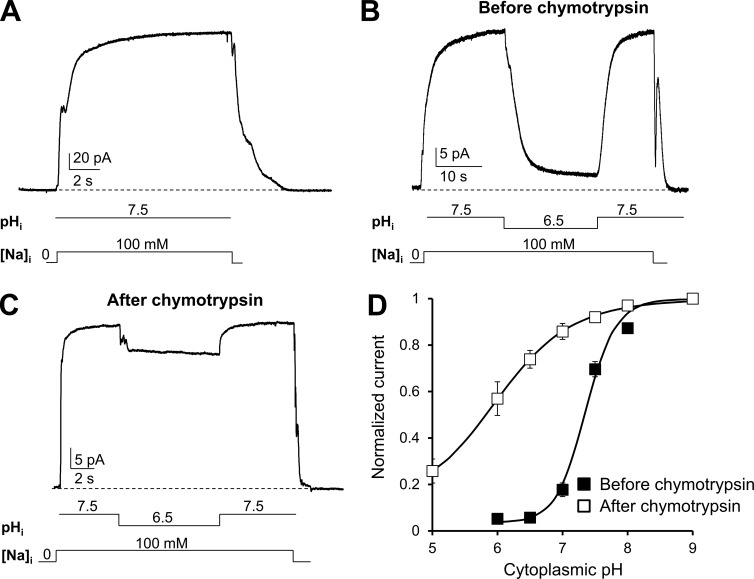
To investigate the modulation of the cardiac exchanger NCX1.1 (Nicoll et al., 1990) by cytoplasmic pH, we used site-directed mutagenesis and electrophysiological measurements. WT or mutant exchanger cRNAs were injected into Xenopus laevis oocytes to record their currents using the giant excised patch technique in the inside-out configuration (Hilgemann and Lu, 1998; Ottolia et al., 2009). Outward currents were generated by rapidly replacing cytoplasmic Cs+ with Na+ (100 mM) at the intracellular surface of the patch. In the presence of Cs+, NCX does not transport whereas application of Na+ allows the exchange cycle to occur. 8 mM Ca2+ was continuously present within the pipette at the extracellular surface. As seen in the traces recorded from the WT exchanger (Fig. 1 A), exchange currents peaked rapidly in the presence of intracellular pH 7.5 and remained stable overtime. Lowering the pH from 7.5 to 6.5 while maintaining a constant cytoplasmic Na+ (100 mM) and Ca2+ (4 µM) decreased the current by ∼90%. This decay occurred over several seconds and could be fully reversed by returning the pH to 7.5 (Fig. 1 B). We examined the current inhibition over a wide range of pH values, and by plotting the steady-state current versus cytoplasmic pH, we determined that 50% of NCX current inhibition occurs at a pH of 7.4 (Fig. 1 D). This agrees with previous studies conducted with cardiac myocytes and other techniques (Hilgemann et al., 1992a; Doering and Lederer, 1993, 1994; Boyman et al., 2011).
Figure 1.
Cytosolic protons regulate NCX current. (A–C) Giant-patch recordings from oocytes expressing the cardiac NCX1.1 in the presence of the indicated intracellular pH before (A and B) and after (C) chymotrypsin digestion. The lines below traces indicate solution changes. Outward currents were generated by rapidly replacing 100 mM intracellular Cs+, pH 7, with 100 mM Na+, pH 7.5. Ca2+ is maintained constant during the recording (contaminant Ca2+ measured at 4 µM). WT current peaked and remains stable until Na+ was removed from the intracellular side of the patch (A). In the presence of pH 7.5 the WT exchanger does not show Na+-dependent inactivation, as alkalization abrogates it (Hilgemann et al., 1992b). A step decrease in pH from 7.5 to 6.5 evoked a slow inhibition of the current (B), which could be reversed by restoring pH 7.5. Cytoplasmic perfusion of 1 mg/ml chymotrypsin removes the regulatory properties of NCX (Hilgemann, 1990). After treatment, cytoplasmic protons are less effective in inhibiting NCX currents, suggesting that proteolytically accessible cytoplasmic components are involved in pH regulation (C). (D) Steady-state currents measured before (■) and after (□) chymotrypsin digestion were plotted versus pH values. Each point corresponds to the mean of five to nine experiments. Error bars represent SEM.
We then investigated the effects of chymotrypsin “deregulation” on the pH sensitivity of NCX. Regions of the exchanger that undergo proteolysis are not well established. However it is believed that mild digestion of NCX with chymotrypsin cleaves a portion of the large cytoplasmic loop between TMSs 5 and 6 (Philipson et al., 1988), eliminating NCX secondary regulation by Na+, Ca2+, and ATP (Hilgemann, 1990). Doering and Lederer (Doering and Lederer, 1993, 1994) showed that NCX activity after deregulation by chymotrypsin became proton insensitive over the pH range 6.5 to 9. This led to the hypothesis that a pH sensor resides within the large intracellular loop (Doering and Lederer, 1993, 1994; Blaustein and Lederer, 1999; Boyman et al., 2011). We have extended these findings by examining NCX’s proton sensitivity over a broader range of pH values to determine if chymotrypsin deregulation merely shifts the pH sensitivity or abrogates it. The cytoplasmic side of the membrane patch was exposed to 1 mg/ml chymotrypsin for ∼1 min with complete deregulation determined experimentally by the removal of both Na+ and Ca2+ regulation (not depicted). Contrary to the untreated WT exchanger, cytoplasmic acidification evoked only a small decrease of current at pH 6.5 (Fig. 1 B). The dependence of the steady-state exchanger current on cytoplasmic pH after chymotrypsin exposure is shown in Fig. 1 C. Our data indicate that deregulation of NCX by chymotrypsin does not completely abrogate pH sensitivity but drastically reduces it. The fit of the H+-dependency curves to a Hill function yielded apparent affinity values of 38 ± 5 nM (n = 9) for WT and 288 ± 50 nM (n = 5) for WT after deregulation by chymotrypsin with corresponding Hill coefficients of 1.81 ± 0.04 and 0.85 ± 0.07. The requirement of stronger acidification to inhibit NCX activity suggests that chymotrypsin removes a high-affinity proton site that senses changes in proton concentrations within the physiological range.
Na+ and Ca2+ regulation modulate the sensitivity of NCX to cytoplasmic pH
Previous studies indicated that NCX proton modulation consists of at least two components (Doering and Lederer, 1993, 1994). One component requires the presence of intracellular Na+ that, by inactivating NCX (Hilgemann et al., 1992b), enhances proton inhibition (Doering and Lederer, 1993, 1994) closely linking pH modulation to Na+-dependent inactivation. The other component is linked to Ca2+ regulation as it originates from the competitive binding of protons to the two CBDs within the large cytoplasmic loop (Boyman et al., 2011). The displacement of Ca2+ from these sites by protons would result in decreased transport activity.
The individual contributions of Na+ and Ca2+ modulation to the pH sensitivity of NCX remain unclear, because it has been technically difficult to dissect their respective roles. In addition, it is unknown whether proton sensitivity occurs in the absence of Na+ or Ca2+ regulation. To address this problem, we used site-specific mutagenesis to remove individually or in combination the Na+ or Ca2+ dependencies of NCX, allowing us to investigate their respective roles in NCX pH sensitivity.
Na+-dependent inactivation was removed with the mutation K229Q. This substitution within the XIP region abolishes the regulation of NCX by intracellular Na+ without altering its transport properties (Matsuoka et al., 1997). Moreover, mutation K229Q does not modify the affinity of NCX for regulatory Ca2+ (Matsuoka et al., 1997) or only mildly affects it (He et al., 2000). Regulation by intracellular Ca2+ was eliminated by mutation E516L. This residue coordinates Ca2+ ions within the primary Ca2+-binding site of CBD2, and its substitution renders NCX insensitive to intracellular Ca2+ while retaining Na+-dependent inactivation (Besserer et al., 2007; Chaptal et al., 2009).
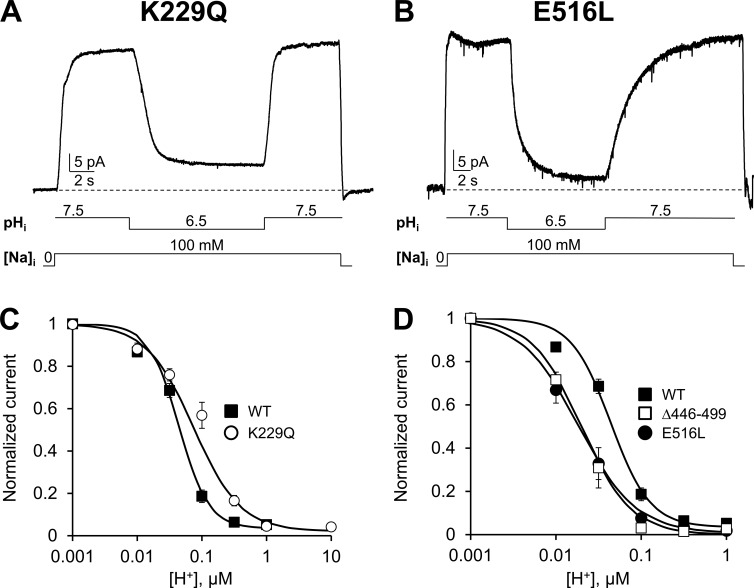
Using a protocol similar to that used for the WT exchanger, we applied various proton concentrations while maintaining cytoplasmic Na+ and Ca2+ constant. The effects of changes in proton concentration on the elicited NCX current of K229Q and E516L exchangers are shown in Fig. 2 (A and B), respectively. The transport activity of both mutant exchangers steeply declines after acidification to pH 6.5 and is fully reversible when pH is returned to 7.5, demonstrating that neither Na+- nor Ca2+-dependent regulation is essential for the pH sensitivity of NCX. Removal of the Na+-dependent inactivation produced a decrease in NCX apparent affinity for cytoplasmic protons (Fig. 2 C; values for the K1/2 are 38 ± 5 nM, n = 9 for WT and 99 ± 6 nM, n = 6 for K229Q). In contrast, disruption of the primary Ca2+ coordinating site within CBD2, which ablates the response of NCX to regulatory Ca2+ (Besserer et al., 2007; Chaptal et al., 2009), had a tendency to increase the sensitivity of NCX to cytoplasmic protons (Fig. 2 D; K1/2 for mutant E516L is 22 ± 5 nM, n = 6), although the difference was not significant (P > 0.0025).
Figure 2.
Effects of Na+ and Ca2+ regulation on NCX pH sensitivity. (A and B) Examples of outward ionic currents recorded from oocytes expressing the indicated mutants. Exchanger K229Q lacks Na+-dependent inactivation (Matsuoka et al., 1997), whereas mutant E516L is not regulated by cytoplasmic Ca2+ (Chaptal et al., 2009). Both mutants retain proton sensitivity indicating that pH regulation can occur in the absence of Na+ or Ca2+ regulation. (C) The dose–response curve for cytoplasmic protons for exchanger K229Q (○) is shown and compared with WT (measured at steady-state, ■). Removal of Na+-dependent inactivation (K229Q) slightly decreases NCX sensitivity to protons and alters the cooperativity of the binding. (D) The pH dependency of steady-state current for mutants E516L (●) and Δ446–499 (□) is shown and compared with WT (■). Disruption of key Ca2+ coordinating sites within CBD1 (Δ446–499) and CBD2 (E516L) does not affect the sensitivity of NCX to cytoplasmic protons. The decrease in the apparent affinity for protons observed is not significant. Error bars represent SEM.
We also examined the role of CBD1 in NCX pH regulation by measuring the response of the deletion mutant Δ446–499 to changes in cytoplasmic protons. This region encompass a large portion of CBD1 (amino acids 371–501), including the Ca2+ coordinating sites 3 and 4 (Hilge et al., 2009), whose affinities for Ca2+ have been proposed to be modulated by pH (Boyman et al., 2011). The sensitivity of this mutant for regulatory Ca2+ is dramatically decreased (Fig. S2). Because of competition between Ca2+ and Na+ at the transport site, it was not possible to increase the intracellular Ca2+ concentration sufficiently to accurately determine its apparent affinity. This result is consistent with previously published data showing that an exchanger with the coordinating sites of CBD1 neutralized responds weakly to changes in intracellular Ca2+ (Ottolia et al., 2009). Importantly, the Δ446–499 (CBD1) and E516L (CBD2) exchanger mutants retain Na+ regulation (Fig. S2).
If protons inhibit the full-length exchanger by binding to the Ca2+ coordination sites within CBD1, then their removal should render NCX H+ insensitive. However, we found that the deletion mutant within CBD1 slightly increased the sensitivity of NCX to protons, as was seen for CBD2 mutant (E516L; Fig. 2 D). The extrapolated apparent H+ affinity under steady-state conditions for Δ446–499 is 19 ± 3 nM (n = 5), which is lower than that of WT (38 ± 5 nM), although the difference is not statistically significant (P > 0.0025).
Overall, these mutagenesis studies show that NCX pH regulation does not require Na+ and Ca2+ regulation and involves a site outside the regions associated with these two allosteric processes.
NCX pH modulation exists in the absence of Na+ and Ca2+ regulation
To further test the relevance of Na+ and Ca2+ regulation in NCX pH sensitivity, we engineered an exchanger lacking both Na+ and Ca2+ ionic modulation by creating the double mutant K229Q-E516L. If NCX pH regulation occurs via H+ modulation of these processes, then this double mutant should generate a pH-insensitive exchanger.
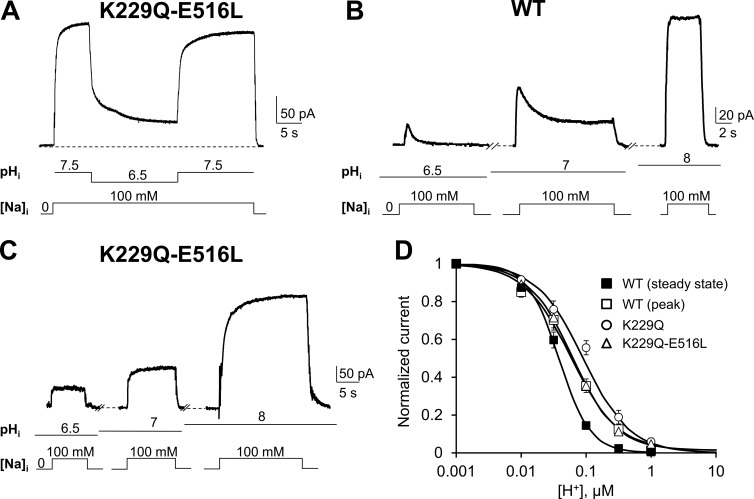
As predicted, K229Q-E516L is insensitive to both cytoplasmic Na+ and Ca2+ (Fig. S2). We first examined the effects of acidification on K229Q-E516L outward currents by lowering the pH from 7.5 to 6.5 while maintaining constant cytoplasmic Na+ (100 mM) and Ca2+ (4 µM; Fig. 3 A). Mutant K229Q-E516L showed an extent of current inhibition in the presence of pH 6.5 similar to that seen for the WT (compare Fig. 3 A with Fig. 1 B) and full reversibility upon reexposure to pH 7.5. Note also that K229Q-E516L ionic currents did not show the slow decay of the current upon application of intracellular Na+, confirming the lack of Na+-dependent inactivation (compare Fig. 3 B with Fig. 3 C).
Figure 3.
Na+ and Ca2+ modulation is not required for proton regulation. (A) Representative outward currents recorded from oocytes expressing mutant K229Q-E516L, which lacks both Na+ and Ca2+ regulation. Acid pH inhibits K229Q-E516L ionic currents, indicating the pH modulation involves regions of NCX not associated with either Na+ or Ca2+ regulation. The lines below the traces indicate solution changes. (B and C) WT and K229Q-E516L currents were recorded after exposing the patch to the indicated pH values for 20 to 25 s before Na+ application. This allows us to investigate proton inhibition before (peak) and after (steady state) the development of Na+-dependent inactivation. Ca2+ is maintained constant throughout the recordings. Note that K229Q-E516L lacks the current decay caused by intracellular Na+ but still responds to changes in H+ concentration. Accordingly, K229Q-E516L peak and steady-state currents are essentially the same. (D) Dose–response curves for cytoplasmic H+ for WT peak and steady-state currents (peak, □; steady state, ■) and mutants K229Q (○) and K229Q-E5 16L (△). The K229Q-E516L H+ dose–response curve is superimposable with that of WT exchanger measured at peak, before the development of the Na+-dependent inactivation. Error bars represent SEM.
The dependence of NCX K229Q-E516L current on [H+] is shown in Fig. 3 D. Remarkably, this mutant has an apparent H+ affinity similar to the one of the WT NCX peak current (i.e., before the development of the slow Na+-dependent inactivation; Hilgemann et al., 1992b; Fig. 3 D, compare △ and □). This demonstrates that pH modulation of NCX does not occur primarily via proton regulation of its Ca2+- and Na+-dependent regulation. Nevertheless, we confirmed our results obtained with mutant K229Q showing that Na+-dependent inactivation can enhance the inhibition of NCX by protons. Indeed, the [H+] dependence of the WT steady-state current (Fig. 3 D, ■), when the Na+-dependent inactivation has fully developed, displays a small decrease in the apparent affinity for protons as compared with WT peak current (□) and K229-E516L (△) and K229Q (○) mutant currents and a significant change in the cooperativity of binding (Fig. 4).
Figure 4.
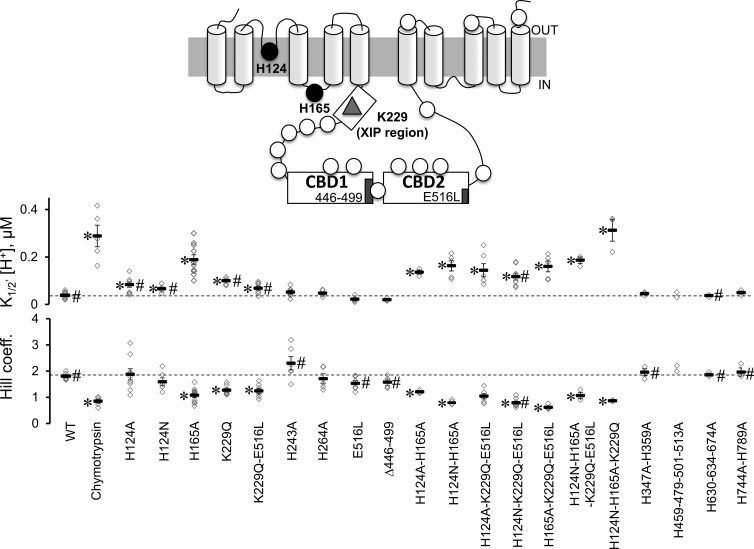
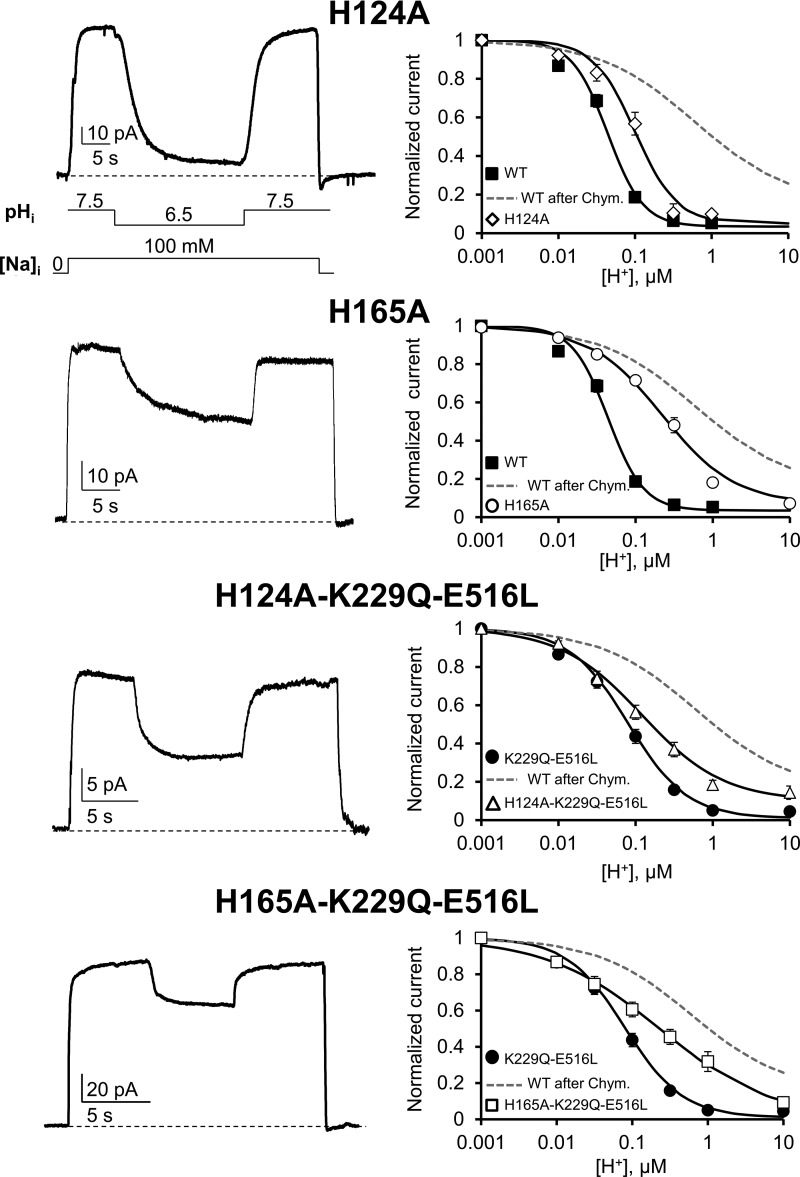
Proton sensitivity of His mutants. The topology of mammalian NCX is shown as predicted by Ren and Philipson (2013). The approximate position of the 18 histidines (circles), Lys 229 (triangle), the two regulatory Ca2+-binding domains (CBD1 and CBD2) and the XIP region are shown. His residues were mutated to Ala, and the sensitivities of the NCX mutants to cytoplasmic protons were measured at steady state. Normalized currents were plotted as a function of proton concentration to extrapolate the apparent affinity for each single experiment. The number of experiments used for each exchanger mutant is shown with the corresponding mean value ± standard error. Values statistically different after a Bonferroni post hoc test from WT are marked with an asterisk (*, P < 0.0025), whereas values statistically different from WT after chymotrypsin deregulation are marked with # (P < 0.0025). Because of the limited number of experimental observations, we excluded mutant H459-479-501-513A from the multiple comparison procedure and reported the values obtained from the single experiments. Note that all exchangers including mutation of either His 124 or His 165 have decreased sensitivity to protons.
Two histidines are important for NCX proton sensitivity
The data presented so far strongly support the view that Na+ and Ca2+ regulatory mechanisms are not required for NCX regulation by H+ and suggest the existence of alternative proton-sensitive sites. As the pKa of the imidazole side chain of His is ∼6.5, we hypothesized that the inhibitory effects of H+ on NCX activity are mediated by cytoplasmically accessible histidines. The cardiac NCX possesses a total of 18 His residues, 13 of which are located within the large intracellular loop (Fig. 4). Because replacement of all 18 His with the pH-insensitive Ala residue abolished NCX activity, we created a family of exchangers with different His substitutions and measured their sensitivity to cytoplasmic pH. The extrapolated values of the apparent H+ affinities of the His mutants are shown in Fig. 4 together with their respective Hill coefficient values. We found that individual substitution of His 124 and His 165 significantly reduced the pH sensitivity of the cardiac NCX (83 ± 10, n = 9, for H124A and 188 ± 20, n = 10, for H165A). Moreover, neutralization of His 165 affected the cooperativity of proton binding (Hill coefficient values for WT and H165A are 1.81 ± 0.04 and 1.07 ± 0.09, respectively; Figs. 4 and 5), indicating an important role of this residue in H+ modulation of NCX.
Figure 5.
His 124 and His 165 are important for NCX pH regulation. Examples of outward currents recorded from the indicated mutants in the presence of different cytoplasmic pH values. Cytoplasmic Na+ and H+ concentrations are indicated under the H124A trace. The normalized steady-state currents versus [H+] for mutants H124A (♢), H165A (○), H124A-K229Q-E516L (△), and H165A-K229Q-E516L (□) are shown on the right. For comparison, the proton sensitivity of WT NCX is shown before (■) and after chymotrypsin treatment (gray dashed line). The dependence of H124A-K229Q-E516L and H165A-K229Q-E516L currents to changes in proton concentration is instead compared with the pH sensitivity of K229Q-E516L (●). Current values were measured at steady state, which in exchangers H165A, H124A-K229Q-E516L and H165A-K229Q-E516L are essentially the same as peak currents because of the absence of Na+-dependent inactivation. Replacement of His 124 and His 165 decreased the exchanger pH sensitivity even in the absence of Na+ and Ca2+ regulation. Error bars represent SEM.
Because His 124 also plays a role in Na+-dependent inactivation (Ottolia et al., 2005) and replacement of His 165 with an Ala abolished both Na+-dependent inactivation and Ca2+ regulation (Fig. S3), we sought to determine whether the altered Na+ and Ca2+ modulation of these mutant exchangers was responsible for their decreased pH sensitivity. We therefore introduced mutations (H124A and H165A) in the K229Q-E516L background (which lacks both Ca2+- and Na+-dependent regulation). If His 124 and His 165 decreased NCX pH sensitivity independently of their effects on Na+ and Ca2+ regulation, then they should alter the response of NCX to cytoplasmic protons, even in the presence of mutations K229Q-E516L.
Indeed, this turns out to be the case. Addition of H165A or H124A mutations into K229Q-E516L background resulted in exchangers with higher apparent affinities for protons (Fig. 5) when compared with both WT and K229Q-E516L exchangers (K1/2 values [nM]: 68 ± 9 for K229Q-E516L, n = 7; 160 ± 22 for H165A-K229Q-E516L, n = 5; and 143 ± 27 for H124A-K229Q-E516L, n = 6). Strikingly, H165A-K229Q-E516L mutant showed a drastic decrease in the cooperativity of proton binding (Hill coefficient values: 1.24 ± 0.09 for K229Q-E516L, n = 7; and 0.61 ± 0.04 for H165A-K229Q-E516L, n = 5; Fig. 5) and biophysical properties that closely resemble those determined for the WT after chymotrypsin deregulation (P < 0.0025). The results emphasized the important role that residues 124 and 165 play in NCX pH modulation and suggest that they decrease exchanger proton sensitivity independently of their effects on Ca2+- and Na+-dependent regulation.
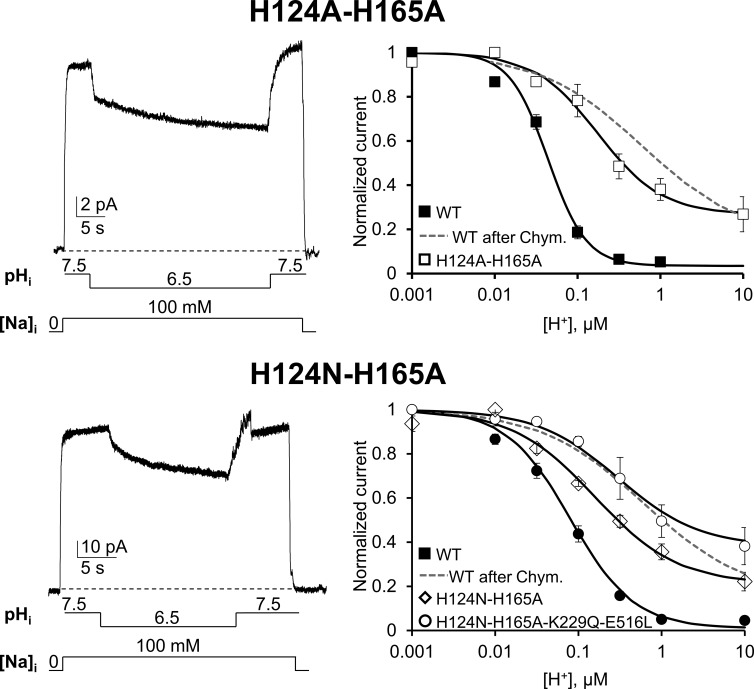
Both His 124 and His 165 are important for NCX pH sensitivity, but His 165 plays a more prominent role (Figs. 4 and 5). We then combined these two mutations to determine whether their effects were additive. Fig. 6 shows that in contrast to the single point mutations, the double histidine mutants (H124A-H165A) could still pass current at acidic pH with a H+ concentration response not significantly different from the one measured from the deregulated WT exchanger (Fig. 6, gray dashed line; P < 0.0025). Similar results were obtained with the mutant H124N-H165A (Fig. 6), where we introduced an Asn at position 124 instead of an Ala to generate greater ionic currents but without affecting the response to protons. Finally, mutations H124N-H165A decreased NCX pH sensitivity when introduced into the K229Q-E516L background, confirming that these mutations perturb NCX pH sensitivity independently of their effects on Na+ and Ca2+ regulation. Similar results were obtained with mutant H124N-H165A-K229Q (Fig. 4).
Figure 6.
His 124 and His 165 alter NCX pH regulation independently of their effects on Na+ and Ca2+ regulation. Recording of outward currents from oocytes expressing the indicated mutants. Normalized currents are plotted as function of proton concentration and shown on the right. Mutants H124A-H165A and H124N-H165A showed a further decrease in pH sensitivity when compared with the single mutants (see Fig. 5), with ∼25% of activity still present at 10 µM H+, pH 5. Introduction of mutations H124N-H165A in an exchanger lacking both Na+ and Ca2+ regulation (K229Q-E516L) did not prevent the decrease in proton sensitivity showing a H+ dependence as H124N-H165A (P > 0.0025; compare ○ with ♢). These results indicate that His 124 and His 165 alter NCX pH sensitivity independently from their effects on Na+ and Ca2+ regulation. Identical results were obtained with mutant H124N-H165A-K229Q (not depicted; see Fig. 4 for K1/2 value). The proton sensitivity of WT before (■) and after (gray dashed line) chymotrypsin deregulation is shown for comparison. Error bars represent SEM.
Our data establish that residues His 124 and 165 are important elements for NCX pH regulation. Their simultaneous substitution resembles the pH dependency and cooperativity of the deregulated exchanger generated by chymotrypsin treatment. This suggests that these residues can account for the pH sensitivity of NCX occurring over the pathophysiological proton concentration range (Orchard and Kentish, 1990; Orchard and Cingolani, 1994; Vaughan-Jones et al., 2009).
Discussion
Modulation of NCX by cytoplasmic protons
Given the steep pH dependency of NCX, any intracellular pH changes will have a dramatic effect upon its activity (Baker and Blaustein, 1968; Philipson et al., 1982; Hilgemann et al., 1992b; Doering and Lederer, 1993, 1994; Boyman et al., 2011). Accordingly, the pH regulation of NCX is likely to play an important role in vivo. Numerous pathological conditions are associated with drops in pH, which in turn affect NCX activity. Notably, ischemia can reduce the intracellular pH by a full unit, providing an inhibitory influence on NCX transport that may contribute to the Ca2+ aberrations associated with this pathology (Orchard and Cingolani, 1994; Vaughan-Jones et al., 2009; Garciarena et al., 2013). Despite its importance, the mechanisms by which cytosolic pH regulates NCX are poorly understood. Both Na+ and Ca2+ modulation have been previously associated with NCX proton sensitivity, suggesting an intricate mechanism of regulation in which multiple allosteric processes interact to fine-tune NCX activity. In this study, we demonstrate that NCX pH regulation is a distinct allosteric mechanism that can develop independently of the Na+ and Ca2+ regulatory processes. We have identified two histidines that are required for the allosteric mechanism responsible for NCX pH modulation.
Role of Na+ regulation in NCX pH sensitivity
It has been proposed that NCX proton modulation consists of two components: a primary one, which occurs in the absence of intracellular Na+, and a secondary form that is enhanced by intracellular Na+ involving Na+-dependent inactivation (Doering and Lederer, 1993, 1994). Our studies demonstrate that it is possible to dissect these two components and establish their relative contribution to pH regulation. This was accomplished by testing the effects of various proton concentrations on an exchanger lacking Na+-dependent inactivation. We abrogated the Na+ modulation by replacing Lys 229 with Glu, a mutation that prevents Na+-dependent inactivation occurring while minimally affecting Ca2+ regulation (Matsuoka et al., 1997). The K229Q exchanger is still strongly modulated by pH, and its H+ dose–response curve overlaps the one of the WT measured from the initial peak current (Fig. 3 D) before inactivation (Hilgemann et al., 1992b). These results demonstrate for the first time that the major contributing component of NCX pH modulation relies on a distinct molecular mechanism from the Na+-dependent inactivation process. Nevertheless, as previously reported (Doering and Lederer, 1994), intracellular Na+ enhances the sensitivity of NCX to cytoplasmic protons. Our data, however, show that the impact of the Na+-dependent inactivation on NCX pH modulation is modest. This is revealed by the small but significant decrease in H+ affinity of mutant K229Q, K229Q-E516L and WT peak current when compared with that of the WT steady-state current after inactivation has occurred. As previously suggested, high intracellular [Na+] may slow the transport cycle, favoring a conformational state more sensitive to protonation (Doering and Lederer, 1994). This is consistent with NCX having different affinities for transported Na+ (Matsuoka et al., 1997) and regulatory Ca2+ (Hilgemann et al., 1992a; Ottolia et al., 2009) before and after Na+-dependent inactivation. Alternatively, Lys 229 may alter the pH dependence of Na+ inactivation and, therefore, NCX activity. We believe that this is not the case, as a previous study demonstrated that Na+ inactivation no longer occurs in mutant K229Q (Matsuoka et al., 1997). We also show that the prominent transient current seen in WT upon rapid application of cytoplasmic Na+ is absent in K229Q-E516L, even at pH values below 6, confirming the lack of Na+-dependent inactivation (Fig. 3 C).
Regardless of the underlying mechanism, our results suggest that by shifting NCX proton affinity, intracellular Na+ may exacerbate the inhibitory effects of H+ in vivo. This effect may be relevant during cardiac ischemia/reperfusion, when the levels of both H+ and Na+ change over time, thereby affecting NCX activity and subsequently contractility.
Role of Ca2+ regulation in NCX pH sensitivity
Intracellular Ca2+ modulates NCX activity by binding either to two regulatory domains (CBD1 and CBD2; Hilge et al., 2006; Nicoll et al., 2006; Wu et al., 2010) or the transport site, which has a lower affinity for Ca2+ than the CBDs. Whether protons interfere with the binding of Ca2+ to these sites remains controversial. Studies investigating the equilibrium properties of the isolated CBDs showed that protons can directly bind to the regulatory domains displacing Ca2+ and therefore, by implication, inhibit NCX (Boyman et al., 2011). This contrasts with earlier investigations, which excluded the CBDs as proton-binding sites and suggested only a weak competition between H+ and Ca2+ at the translocation site, based on the observation that high concentrations of Ca2+ (mM) were required to relieve proton inhibition (Philipson et al., 1982; Doering and Lederer, 1993, 1994).
To clarify how cytoplasmic Ca2+ affects NCX pH modulation, we mutated strategic residues of the full-length exchanger that directly contribute to the binding of Ca2+. Specifically, we created an exchanger with the Ca2+ coordinating sites 3 and 4 of CBD1 deleted (Δ446–499). These sites set the affinity of the exchanger for cytoplasmic Ca2+ (Matsuoka et al., 1995; Hilge et al., 2006; Chaptal et al., 2009; Ottolia et al., 2009), and their removal or replacement drastically decreases the sensitivity of NCX to cytoplasmic Ca2+ (Fig. S2; Ottolia et al., 2009). Experiments conducted with the isolated Ca2+-binding domain suggest that they may also coordinate protons (Boyman et al., 2011). The role of CBD2 in proton coordination was instead evaluated by mutating Glu 516 (E516L), which coordinates Ca2+ at the primary site of CBD2. The secondary Ca2+ coordinating site within CBD2 appears to have no function (Hilge et al., 2006; Besserer et al., 2007; Ottolia et al., 2009). If protons bind to any of these sites to displace Ca2+ and inhibit NCX, then the prediction is that their disruption should decrease the sensitivity of NCX to cytoplasmic protons. However, our results show that removal of the residues coordinating Ca2+ did not significantly alter the apparent affinity of NCX for protons. In contrast, mutants E516L and Δ446–499 showed the tendency to respond to protons more efficiently (Figs. 2 and 4). This may be caused by their enhanced Na+-dependent inactivation at high Ca2+ concentrations (Fig. S2), which in turn will facilitate proton regulation. Consistent with this hypothesis, the mutation E516L failed to increase the sensitivity of NCX to protons in the absence of Na+-dependent inactivation, as seen in mutant K229Q-E516L (Fig. 3). This supports the hypothesis that the slightly increased sensitivity to protons seen in the CBD mutants is caused by the modulatory effect of Ca2+ upon the Na+-dependent inactivation process.
Overall, the data indicate that protons bind to a site outside of the CBDs to inhibit NCX. It is important to note that our experiments were conducted using the full-length exchanger and not the isolated domains (Boyman et al., 2011). Our data do not exclude the possibility that protons bind directly to the CBD domains, as previously reported (Boyman et al., 2011). However, they demonstrate that this process cannot account for the inhibition of the current by H+ in the full-length exchanger.
His 124 and His 165 are involved in NCX pH modulation
The mutagenesis results presented herein suggests that His 124 and His 165 contribute to the molecular mechanisms linked to NCX pH sensitivity. This is the first study of specific residues affecting exchanger pH regulation.
In the cardiac NCX, His 124 has been previously found to play a role both in ion translocation and Na+-regulation (Ottolia et al., 2005). This residue is found in the loop connecting transmembrane segments 2–3 (Fig. 4), which are involved in ion transport (Nicoll et al., 1996, 1999; Iwamoto et al., 2000; Ottolia et al., 2005; Liao et al., 2012; John et al., 2013). Cysteine scanning mutagenesis studies indicate that this region forms a reentrant loop in the cardiac isoform NCX1 (Iwamoto et al., 2000; Nicoll et al., 2002), suggesting that His 124 may be exposed to cytoplasmic protons. However, the crystal structure of the archaebacterial NCX_Mj does not support this finding, as it shows that the corresponding region is shorter, forming an extracellular loop (Liao et al., 2012). This divergence of biochemical and structural data makes defining the position of His 124 ambiguous. We attempted to investigate the accessibility of His 124 from the intracellular environment by introduction of a cysteine at this position. However, mutant H124C resulted in an inactive exchanger preventing such efforts (unpublished data). Given its undefined location, the mechanism by which this residue modulates NCX pH sensitivity remains elusive. The imidazole side chain at position 124 may be accessible to cytoplasmic protons, therefore playing a direct role in pH regulation. Alternatively, this residue may be involved in long-range conformational changes, which indirectly affect proton sensitivity. This is consistent with the observation that replacement of this residue slows down conformational changes involved in the Na+-dependent inactivation process, supporting a role of His 124 in NCX regulation (Ottolia et al., 2005). Further investigations are required to determine the mechanistic contribution of His 124 to pH modulation.
His 165 is modeled to reside in the cytoplasmic loop connecting TMSs 3–4 in the cardiac NCX, whereas in the NCX_Mj structure, the equivalent residue, Lys 103, is found near the intracellular surface of TMS 4 (Liao et al., 2012). Replacement of His 165 with an Ala decreased the apparent H+ affinity of NCX by approximately fourfold and drastically altered the cooperativity of the binding. These observations suggest that this residue is an important component of the NCX pH sensor.
Mutant H165A rendered NCX insensitive to either Na+ or Ca2+ regulation (Fig. S3; Ottolia et al., 2002). This is a striking result indicating that this residue is a key element of NCX allosteric ionic regulation, as it appears to transduce pH and Na+, and Ca2+ signals. Possibly, His 165 mediates the propagation of information from the XIP region (Na+-dependent inactivation) and the Ca2+-binding domains to the transport sites, functionally connecting them. A change in the imidazole side-chain charge of His 165 may affect this interfacial coupling, altering Na+ and Ca2+ signaling. Consistent with this hypothesis is the observation that alkaline pH abolishes both Na+ and Ca2+ regulation (Hilgemann et al., 1992b), mimicking the phenotype seen in H165A exchanger. In addition, protonation of His 165 seems to inhibit NCX activity directly via a mechanism that does not involve the molecular processes linked to Na+ and Ca2+ regulation. Several observations support this hypothesis and suggest that the reduced response of H165A to cytoplasmic acidification is not linked to the effects of this mutation on Na+ and Ca2+ modulation. First, we have demonstrated that the removal of Na+ and Ca2+ regulation (K229Q-E516L) minimally affects the response of the exchanger to pH, indicating that proton inhibition of NCX involves an additional site and mechanism. Second, H165A decreased both the apparent proton affinity and the cooperativity of proton binding in the presence of mutations K229Q and E516L, which disrupt the cytoplasmic sites involved in Na+ and Ca2+ regulation, respectively. This further supports the hypothesis that the effects of His 165 mutation on Na+ and/or Ca2+ regulation do not cause its decreased response to cytoplasmic pH. Finally, replacement of His 165 with amino acids whose pKa values are not within the pathophysiological pH range (H165K, H165R, H165Q, and H165E) mimics the response of mutant H165A (Fig. S3 B). This indicates that the effects of pH are independent of properties of the side chain at this location and the remaining pH sensitivity comes from other sites, as seen in the deregulated exchanger by chymotrypsin treatment. These observations point to the conclusion that His 165 is a key component of the NCX pH sensor.
In summary, we report for the first time individual residues that alter the response of NCX to cytoplasmic pH. The simultaneous replacement of His 124 and His 165 drastically decrease the sensitivity of NCX to cytoplasmic H+, and the resulting mutant behaves similarly as the WT exchanger after chymotrypsin treatment (P < 0.0025). As previously mentioned, chymotrypsin removes the component of proton regulation occurring within pathophysiological ranges of pH, and our results suggest that His 124 and 165 may be responsible for this component of NCX pH modulation. The residual proton sensitivity observed after chymotrypsin deregulation and mutagenesis of His 124 and 165 may originate from a competition between protons and Na+ or Ca2+ ions at their transport sites, consistent with previous studies (Philipson et al., 1982; Doering and Lederer, 1993).
Collectively, the mutagenesis studies presented herein indicate that regulation of NCX by cytoplasmic protons, which occurs within the physiological range, is a distinct allosteric modulation, independent from Na+ and Ca2+ regulation. This ionic regulation requires His 124 and 165. These residues may be a direct site of protonation or alternatively alter NCX pH sensitivity via indirect allosteric mechanisms, for example by perturbing distantly located regions involved in pH inhibition. Further effort is needed to determine how these two histidines affect NCX activity.
Supplementary Material
Acknowledgments
We would like to thank Tristan Grogan MS (Statistics Core, Department of Medicine, David Geffen School of Medicine at UCLA) for his help with the statistical analysis. We thank Dr. Kenneth D. Philipson for insightful discussions.
This research was supported by the American Heart Association, Western States Affiliate (grant 13GRNT17210044 to M. Ottolia) and the National Institutes of Health (grant R01HL130308 to M. Ottolia, grant R01HL048509 to J.I. Goldhaber, and grant R01GM110276 to R. Olcese).
The authors declare no competing financial interests.
Author contributions: M. Ottolia, S. John, R. Olcese, and J.I. Goldhaber conceived, designed, and interpreted this research; M. Ottolia, S. John, and B. Kim collected the data, and M. Ottolia and S. John analyzed the data; M. Ottolia, S. John, R. Olcese, and J.I. Goldhaber wrote the paper.
José D. Faraldo-Gómez served as editor.
References
- Baker P.F., and Blaustein M.P.. 1968. Sodium-dependent uptake of calcium by crab nerve. Biochim. Biophys. Acta. 150:167–170. 10.1016/0005-2736(68)90023-0 [DOI] [PubMed] [Google Scholar]
- Besserer G.M., Ottolia M., Nicoll D.A., Chaptal V., Cascio D., Philipson K.D., and Abramson J.. 2007. The second Ca2+-binding domain of the Na+ Ca2+ exchanger is essential for regulation: crystal structures and mutational analysis. Proc. Natl. Acad. Sci. USA. 104:18467–18472. 10.1073/pnas.0707417104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M.P., and Lederer W.J.. 1999. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79:763–854. [DOI] [PubMed] [Google Scholar]
- Boyman L., Hagen B.M., Giladi M., Hiller R., Lederer W.J., and Khananshvili D.. 2011. Proton-sensing Ca2+ binding domains regulate the cardiac Na+/Ca2+ exchanger. J. Biol. Chem. 286:28811–28820. 10.1074/jbc.M110.214106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaptal V., Ottolia M., Mercado-Besserer G., Nicoll D.A., Philipson K.D., and Abramson J.. 2009. Structure and functional analysis of a Ca2+ sensor mutant of the Na+/Ca2+ exchanger. J. Biol. Chem. 284:14688–14692. 10.1074/jbc.C900037200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering A.E., and Lederer W.J.. 1993. The mechanism by which cytoplasmic protons inhibit the sodium-calcium exchanger in guinea-pig heart cells. J. Physiol. 466:481–499. [PMC free article] [PubMed] [Google Scholar]
- Doering A.E., and Lederer W.J.. 1994. The action of Na+ as a cofactor in the inhibition by cytoplasmic protons of the cardiac Na+-Ca2+ exchanger in the guinea-pig. J. Physiol. 480:9–20. 10.1113/jphysiol.1994.sp020336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciarena C.D., Youm J.B., Swietach P., and Vaughan-Jones R.D.. 2013. H+-activated Na+ influx in the ventricular myocyte couples Ca2+-signalling to intracellular pH. J. Mol. Cell. Cardiol. 61:51–59. 10.1016/j.yjmcc.2013.04.008 [DOI] [PubMed] [Google Scholar]
- Giladi M., Sasson Y., Fang X., Hiller R., Buki T., Wang Y.X., Hirsch J.A., and Khananshvili D.. 2012. A common Ca2+-driven interdomain module governs eukaryotic NCX regulation. PLoS One. 7:e39985 10.1371/journal.pone.0039985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Feng S., Tong Q., Hilgemann D.W., and Philipson K.D.. 2000. Interaction of PIP(2) with the XIP region of the cardiac Na/Ca exchanger. Am. J. Physiol. Cell Physiol. 278:C661–C666. [DOI] [PubMed] [Google Scholar]
- Hilge M., Aelen J., and Vuister G.W.. 2006. Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol. Cell. 22:15–25. 10.1016/j.molcel.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Hilge M., Aelen J., Foarce A., Perrakis A., and Vuister G.W.. 2009. Ca2+ regulation in the Na+/Ca2+ exchanger features a dual electrostatic switch mechanism. Proc. Natl. Acad. Sci. USA. 106:14333–14338. 10.1073/pnas.0902171106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W. 1990. Regulation and deregulation of cardiac Na+-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 344:242–245. 10.1038/344242a0 [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W., and Lu C.C.. 1998. Giant membrane patches: improvements and applications. Methods Enzymol. 293:267–280. 10.1016/S0076-6879(98)93018-X [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W., Collins A., and Matsuoka S.. 1992a Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J. Gen. Physiol. 100:933–961. 10.1085/jgp.100.6.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W., Matsuoka S., Nagel G.A., and Collins A.. 1992b Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J. Gen. Physiol. 100:905–932. 10.1085/jgp.100.6.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T., Uehara A., Imanaga I., and Shigekawa M.. 2000. The Na+/Ca2+ exchanger NCX1 has oppositely oriented reentrant loop domains that contain conserved aspartic acids whose mutation alters its apparent Ca2+ affinity. J. Biol. Chem. 275:38571–38580. 10.1074/jbc.M003788200 [DOI] [PubMed] [Google Scholar]
- John S.A., Liao J., Jiang Y., and Ottolia M.. 2013. The cardiac Na+-Ca2+ exchanger has two cytoplasmic ion permeation pathways. Proc. Natl. Acad. Sci. USA. 110:7500–7505. 10.1073/pnas.1218751110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Nicoll D.A., Collins A., Hilgemann D.W., Filoteo A.G., Penniston J.T., Weiss J.N., Tomich J.M., and Philipson K.D.. 1991. Identification of a peptide inhibitor of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. J. Biol. Chem. 266:1014–1020. [PubMed] [Google Scholar]
- Liao J., Li H., Zeng W., Sauer D.B., Belmares R., and Jiang Y.. 2012. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science. 335:686–690. 10.1126/science.1215759 [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Nicoll D.A., Hryshko L.V., Levitsky D.O., Weiss J.N., and Philipson K.D.. 1995. Regulation of the cardiac Na+-Ca2+ exchanger by Ca2+. Mutational analysis of the Ca2+-binding domain. J. Gen. Physiol. 105:403–420. 10.1085/jgp.105.3.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Nicoll D.A., He Z., and Philipson K.D.. 1997. Regulation of cardiac Na+-Ca2+ exchanger by the endogenous XIP region. J. Gen. Physiol. 109:273–286. 10.1085/jgp.109.2.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll D.A., Longoni S., and Philipson K.D.. 1990. Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger. Science. 250:562–565. 10.1126/science.1700476 [DOI] [PubMed] [Google Scholar]
- Nicoll D.A., Hryshko L.V., Matsuoka S., Frank J.S., and Philipson K.D.. 1996. Mutation of amino acid residues in the putative transmembrane segments of the cardiac sarcolemmal Na+-Ca2+ exchanger. J. Biol. Chem. 271:13385–13391. 10.1074/jbc.271.23.13385 [DOI] [PubMed] [Google Scholar]
- Nicoll D.A., Ottolia M., Lu L., Lu Y., and Philipson K.D.. 1999. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J. Biol. Chem. 274:910–917. 10.1074/jbc.274.2.910 [DOI] [PubMed] [Google Scholar]
- Nicoll D.A., Ottolia M., and Philipson K.D.. 2002. Toward a topological model of the NCX1 exchanger. Ann. N. Y. Acad. Sci. 976:11–18. 10.1111/j.1749-6632.2002.tb04709.x [DOI] [PubMed] [Google Scholar]
- Nicoll D.A., Sawaya M.R., Kwon S., Cascio D., Philipson K.D., and Abramson J.. 2006. The crystal structure of the primary Ca2+ sensor of the Na+/Ca2+ exchanger reveals a novel Ca2+ binding motif. J. Biol. Chem. 281:21577–21581. 10.1074/jbc.C600117200 [DOI] [PubMed] [Google Scholar]
- Nicoll D.A., Ottolia M., Goldhaber J.I., and Philipson K.D.. 2013. 20 years from NCX purification and cloning: milestones. Adv. Exp. Med. Biol. 961:17–23. 10.1007/978-1-4614-4756-6_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C.H., and Cingolani H.E.. 1994. Acidosis and arrhythmias in cardiac muscle. Cardiovasc. Res. 28:1312–1319. 10.1093/cvr/28.9.1312 [DOI] [PubMed] [Google Scholar]
- Orchard C.H., and Kentish J.C.. 1990. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. 258:C967–C981. [DOI] [PubMed] [Google Scholar]
- Ottolia M., Schumann S., Nicoll D.A., and Philipson K.D.. 2002. Activation of the cardiac Na+/Ca2+ exchanger by DEPC. Ann. N. Y. Acad. Sci. 976:85–88. 10.1111/j.1749-6632.2002.tb04720.x [DOI] [PubMed] [Google Scholar]
- Ottolia M., Nicoll D.A., and Philipson K.D.. 2005. Mutational analysis of the alpha-1 repeat of the cardiac Na+-Ca2+ exchanger. J. Biol. Chem. 280:1061–1069. 10.1074/jbc.M411899200 [DOI] [PubMed] [Google Scholar]
- Ottolia M., Nicoll D.A., and Philipson K.D.. 2009. Roles of two Ca2+-binding domains in regulation of the cardiac Na+-Ca2+ exchanger. J. Biol. Chem. 284:32735–32741. 10.1074/jbc.M109.055434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolia M., Torres N., Bridge J.H., Philipson K.D., and Goldhaber J.I.. 2013. Na/Ca exchange and contraction of the heart. J. Mol. Cell. Cardiol. 61:28–33. 10.1016/j.yjmcc.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C., Thompson S., and Epel D.. 2004. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 35:427–431. 10.1016/j.ceca.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Philipson K.D., Bersohn M.M., and Nishimoto A.Y.. 1982. Effects of pH on Na+-Ca2+ exchange in canine cardiac sarcolemmal vesicles. Circ. Res. 50:287–293. 10.1161/01.RES.50.2.287 [DOI] [PubMed] [Google Scholar]
- Philipson K.D., Longoni S., and Ward R.. 1988. Purification of the cardiac Na+-Ca2+ exchange protein. Biochim. Biophys. Acta. 945:298–306. 10.1016/0005-2736(88)90492-0 [DOI] [PubMed] [Google Scholar]
- Ren X., and Philipson K.D.. 2013. The topology of the cardiac Na+/Ca2+ exchanger, NCX1. J. Mol. Cell. Cardiol. 57:68–71. 10.1016/j.yjmcc.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock M.J., Ottolia M., Bers D.M., Blaustein M.P., Boguslavskyi A., Bossuyt J., Bridge J.H., Chen-Izu Y., Clancy C.E., Edwards A., et al. 2015. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J. Physiol. 593:1361–1382. 10.1113/jphysiol.2014.282319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R.D., Spitzer K.W., and Swietach P.. 2009. Intracellular pH regulation in heart. J. Mol. Cell. Cardiol. 46:318–331. 10.1016/j.yjmcc.2008.10.024 [DOI] [PubMed] [Google Scholar]
- Wu M., Wang M., Nix J., Hryshko L.V., and Zheng L.. 2009. Crystal structure of CBD2 from the Drosophila Na+/Ca2+ exchanger: diversity of Ca2+ regulation and its alternative splicing modification. J. Mol. Biol. 387:104–112. 10.1016/j.jmb.2009.01.045 [DOI] [PubMed] [Google Scholar]
- Wu M., Le H.D., Wang M., Yurkov V., Omelchenko A., Hnatowich M., Nix J., Hryshko L.V., and Zheng L.. 2010. Crystal structures of progressive Ca2+ binding states of the Ca2+ sensor Ca2+ binding domain 1 (CBD1) from the CALX Na+/Ca2+ exchanger reveal incremental conformational transitions. J. Biol. Chem. 285:2554–2561. 10.1074/jbc.M109.059162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.