“Anything that Just Costs Money is Cheap”
– (attributed to) John Steinbeck
Introduction
The understanding of “value” in cancer care has changed substantially (and rapidly) over time. Historically, value has been defined from a number of perspectives, including those in disciplines as broad as economics, anthropology, ethics, and healthcare. The concept of value is deeply rooted in economic theory, dating back to the mid seventeenth century, and formally applied in 1776 by the Scottish economist Adam Smith in “An Inquiry into the Nature and Causes of the Wealth of Nations” [1]. As our economy has diversified from one based in commodities, revisions of the theory of value have come often. In our modern economy, value has been tied directly to the cost of an object (which can include the cost of its production, distribution, exchange and consumption). However, this strict economic definition of value has been refined, and in no sector is this more relevant than in healthcare. Value involves preferences (for example, those of a patient or a consumer), a cost (for example, financial risk or toxicity), an exchange (between individuals, or a tradeoff within an individual), and the context of such an exchange (for example, in a patient with a terminal illness). And, as will be seen in this discussion, value, regardless of perspective, has political and societal implications. In this review, we aim to define value in gynecologic cancer care, to demonstrate the importance of value in the provision of this care, discuss how value impacts government relations and payors, and to provide examples of the intersection of value in the delivery of gynecologic cancer care.
Defining Value
In its most elemental form, health care value can be distilled into a simple equation: outcomes (numerator) divided by the total costs (denominator) [2]. However the prioritization and designation of what outcomes are relevant may vary depending on one’s perspective. There are a multitude of examples within gynecologic oncology where the value equation may shift based on whether the outcomes are framed by the societal, payor, provider, or patient perspective.
Michael Porter’s value proposition
Michael E. Porter, a renowned Harvard Business School economist, has argued that “improving health and health care value for patients” is integral not only to controlling costs, but also to overall health care reform [3]. The definition and the demonstration of value as “health outcomes achieved per dollar spent” is a key component to Porter’s reformed value-based system [4].
Porter argues that defining value as the goal, rather than “reducing cost, maximizing revenue, or providing every service,” is the essential principle to delivering a high-value system [5]. Central to this idea is the argument that to improve value, one has to improve outcomes; and to improve outcomes one must provide high-quality care. However, surrogate measures of quality, such as adherence to guidelines and patient satisfaction, do not accurately assess quality of care. In order to truly assess quality and improve value, the outcomes important to each disease must be specifically defined and measured. Porter argues that “the absence of comprehensive and rigorous outcome and cost measurement is arguably the biggest “weakness standing in the way of health care improvement” [2].
In an effort to define high quality outcomes, Porter developed an outcome measures hierarchy, in which specific health outcomes are multi-tiered and disease-specific. In oncology, the highest tier is assigned to survival, followed by functional status achieved, recovery times following treatment, effects of the treatment process on function and quality of life, and sustainability of the cancer-free state. Similarly, improving cost measurement is critical to Porter’s value framework, where costs are defined based on a “cycle of care” for a given medical condition, sometimes involving periods of a year or more. In Porter’s value-based system, the responsibility for outcomes and costs are shared by all participating providers for a given medical condition, regardless of rank or specialty.
Ultimately, Porter argues, “Improving value requires either improving one or more outcomes without raising costs, or lowering costs without compromising outcomes, or both” [6]. Porter’s strategic agenda for a value-based health care delivery system includes six components: 1) Organization of care into disease-related integrated practice units; 2) Measurement of outcomes and costs for every patient; 3) Bundled payments for care cycles, 4) Integration of care delivery across facilities; 5) Expansion of services across geography; and 6) Building an enabling IT platform. Recently, other definitions of value have been proposed that focus more directly on the balance between clinical benefit, toxicity, and cost in healthcare.
Measurement of Outcomes and Perspective of Value
Crucial to the dialogue on value-based care in oncology is the ability to measure and compare outcomes and cost. In order to successfully accomplish this, standardized methods are essential. To this end, there are national and international efforts to bring together stakeholders to define relevant and patient-centered outcome measures for specific disease sites. One notable example is the International Consortium for Health Outcomes (ICHOM), which has brought together clinical experts along with patient representatives to develop minimum standard outcome-measures, with oncologic sets already developed for prostate (localized and advanced), breast, colorectal, and lung cancers [7, 8].
The patient perspective can add to the value equation and to the broader dialogue surrounding value-based care in a myriad of ways, and can be achieved by assessing patient reported outcomes (PROs, including patient symptom burden, quality of life measures and patient preferences), as well as patient centered outcomes such as financial toxicity.
Similar to the situation with defining outcomes, what costs are included in the denominator may vary depending on one’s perspective. For example, cost from a payor perspective might include all costs for a cycle of care for any particular medical condition [2]. Lost wages or productivity from an affected patient or caregivers are notable additions to cost analyses from a societal and patient perspective. Additionally, out of pocket costs for medical care is an important additional consideration from the patient perspective.
The monumental shift from the focus on the volume of services delivered to value-based health care delivery is hindered by the challenge of defining and measuring outcomes that matter to patients [7]. The number of clinical trials in oncology that have incorporated patient reported outcomes is low. However, when performed, such outcomes can influence the value assessment of new agents, even without overall survival benefit. Similarly, PROs are in the minority in standardized quality metrics. For example, within the National Quality Measures Clearinghouse, only 139/1958 (7%) of quality indicators are considered outcome-measures, and less than 2% of quality indicators are specifically patient reported outcomes [7]. Given the challenges of uniformly measuring and reporting PROs, integration of electronic patient reported outcomes systems into practice is increasingly common, and has the potential to contribute to the assessment of value in oncologic care [9]. Likewise, increased efforts to include relevant patient centered outcomes in clinical trials have the potential to greatly influence value assessments for cancer therapies. In a health care environment where resources are limited, understanding patient preferences can help organizations understand how to organize cancer care delivery to meet the challenges of delivering high value and patient-centered care. Continued movement towards the categorical incorporation of patient reported outcomes, preferences and perspectives into the metrics of quality and cost will ultimately drive and shape the value discussion for women with gynecologic malignancies and ensure alignment with the goals of patient-centered care.
ASCO value framework
In the United States, cancer is ranked second among the most expensive diseases to treat [https://meps.ahrq.gov/data_files/publications/st331/stat331.shtml]. As a high proportion of health care expenditure occurs in oncology, and with an increasing proportion of cost incurred to patients as direct out of pocket co-payments or shared-payment plans, cancer patients are increasingly experiencing financial toxicity [10]. This increasing financial burden for cancer patients has resulted in a lower quality of life, impedance of access to the highest quality care, and an increasing rate of bankruptcy [10, 11]. Evidence suggests that cancer patients want not only information regarding the effectiveness of available treatments, but also information on their relative costs [12, 13].
In 2007, the American Society of Clinical Oncology convened its first Value in Cancer Care Task Force (formerly Cost of Cancer Care Task Force) to address the issue of the rising costs of cancer care. In 2009, ASCO released a guidance statement with the following recommendations: “(1) recognition that patient-physician discussions regarding the cost of care are an important component of high-quality care; (2) the design of educational and support tools for oncology providers to promote effective communication about costs with patients; (3) and the development of resources to help educate patients about the high cost of cancer care to help guide their decision making regarding treatment options” [14].
In 2015, ASCO presented a value framework “for comparing the relative clinical benefit, toxicity, and cost of treatment in the medical oncology setting”; and to “provide a standardized approach to assist physicians and patients in assessing the value of a new drug treatment for cancer as compared with one or several prevailing standards of care” [15]. The framework was designed to assess the relative value of novel therapies based on outcomes published in phase III randomized controlled trials. ASCO envisioned this framework as a physician-guided tool not only to assess the relative value of a treatment, but also to aid in shared-decision making with patients. In May of 2016, ASCO presented a revised value framework following a period of public comment [16].
There are two versions of the value framework, the advanced disease and the adjuvant/curative framework. The advanced-disease framework is best suited to assessing the relative value of treatments, for example in a case of newly diagnosed advanced or recurrent ovarian cancer, and consists of six measurable aspects of treatment used to calculate the Net Health Benefit (NHB) of a novel therapy compared to a standard of care: 1) clinical benefit based on improvement in overall survival (OS), progression-free survival (PFS), or response rate (RR); 2) toxicity; 3) probability of long-term survival; 4) palliation of cancer-related symptoms; 5) quality of life (QOL); and 6) treatment-free interval (TFI). The ASCO task force has assigned relative importance weights to each of these aspects, as well as to each component of clinical benefit (OS, PFS, and RR). The maximum possible NHB score for the advanced disease framework is 180 points; with the following possible total points for each aspect of the framework: 1) clinical benefit = 100 points; 2) toxicity = −20 to 20 points; 3) long-term survival = 20 points; 4) palliation of symptoms = 10 points; 5) QOL = 10 points; 6) TFI = 20 points. For each component of clinical benefit, ASCO assigned the following importance weight multipliers: OS, 1; PFS, 0.8; and RR, 0.7. After calculating NHB for a given novel therapy versus a standard of care, a value snapshot is used to graphically illustrate the improvement in clinical benefit, the difference in toxicity, and the NHB score of a novel therapy over a standard therapy. Alongside the NHB, the value snapshot compares the drug-acquisition cost (and, if available, the patient’s out of pocket cost) per cycle of the novel therapy versus the standard therapy. The second framework is directed at adjuvant therapy, and incorporates similar principles as the advanced disease framework above, but in a more abbreviated algorithm. It also derives an overall Net Health Benefit score [16]. Examples of the ASCO value framework relative to ovarian cancer are presented later in this document.
New Models of Healthcare Delivery
Accountable care organizations (ACOs)
ACOs were designed to align patient centered care with value based care. Introduced in 2010, ACOs attempt to incentivize high-value care using the following key principles: (1) coordination of care through integrating services and (2) providing financial incentives for clinicians and hospitals that efficiently manage patient care. Providers who join ACOs assume responsibility for patient outcomes and share savings if quality and cost benchmarks are met as measured by quality metrics that are reported to CMS annually. In 2015, more than 400 ACOs had been created serving 7.2 million beneficiaries. Results to date are mixed – in 2012, 52 of 220 ACOs were able to meet quality benchmarks and budget targets [http://healthaffairs.org/blog/2016/09/21/medicare-acos-incremental-progress-but-performance-varies]. Sustained participation with ACOs accepting shared financial risks may be a challenge as new models continue to be tested.
The ACO structure is primarily a primary care model, but its extension to oncology care is expected. The oncology ACO has the potential to standardize care pathways, integrate care, and improve patient/provider communication and patient education [17]. An analysis of cancer patients included in the Physician Group Practice Demonstration (PGPD) from 2005–2010 suggests that an oncology ACO model may improve the value of care provided. Decreased spending (primarily in acute care settings) without changes in mortality were observed [18]. Additionally, health insurance plans have teamed with health systems in piloting oncology ACO programs [19].
Oncology Patient Centered Medical Home (OCPMH)
The OCPMH is another model of care designed to improve cancer care delivery efficiency and value. In this model a comprehensive team of providers (eg. oncologist, pharmacists, nurses, primary care physicians) coordinate sustained care for a per beneficiary per month fee [20]. Reductions in hospital admissions, emergency room visits and length of stay have been demonstrated [21, 22].
The Future of Physician Payment Reform: Shifting Reimbursement
Healthcare in the United States is most commonly reimbursed under the fee-for-service model, which incentivizes procedures and interventions that are typically more highly reimbursed and provides little or no reimbursement for services such as patient education and teaching, service coordination, and lifestyle modification. This strategy can promote overuse of unnecessary interventions and underuse of other evidence-based strategies that are not rewarded [19]. The Affordable Care Act (ACA) contains numerous provisions that directly address how health care is organized, delivered, and paid for in the United States. Shifting the focus of health care from volume to value is a central pillar of the ACA. Improving value by increasing efficiency and decreasing the high cost of a predominantly fee-for-service system is a central initiative. The ACA anticipates the realization of this goal by: (1) shifting from a reimbursement system based on the volume of services provided to one based on the value of care; and (2) testing new models of health care delivery.
Although quality and cost are often thought to increase in parallel, it is important to remember that low quality care can be very expensive, and high quality care can be delivered at low cost; quality and cost are not mutually exclusive. Tying physician payments to the quality of care provided is an overarching goal of the ACA. The Department of Health and Human Services (HHS) aims “to have 30% of Medicare payments tied to quality or value through alternative payment models by the end of 2016 and 50% of payments by 2018” [23]. Physician payments are being modified so that those who provide higher value care will receive higher payments than those who provide lower quality care.
The most common methods to reform fee-for-service have focused on pay-for-performance (P4P) programs. These programs provide payment incentives when the care that is rendered meets a specified goal and, alternatively, may result in a penalty or disincentive if certain targets are not met. P4P initiatives are meant to promote high quality care and align the goals of care across physicians and hospitals [19].
The Centers for Medicare and Medicaid Services (CMS) has developed and tested a number of P4P programs over the last decade. The Physician Quality Reporting System (PQRS) program requires physician reporting of specific quality metrics; it was initially associated with incentives and now also includes penalties [https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html?redirect=/pqri]. The electronic health record incentive program (“meaningful use”, MU) was instituted in 2011 as a part of the American Recovery and Reinvestment Act (ARRA) to promote use of electronic health records and has a number stages that require more sophisticated use of electronic health records [https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/index.html?redirect=/ehrincentiveprograms] [24]. Finally, the Physician Value-Based Payment Modifier (PVBM) program is an initiative stemming from the ACA that adjusts Medicare reimbursements and aims to improve value by rewarding both higher quality and reduced costs [https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/Value-Based-Programs.html].
Medicare Access and Children’s Health Insurance Program (CHIP) Reauthorization Act (MACRA)
The development and implementation of financial rewards to physicians who provide value-based health care is ongoing through changes to Medicare reimbursement strategies. In April 2015, passage of the Medicare Access and CHIP Reauthorization Act (MACRA) repealed the long-standing, unsuccessful Sustainable Growth Rate (SGR) formula for Medicare and sunset the PQRS, Value-based Payment Modifier and Meaningful Use of Electronic Health Records programs for physicians. In their place, CMS put in annual updates to the money available from Medicare to pay physicians and two value-based payment alternatives that physicians need be in to avoid payment penalties [25]. Under MACRA, now known as the Quality Payment Program (QPP), CMS will collect data from physician services in 2017 with payment adjustments being made based upon this data starting in 2019. One of the value-based payment alternatives under the QPP is the Merit-based Incentive Payment System (MIPS), which looks very similar to the existing federal quality programs [25].
Merit-based Incentive Payment System (MIPS)
MIPS is a fee-for-service–based program with reporting beginning in 2017 with payment adjustments in 2019 linked to a composite score of quality, resource use, clinical improvement, and the use of technology including EHR. In large part, MIPS is a modification of P4P programs and incorporates elements of PQRS, MU and PVBM. Initial adjustments of 4% (either positive or negative) based on performance will increase to 9% in 2022. In addition, during the first six payment years of the program (2019–2024), MACRA allows for up to $500 million each year in additional positive adjustments for exceptional performance. The overall program is designed to be budget neutral, so provider bonuses will be offset by provider penalties. It is believed that most clinicians will initially participate in MIPS.
The Centers for Medicare and Medicaid have announced that the first year of reporting, 2017, physicians will be able to “Pick their Own Pace,” for reporting. If a physician is ready, he or she can begin January 1, 2017 and start collecting performance data. For those not ready on January 1, start can be anytime between January 1 and October 2, 2017; regardless of the start date, performance data must be sent by March 31, 2018. If a physician chooses not to report anything, they will receive a 4% negative adjustment to their Medicare reimbursements in 2019. If a physician chooses to submit something in 2017 (for example, information on a single quality measure or clinical practice improvement activity), the negative payment adjustment will not be applied. If a physician chooses to submit more quality, clinical practice improvement, and electronic health record information (now known as Advancing Care Information), they will qualify for various bonus pools, depending on how much they report. The quality domain will rely on reporting of six measures from a range of options that vary across specialties and practices. In the first year of roll out quality will account for 60% of the total MIPS score. Advancing care information is a measure that reflects how physicians use technology in their daily practice. This measure has a particular emphasis on information exchange and is not merely a measure of EHR use. Advancing care information will account for 25% of the total MIPS score in the first year. Clinical practice improvement activities are meant to incentivize actions that enhance quality of care, including coordination of care, patient engagement and safety. A list of 90 potential options are available to fulfill this metric. Clinical practice improvement activities will account for 15% of the total MIPS score in year one. Finally in the first year, cost of care will contribute 0% to the total score. However in subsequent years, it will be at 10%, increasing over time to 30% of the total MIPS score. This metric is based on Medicare claims data of 40 episode-specific measures [http://www.hhs.gov/about/news/2016/04/27/administration-takes-first-step-implement-legislation-modernizing-how-medicare-pays-physicians.html].
Alternative Payment Models (APMs)
CMS is promoting a number of novel alternative payment models (APMs) aimed at reducing unwarranted expenditures and improving quality. These include accountable care organizations (ACOs), advanced primary care medical homes, and bundled payments. It is estimated that approximately 20% of Medicare fee-for-service expenditures currently are distributed through some type of APM. The goal is to increase APMs to 30% by 2016 and 50% by 2018 [CMS.gov Centers for Medicare and Medicaid Services]. Physicians participating in APMs that qualify as “Advanced APMs” are eligible for bonuses initially and then potentially increased reimbursement [19], assuming they meet the criteria to be a qualified provider in that Advanced APM. The underlying principle of episode-based, bundled payment is to link the payment of services by providers, hospitals and other facilities that occur during a given episode of care or intervention. By linking payments there is an incentive to provide value and minimize waste and utilization of unnecessary services. Through a bundled payment, providers and systems that can most efficiently allocate resources and provide high quality care will be rewarded [CMS.gov Centers for Medicare and Medicaid Services].
A number of bundled payment models have been developed from a variety of sources including collaborations with physicians that are currently undergoing evaluation [19, 26] [27]. The CMS has begun testing 3 bundled payment models: Bundled Payments for Care Improvement (BPCI), the Comprehensive Care for Joint Replacement (CCJR) Model, and the Oncology Care Model (OCM) [26]. While all of the models differ in certain aspects, the underlying principle is that services around a defined period of time are bundled and a single organization is accountable for the care that is rendered [CMS.gov Centers for Medicare and Medicaid Services]. The CCJR program encompasses Medicare beneficiaries who undergo hip or knee replacement and is the first mandatory bundled payment program [26, 27]. The CCJR program captures care from the time of hospitalization for joint replacement until 90 days after discharge [26].
The OCM program provides an APM for cancer patients receiving chemotherapy. The episode of care begins when a patient initiates chemotherapy and spans the following 6 months. It encompasses all care that is rendered including physician costs, facility charges, drug charges, laboratory and radiology charges and end of life care. The OCM program began in 2016 and contains a number of quality reporting requirements aimed at reducing unnecessary care [https://innovation.cms.gov/Files/slides/ocm-performancemethod-slides.pdf]. Participating oncologists receive a monthly care management fee to reward coordinated care that reduces unnecessary testing and hospitalizations. Additional bonus payments are possible for practices that meet cost and quality targets [26]. ASCO has developed a program that relies on several of the same tenets [http://www.asco.org/sites/www.asco.org/files/asco_patient-centered_oncology_payment_final_2.pdf].
A list of the currently approved Advanced APMS under the Quality Payment Program for 2017 and 2018, are at https://qpp.cms.gov/education.
Efforts to Address and Assess Value-Based Gynecologic Cancer Care
Given the increasing focus of value in healthcare, the SGO and its members have been actively involved in projects that address value in the delivery of gynecologic cancer care. Examples of these efforts including addressing interventions that have a low value for money (“Choosing Wisely” measures) and applying the ASCO value framework to ovarian cancer chemotherapy and developing gynecologic cancer APMs.
SGO Choosing Wisely Measures
The American Board of Internal Medicine (ABIM) Foundation has identified a growing need to address the cost of healthcare delivery and overuse of healthcare resources in the United States. In 2012 the ABIM Foundation launched the “Choosing Wisely” campaign as a means to address wasteful and unnecessary medical treatment. In an setting of finite health care resources, the Foundation aims to improve delivery of high quality, affordable care while minimizing the overuse of medical tests and procedures [28], and supported by evidence. Each participating organization has provided a list of “Things to Question”, which include specific, evidence-based recommendations that patients and providers are encouraged to discuss. As a result, medical organizations and the Foundation have published hundreds of recommendations on medical resource utilization to improve the safety and quality of health care.
The Society of Gynecologic Oncology (SGO) recommendations are as follows: “1) Don’t screen low risk women with CA-125 or ultrasound for ovarian cancer, 2) Don’t perform Pap tests for surveillance of women with a history of endometrial cancer, 3) Don’t perform colposcopy in patients treated for cervical cancer with Pap tests of low-grade squamous intraepithelial lesion (LGSIL) or less, 4) Avoid routine imaging for cancer surveillance in women with gynecologic cancer, specifically ovarian, endometrial, cervical, vulvar and vaginal cancer, and 5) Don’t delay basic level palliative care for women with advanced or relapsed gynecologic cancer, and when appropriate, refer to specialty level palliative medicine” [29]. These recommendations provide a basic framework for value-based care in gynecologic oncology, however further research is warranted to measure the implementation and uptake of these recommendations.
Applying the ASCO value framework to ovarian cancer
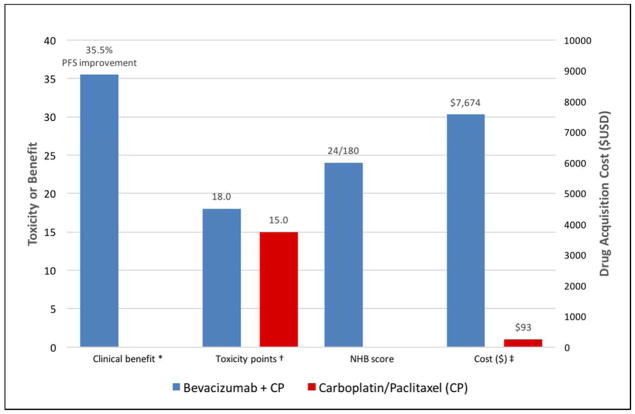
SGO members have also begun to demonstrate the potential clinical utility of the 2015–2016 ASCO value framework. In one example, the advanced disease framework was applied to the use of concurrent chemotherapy plus maintenance bevacizumab in the primary treatment of ovarian cancer (based on Gynecologic Oncology Group trial 218, a comparison of standard carboplatin+paclitaxel (CP) chemotherapy versus concurrent plus maintenance bevacizumab with CP (CP+B)) [30]. As PFS was the primary end point in GOG 218, the PFS HR of 0.65 (p<0.001) for CP+B versus CP alone was used to calculate a clinical benefit score of 28. Using the GOG 218 reporting of select grade ≥2 adverse events (including grade 2 GI and hypertensive events) together, an overall toxicity score of −4 was calculated for CP + B versus CP alone. Long-term survival, palliation of symptoms, and treatment-free interval data were not available, and there was no difference in QOL between CP+B and CP alone. Therefore, the final NHB score (as defined previously) for CP+B compared to CP alone was 24. Figure 1a illustrates the application of ASCO’s graphical value snapshot for CP+B versus CP alone, with comparisons of clinical benefit, toxicity, NHB, and cost. In this case, the drug acquisition cost resulted in a cost comparison of $7,674 per cycle for CP+B compared to $93 per cycle for CP alone, a cost difference of $7,581. Ideally, out of pocket cost differences between regimens would also be presented. This example demonstrates the ability to use the ASCO Value Framework to calculate relative net health benefit scores when discussing options for therapy in a clinical scenario.
Figure 1.
Value snapshot illustrating ASCO’s comparison of clinical benefit, toxicity, NHB, and cost of novel therapy versus standard therapy. Comparison of bevacizumab + carboplatin/paclitaxel versus carboplatin/paclitaxel alone (GOG 218)
PFS = progression free survival; NHB = Net Health Benefit; CP = Carboplatin/Paclitaxel; USD = 2016 United States dollars
* In accordance with ASCO’s value snapshot, clinical benefit is presented as a percent (%) reduction in risk of progression during the study period based on the hazard ratio (HR) for progression free survival (PFS). Clinical benefit calculations involving a HR rely on the following formula: (1-HR) × 100. For PFS, a multiplier of 0.8 is also applied to this formula.
† The difference in toxicity is graphically shown as the difference in the total point score based on ASCO’s toxicity calculations. ASCO’s revised framework applies different importance weights to all grade 1 or 2 versus grade 3 or 4 adverse events. Toxicity data in GOG 218 reported combined select grade ≥2 adverse events (including grade 2 GI and hypertensive events); therefore, we assigned importance weights based on ASCO’s weighting of grade 3 or 4 adverse events: 1.5 points for each grade 3 or 4 adverse event with <5% frequency; and 2.0 points for each toxicity with a ≥5% frequency. In accordance with ASCO’s framework, ‘laboratory only’ toxicities were excluded. A toxicity score of 18 was calculated for CP + B compared to a score of 15 for CP alone; and a percent difference was calculated and multiplied by 20 in accordance with ASCO’s toxicity calculation. Given CP+B had a higher frequency of toxicities versus CP alone, the overall toxicity score was negative: −4.
‡ The cost estimates are based on Medicare J-code reimbursements; and calculated for a 170cm/70kg female patient with a creatinine of 0.8.
SGO Efforts in Payment Reform
The SGO Health Policy and Socioeconomic Committee Future of Physician Payment Reform (PPR) Taskforce has focused efforts over the past two years to tackle forthcoming changes under MACRA. These include developing models that reflect best care practices for the gynecologic cancer care of women, and guidance for SGO members on how to incorporate and adapt to new MACRA based reporting and payment systems imposed by CMS. The SGO is currently developing a number of APMs specific to women with gynecologic malignancies. Input is clearly needed by providers of women’s cancer care to help develop models that can best improve the quality of care for women with gynecologic malignancies.
This taskforce began with endometrial cancer (EC) care as the initial prototype for designing an APM for gynecologic cancer care.
The endometrial cancer care model will form the basis of a bundled care pathway, and secondarily, form components of care upon which quality metrics will be built. As the treatment of EC includes surgery, chemotherapy, radiation, genetics, surveillance and other components of care, the APM model for EC has been divided into episodes of care, with a focus first on the surgical treatment of EC.
The taskforce has taken a step-wise approach towards developing this model. First, it gathered input from a multi-disciplinary panel of providers involved in EC care to identify services provided from the time of diagnosis through completion of treatment for EC, including services that were reimbursed as well as uncompensated activities. After mapping this pathway of care, data from national population-based claims and cancer registry sources were abstracted to identify actual treatment patterns rendered (i.e. modality of surgeries) and their associated outcomes. These baseline estimates of “real world” care and their associated costs were then applied towards simulation models.
Initial models have suggested that costs during the perioperative period are driven in large part by the route of surgery, as well as postoperative readmissions and emergency department visits. Initial results have shown a cost savings if 70% of low risk EC cases were to undergo minimally invasive surgery (as a result of lower rates of complications and utilization of urgent and emergent services). Further efforts are currently underway to determine which specific tests, procedures or baseline conditions are the biggest drivers of overall cost. Next, the taskforce will validate the EC model using registry-based, institutional or individual practice-based data to identify variations of practice (and potential cost-savings differences) compared to that based on averages from national data. Once the EC model is finalized, the APM will be presented to public or private payers for consideration of piloting this bundled payment model.
Subsequent steps will include rolling the APM out to providers of EC care for use in discussions with their individual payers or participation in MACRA. Forthcoming efforts will include development of episodes of care involving adjuvant therapy, recurrence treatment, and survivorship care, as well as development of models for other cancer sites.
In conjunction with the SGO Quality and Outcomes Taskforce, the SGO Education Committee, and the SGO PPR taskforce, joint efforts have been directed towards defining quality metrics, including process-based as well as outcome-based metrics. It is important to ensure alignment of proposed metrics for the National Quality Forum (NQF) and PQRS, with the treatments rendered in the EC model. The Clinical Outcomes Registry will include collection of additional outcome metrics which will potentially reportable to the PQRS in order to ensure that quality of care is maximized. Simultaneously the SGO education committee has been developing and designing an educational platform through web-based resources, webinars and meeting conferences to disseminate this information. Certainly there will be heterogeneity amongst gynecologic oncology practices across the country and individual payers respectively. Reimbursement rates are known to differ locally, regionally, and nationally. Nonetheless, the SGO seeks to provide healthcare providers engaged in treating women with gynecologic cancer the resources to examine their own practices, negotiate payment models, and continue to provide the best care in the era of payment reform.
CONCLUSIONS
Understanding value in healthcare is critical given its relationship to the provision of cost effective and quality patient care. Recent changes in healthcare law have mandated the inclusion of value-based care in payment models that will impact hospital and provider reimbursement. Being prepared to respond to these changes will position the members of the Society of Gynecologic Oncology favorably as healthcare delivery adapts to the current environment.
Contributor Information
David E. Cohn, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Ohio State University College of Medicine, Columbus, OH.
Emily Ko, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Pennsylvania Health System, Pennsylvania Hospital, Philadelphia, PA.
Larissa A Meyer, Department of Gynecologic Oncology and Reproductive Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX.
Jason D. Wright, Division of Gynecologic Oncology, Columbia University College of Physicians and Surgeons and New York Presbyterian Hospital, New York, NY.
Sarah Temkin, Johns Hopkins Medicine Department of Gynecology and Obstetrics, Baltimore, MD.
Jonathan Foote, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Duke University, Durham, NC.
Nate Jones, Division of Gynecologic Oncology, Columbia University College of Physicians and Surgeons and New York Presbyterian Hospital, New York, NY.
Laura J. Havrilesky, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Duke University, Durham, NC.
References
- 1.Smith A. An inquiry into the nature and causes of the wealth of nations. London: 1776. Printed for W. Strahan; and T. Cadell. [Google Scholar]
- 2.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 3.Porter ME, Teisberg EO. How physicians can change the future of health care. JAMA. 2007;297(10):1103–11. doi: 10.1001/jama.297.10.1103. [DOI] [PubMed] [Google Scholar]
- 4.Porter ME. A strategy for health care reform--toward a value-based system. N Engl J Med. 2009;361(2):109–12. doi: 10.1056/NEJMp0904131. [DOI] [PubMed] [Google Scholar]
- 5.Porter ME. Value-based health care delivery. Ann Surg. 2008;248(4):503–9. doi: 10.1097/SLA.0b013e31818a43af. [DOI] [PubMed] [Google Scholar]
- 6.Porter ME, Lee TH. The Strategy That Will Fix Health Care. Harvard Business Review. 2013;91(12):24–24. [Google Scholar]
- 7.Porter ME, Larsson S, Lee TH. Standardizing Patient Outcomes Measurement. N Engl J Med. 2016;374(6):504–6. doi: 10.1056/NEJMp1511701. [DOI] [PubMed] [Google Scholar]
- 8.Mak KS, et al. Defining a standard set of patient-centred outcomes for lung cancer. Eur Respir J. 2016 doi: 10.1183/13993003.02049-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen RE, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract. 2014;10(4):e215–22. doi: 10.1200/JOP.2013.001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zafar SY, Abernethy AP. Financial toxicity, Part I: a new name for a growing problem. Oncology (Williston Park) 2013;27(2):80–1. 149. [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsey S, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32(6):1143–52. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zafar SY, et al. The utility of cost discussions between patients with cancer and oncologists. Am J Manag Care. 2015;21(9):607–15. [PubMed] [Google Scholar]
- 13.Bullock AJ, et al. Understanding patients’ attitudes toward communication about the cost of cancer care. J Oncol Pract. 2012;8(4):e50–8. doi: 10.1200/JOP.2011.000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meropol NJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27(23):3868–74. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 15.Schnipper LE, et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J Clin Oncol. 2015;33(23):2563–77. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnipper LE, et al. Updating the American Society of Clinical Oncology Value Framework: Revisions and Reflections in Response to Comments Received. J Clin Oncol. 2016;34(24):2925–34. doi: 10.1200/JCO.2016.68.2518. [DOI] [PubMed] [Google Scholar]
- 17.Mehr S. Applying Accountable Care to Oncology: Developing an Oncology ACO. Am J Manag Care. 2013;19:E3. [PubMed] [Google Scholar]
- 18.Colla CH, et al. Cancer spending and accountable care organizations: Evidence from the Physician Group Practice Demonstration. Healthcare : the journal of delivery science and innovation. 2013;1(3–4):100–107. doi: 10.1016/j.hjdsi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apte SM, Patel K. Payment Reform: Unprecedented and Evolving Impact on Gynecologic Oncology. Front Oncol. 2016;6:84. doi: 10.3389/fonc.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel K, et al. Transforming Oncology Care: Payment and Delivery Reform for Person-Centered Care. Am J Manag Care. 2015;21(5):388–93. [PubMed] [Google Scholar]
- 21.Sprandio JD. Oncology Patient–Centered Medical Home. Journal of Oncology Practice. 2012;8(3S):47s–49s. doi: 10.1200/JOP.2012.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoverman JR, et al. Opening the Black Box: The Impact of an Oncology Management Program Consisting of Level I Pathways and an Outbound Nurse Call System. Journal of Oncology Practice. 2014;10(1):63–67. doi: 10.1200/JOP.2013.001210. [DOI] [PubMed] [Google Scholar]
- 23.Burwell SM. Setting Value-Based Payment Goals — HHS Efforts to Improve U.S. Health Care. New England Journal of Medicine. 2015;372(10):897–899. doi: 10.1056/NEJMp1500445. [DOI] [PubMed] [Google Scholar]
- 24.Wright A, et al. Early results of the meaningful use program for electronic health records. N Engl J Med. 2013;368(8):779–80. doi: 10.1056/NEJMc1213481. [DOI] [PubMed] [Google Scholar]
- 25.COMPUTATION OF THE 2016 VALUE MODIFIER. 2015 Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/2016-VM-Fact-Sheet.pdf.
- 26.Press MJ, Rajkumar R, Conway PH. Medicare’s New Bundled Payments: Design, Strategy, and Evolution. JAMA. 2016;315(2):131–2. doi: 10.1001/jama.2015.18161. [DOI] [PubMed] [Google Scholar]
- 27.Mechanic RE. Mandatory Medicare Bundled Payment--Is It Ready for Prime Time? N Engl J Med. 2015;373(14):1291–3. doi: 10.1056/NEJMp1509155. [DOI] [PubMed] [Google Scholar]
- 28.Lynch T, Wolfson D. Choosing Wisely in an Era of Limited Resources. ABIM Foundation Forum; 2012. [Google Scholar]
- 29.Five Things Physicians and Patients Should Question: The Society of Gynecologic Oncology Choosing Wisely Campaign in collaboration with the ABIM Foundation. 2013.
- 30.Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]