ABSTRACT
World Health Organization recommends oral cholera vaccine (OCV) to prevent and control cholera, but requires cost-effectiveness evidence. This review aimed to provide a critical appraisal and summary of global economic evaluation (EE) studies involving OCV to guide future EE study. Full EE studies, published from inception to December 2015, evaluating OCV against cholera disease were included. The included studies were appraised using WHO guide for standardization of EE of immunization programs. Out of 14 included studies, almost all (13/14) were in low- and middle-income countries. Most studies (11/14) evaluated mass vaccination program. Most of the studies (9/14) incorporated herd protective effect. The most common influential parameters were cholera incidence, OCV coverage, herd protection and OCV price. OCV vaccination is likely to be cost-effective when targeted at the population with high-risk of cholera and poor access to health care facilities when herd protection effect is incorporated and OCV price is low.
KEYWORDS: Economic Evaluation, Oral Cholera Vaccine, Systematic Review
Introduction
Cholera is an acute infectious disease caused by the ingestion of bacteria Vibrio cholera O1 or less commonly O139, and transmitted via a direct fecal-oral contamination or ingestion of contaminated water or food.1 Cholera remains as a significant but neglected disease, disproportionately affects the health of impoverished populations in low- and middle-income countries (LMICs).2 In 2014, 190,549 cholera cases with 2231 deaths were reported to World Health Organization (WHO), with 55% of cases originated from Africa, 30% from Asia and 15% from Hispaniola.3 The officially reported cases represent only around 5–10% of actual cases worldwide due to the poor surveillance systems and under-reporting motivated by fear of trade sanctions and lost tourism.3 Indeed, a recent analysis estimated an annual global cholera burden of 2.9 million cases and 95,000 deaths in endemic countries, resulting an overall case-fatality ratio (CFR) of 3.33%.4 A further 87,000 cases and 2,500 deaths, with 2.87% CFR was estimated in non-endemic countries.5
Only about 25% of cholera cases are symptomatic where 80–90% develop acute watery diarrhea and 10–20% develop severe watery diarrhea with vomiting.1 Diagnosis is confirmed by isolating Vibrio cholera O1 or O139 from feces through laboratory testing,1 and new rapid diagnostics are becoming available in places where there is a lack of laboratory facility.3 With proper treatment usually through administration of oral rehydration salts or with antibiotics in severe cases, the CFR is below 1%.1 If untreated, however, the CFR may reach up to 40%.1 To prevent and control cholera, WHO recommends a multidisciplinary approach which involves key measures of water and sanitation (W&S) interventions, health and hygiene education, strengthening disease surveillance, and oral cholera vaccine (OCV) vaccination campaigns in high-risk areas.2 As for most waterborne diseases, the provision of safe water and sanitation is critical to control cholera to provide a longer-term solution.6 However, it requires substantial long-term investments and high maintenance costs which are difficult to fund and sustain by these countries, especially in LMICs.7 In response to that, WHO recommends OCV vaccination as it provides a short-term solution to bring about an immediate effect to a potential cholera outbreak while other longer term interventions are put into place.6 To help control cholera, WHO has created a global stockpile of OCV to be deployed for outbreak response and recommended that all age groups to be vaccinated.6 However, given the high price of OCV, cost-effectiveness is among other considerations to define the vaccination strategy including timing (e.g. before outbreak), target population (e.g. targeting children only) and the setting (e.g. school-based).6
Two types of OCVs, namely Shanchol™ and Dukoral®, administered through two-doses, are available internationally and pre-qualified by WHO.6 Shanchol™ contains killed whole cells of V.cholera serogroups O1 and O139. It is distributed through the stockpile for cholera emergency response, as it is easier to use without the need of buffer and is less expensive than Dukoral®.6 Dukoral® consists of killed whole cells of V. cholerae serogroup O1, as well as recombinant B-subunit of the cholera toxin. The cholera toxin component also provides short-term protection against enterotoxigenic E. coli. Therefore, Dukoral® is used mainly as a traveler's vaccine. Another vaccine named as Vaxchora™, an oral single-dose vaccine consisting of an attenuated live V.cholera O1 strain (CVD 103-HgR), has been recently approved as traveler's vaccine in the United States. In addition, two other cholera vaccines are also licensed in Vietnam (mORC-Vax™) and Korea (Euvichol), which both of them are nearly identical to Shanchol™.6
Prior to implementation of OCV vaccination, policy-makers would need information on economic evaluation (EE), amongst many other criteria, to assess the costs and benefits of adopting the new intervention. A number of EE studies have been conducted, but there is a lack of critical appraisal and summary. Previous systematic review.8 conducted on all types of diarrheal vaccines only explored the general economic value of diarrheal vaccines but did not go in depth to appraise the analytical methods and assumptions applied in the EE studies. Therefore, this review aimed to provide a critical assessment of global EE studies, which can be used by WHO to encourage vaccine uptake and also to guide future EE study with sound methodology to help clinicians and policy makers make the evidence-informed decision for adopting OCV vaccination implementation.
Methods
Data sources and search strategy
We electronically searched for relevant articles published from inception to 31st December 2015. We searched MEDLINE, EMBASE, Centre for Reviews and Dissemination (Database of Abstracts of reviews of Effects, NHS Economic Evaluation Database, Health Technology Assessment Database), EconLit, Lilacs, Scielo, Red de Revistas Científicas de América Latina y El Caribe, España y Portugal (Redalyc), Research Papers in Economics (RePEc), CEA Registry, CABI (Centre for Agriculture and Biosciences International) Global Health Database, WHO Library Database (WHOLIS) and World Bank e-Library. The search strategy was based on a broad combined search string “cholera” AND “vaccin*” AND “cost*” OR “econom*”. There was no language restriction. In addition, bibliographies of relevant articles were examined to identify potential studies not indexed in the aforementioned databases.
Study selection
Studies were included if they were full EEs evaluating OCV against cholera disease only. Studies evaluating OCV for prevention of other diarrheal diseases (e.g. traveler's diarrhea) were not included. Studies were screened by two independent reviewers (SLT and SK). Initially, title and abstract of articles were screened to identify potentially relevant studies. Thereafter, full-text of relevant studies were retrieved and reviewed.
Data extraction and quality assessment
Methods, assumptions, results and conclusions of the studies were extracted by two independent reviewers (SLT and SK) using a standardized data extraction sheet. Any disagreement was resolved by discussion.
The methodological quality of each study was assessed by two independent reviewers (SLT and SK) using WHO's checklist for appraising the quality of EE of immunization programs.10 The main aspects assessed included analysis framing, costs and effects estimation, modeling, discounting, uncertainty, and conclusion.
Data analysis
Data extracted was analyzed in accordance to the WHO guide for standardization of EE of immunization programs.10 The costs were presented in USD2015 based on currency exchange rate11 and average consumer price index.12 when reported before 2015. The currency year was assumed to be the same as the publication year if not stated.13
Results
Study selection
Our search yielded a total of 1262 potential from electronic databases. 636 duplicates were removed. Of the remaining 626 studies screened, only 27 were relevant and retrieved to be reviewed in full-text. During the full-text screening, only 14 studies met the inclusion criteria. The excluded studies were not full EE studies (n = 10), and not evaluating on cholera disease (n = 3). The flow of study selection was illustrated in Fig. 1. As a result, a total of 14 studies were included.
Figure 1.
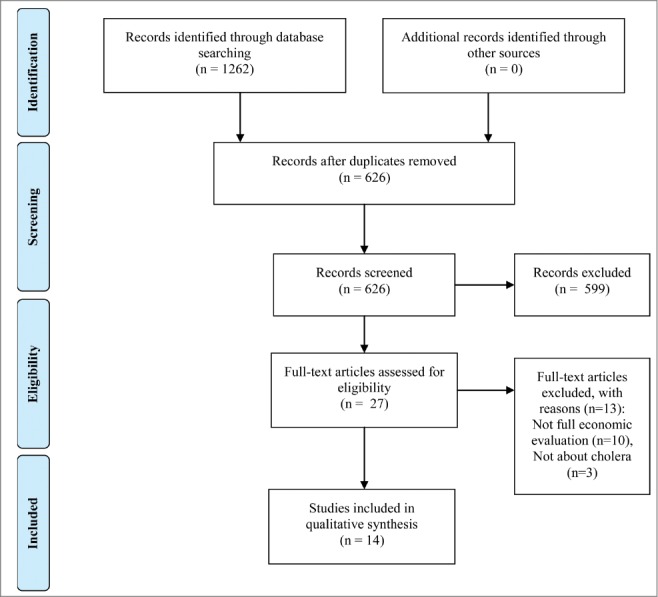
Flow of study selection.
Country, funding and authorship
Almost all the studies (13/14)14–26 were conducted in LMICs while one study (1/14)27 was conducted in high-income countries (HICs). Most studies (9/14)14,15,18–22,24,27 targeted a single country, while a few studies (2/14)17,26 targeted a cluster of countries, and three studies (3/14).16,23,25 with no specified country. Of the studies with specified countries, they were focused in Africa (6/11).15,17,19,20,24,26 and South Asia (5/11)14,18,21,22,26 regions, with Bangladesh (4/11),18,21,22,26 India (2/11).14-26 and Mozambique (2/11)24,26 being the most frequently studied countries.
More than half of the studies (8/14) were funded by non-government organizations where Bill and Melinda Gates Foundation was the sole funder for 6 studies.14,15,20,22,24,26 while the other 2 studies were funded by World Health Organization.17 and FUNCEI.27 respectively. One study (1/14)16 was funded by a governmental agency (i.e. United States Agency for International Development). The remaining studies (5/14)18,19,21,23,25 were not funded.
Study type and perspective
The type of EEs were cost-utility analysis (CUA) (6/14)15,16,20–22,26 and cost-benefit analysis (CBA) (5/14),14,23–25,27 and cost-effectiveness analysis (CEA) (3/14).17-19 The perspective taken were societal (6/14),14,20,22,24–26 healthcare provider (4/14),14,16,20,27 national (2/14),17,18 and not specified (4/14)15,19,21,23 (Note: Two studies.14,20 adopted more than 1 perspectives). Of the CUAs.14-16,20-22 all employed the outcome measure of Disability-Adjusted Life Year (DALY), with the disability weight mainly adopted from diarrhea28,29 as it was not available for cholera. The most recently reported disability weight was 0.2.22, while 0.1.15,20,26 was most commonly used in the EEs; an anomaly 0.9 was used in one EE16 and not reported for another EE.21 Of the CBAs, 3.14,23,25 included value of statistical life (VSL).30 to capture the outcome measure. The characteristics of the included studies were summarized in Table 1.
Table 1.
General characteristics of included economic evaluation studies.
| Author | Country | Target population; Population/setting characteristics | Vaccine type; No. of dose(s) | Funding sources | Study type | Perspective | Time horizon (years) | Vaccine protection period (years) | Discount rate (%) | Modelling approach | Herd protection included? | Sensitivity analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cookson 199727 | Argentina; HIC | Adults (Age>14 years); High-risk for epidemic | CVD 103-HgR; 1 | FUNCEI | CBA | Healthcare provider | 3 | 3 | C:5 | Static | – | One-way, Threshold |
| Murray 199816 | NS; LIC, MIC | Mass; High-risk for epidemic (refugee), Population with endemic cholera | Dukoral; 2 | United States Agency for International Development | CUA | Healthcare provider | 1 | 1 | – | Static | – | One-way |
| Naficy 199817 | Sub-Saharan Africa; LIC, MIC | Mass (Age> 1 year); Refugee camp | Dukoral; 2 | World Health Organization | CEA | National | 2 | 2 | C:10 | Static | – | One-way |
| Sack 200318 | Bangladesh; MIC | NS; Endemic | Dukoral; NS | – | CEA | National | NS | 3 | – | Static | – | One-way |
| Cook 200914 | India; MIC | Mass; Endemic | mORC-Vax™; 2 | Bill and Melinda Gates Foundation | CBA* | Healthcare provider, Societal | 3 | 3 | C: 8 | Static | Yes | One-way |
| Jeuland and Cook 200926 | Bangladesh, India, Indonesia, Mozambique; LIC | Children, Adolescent and Mass; Endemic | mORC-Vax™; 2 | Bill and Melinda Gates Foundation | CUA | Societal | 3 | 3 | C:3 | Static | Yes | Threshold |
| Jeuland and Lucas 200924 | Mozambique; LIC | Children, Adolescent and Mass; Endemic | mORC-Vax™; 2 | Bill and Melinda Gates Foundation | CBA | Societal | 3 | 3 | C:3 | Static | Yes | One-way |
| Jeuland and Whittington 200925 | NS; LIC, MIC | Mass; Endemic | mORC-Vax™; 2 | – | CBA* | Societal | NS | 2–4 | C:3–6 | Static | Yes | One-way, PSA |
| Kim 201115 | Zimbabwe; LIC | Mass; Outbreak | Hypothetical; 2 | Bill and Melinda Gates Foundation | CUA | NS | NS | 6 months (age 2–4 years), 2 years (age = >5 years) | C:3 | Static | Yes | One-way, Multivariate, Threshold, Scenario |
| Schaetti 201220 | Tanzania; LIC | Mass; Endemic | Dukoral; 2 | Bill and Melinda Gates Foundation | CUA | Healthcare provider, Societal | 3 | 3 | B:3 | Static | Yes | One-way |
| Whittington 201223 | NS; LIC, MIC | Mass; NS | NS; NS | – | CBA* | NS | NS | NS | C:4.5 | Static | Yes | PSA |
| Sardar 201319 | Zimbabwe; LIC | NS; Outbreak | NS; NS | – | CEA | NS | Life | NS | – | Dynamic transmission | Yes | One-way |
| Troeger 201422 | Bangladesh; MIC | Children <15 years, Mass (Age>1 year); Endemic | Shanchol; 2 | Bill and Melinda Gates Foundation | CUA | Societal | 1 | 3 | C:3 | Dynamic transmission | Yes | One-way |
| Smalley 201521 | Bangladesh; MIC | Mass (Age>1 year); Low- to high- risk regions | Shanchol; 2 | – | CUA | NS | 8 | 5 | – | Mixed integer programming model | – | – |
HIC, High-income country; LIC, Low-income country; MIC, Middle-income country; -, No; NS, Not specified; CBA, Cost-benefit analysis; CUA, Cost-utility analysis; CEA, Cost-effectiveness analysis; C, Cost; B, Benefit.
*Value of statistical life was used.
Vaccine type and study question
MORC-Vax™ (4/14)14,24–26 and Dukoral® (4/14)16–18,20 were the two most commonly used vaccine in the studies, followed with Shanchol™ (2/14),21,22 CVD 103-HgR (1/14),27 and not specified in 3/14 of the studies.15,19,23 Most of the studies (11/14)14–17,20-26 targeted vaccination for mass population involving children and adults, followed with adults only in one study (1/14),27, and not specified in two studies (2/14)18,19 Population included in the studies were those at risk of endemic cholera (7/14).14,18,20,22,24-26 affected by outbreak of cholera (2/14)15,19 at risk of epidemic cholera (1/14),27 refugee residing in camp (1/14)17, and not stated (1/14).23 One study (1/14)16 evaluated population at risk of both endemic and refugee with epidemic risk, and another study (1/14)21 assessed population at regions of low- to high- risk of cholera.
Five studies (5/14).14,15,18,20,27 compared OCV vaccination with no vaccination, while four studies (4/14)16,19,23,25 compared OCV vaccination to a single or multiple interventions of W&S interventions. In addition, five studies (5/14) investigated the EE of OCV vaccination by comparing different vaccination strategies. The studies varied and compared the OCV vaccination strategies either by targeting population with different age-cohorts and access to vaccination (3/5)22,24,26 or implementing OCV vaccination at different timing (i.e. as preemptive (before outbreak) or reactive (after outbreak)) (1/5)17. Another study (1/5).21 compared the vaccination strategies by targeting specific population groups by i) age and region, ii) age only, or iii) region only.
Cost-effectiveness thresholds
Of the CUA and CEA studies (9/14), five studies (5/9)18,20–22,26 clearly defined the criteria for cost-effectiveness, which the majority (4/5).20–22,26 used the criteria set out by the WHO Commission on Macroeconomics and Health.31 where the ratio of cost to DALY averted below 1 per-capita gross domestic product (GDP) was considered ‘very cost-effective’ and under 3 per-capita GDP ‘cost-effective’, and one (1/5)18 used the cost per death averted of comparator as a ‘cost-saving’ benchmark. The remaining studies (4/9)15–17,19 did not mention about the cost-effectiveness threshold criteria.
Modeling structure
Only two studies (2/14)19,22 used a dynamic transmission model to assess the EE of OCV vaccination. One study (1/14)21 used mixed integer programming model, where CUA was conducted in addition to the analysis determining the optimized OCV distribution strategies. The remaining studies (11/14) applied static models where one (1/11)17 was decision tree model, and the others (10/11).14–16,18,20,23–27 were not clearly stated, but likely to be simple calculation method.
Herd protection effect
Herd protection effect is the indirect protection effect whereby if there are relatively more people being vaccinated, there is a decrease in the proportion of infectious people who will come into contact with a susceptible.10 Herd protection effect of OCV vaccination was incorporated in nine studies (9/14).14,15,19,20,22–26 Five studies (5/9).15,19,22,25,26 used the data reported in previous dynamic model to estimate the herd protection effect, three studies (3/9).24–26 used surveillance data, one study (1/9).20 used observational study, one study (1/9).25 used previous EE study, while the other two studies (2/9).14,23 did not specify the data source (Note: Two studies.25,26 used more than one data sources). The methods used to incorporate herd protection effects was either by dynamic transmission model (2/9),19,22 additional scenario (assumed a certain proportion of case averted in addition to the direct effect from vaccination program) either in the model (5/9).14,15,20,23,24 or in sensitivity analysis (2/9).25,26 Only two studies (2/9) clearly reported herd protection effect estimations which were 0–33% 26 and 60–100%.25 cases averted in addition to the number of cases prevented from the OCV direct effect.
Epidemiological parameters
The incidence of cholera assumed in the studies ranged from 0.1.25 to 11.21 per 1,000 persons per year. One study varied the incidence of cholera from 0 to 4.18 to assess the cost-effectiveness of vaccination. Most studies (4/14).14,20,23,27 presented the estimates in general, by age-cohort (3/14).24-26 by different areas (1/14).22 by different age-cohorts and areas (1/14),21 and by different population characteristics and age-cohorts (i.e. refugee or population at risk) (1/14).16 Four studies (4/14).15,17-19 did not report about incidence. CFR was reported in almost all studies (12/14).14–18,20–26 which ranged from 0.01%.17 to 20%.15,18 while two studies (2/14).19,27 did not report about the CFR estimate. The most common data source used to derive the incidence rate and CFR was literature calibrated to local data, followed with local data, and adaption from previous EE. In addition to cholera, there were 3 studies (3/14).16,23,25 which also included the incidence rate and the CFR for diarrhea where the incidence rate ranged from 0.2.16 to 1.4.25 per person per year, and CFR ranged from 0.04%.25 to 0.12%.25. The cost of cholera per case was reported in 9 studies14,19,20,22-27 which ranged from USD6.0914 to 88427 (See full details in Appendix 1 and Appendix 2; Note: All studies reported cost in USD, therefore there was no need to convert currency).
Vaccine coverage
Most of the studies (12/14).14-17,19,20,22-27 specified the vaccine coverage where the estimate varied considerably while the other two studies (2/14).18,21 did not specify. One study (1/12).22 varied the vaccine coverage from 0 to 100% to assess the cost-effectiveness of vaccination while the others (11/12).14–17,19,20,23-27 used the range from 10 to 80% based on the data sources used including literature, local data and previous models or assumptions. (See full details in Appendix 1)
Vaccine effectiveness
For vaccine effectiveness, the majority of the studies (9/14).15-17,19-22,24,25 clearly specified the data sources used to derive the estimate, three studies (3/14).18,26,27 derived the estimate based on assumption, while the remaining studies (2/14).14,23 did not specify. The estimate derived from randomized controlled trials (RCTs) and systematic review of RCTs was 65% overall for 2 studies.21,22 The effectiveness estimate derived from observational studies.16,17,24, field trials16,17,24 and previous models.15,25 or based on assumptions.18,26,27 ranged from 25 – 93%. (See full details in Appendix 1)
Vaccine duration
Most studies (5/14).14,20,24,26,27 applied the analytical time horizon of 3 years, followed by two studies (2/14) for 1 year,16,22 and one study each for 8 years (1/14)21, 2 years (1/14)17, and lifetime (1/14)19, while four studies (4/14).15,18,23,25 did not specify the time horizon. The analytical time horizon was the same as the vaccine protection years for half of all studies (7/14).14,16,17,20,24,26,27 ranging from 1–3 years.
Vaccination costs
Of all the included studies, the cost per fully immunized person ranged from USD0.52.18 to 18.2719 Vaccine cost per dose estimated in the studies ranged from USD0.55.14 to 5.52.20 with administration cost per dose ranged from USD0.37.27 to 2.95.16. Four studies (5/14).14–16,24,26 included wastage rate of vaccine in the cost estimates with an estimated 10% of wastage (4/5)14–16,24,26 while one study (1/5)14 did not specify how the wastage rate was accounted for. The remaining studies (9/14)17-23,25,27 did not consider wastage rate of vaccine. Three studies (3/14)14,24,25 also captured the costs for patients and their family to obtain vaccination which ranged from USD0.07.14 to 0.44.24 per dose, and another study which expressed in 0.25 – 1.25 hour per dose.,25 both of which were based on unpublished data and assumptions (See full details in Appendix 2).
Discount rate
Of the studies which specified the time horizon of longer than a year (8/14), five studies (5/8) applied.14,17,24,26,27 a discount rate between 3 to 10% to costs only, while one (1/8)20 applied 3% discount rate to the benefits only and one (2/8)19,21 did not apply discount rate to either cost or benefit.
Quality of studies
Out of the 14 studies, 13.14–17,19–27 has clearly described the study question. Most studies clearly described the measurements and methodology used for cost (12/14)14,16,17,19–27 and effects (10/14)14–16,19,20,22–26. For the methods used for data analysis, most of the studies (10/14)14,17,19–26 described clearly the methods and the structure of the model. Almost all studies (13/14)14–18,20–27 had conducted sensitivity analyses. All studies.14–27 clearly justified the conclusion of the study based on the study results.
Economic value of OCV vaccination
Compared to no intervention
Of the 5 studies which compared OCV vaccination to no vaccination.14,15,18,20,27 four (4/5) found OCV vaccination to be either cost-saving.15,18 or with positive benefit-cost ratio14,27 while one (1/5)20 found that it was not cost-effective. The study 20 which found vaccination to be not cost-effective had a much higher cost of vaccination with USD7.01 per fully immunized person while it ranged from USD0.52 to 6.05 in other studies.14,15,18,27 (See Table 2 for the details of the results).
Table 2.
The characteristics and results of studies which compared oral cholera vaccine vaccination with no vaccination.
| Cost-effective/ Cost-saving | Not cost-effective | ||||
|---|---|---|---|---|---|
| Author |
Cookson 199727 |
Sack 200318 |
Cook 200914 |
Kim 201115 |
Schaetti 201220 |
| Type of model; Herd effect | Static; No | Static; No | Static; No | Static; Yes | Static; Yes |
| Cost-effectiveness threshold defined? | NA | Yes | NA | No | Yes |
| Results | Vaccination was cost-saving with savings of prevented cholera outweighing cost of vaccination. | 1) Cost-effectiveness depended on the price of vaccines and incidence of cholera. It was cost-saving when the price was low and incidence was high. | 1) For private healthcare provider's perspective, the benefit was maximized when full marginal cost was offered as user fee. | When price of vaccine was USD1.10/dose and herd effect was incorporated, vaccination was found to be cost-saving. | Not cost-effective even if vaccines were donated, for both healthcare provider and societal perspectives. |
| 2) For societal perspective, the benefit was achieved with lower user fee. | |||||
| Incidence (per 1,000 population per year) | 2.5 | 0 – 4 | 1.64 | NS | 2.3 (0.5 – 4.0) |
| Coverage | 75% | NS | 65% | 50% | 50% |
| Effectiveness | 75% | 25% for <5 years | Cholera reduction is a function of uptake | 93% | Among vaccinated people = 79% Among unvaccinated people = 45% |
| 75% for adults and older children | |||||
| Cost per fully immunized person (USD 2015) | 2.22 | 0.52 | 2.66 | 3.3 or 12.1 | 14.06 |
NA, Not applicable; NS, Not specified.
Compared to other interventions
Of the 4 studies which used the comparators of W&S interventions, all.16,19,23,25 found that OCV vaccination was not cost-effective. One study.25 found that, if OCV vaccination was followed with W&S intervention, the result turned out to be cost-effective. However, notably, a higher end of vaccination cost per fully immunized person were used in the studies.16,19,23,25 which ranged from 6.81.23 to 18.31.19 (See Table 3 for the details of the results), compared to those studies.14,15,18,27 which have found OCV vaccination to be cost-effective.
Table 3.
The characteristics and results of studies which compared oral cholera vaccine vaccination with other interventions.
| Author | Murray 199816 | Whittington 201223 | Jeuland and Whittington 200925 | Sardar 201319 |
|---|---|---|---|---|
| Comparator | 1) Vaccination alone | 1) Cholera vaccination | 1) School-based vaccination (SV) | 1) Vaccination |
| 2) Drinking-water and sanitation (W&S) | 2) Handwashing | 2) Community-based vaccination (CV) | 2) Promoting hand-hygiene and clean water distribution | |
| 3) Outpatient treatment | 3) Total sanitation campaign | 3) Borehole + hand pump | 3) Treatment | |
| 4) Inpatient treatment | 4) Chlorination | 4) Biosand filter | 4) Sanitation | |
| 5) Vaccination + W&S | 5) Biosand filters | |||
| 6) W&S + Treatment | 6) Long-lived insecticide-treated bed nets | |||
| Type of model; Herd effect | Static; No | Static; Yes | Static; Yes | Dynamic; Yes |
| Cost-effectiveness threshold defined? | No | NA | NA | No |
| Results | Not cost-effective | Not cost-effective | Not cost-effective (Cost-effective if SV or CV was followed with either one of the W&S interventions.) | Not cost-effective |
| Incidence (per 1,000 population per year) | 1) Refugee: | 2 | All cases: 0.1 -4 | NS |
| Age <5 years: 40%; | Children only: 0.1 – 9 | |||
| Age = >5 years: 56% | Diarrhea: 0.4 – 1.4 per person-year | |||
| 2) Population at risk: | ||||
| Age <5 years: 40%; | ||||
| Age = >5 years: 35% | ||||
| Simple diarrhea for both population: | ||||
| Age <5 years: 2.6 | ||||
| Age = >5years: 0.2 | ||||
| Coverage | 50–80% | 10 – 80% | 10–80% | 35% |
| Effectiveness | 1) Refugee: | Cholera reduction is a function of uptake | Cholera reduction is a function of uptake | 67% |
| Age <5 years: 40%; | ||||
| Age = >5 years: 56% | ||||
| 2) Population at risk: | ||||
| Age <5 years: 40%; | ||||
| Age = >5 years: 35% | ||||
| Cost per fully immunized person (USD 2015) | 11.35 (Refugee), 9.47 (Population with endemic cholera) | 1.44 – 6.81 | 1.54–7.2 | 7.10, 18.27 (high emergency) |
NA, Not applicable.
Different vaccination strategies
Five studies investigated the EE of OCV vaccination with different vaccination strategies and with varying conditions. One study.22 found that OCV vaccination was cost-effective when targeted to specific groups including population in districts or hotspots with high-risk of cholera, children below 15 years old, and area where the population had poor access to health care facilities. Another study.17 which investigated the population in refugee camp, found the incremental cost per case of cholera was lower when vaccination was implemented preemptively (at the inception of refugee camp in this case) compared to after the outbreak. Two studies found that when herd protection was included, vaccination was cost-effective.26 or yielded positive benefit-cost ratio.24 either by implementation in the school or community with specific age-groups targeted or including all ages. One study.21 found that when CFR was 0.5% (the lowest), with vaccine price of USD0.9 per dose (the maximum) and when the investment was fewer than 8 years (assuming 20 million doses annually), OCV vaccination targeted at i) specific age-group and region, ii) specific age-group, and iii) specific region were all cost-effective. The details of the results were summarized in Table 4 while the full extended results for all comparisons can be found in Appendix 3.
Table 4.
The characteristics and results of studies which compared oral cholera vaccine vaccination in different strategies.
| Author | Naficy 199817 | Jeuland and Cook 200926 | Jeuland and Lucas 200924 | Troeger 201422 | Smalley 201521 |
|---|---|---|---|---|---|
| Comparator | 1) Preemptive treatment (PT) + Preemptive vaccination (PV) | 1) School-based vaccination for age 5–14 | With or without user fee for: | 1) Non-selective countrywide | Vaccination target strategies: |
| 2) Reactive treatment (RT) + Reactive vaccination (RV) | 2) School-based vaccination for age 1–14 | 1) School-based vaccination for age 5–14 | 2) Non-selective high -risk districts | 1) By age and region | |
| 3) PT + RV | 3) Community-based for all age groups | 2) School-based vaccination for age 1–14 | 3) Children <15 years | 2) Age only | |
| 4) RT + PV | 3) Community-based for all age groups | 4) Children < 15 years (with differing vaccine efficacy) | 3) Region only | ||
| 5) Hotspot targeted | by varying: | ||||
| 6) Poor access to treatment population targeted | Vaccine price, CFR, Years of investment | ||||
| Type of model; Herd effect | Static; No | Static; Yes | Static; Yes | Dynamic; Yes | Mixed integer programming model; No |
| Cost-effectiveness threshold defined? | No | Yes | NA | Yes | Yes |
| Results | The incremental cost per case was lower when vaccination started at the inception of refugee camp (preemptively) compared to after outbreak (reactively). | Cost-effective for all options when herd protection was included. | Positive benefit-cost ratio for all options when herd protection was included. | All except option 1 was cost-effective. | When CFR was 0.5%, all options were cost-effective when vaccine price was USD0.9/dose and the investment was < 8 years (20million doses/year) |
| Incidence (per 1,000 population per year) | NS | Varied by age: | Varied by age: | Varied by risk: | Varied by age and risk: |
| 0.3 – 7.2 | 1.4 – 17.6 | 2.1 – 10 | 0.6 – 11 | ||
| Coverage | 70–80% | 80% | 53–61% | 0–100% | NS |
| Effectiveness | 80% in the first 6 months and 0–50% thereafter (varied by age) | 60% in year 1 and 2, 50% in year 3 | 55%, reduced by 17% in year 3 | Overall 65% (42–74% by age) | Overall 65% (42–74% by age) |
| Cost per fully immunized person (USD 2015) | 1.56 | 2.54–3.68 | 2.70 | 5.16, 10.32 (Poor access to treatment) | 4 |
NA, Not applicable; NS, Not specified.
Sensitivity analysis
Most studies (11/14)14-20,22,24,25,27 performed one-way sensitivity analysis, followed with threshold analysis (3/14)15,26,27, probabilistic sensitivity analysis (PSA) (2/14)23,25, and no sensitivity analysis (1/14)21 (Note: Three studies.15,25,27 conducted more than 1 sensitivity analysis). The most commonly reported influential input parameters were cholera incidence (11/14)14–18,20–22,24,25,27, vaccine coverage (7/14)14,16,17,21-24, herd protection and vaccine price (6/14)15-18,20,21 (Appendix 4).
Discussions
Our review revealed that the data and model structure used in the EE sources varied considerably, therefore comparison across studies is difficult. However, EEs which compared OCV vaccination with no intervention were most likely to report cost-effective results even when herd effect was not incorporated. When comparing between different vaccination strategies, OCV vaccination was more likely to be cost-effective when targeted at area with high risk of cholera, population with poor access to health care facilities, or children below 15 years old, herd protection effect was incorporated, or/and vaccine price was low. All EEs which compared OCV vaccination with W&S interventions found OCV vaccination to be not cost-effective. Therefore, the results support the WHO recommendation where a multidisciplinary approach is crucial to control cholera in a long-run but in case of potential outbreak, OCV vaccination with aforementioned strategies is a cost-effective approach to provide immediate effect. As this systematic review is aimed to serve as a guidance for future EE on OCV, a number of methodological issues are discussed in the following sections.
In order to perform EE in a way to represent real-world scenario, EE studies should be designed in accordance with WHO's guidance for planning and use OCV in mass immunization campaigns.1 For example, in area with endemic cholera, it is recommended that all population at risk (> 2 years for Dukoral®, and >1 year for Shanchol™) should be vaccinated. EE would be meaningful if comparison is made between age-cohorts (i.e. children vs adults or children vs all ages) or between settings with different access of health care facilities in order to provide information which population is with higher priority especially when budget is in constraint. Otherwise, comparison made only between OCV or no vaccination approach without considering different population would lead to a result which cannot be applied in real-world scenario.
Performing sensitivity analysis in the EE studies is important as the real-world data in terms of burden of disease of cholera and the impact of OCV are scarce and hence the input assumed carries substantial uncertainty. According to our review, we identified the most influential input parameters to be cholera incidence, vaccine coverage, herd protection and vaccine price. However, this finding should be interpreted with caution as it was based on only those parameters tested and reported in the studies. Nevertheless, performing sensitivity analysis with these input parameters is essential to produce robust results. The range of estimates of these input parameters can be referred to the estimates reported in this review.
The herd protection effect of OCV vaccination has been evidenced especially when it is implemented as a mass vaccination program.32 The use of dynamic transmission model to capture the effect would be ideal but it requires complex technical expertise. However, in this review, we identified studies which incorporated herd protection effect by performing sensitivity analysis by adding additional cases averted in addition to the number of cases prevented from the OCV direct effect. In fact, the assumption is not realistic as it does not mimic the trend of transmission reduction. Nevertheless, incorporating herd protect effect is crucial to prevent the underestimation of the benefits of vaccination. Therefore, estimating the herd protection effect in this way is a potential solution when there is a constraint in the expertise capacity.
The conventional EE normally captures the benefits of intervention specific to health outcomes only which have been observed in this review. However, recent guidelines have stressed the importance to include the broader benefits in terms of child development, household financial security and economic development especially when childhood vaccination is involved (which applies to OCV vaccination).33 In addition, there has been an argument that CBA with the incorporation of VSL is advantageous to capture the non-health benefits and financial risk protection offered by vaccination.30 in contrast to the preference of the WHO's recommendation.10 to use cost-utility analysis (CUA) for EE of immunization programmes. Nevertheless, future EE should consider incorporating the broader benefits issue to prevent the underestimation of OCV vaccination.
The most commonly employed cost-effectiveness threshold of the included studies was the GDP-based thresholds of 1 time and 3 times of GDP per capita.31 This method gives the value for money indication of the intervention with the specified setting in the model. Therefore, using it as a stand-alone criterion as a decision rule to fund the intervention or not has been largely discouraged.34 as it lacks other considerations relevant to local settings for decision-making. Therefore, future studies are encouraged to use a multi-criteria decision analysis considering not only the cost-effectiveness information but also budget impact, fairness, and feasibility among other considerations considered important in the local context.34
Despite performing an exhaustive literature search, there might be unpublished study that we were unaware of. Although we did not apply language restriction, we have identified only English literature. There are potential studies which were published in other languages which could be identified, if we have broadened our search term using other language or searched in non-English journals.
In conclusion, our review found that OCV vaccination is potentially cost-effective as part of the prevention and control measure of cholera when targeted at the population with high risk of cholera and poor access to health care facilities and when OCV price is low. Nevertheless, when applying this result in specific context, attention should be paid to the EEs which have employed the input parameter and methodology which are applicable to the corresponding context. Methodological issues in terms of using the appropriate comparator, addressing uncertainty of input parameters, incorporation of herd effect and broader benefits of vaccination should be addressed. This review provides supporting information to policy-makers when considering OCV vaccination as a sound intervention and to prevent the underutilization of OCV. The findings in this review will be used as foundation for development of WHO guidance on EE study for OCV to guide future EE study.
Supplementary Material
Funding Statement
This study was funded by Initiative for Vaccine Research, World Health Organization, Geneva, Switzerland.
Disclosure of potential conflicts of interest
Raymond Hutubessy is a staff member of Initiative for Vaccine Research, World Health Organization, Geneva, Switzerland and the opinions expressed in this article are the author's own and do not reflect the view of the organization. Other authors have no other conflict of interest to declare.
References
- 1.World Health Organization Oral cholera vaccines in mass immunization campaigns: Guidance for planning and use. 2010. [Google Scholar]
- 2.World Health Organization Editorial note: Cholera, a public health priority. Weekly epidemiological record. 2015;40(90):517-44. [Google Scholar]
- 3.World Health Organization. Cholera, 2014. Weekly epidemiological record. 2015;40(90):517-44. [Google Scholar]
- 4.Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9(6):e0003832. doi: 10.1371/journal.pntd.0003832. PMID:26043000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali M, Lopez AL, You Y, et al.. The global burden of cholera. Bulletin of the World Health Organization. 2012;90(3):209-18. doi: 10.2471/BLT.11.093427. PMID:22461716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Cholera vaccines: WHO position paper. Weekly epidemiological record. 2010;13(85):117-28. [Google Scholar]
- 7.Organization World Health. The potential role of new cholera vaccines in the prevention and control of cholera outbreaks during acute emergencies. Report of a meeting, Geneva, 13-14 February, 1995; 1995. [Google Scholar]
- 8.Rheingans R, Amaya M, Anderson J, Chakraborty P, Atem J. Systematic review of the economic value of diarrheal vaccines. Human vaccines & immunotherapeutics. 2014;10(6):1582-94. doi: 10.4161/hv.29352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organization World Health. Implementing the WHO Stop TB Strategy: A handbook for national TB control programmes. World Health Organization; 2008. [PubMed] [Google Scholar]
- 10.Walker DG, Hutubessy R, Beutels P. WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine. 2010;28(11):2356-59. doi: 10.1016/j.vaccine.2009.06.035. PMID:19567247 [DOI] [PubMed] [Google Scholar]
- 11.FxTop Currency converter. http://fxtop.com/en/currency-converter-past.php.
- 12.US Bureau of Labor Statistics CPI Inflation Calculator. 2016; Accessed 18/6/2016 http://www.bls.gov/data/inflation_calculator.htm
- 13.Chao TE, Sharma K, Mandigo M, Hagander L, Resch SC, Weiser TG, Meara JG. Cost-effectiveness of surgery and its policy implications for global health: a systematic review and analysis. Lancet Global Health. 2014;2(6):e334-45. doi: 10.1016/S2214-109X(14)70213-X. PMID:25103302 [DOI] [PubMed] [Google Scholar]
- 14.Cook J, Jeuland M, Maskery B, Lauria D, Sur D, Clemens J, Whittington D. Using private demand studies to calculate socially optimal vaccine subsidies in developing countries. J Policy Anal Manage. 2009;28(1):6-28. doi: 10.1002/pam.20401. PMID:19090047 [DOI] [PubMed] [Google Scholar]
- 15.Kim S-Y, Choi Y, Mason PR, Rusakaniko S, Goldie SJ. Potential impact of reactive vaccination in controlling cholera outbreaks: An exploratory analysis using a Zimbabwean experience. S Afr Med J. 2011;101(9):659-64. PMID:21920160 [PubMed] [Google Scholar]
- 16.Murray J, McFarland D, Waldman RJ. Cost-effectiveness of oral cholera vaccine in a stable refugee population at risk for epidemic cholera and in a population with endemic cholera. Bull World Health Organ. 1998;76(4):343. PMID:9803585 [PMC free article] [PubMed] [Google Scholar]
- 17.Naficy A, Rao MR, Paquet C, Antona D, Sorkin A, Clemens JD. Treatment and vaccination strategies to control cholera in sub-Saharan refugee settings: A cost-effectiveness analysis. Jama. 1998;279(7):521-25. doi: 10.1001/jama.279.7.521. PMID:9480362 [DOI] [PubMed] [Google Scholar]
- 18.Sack DA. When should cholera vaccine be used in cholera-endemic areas? J Health Popul Nutr. 2003;21(4):299-303. PMID:15038584 [PubMed] [Google Scholar]
- 19.Sardar T, Mukhopadhyay S, Bhowmick AR, Chattopadhyay J. An optimal cost effectiveness study on Zimbabwe cholera seasonal data from 2008–2011. 2013;8(12):e81231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaetti C, Weiss MG, Ali SM, Chaignat CL, Khatib AM, Reyburn R, Duintjer Tebbens RJ, Hutubessy R. Costs of illness due to cholera, costs of immunization and cost-effectiveness of an oral cholera mass vaccination campaign in Zanzibar. PLoS Negl Trop Dis. 2012;8(12):e81231. doi: 10.1371/journal.pntd.0001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smalley HK, Keskinocak P, Swann J, Hinman A. Optimized oral cholera vaccine distribution strategies to minimize disease incidence: A mixed integer programming model and analysis of a Bangladesh scenario. Vaccine. 2015;33(46):6218-23. doi: 10.1016/j.vaccine.2015.09.088. PMID:26458806 [DOI] [PubMed] [Google Scholar]
- 22.Troeger C, Sack DA, Chao DL. Evaluation of targeted mass cholera vaccination strategies in Bangladesh: A demonstration of a new cost-effectiveness calculator. Am J Trop Med Hyg. 2014;91(6):1181-89. doi: 10.4269/ajtmh.14-0159. PMID:25294614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittington D, Jeuland M, Barker K, Yuen Y. Setting priorities, targeting subsidies among water, sanitation, and preventive health interventions in developing countries. World Development. 2012;40(8):1546-68. doi: 10.1016/j.worlddev.2012.03.004. [DOI] [Google Scholar]
- 24.Jeuland M, Lucas M, Clemens J, Whittington D, DOMI Cholera Economics Study Group A cost–benefit analysis of cholera vaccination programs in Beira, Mozambique. World Bank Economic Review. 2009;23(2):235-67. doi: 10.1093/wber/lhp006. [DOI] [Google Scholar]
- 25.Jeuland M, Whittington D. Cost–benefit comparisons of investments in improved water supply and cholera vaccination programs. Vaccine. 2009;27(23):3109-20. doi: 10.1016/j.vaccine.2009.02.104. PMID:19428925 [DOI] [PubMed] [Google Scholar]
- 26.Jeuland M, Cook J, Poulos C, Clemens J, Whittington D. Cost‐Effectiveness of New‐Generation Oral Cholera Vaccines: A Multisite Analysis. Value in Health. 2009;12(6):899-908. doi: 10.1111/j.1524-4733.2009.00562.x. PMID:19824189 [DOI] [PubMed] [Google Scholar]
- 27.Cookson ST, Stamboulian D, Demonte J, Quero L, Martinez de Arquiza C, Aleman A, Lepetic A, Levine MM. A cost-benefit analysis of programmatic use of CVD 103-HgR live oral cholera vaccine in a high-risk population. International journal of epidemiology. 1997;26(1):212-9. doi: 10.1093/ije/26.1.212. PMID:9126522 [DOI] [PubMed] [Google Scholar]
- 28.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, et al.. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2129-43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Global burden of disease 2004 update: Disability weights for diseases and conditions. World Health Organization. 2004. http://www.who.int/healthinfo/global_burden_disease/GBD2004_DisabilityWeights.pdf. [Google Scholar]
- 30.Laxminarayan R, Jamison DT, Krupnick AJ, Norheim OF. Valuing vaccines using value of statistical life measures. Vaccine. 2014;32(39):5065-70. doi: 10.1016/j.vaccine.2014.07.003. PMID:25045822 [DOI] [PubMed] [Google Scholar]
- 31.WHO Commission on Macroeconomics and Health Investing in health for economic development Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001. [Google Scholar]
- 32.Longini IM Jr, Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. Controlling endemic cholera with oral vaccines. PLoS medicine. 2007;4(11):e336. doi: 10.1371/journal.pmed.0040336. PMID:18044983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jit M, Hutubessy R. Methodological Challenges to Economic Evaluations of Vaccines: Is a Common Approach Still Possible? Appl Health Econ Health Policy. 2016;14(3):245-52. doi: 10.1007/s40258-016-0224-7. PMID:26832145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, Hill SR. Cost–effectiveness thresholds: pros and cons. Bulletin of the World Health Organization. 2016;94(12):925. doi: 10.2471/BLT.15.164418. PMID:27994285 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


