Abstract
Protective effects of pregnancy during MS have led to clinical trials of estriol, the pregnancy estrogen, in MS. Since estriol binds to estrogen receptor (ER) beta, ER beta ligand could represent a “next generation estriol” treatment. Here, ER beta ligand treatment was protective in EAE in both sexes and across genetic backgrounds. Neuroprotection was shown in spinal cord, sparing myelin and axons, and in brain, sparing neurons and synapses. Longitudinal in vivo MRIs showed decreased brain atrophy in cerebral cortex gray matter and cerebellum during EAE. Investigation of ER beta ligand as a neuroprotective treatment for MS is warranted.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, estrogen, pregnancy, neuroprotection
1. Introduction
Multiple sclerosis (MS) is a putative autoimmune disease targeting the central nervous system (CNS) leading to neurodegeneration. Currently approved treatments for MS were designed to modulate peripheral immune responses to decrease CNS inflammation. These treatments reduce relapse rates by about half compared to placebo treatment in clinical trials, with only modest effects on slowing permanent disability accumulation. Many MS treatments are relatively safe and well tolerated. More aggressive anti-inflammatory treatments reduce relapse rates further, but are associated with toxicities related in part to immunosuppression. Rather than escalating to more aggressive anti-inflammatory treatments for better long term disability outcomes, an alternative would be to combine relatively safe anti-inflammatory treatments with a neuroprotective treatment in patients with relapsing remitting MS (Voskuhl, 2016). Such neuroprotective treatments may also benefit progressive MS patients.
“Bedside to Bench to Bedside” research is a way to capitalize on a known clinical observation, mechanistically dissect it at the laboratory bench, then translate basic findings back to the clinic in the form of a novel clinical trial (Voskuhl and Gold, 2012). This approach has a clinical observation as its foundation. In contrast, “Bench to Bedside” research is based on a molecule or pathway thought to be involved in a disease mechanism, with trials designed to block this molecule. The latter approach carries risk that the molecule or pathway of interest may not ultimately be physiologically significant in humans with disease, since most biological processes involve redundant mechanisms and compensatory pathways. Blocking one molecule or pathway may not have a significant effect in complex diseases in humans. This is one reason why pharmaceutical company success rates are low compared to the number of lead candidates initially passing in vitro screening and in vivo preclinical models. The “Bedside to Bench to Bedside” approach mitigates this risk since it starts with a clinical observation known to be physiologically relevant. Mechanisms underlying clinical observations may involve several molecules and complementary pathways. A multifaceted approach may indeed be what is required to impact complex diseases. The “Bedside to Bench to Bedside” approach in drug development is a conceptual shift, since the “Bench to Bedside” approach has focused on the molecule first, with questions of physiologic relevance coming later.
A major clinical observation in MS is that pregnancy is protective (Confavreux et al. , 1998). Relapses are decreased by over 70% in the last trimester. Hormones and other factors change during pregnancy, each warranting consideration for mediating this protection. Estriol is an estrogen of pregnancy, distinct from estradiol of ovulatory cycles. It is made by the fetal placental unit and rises progressively during pregnancy to reach high levels in the last trimester (Lindberg et al. , 1974). Estriol was given to mice with experimental autoimmune encephalomyelitis (EAE) at doses to induce a level in the blood physiologic with mouse pregnancy, and disease protection was observed (Kim et al. , 1999). This disease protection was found in both female and male mice, in both relapsing and progressive EAE models, and when administered either before or after EAE onset, reviewed in (Spence and Voskuhl, 2012). Estriol is a relatively weak estrogen, acting on estrogen receptor (ER) alpha and ER beta, with higher affinity for ER beta (Katzenellenbogen, 1984, Kuiper et al. , 1997). ER alpha ligand treatment during EAE was protective early in EAE (Morales et al. , 2006), was shown to be anti-inflammatory during peripheral immune responses (Lelu et al. , 2011, Morales, Loo, 2006), and neuroprotective by binding to astrocytes to reduce CCL2 and immune infiltration in the CNS (Kim et al. , 2014, Spence et al. , 2011). ER beta ligand treatment was protective later during EAE (Tiwari-Woodruff et al. , 2007), did not alter peripheral immune responses (Tiwari-Woodruff, Morales, 2007), and did not target astrocytes (Spence et al. , 2013). Instead ER beta ligand and estriol each had protective effects on microglia and dendritic cells (Drew and Chavis, 2000, Du et al. , 2011, Papenfuss et al. , 2011, Saijo et al. , 2011). Also, ER beta ligand was shown to act on oligodendrocytes to increase remyelination (Crawford et al. , 2010, Khalaj et al. , 2013). In EAE (Ziehn et al. , 2012, Ziehn et al. , 2010) and non-EAE (Kramar et al. , 2009, Liu et al. , 2008) ovariectomized mice, estriol and estrogen receptor beta ligand treatment improves cognitive behavioral testing and hippocampal synaptic plasticity, respectively. Coming full circle, these latter preclinical data are consistent with another clinical observation in humans, namely cognitive dysfunction occurs in healthy women after surgical ovariectomy (Sherwin, 1988).
Two trials have been completed, and one is ongoing (www.clinicaltrials.gov), with oral estriol treatment of 8 mg per day in women with MS to induce an estriol level in blood that recapitulates pregnancy. The first pilot clinical trial used the biomarker of enhancing lesions on monthly MRIs as the primary outcome measure. There was an over 70% reduction in enhancing lesions with estriol treatment compared to pretreatment in a single arm crossover design (Sicotte et al. , 2002). The second trial was a Phase 2b, placebo-controlled, multicenter trial with relapse rate reduction as the primary outcome measure (Voskuhl et al. , 2016). Relapses were reduced by a third to a half more in the estriol plus glatiramer acetate group compared to the glatiramer acetate plus placebo. Exploratory analyses showed that higher estriol blood levels correlated with reduced relapses and with reduced enhancing lesion positive scans. Higher estriol blood levels also correlated with improved cognitive testing performance. Trends for protective effects on cerebral cortical gray matter atrophy by MRI were also observed, particularly in patients who were enhancing lesion negative, suggesting neuroprotective effects (Voskuhl, Wang, 2016).
Oral estriol is taken at doses of 1-2 mg per day in Europe and Asia to alleviate menopausal symptoms. Estriol has been considered the safest of the estrogens for decades, likely due to its preferential binding to ER beta over ER alpha, since toxicities related to breast and uterus are mediated by ER alpha, not ER beta (Head, 1998, Lauritzen, 1987, Takahashi et al. , 2000). While safety was shown in the two completed MS clinical trials (Sicotte, Liva, 2002, Voskuhl, Wang, 2016), the search for a next generation estriol has begun that entails the use of selective estrogen receptor modifiers (SERMs). An ideal candidate would be an ER beta ligand. Here, we will investigate neuroprotective effects of ER beta ligand treatment in EAE, using a ligand that previously showed promise in other neurodegenerative disease models of Alzheimer's disease (George et al. , 2013) and Parkinson's disease (McFarland et al., 2013). Beneficial effects in EAE will be shown that go beyond preservation of spinal cord white matter myelin and axons. We will show reduction of atrophy of cerebral cortex and cerebellum by in vivo longitudinal MRIs and preservation of neurons and synapses in gray matter by neuropathology.
2. Materials and Methods
2.1. Animals
C57BL/6 and NOD mice, 8 weeks of age, were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were maintained under environmentally controlled conditions in a 12-hour light/dark cycle with access to food and water ad libitum. All procedures were done in accordance with the guidelines of the National Institutes of Health and the Chancellor's Animal Research Committee of the University of California, Los Angeles Office for the Protection of Research Subjects.
2.2. Reagents / Treatments
ER beta ligand (AC186) was provided by Acadia Pharmaceuticals (McFarland, Price, 2013). It was dissolved in Miglyol 812N liquid oil (Sasol North America) or sesame oil (Sigma Aldrich) at 15 mg/mL for delivery of a 30 mg/kg treatment dose which was administered subcutaneously every other day, achieving a final dose of 15 mg/kg/day. The generic ER beta ligand, diarylpropionitrile (DPN, Tocris Biosciences), was dissolved in 10% molecular-grade ethanol and diluted with 90% Miglyol 812N liquid oil (Sasol North America) to achieve a final dose of 8/mg/kg per day, as described (Tiwari-Woodruff, Morales, 2007).
2.3. EAE
C57BL/6 and NOD mice were injected subcutaneously with Myelin Oligodendrocyte Glycoprotein (MOG), amino acids 35–55 (200 μg/animal, American Peptides), emulsified in complete Freund's adjuvant (CFA) and supplemented with Mycobacterium Tuberculosis H37ra (300 μg/animal, Difco Laboratories), over two sites drained by left inguinal and auxiliary lymph nodes in a total volume of 0.1 ml/mouse. One week later, a booster immunization was delivered over contra lateral lymph nodes. Pertussis toxin (500 ng/mouse) (List Biological Laboratories, Inc.) was injected intraperitoneally on days 0 and 2. Animals were monitored daily for EAE signs based on a standard EAE 0–5 scale scoring system: 0—healthy, 1—complete loss of tail tonicity, 2—loss of righting reflex, 3—partial paralysis, 4—complete paralysis of one or both hind limbs, and 5—moribund. Treatments with ER beta ligand or vehicle were initiated at the first clear signs of clinical disease (EAE grade 2 at day 13-15) and continued to the endpoint of the experiment, as described (Wisdom et al. , 2013).
2.4. Rotarod Testing
Motor behavior was tested up to two times per week for each mouse using a rotarod apparatus (Med Associates Inc., St. Albans, VT). Briefly, animals were placed on a rotating horizontal cylinder for a maximum of 200 seconds. The amount of time the mouse remained walking on the cylinder, without falling, was recorded. Each mouse was tested on a speed of 3-30 rpm and given three trials for any given day. The three trials were averaged to report a single value for an individual mouse, and then averages were calculated for all animals within a given treatment group, as described (Du et al. , 2014).
2.5. Histological Preparation
Mice were exposed to a lethal dose of isoflurane and perfused transcardially with ice-cold 1× PBS for 8–15 min, followed by 10% formalin for 10-15 min. Spinal cords and brains were dissected and submerged in 10% formalin overnight at 4°C, followed by 30% sucrose in PBS for 24 h. Tissues were embedded in 75% gelatin/15% sucrose solution for cryostat sectioning then post-fixed overnight in 10% formalin and cryoprotected in 30% sucrose. The embedded tissues were stored in − 80 °C after flash frozen in dry ice. 40 μm thick free-floating spinal cord cross-sections, and sagittal brain sections were obtained with a microtome b cryostat (model HM505E) at −20 °C. Tissues were collected serially and stored in 1× PBS with 1% sodium azide in 4°C until immunohistochemistry (Spence, Wisdom, 2013).
2.6. Immunofluorescence
Prior to histological staining, 40-mm thick free-floating sections were thoroughly washed with 1X PBS to remove residual sodium azide. In the case of anti-MBP labeling, tissue sections were processed with an additional 1 h incubation with 5% glacial acetic acid in 100-proof ethanol at room temperature (RT). After washing tissue sections were permeabilized with 0.3% TritonX-100 and 2% normal goat serum in 1X PBS for 30 min at RT and blocked with 10% normal goat serum in 1X PBS for 1 hr. Tissues were then incubated with primary antibodies overnight in 4 °C. The following primary antibody (Ab) were used: Rat anti-MBP (Millipore) at 1:1000 dilutions, Rabbit anti-NF200 (Sigma Aldrich) at 1:750 dilutions, Rabbit anti- beta-APP (Life Technologies) at 1:200 dilutions, Mouse anti-NeuN at 1:1000 dilutions (Millipore), Rabbit anti-PSD95, and Rat anti-CD45 at 1:1500 dilutions (Millipore). The next day tissues were washed and incubated with secondary antibodies conjugated to Cy5 or Cy3 (Millipore) for 1 hr at RT. A nuclear stain DAPI (2 ng/mL; Molecular Probes) was added 10 min prior to final washes after secondary Ab incubation. Sections were mounted on slides, allowed to semi-dry, and cover slipped in fluoromount G (Fisher Scientific). IgG-control experiments were performed for all primary Ab, and only non-immunoreactive tissues under these conditions were analyzed (Spence, Wisdom, 2013).
2.7. Chromagen Immunohistochemistry
Tissue sections were thoroughly washed with 1x PBS to remove residual sodium azide and treated with 3% hydrogen peroxide for 30 min at RT and then simultaneously blocked with 10% NGS and permeabilized with 0.3% Triton X-100 in 1x PBS for 1 h at room temperature. Tissues were then incubated with primary antibodies overnight in 4 °C. The following primary antibodies were used: Rat anti-CD3 at 1:2000 dilutions (BD Pharmigen), anti-Calbindin D28K at 1:1000 dilutions (Millipore), and Rabbit anti-Iba1 at 1:10000 dilutions (Wako Chemicals), were added for 2 h at RT, and then placed in 4°C overnight. Tissue sections then followed with secondary Ab labeling at 1:1000 dilutions (Vector labs) for 1 h at room temperature and then with Avidin-Biotin Conjugation solution (Vector Labs) for 1 h at RT. Tissue sections were treated with DAB peroxidase substrate (Vector labs) according to manufacturer instructions. IgG-control experiments were performed for all primary Ab, and only non-immunoreactive tissues under these conditions were analyzed(MacKenzie-Graham et al., 2009).
2.8. Microscopy and Quantification
Spinal cord cross-sections from each mouse were captured under microscope at 10x magnification. To quantify demyelination in the spinal cord and cerebellum, white matter was manually delineated on the basis of DAPI staining, and MBP staining intensity was calculated and reported in the sampled area. Axonal damage was assessed by counting beta-APP+ cells in a confocal 10x microscope in spinal cord. Axonal densities were calculated by counting the number of NF200+ or NeuN+ neuronal cells in a 10x confocal image in the sampled area of the captured tissue section. Cerebellar Purkinje (Calbindin+) cells were manually counted using a brightfield 10x microscope over the entire sagittal cerebellum. CD3+, and Iba1+ cells were manually quantified under a confocal 10x microscope for CD45+ cells or a brightfield 10x microscope for CD3+ and Iba1+ cells (Spence, Wisdom, 2013).
2.9. MRI Acquisition
Mice were anesthetized with isofluorane, respiration rate was monitored, and temperature was maintained at 37°C. In vivo magnetic resonance imaging was performed on a 200 mm horizontal bore 7.0 T Bruker imaging spectrometer with a micro-imaging gradient insert with a maximum gradient strength of 100 G/cm and 30 mm birdcage RF coil (Bruker Instruments, Billerica, MA). Imaging parameters were as follows: rapid-acquisition with relaxation enhancement (RARE) sequence, matrix dimensions = 256 × 128 × 64; field of view = 3.84 cm × 1.92 cm × 0.96 cm; repetition time (TR) = 3500 ms; apparent time to echo (apparent TE) = 32 ms; echo train length = 16; total scan time = 37 mins. Spatial resolution was 150 μm3 per voxel (MacKenzie-Graham et al., 2012a).
2.10. MRI Analysis
In vivo magnetic resonance images were analyzed as previously described (Spence et al., 2014). Briefly, images were skull-stripped using the Brain Surface Extractor (BSE) in BrainSuite 11a (Shattuck and Leahy, 2001) and bias-field inhomogeneities removed using the N3 correction (Sled et al., 1998). A minimum deformation atlas (MDA) was produced and images were spatially and intensity normalized to it using a rigid-body transformation. Cerebral cortices and cerebella were manually labeled on the atlas as described (Spence, Kurth, 2014) and the labels were then warped onto the individual spatially-normalized images to produce standardized estimates of gray matter volumes in individual subjects. All automated image processing was performed using the LONI Pipeline Processing Environment (Rex et al., 2003) on an 8-processor core Mac Pro computer (Apple, Cupertino, CA (MacKenzie-Graham, Rinek, 2012a).
2.11. Statistical Analysis
The significance of EAE severity and rotarod performance were determined by repeated measures ANOVA. Immunohistochemical data was analyzed by one-way ANOVA. Bonferroni tests (GraphPad Prism6) were used to adjust for for multiple comparisons. Global and regional brain volume changes in EAE mice and healthy controls were compared with a repeated measures ANOVA in SPSS 22 (IBM, Armonk, NY). If Mauchly's test indicated that the assumption of sphericity had been violated (p < 0.05), then the degrees of freedom were corrected using Huynh-Feldt estimates of sphericity. Regression analysis and Welch's t-tests were performed in Excel 2011 (Microsoft, Redmond, WA). All results are presented as mean ± standard error.
3. Theory
To address the need for development of a neuroprotective treatment for MS, this work will focus on a “next generation estriol treatment”, namely ER beta ligand treatment. Potential neuroprotective effects will be investigated in EAE, not only in spinal cord, but also in cerebral cortex and cerebellum, using in vivo longitudinal MRI and neuropathology.
4. Results
4.1. ER beta ligand treatment reduces chronic EAE in females and males across genetic backgrounds
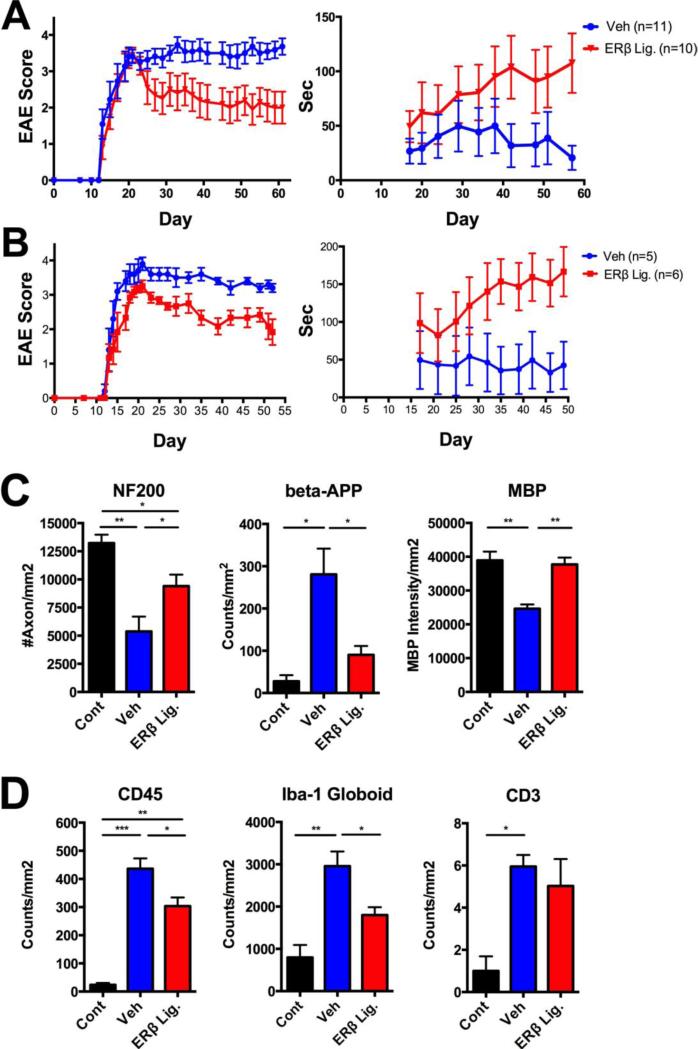
Active EAE was induced using MOG 35-55 peptide in female C57BL/6 mice. Treatment with ER beta ligand or vehicle control was initiated after EAE induction, at the first signs of clinical disease and continued every other day to the end of experiment. Clinical EAE severity scores were reduced in mice treated with ER beta ligand as compared to vehicle control (Table 1, Fig 1A, left). Furthermore, when mice were assessed for the number of seconds they could remain on the rotarod, the ER beta ligand treated group performed better than the vehicle control group (Fig 1A, right). These data showing clinical disease protection using the novel ER beta ligand were consistent with previously reported protective effects of a generic ER beta ligand (DPN) in female C57BL/6 mice with EAE.
Table 1.
Effects of ERβ ligand treatment on clinical disease severity scores: Efficacy in both sexes and across genetic backgrounds.
| Strain and Sex | Experimenta | Treatment | Mean Disease Scoreb | P-value | Cumulative Disease Scorec | P-value |
|---|---|---|---|---|---|---|
| C57BL/6 Females | Exp.1 (Day 61) | Vehicle ER Beta Lig. | 3.0 ± 0.3 2.0 ± 0.4 |
<0.0001 | 142.3 ± 12.7 93.5 ± 20.0 |
0.0195 |
| Exp.2 (Day 51) | Vehicle ER Beta Lig. | 2.2± 0.2 1.3± 0.1 |
0.0299 | 82.8± 6.3 46.9± 2.0 |
0.0003 | |
| Exp.3 (Day 50) | Vehicle ER Beta Lig. | 2.7 ± 0.1 1.7 ± 0.5 |
<0.0001 | 96.1 ± 3.1 61.1 ± 16.4 |
0.0582 | |
| C57BL/6 Males | Exp.4 (Day 52) | Vehicle ER Beta Lig. | 2.9 ± 0.1 2.1 ± 0.1 |
<0.0001 | 109.8 ± 3.6 79.0 ± 5.1 |
0.0020 |
| NOD Females | Exp.5 (Day 51) | Vehicle ER Beta Lig. | 2.7 ± 0.2 1.9 ± 0.1 |
<0.0001 | 99.3 ± 8.0 71.0 ± 4.8 |
0.0103 |
Each experiment and its duration.
Mean Disease Score, is the average EAE clinical score calculated one week after the initiation of treatment (day 21) through the duration of the study for the mean score for each group. Mean ± SEM. P-values are calculated using One-way ANOVA with post-hoc Bonferroni test.
Cumulative Disease Score is calculated by summing the daily EAE clinical scores for each mouse for the mean cumulative score for each group over the same duration as the mean disease scores. Mean ± SEM. P-values are calculated using Unpaired Student's t-test.
Figure 1. Protective effects of ER beta ligand treatment on female and male mice with chronic EAE.
ER beta ligand treatment (30 mg/kg/every other day) or vehicle treatment initiated after EAE onset (day 13) resulted in significant reductions in EAE clinical severity scores (Table 1) and an increase in the number of seconds on the rotarod, p < 0.0001 in female (A) and male (B) mice with EAE. Immunohistochemistry of spinal cord white matter showed axonal loss (reduced NF200 staining), axonal damage (increased beta-APP staining) and demyelination (decreased MBP staining) in vehicle (blue) treated EAE compared to healthy controls (black), while ER beta ligand treated EAE mice (red) compared to vehicle treated had preserved axons and myelin (Fig 1C). Vehicle treated EAE (blue) compared to healthy controls (black) showed increased inflammation (CD45 staining), increased macrophages/activated microglia (Iba-1 staining with globoid morphology) and T lymphocytes (CD3 staining). ER beta ligand treated EAE mice (red) had reduced pan-immune (CD45) and macrophage/activated microglia (Iba-1 globoid) staining, with no decrease in T lymphocyte (CD3) staining. For neuropathology, three to five mice were examined per treatment group. CD45 counts included dorsal column of spinal cord, while Iba-1 and CD3 counts were done for whole spinal cord white matter including dorsal, lateral and ventral regions. *** p < 0.0001, ** p < 0.005, * p < 0.05, with p-values determined by one-way ANOVA.
To ascertain whether ER beta ligand treatment was only beneficial in female mice or could also be beneficial in males, we then induced active EAE in male C57BL/6 mice, and again observed significant protection in both standard EAE and rotarod clinical outcomes (Table 1, Fig 1B). Next, to ascertain whether beneficial effects of ER beta ligand treatment in chronic EAE were limited to one genetic background or could also be protective across genetic backgrounds, active EAE was induced in female NOD mice with chronic EAE. ER beta ligand treatment was also protective in NOD females with chronic EAE (Table 1). Together these results demonstrate that beneficial effects of ER beta ligand treatment are not limited to one sex or one genetic background, but can be observed in both sexes across various genetic backgrounds.
4.2 Effect of ER beta ligand treatment on spinal cord neuropathology
Classic immunohistochemistry of spinal cord white matter showed axonal loss (by reduced NF200 staining) p<0.005, axonal damage (by increased beta-APP staining) p<0.05, and demyelination (by decreased MBP staining) p < 0.005, in vehicle treated EAE compared to healthy controls. ER beta ligand treatment of EAE mice after disease onset preserved axons and myelin (ER beta ligand EAE vs, vehicle EAE: axons p<0.05; axonal damage p<0.05; myelin p<0.005), (Fig 1C). Assessment of spinal cord white matter inflammation in vehicle treated EAE compared to healthy controls showed increased inflammation (by CD45 staining) p<0.0001, increased macrophages/activated microglia (by Iba-1 staining with globoid morphology) p<0.005, and increased T lymphocytes (by CD3 staining) p < 0.05. ER beta ligand treatment of EAE partially reduced inflammation as detected by CD45 staining, p<0.05, with this driven by the reduction in macrophages/activated microglia, p<0.05, not T lymphocytes.
4.3 ER beta ligand treatment reduces brain atrophy on MRI during chronic EAE
Chronic EAE in C57BL/6 mice entails not only demyelination and axonal loss in spinal cord, but also neuronal and synaptic loss in cerebellum and cerebral cortex (Brown and Sawchenko, 2007, MacKenzie-Graham, Rinek, 2012a, Mangiardi et al. , 2011, Ziehn, Avedisian, 2010), and previous MRI studies have shown atrophy of brain, cerebral cortex, and cerebellum during chronic EAE (Aharoni et al. , 2013, Lepore et al. , 2013, MacKenzie-Graham, Rinek, 2012a, MacKenzie-Graham, Tiwari-Wooddruff, 2009, Spence, Kurth, 2014). Here, we determined whether treatment with ER beta ligand during EAE could spare brain atrophy by MRI.
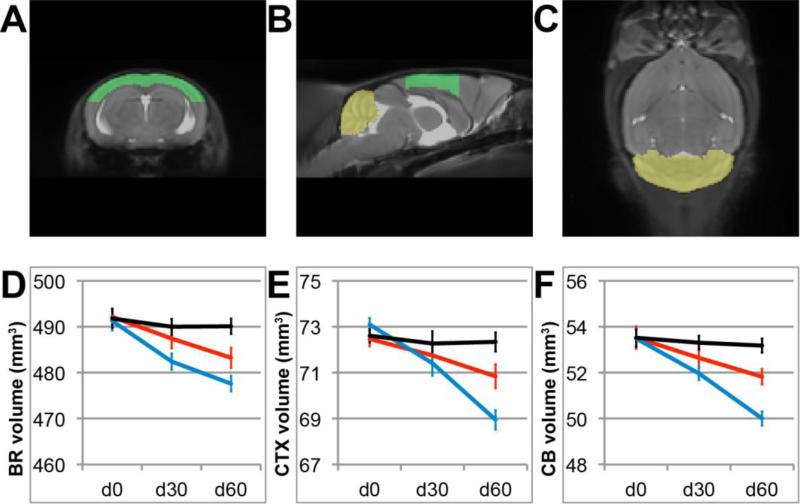
ER beta ligand treated or vehicle treated EAE mice underwent in vivo, longitudinal MRI scanning at day 0, 30 and 60 of EAE. Treatments were initiated after clinical disease onset. Age- and sex-matched healthy controls were scanned in parallel. Whole brain, cerebral cortex and cerebellar volumes were determined at each time point (Fig. 2). Brain volume remained stable over time in the healthy control group, but showed a gradual decrease in the vehicle treated EAE group (time × group interaction p = 1.1 × 10−7). The mean volume of whole brain of mice sixty days after disease induction was 490 mm3 ± 1.7 mm3 (mean ± SEM) in healthy mice and 478 mm3 ± 1.2 mm3 in EAE mice, indicating a 2.4% decrease (p = 5.3 × 10−6) in volume. To assess the effect of ER beta ligand treatment on whole brain atrophy, we then determined the rate of whole brain atrophy in ER beta ligand-treated mice compared to vehicle treated-EAE mice, and found that the mean brain volume decrease was less in ER beta ligand-treated EAE mice (time × group interaction p = 0.0013). The mean volume of the whole brain sixty days after disease induction was 483 mm3 ± 2.2 mm3 in ER beta ligand-treated EAE, indicating a 1.0% difference (p = 0.021) in volume between ER beta ligand treated and vehicle treated EAE mice (Fig. 2D).
Figure 2. Whole brain, cerebral cortical and cerebellar atrophy in EAE are reduced by ER beta ligand treatment.
(A-C) Anatomic delineations of cerebral cortex (green) and cerebellum (yellow) overlaid onto the coronal, sagittal and horizontal planes of a minimum deformation atlas comprising all images from all groups of mice. (D) Mean whole brain volume over time in healthy controls (black), ER beta ligand-treated mice with EAE (red) and vehicle-treated mice with EAE (blue) at d0, d30 and d60. ER beta ligand-treated EAE mice exhibit less brain atrophy than vehicle-treated EAE mice as early as d30. (E) Mean cerebral cortex volume in the same groups. ER beta ligand-treated EAE mice exhibit less atrophy in the cerebral cortex than vehicle-treated EAE mice by d60. (F) Mean cerebellar volumes in the same groups. ER beta ligand-treated EAE mice exhibit less cerebellar atrophy than vehicle-treated EAE mice by d60.
Cerebral cortex volumes of healthy control and EAE mice showed a similar pattern. Cerebral cortex volumes were stable in the healthy control group, while gradually decreasing in the vehicle treated EAE group (time × group interaction p = 4.0 × 10−6). The mean volume of the cerebral cortex at sixty days after disease induction was 72.3 mm3 ± 0.41 mm3 in healthy mice and 68.9 mm3 ± 0.6 mm3 in EAE mice, a 4.6% decrease (p = 1.5 × 10−4) in volume. Cerebral cortex atrophy rates were less in ER beta ligand-treated EAE mice compared to vehicle-treated EAE mice (time × group interaction p = 0.0035). The mean volume of the cerebral cortex at sixty days after disease induction was 70.8 mm3 ± 0.5 mm3 in ER beta ligand-treated EAE, a 2.7% difference (p = 0.014) in volume between ER beta ligand treated and vehicle treated EAE mice (Fig. 2E)
Similarly, a progressive loss of cerebellar volume during EAE was observed in vehicle treated EAE mice compared to healthy mice (time × group interaction p = 1.1 × 10−5). The volume of the whole cerebellum at sixty days after disease induction was 53.2 mm3 ± 0.3 mm3 in healthy mice and 50.0 mm3 ± 0.4 mm3 in EAE mice, a 6.0% decrease (p = 3.1 × 10−6) in volume. ER beta ligand treatment decreased the rate of volume loss compared to vehicle treatment (time x group interaction p = 9.5 × 10−4). The mean volume of the whole cerebellum at sixty days after disease induction was 51.8 mm3 ± 0.4 mm3 in ER beta ligand-treated EAE mice, a 3.6% difference (p = 0.0013) in volume between ER beta ligand treated and vehicle treated EAE mice (Fig. 2F). These in vivo longitudinal MRI results on cerebellar atrophy in gonadally intact mice with EAE extend previous cross-sectional, ex vivo imaging studies in ovariectomized EAE mice that demonstrated preservation of cerebellar cortex volumes during treatment with estradiol, ER alpha ligand, or ER beta ligand treatment (Mackenzie-Graham et al. , 2012b).
4.4. ER beta ligand treatment reduces neuropathology in cerebral cortex during chronic EAE
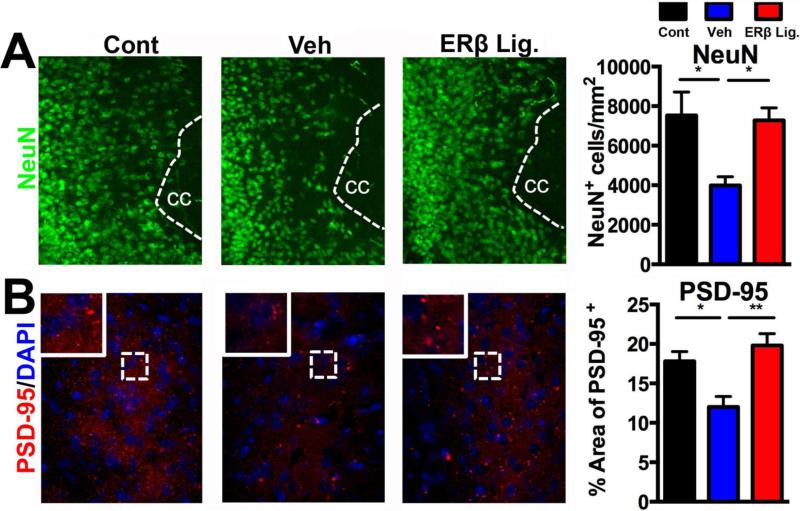
Since longitudinal MRIs showed that ER beta ligand treatment significantly reduced gray matter atrophy in the cerebral cortex during EAE (Fig. 2E), we next determined the effect of ER beta ligand treatment on underlying pathology in cerebral cortical gray matter. Immunofluorescence analysis of cerebral cortical gray matter in vehicle treated EAE compared to healthy controls showed significant decreases in neurons (by NeuN staining) p<0.05 and synapses (by PSD-95 staining) p<0.05 (Fig. 3). ER beta ligand treatment of EAE preserved neurons and synapses in cerebral cortical gray matter (ER beta ligand EAE vs, vehicle EAE: neurons p<0.05; synapses p<0.005) (Fig 3).
Figure 3. ER beta ligand treatment of EAE: protective effects in cerebral cortical gray matter.
Representative 10X images of cerebral cortical gray matter neurons stained for NeuN in green (A) and synapses stained for the postsynaptic protein PSD-95 in red (B) in healthy control (left), vehicle treated EAE (middle), and ER beta ligand treated EAE (right). Quantification of immunofluorescence staining is shown in bar graphs. Vehicle treated EAE mice (blue bars) had fewer NeuN+ cortical neurons and less PSD-95+ synapses in the cerebral cortex as compared to age-matched healthy controls (black bars). ER beta ligand treated EAE mice (red bars), as compared to vehicle treated EAE mice (blue bars), had higher numbers of NeuN+ cortical neurons and PSD-95+ synapses in the cerebral cortex, with values comparable to age-matched healthy controls. In A, cc indicates corpus callosum adjacent to cerebral cortex. In B, tissues were counter stained with the nuclear stain DAPI (blue). Three to five mice were examined for each treatment group. ** p < 0.005, * p < 0.05, with p-values determined by one-way ANOVA.
4.5. ER beta ligand treatment reduces cerebellar neuropathology during chronic EAE
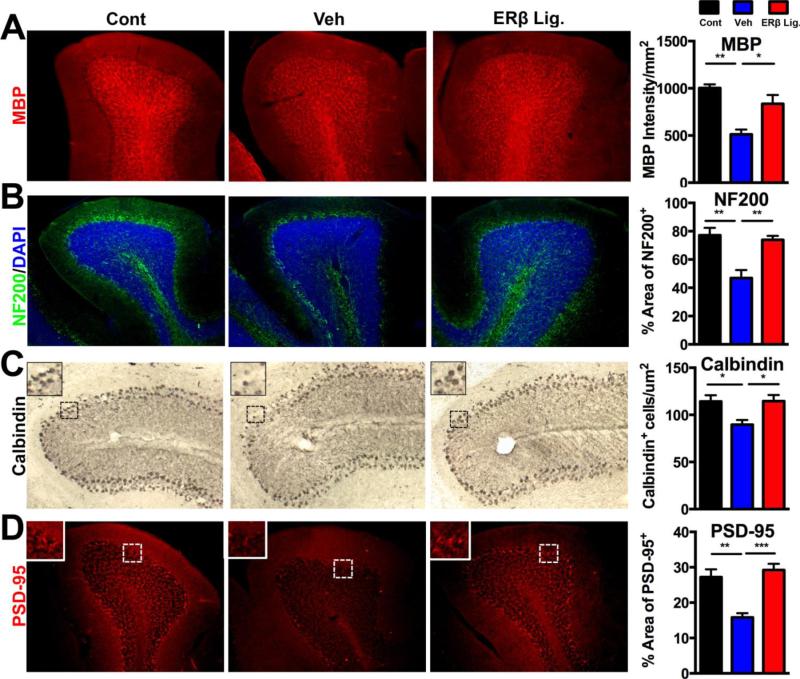
Since longitudinal MRIs showed that ER beta ligand treatment significantly reduced cerebellar atrophy during EAE (Fig. 2F), we determined the effect of ER beta ligand treatment on neuropathology in white and gray matter of cerebellum. Cerebellar white matter showed demyelination, p<0.005, and axonal loss, p<0.005, in vehicle treated EAE compared to healthy controls. ER beta ligand treatment of EAE mice restored myelin and axons (ER beta ligand EAE vs, vehicle EAE: myelin p<0.05, axons p<0.005) to levels near those in healthy controls (Fig. 4 A,B). Cerebellar gray matter showed decreased Purkinje numbers in the Purkinje cells layer, p<0.05, and decreased PSD-95 synaptic staining in the molecular layer, p<0.005, in vehicle treated EAE compared to healthy controls (Fig. 4 C,D). ER beta ligand treated EAE mice showed increased Purkinje cells and synapses (ER beta ligand EAE vs, vehicle EAE: Purkinje cells, p<0.005, synapses, p<0.005) to levels near those in healthy controls (Fig. 4).
Figure 4. ER beta ligand treatment of EAE: protective effects in cerebellar white and gray matter.
(A) Representative 10X images of cerebellar white matter immunofluorescence stained with MBP (red, top) and Neurofilament-200 (green, bottom) in healthy control (left), vehicle treated EAE (middle), and ER beta ligand treated EAE (right). Quantification of myelin staining intensity by MBP and axonal densities by NF200 staining is shown by bar graphs. Vehicle treated EAE (blue bars) as compared to healthy controls (black bars) showed reduced myelin staining, while levels of MBP staining in ER beta ligand treated were increased and similar to those in healthy controls. Axon numbers were reduced in vehicle treated EAE compared to healthy controls, wile they were increased in ER beta ligand treated EAE mice. (B) Representative 10X images of cerebellum including the Purkinje layer, stained for Calbindin using chromogen immunohistochemistry (brown, top) and PSD-95 using immunofluorescence (red, bottom) in the same groups of mice. Vehicle treated EAE mice, had fewer Purkinje cells in the Purkinje layer and less PSD-95 staining in the molecular layer of the cerebellum as compared to age-matched healthy controls. ER beta ligand treated EAE mice, as compared to vehicle treated EAE mice, had higher numbers of Purkinje cells and higher levels of PSD-95 staining. IN B, tissues were counter stained with the nuclear stain DAPI (blue). Three to five mice were examined for each treatment group. *** p < 0.0001, ** p < 0.005, * p < 0.05, with p-values determined by one-way ANOVA.
5. Discussion
ER beta ligand treatment provides protection from chronic EAE including, and extending beyond, the spinal cord (Fig. 1). Specifically, ER beta ligand treatment initiated after disease onset was shown to slow brain atrophy by MRI in cerebral cortex and cerebellum (Fig. 2), preserve myelin and axons in white matter and restore neuronal and synaptic loss in gray matter (Fig. 3,4). These preclinical data warrant consideration of ER beta ligand treatment as a novel neuroprotective treatment for MS.
ER beta ligand can be considered a “next generation estriol” treatment, since estriol is a naturally occurring estrogen with higher affinity for ER beta than ER alpha (Katzenellenbogen, 1984, Kuiper, Carlsson, 1997). Comparing and contrasting effects of estriol versus ER beta ligand treatment in EAE can disentangle which effects of estriol treatment may be mediated by ER beta. Preclinical studies using estriol treatment in EAE, like those using ER beta ligand treatment, have shown beneficial effects in spinal cord including sparing of myelin and axons and improved standard EAE severity scores (Kim, Liva, 1999). Beyond spinal cord in EAE, estriol treatment spared hippocampal CA1 atrophy, reduced neuronal and synaptic loss, and improved cognitive function as assessed by electrophysiology (Ziehn, Avedisian, 2012). Here, ER beta ligand treatment in EAE spared cerebral cortical atrophy by MRI (Fig. 2) and reduced neuronal and synaptic loss (Fig. 3). A major difference between estriol and ER beta ligand treatment in EAE is that estriol was anti-inflammatory during peripheral autoantigen specific responses (Bebo et al. , 2001, Kim, Liva, 1999, Papenfuss, Powell, 2011), while ER beta ligand treatment was not (Du, Sandoval, 2011, Tiwari-Woodruff, Morales, 2007). Since a role for ER alpha in estrogen mediated anti-inflammatory effects in the immune system have been shown using take away (ER alpha knock out mice)(Liu et al. , 2003, Polanczyk et al. , 2003) and add back (treatments with ER alpha specific ligands) experiments (Morales, Loo, 2006), this is likely due to low level ER alpha ligation in the immune system with estriol, but not with ER beta ligand, treatment.
Results in MS clinical trials using estriol treatment, combined with known differences in properties of estriol versus ER beta ligand, can be use to hypothesize what might occur in an MS trial using ER beta ligand treatment. Estriol treatment in MS trials had anti-inflammatory effects in peripheral blood mononuclear cells (Gold et al., 2009, Soldan et al. , 2003), but this may not occur with ER beta ligand treatment due to the lack of ER alpha ligand binding. Estriol treatment reduced gadolinium enhancing lesions in an MS trial with enhancing lesions on monthly MRIs as the primary outcome (Sicotte, Liva, 2002), but this too may not occur in a trial using ER beta ligand treatment since enhancing lesions are linked to peripheral immune activation. Estriol treatment reduced relapse rates in an MS trial with relapses as the primary outcome (Voskuhl, Wang, 2016). This also may not occur in a trial using ER beta ligand treatment given that enhancing lesions are a biomarker for relapses. However, exploratory outcomes in the estriol Phase 2b trial, designed to detect a decrease in relapse rates, showed that higher estriol levels correlated with improved cognitive function as compared to placebo treatment (Voskuhl, Wang, 2016). This was unlikely due to anti-inflammatory effects of estriol since FDA approved disease modifying treatments in MS have not shown significant cognitive improvement over and above placebo treatment during trials. Instead this suggests a direct neuroprotective effect of estriol treatment on cognition (Voskuhl, Wang, 2016), as was previously observed in EAE (Ziehn, Avedisian, 2012). Given the neuroprotective effects of ER beta ligand treatment in cerebral cortex in EAE (Fig. 3), it is likely that ER beta ligand treatment would also improve cognitive function in an MS trial. Another interesting exploratory finding in the estriol Phase 2b trial was that there was less cerebral cortical gray matter atrophy at the MRI timepoint when estriol levels were highest. This sparing of atrophy in cerebral cortex gray matter correlated with improved cognitive performance. Also, it was found in subjects that were enhancing lesion negative, not enhancing lesion positive. Together, these findings suggested direct neuroprotective effects of estriol treatment in the MS trial (Voskuhl, Wang, 2016). Based on the above, we hypothesize that sparing of cerebral cortical gray matter atrophy may also occur with ER beta ligand treatment in an MS clinical trial. This hypothesis is supported by the observation here that ER beta ligand treatment spared cerebral cortical atrophy in EAE using in vivo longitudinal MRIs (Fig. 2).
Direct neuroprotective effects of treatment with the generic ER beta ligand have been shown using CNS cell specific conditional knock outs of ER beta receptor on oligodendrocytes during EAE, with beneficial effects on remyelination (Crawford, Mangiardi, 2010, Khalaj, Yoon, 2013). This is however not mutually exclusive of other neuroprotective effects. Striking effects of ER beta ligand treatment were observed herein on both Purkinje cell number and PSD-95, a post synaptic marker on Purkinje cells receiving input from incoming fibers from deep cerebellar nuclei (Fig. 4). This is consistent with expression of ER beta receptor on Purkinje cells (Andreescu et al. , 2007, Shughrue et al. , 1997), and together suggests direct effects of ER beta ligand treatment on Purkinje cells in the cerebellum. Regarding other potential mechanisms, previous treatment with generic ER beta ligand did not modulate peripheral immune responses or reduce quantitative levels of inflammation in the CNS during EAE (Tiwari-Woodruff, Morales, 2007). However, qualitative effects on microglia and dendritic cells have been shown (Drew and Chavis, 2000, Du, Sandoval, 2011, Papenfuss, Powell, 2011, Saijo, Collier, 2011). Whether direct neuroprotective effects of ER beta ligand treatment on microglia/dendritic cells occur in EAE is unknown. Use of conditional knock outs targeting removal of ER beta receptor in cells of the monocyte/dendritic cell lineage are needed to distinguish between direct effects of ER beta ligand binding to these cells versus downstream indirect effects.
Mechanisms underlying clinical disease protection in EAE differ depending on the ER beta ligand used. In the current study using the ER beta ligand AC186 (McFarland, Price, 2013), a reduction in quantitative levels of macrophages/activated microglia, but not T lymphocytes, in the CNS was observed (Fig. 1). This observation was distinct from results using the generic ER beta ligand DPN, which did not induce quantitative decreases in CD45 or Iba-1 staining in the CNS (Tiwari-Woodruff, Morales, 2007), even though both treatments spared axons and myelin in spinal cord white matter. The effect of the AC186 treatment on reducing macrophages/activated microglia in spinal cord, with no effect T lymphocytes, would suggest an effect of this particular ER beta ligand on the innate immune system, but not the adaptive immune system. An effect on the innate immune system could represent an additional beneficial mechanism with relevance to chronic progressive disease. Treatment of EAE with another ER beta ligand, LY3201, reduced iNOS and NFkB expression in microglia and infiltrating T cells (Wu et al., 2013). Also, treatment of EAE with yet another ER beta ligand (Indazole-Cl) was anti-inflammatory in peripheral immune responses with subsequent decreases in infiltration of both T cell and macrophages into the CNS (Moore et al., 2014). Thus, while protective effects of treatment with several ER beta ligands have been shown in chronic EAE, the degree to which this is mediated through direct effects on CNS cells versus indirect effects on peripheral immune cells varies based on which ER beta ligand is used. What mediates unique properties of each ER beta ligand remains unknown. The multifaceted nature of mechanisms underlying beneficial effects of ER beta ligand treatments are not surprising given its relationship to estrogen, a hormone with a vast array of biological effects. It is likely that a treatment with multifaceted effects is what is required to impact neurodegeneration in a complex neurodegenerative disease such as MS. The current challenge is to identify the most physiologically relevant biological properties for the design of an ER beta ligand treatment optimized for neuroprotective efficacy with minimal off target effects.
6. Conclusions
Treatment with ER beta ligand as a novel neuroprotective agent for MS is expected to be well tolerated since most deleterious effects of estrogen treatment on breast and uterus are mediated by ER alpha, not ER beta. Standard pharmacokinetic, toxicology, and dose finding studies are needed comparing various ER beta ligands in preclinical models as a prelude to the design of a pilot clinical trial in women and men with MS. Given ER beta ligand's neuroprotective properties, such trials would not be limited to RRMS, but would also include progressive MS. ER beta ligand treatment may slow cerebellar atrophy and brain gray matter atrophy, particularly in the cerebral cortex, suggesting use of these outcomes as biomarkers to detect neuroprotective effects in MS clinical trials testing ER beta ligands.
Highlights.
MS protection during pregnancy led to clinical trials of estriol treatment in MS.
Estrogen receptor (ER) beta ligand could be a “next generation estriol” treatment.
ER beta ligand treatment is neuroprotective in spinal cord and brain in EAE.
ER beta ligand treatment spares gray matter atrophy on longitudinal in vivo MRIs.
Treatment preserves not only myelin and axons in WM, also neurons and synapses in GM.
Acknowledgements
We thank Diana Bok, Paula G. Mendoza, Alex Hoffman, and Rojan Kavosh for technical laboratory assistance.
Funding sources
This work was supported by the National Multiple Sclerosis Society's Fast Forward Program, the Conrad N. Hilton Foundation (#20150232), the California Community Foundation (#BAPP-15-118094), the Jack H. Skirball Foundation and the Tom Sherak MS Hope Foundation, as well as by the UCLA Laboratories of Neuroendocrinology Training Grant (5T32HD007228).
Abbreviations
- MS
Multiple sclerosis
- EAE
experimental autoimmune encephalomyelitis
- ER
estrogen receptor
- MBP
myelin basic protein
- NF200
neurofilament 200
- PSD-95
post synaptic density-95
- WM
white matter
- GM
gray matter
- CNS
central nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure.
Dr. Voskuhl in an inventor on patents owned by UCLA for estriol treatment in MS. No other authors have conflicts to disclose.
References
- Aharoni R, Sasson E, Blumenfeld-Katzir T, Eilam R, Sela M, Assaf Y, et al. Magnetic resonance imaging characterization of different experimental autoimmune encephalomyelitis models and the therapeutic effect of glatiramer acetate. Exp Neurol. 2013;240:130–44. doi: 10.1016/j.expneurol.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, et al. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. J Neurosci. 2007;27:10832–9. doi: 10.1523/JNEUROSCI.2588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebo BF, Jr., Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–9. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–60. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–91. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Crawford DK, Mangiardi M, Song B, Patel R, Du S, Sofroniew MV, et al. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010;133:2999–3016. doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2014;111:2806–11. doi: 10.1073/pnas.1307091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Sandoval F, Trinh P, Umeda E, Voskuhl R. Estrogen receptor-beta ligand treatment modulates dendritic cells in the target organ during autoimmune demyelinating disease. Eur J Immunol. 2011;41:140–50. doi: 10.1002/eji.201040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Petit GH, Gouras GK, Brundin P, Olsson R. Nonsteroidal selective androgen receptor modulators and selective estrogen receptor beta agonists moderate cognitive deficits and amyloid-beta levels in a mouse model of Alzheimer's disease. ACS Chem Neurosci. 2013;4:1537–48. doi: 10.1021/cn400133s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Sasidhar MV, Morales LB, Du S, Sicotte NL, Tiwari-Woodruff SK, et al. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERalpha). Lab Invest. 2009;89:1076–83. doi: 10.1038/labinvest.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head KA. Estriol: safety and efficacy. Altern Med Rev. 1998;3:101–13. [PubMed] [Google Scholar]
- Katzenellenbogen BS. Biology and receptor interactions of estriol and estriol derivatives in vitro and in vivo. Journal of Steroid Biochemistry. 1984;20:1033–7. doi: 10.1016/0022-4731(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Khalaj AJ, Yoon J, Nakai J, Winchester Z, Moore SM, Yoo T, et al. Estrogen receptor (ER) beta expression in oligodendrocytes is required for attenuation of clinical disease by an ERbeta ligand. Proc Natl Acad Sci U S A. 2013;110:19125–30. doi: 10.1073/pnas.1311763110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RY, Hoffman AS, Itoh N, Ao Y, Spence R, Sofroniew MV, et al. Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;274:53–61. doi: 10.1016/j.jneuroim.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52:1230–8. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, et al. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–93. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Lauritzen C. Results of a 5 years prospective study of estriol succinate treatment in patients with climacteric complaints. Hormone and Metabolic Research. 1987;19:579–84. doi: 10.1055/s-2007-1011886. [DOI] [PubMed] [Google Scholar]
- Lelu K, Laffont S, Delpy L, Paulet PE, Perinat T, Tschanz SA, et al. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2386–93. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- Lepore S, Waiczies H, Hentschel J, Ji Y, Skodowski J, Pohlmann A, et al. Enlargement of cerebral ventricles as an early indicator of encephalomyelitis. PLoS One. 2013;8:e72841. doi: 10.1371/journal.pone.0072841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg BS, Johansson ED, Nilsson BA. Plasma levels of nonconjugated oestrone, oestradiol-17beta and oestriol during uncomplicated pregnancy. Acta Obstet Gynecol Scand Suppl. 1974;32:21–36. doi: 10.3109/00016347409156390. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–43. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Liu HB, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor alpha mediates estrogen's immune protection in autoimmune disease. J Immunol. 2003;171:6936–40. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Rinek GA, Avedisian A, Gold SM, Frew AJ, Aguilar C, et al. Cortical atrophy in experimental autoimmune encephalomyelitis: in vivo imaging. Neuroimage. 2012a;60:95–104. doi: 10.1016/j.neuroimage.2011.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Tiwari-Wooddruff S, Sharma G, Aguilar C, Vo KT, Strickland LV, et al. Purkinje cell loss in experimental autoimmune encephalomyelitis. Neuroimage. 2009;48:637–51. doi: 10.1016/j.neuroimage.2009.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie-Graham AJ, Rinek GA, Avedisian A, Morales LB, Umeda E, Boulat B, et al. Estrogen treatment prevents gray matter atrophy in experimental autoimmune encephalomyelitis. J Neurosci Res. 2012b;90:1310–23. doi: 10.1002/jnr.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiardi M, Crawford DK, Xia X, Du S, Simon-Freeman R, Voskuhl RR, et al. An animal model of cortical and callosal pathology in multiple sclerosis. Brain Pathol. 2011;21:263–78. doi: 10.1111/j.1750-3639.2010.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Price DL, Davis CN, Ma JN, Bonhaus DW, Burstein ES, et al. AC-186, a selective nonsteroidal estrogen receptor beta agonist, shows gender specific neuroprotection in a Parkinson's disease rat model. ACS Chem Neurosci. 2013;4:1249–55. doi: 10.1021/cn400132u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SM, Khalaj AJ, Kumar S, Winchester Z, Yoon J, Yoo T, et al. Multiple functional therapeutic effects of the estrogen receptor beta agonist indazole-Cl in a mouse model of multiple sclerosis. Proc Natl Acad Sci U S A. 2014;111:18061–6. doi: 10.1073/pnas.1411294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26:6823–33. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfuss TL, Powell ND, McClain MA, Bedarf A, Singh A, Gienapp IE, et al. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J Immunol. 2011;186:3346–55. doi: 10.4049/jimmunol.1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, et al. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003;163:1599–605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19:1033–48. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–95. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. Automated graph-based analysis and correction of cortical volume topology. IEEE Trans Med Imaging. 2001;20:1167–77. doi: 10.1109/42.963819. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52:421–8. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003;171:6267–74. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, et al. Neuroprotection mediated through estrogen receptor-{alpha} in astrocytes. Proc Natl Acad Sci U S A. 2011;108:8867–72. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Kurth F, Itoh N, Mongerson CR, Wailes SH, Peng MS, et al. Bringing CLARITY to gray matter atrophy. Neuroimage. 2014;101:625–32. doi: 10.1016/j.neuroimage.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33:105–15. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, et al. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. J Neurosci. 2013;33:10924–33. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Manabe A, Okada M, Kurioka H, Kanasaki H, Miyazaki K. Efficacy and safety of oral estriol for managing postmenopausal symptoms. Maturitas. 2000;34:169–77. doi: 10.1016/s0378-5122(99)00108-5. [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104:14813–8. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl R. Rebound Relapses After Ceasing Another Disease-Modifying Treatment in Patients With Multiple Sclerosis: Are There Lessons to Be Learned? JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.0934. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. 2012;8:255–63. doi: 10.1038/nrneurol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Wang H, Wu TC, Sicotte NL, Nakamura K, Kurth F, et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:35–46. doi: 10.1016/S1474-4422(15)00322-1. [DOI] [PubMed] [Google Scholar]
- Wisdom AJ, Cao Y, Itoh N, Spence RD, Voskuhl RR. Estrogen receptor-beta ligand treatment after disease onset is neuroprotective in the multiple sclerosis model. J Neurosci Res. 2013;91:901–8. doi: 10.1002/jnr.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WF, Tan XJ, Dai YB, Krishnan V, Warner M, Gustafsson JA. Targeting estrogen receptor beta in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2013;110:3543–8. doi: 10.1073/pnas.1300313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Dervin SM, O'Dell TJ, Voskuhl RR. Estriol preserves synaptic transmission in the hippocampus during autoimmune demyelinating disease. Lab Invest. 2012;92:1234–45. doi: 10.1038/labinvest.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–86. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]