Abstract
Aim
Statins reduce morbidity and mortality among patients with diabetes, but their use remains suboptimal. Understanding trends in statin use may inform strategies for improvement.
Methods
We enrolled a national, retrospective cohort of 899,664 veterans aged ≥40 years with diabetes in 2003. We followed them through 2011, dividing the nine-year follow-up into 90-day periods. For each period, we determined statin use, defined as possession of ≥30-day supply. We examine factors associated with statin uptake among baseline non-users with a multivariate model.
Results
Baseline prevalence of statin use was 43%, increased by 1.8% per period (p for trend < 0.001), and reached a maximum of ~59%. Statin use among non-Hispanic racial/ethnic minorities lagged behind their white counterparts. Among baseline non-users, statin use was 9% after Year 1 and reached 36% by Year 9. Factors associated with statin uptake included use of hypoglycemic agents, HbA1c between 7 and 8.9% (53 – 74 mmol/mol), hypertension, heart failure, peripheral vascular disease, and Hispanic ethnicity.
Conclusion
Statin use is slowly increasing among patients with diabetes, and at varying rates within subgroups of this population. Policies that prioritize these subgroups for statin promotion may help guide future, intervention-based research to increase compliance with current guidelines.
Keywords: HMG-CoA reductase inhibitors, racial disparities, cholesterol treatment guidelines, temporal trends
1. INTRODUCTION
Patients with diabetes have a well-established elevated risk of cardiovascular disease. Statins reduce the risk of all-cause mortality, cardiovascular mortality, stroke, and myocardial infarction for patients with diabetes.1–3 Statins may also reduce the risk of diabetic complications, such as foot ulcers and amputations.4–6 Benefits of statins are not restricted to patients with marked hyperlipidemia or known cardiovascular disease.7
Recommendations regarding statin use have been relatively constant for patients with diabetes over the past 15 years. The Adult Treatment Panel (ATP) III guideline published in 2002 recommend statins for patients with diabetes and an additional risk factor: hypertension, older age (≥45 years for men and ≥55 year for women), a family history of cardiovascular disease, or active tobacco use.8 Given the prevalence of these risk factors, the vast majority of patients with diabetes would have qualified for statin use while ATP III guidelines were in effect even if low density lipoprotein cholesterol (LDL-c) levels were not elevated.9 Recommendations recently simplified. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines now advocate for statin use among patients with diabetes regardless of additional risk factors or hypercholesterolemia.10 Despite guideline updates, the core message remains unchanged: the vast majority of patients with diabetes would benefit from statin use.
Despite consistent core of recommendations since 2002, less than 60% of the current population of patients with diabetes is taking a statin.11,12 While these cross-sectional estimates are informative, the adoption of statins has likely been dynamic, changing over time and in different subgroups. Understanding the real-world trends in statin use in a national cohort may help inform initiatives to optimize statin use for patients with diabetes and increase compliance with guideline recommendations.
In this paper, we describe longitudinal trends in statin use within a national cohort of patients with diabetes while the ATP III guidelines were in effect (2003 – 2011). We also investigated clinical factors and patient demographics associated with statin use among baseline non-users.
2. METHODS
2.1 Study Design and Data Sources
This is a national, retrospective cohort study of all patients with diabetes who were at least 40 years old and treated in the U.S. Department of Veterans Affairs (VA) healthcare system during 2003. VA and Medicare data was used to identify patients with diabetes based on whether 1) they received at least one prescription for a diabetes medication in 2003, or 2) two or more International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 250.xx for diabetes were present in any in-patient or out-patient visits in 2001 – 2002.13 Patients were followed from 2003 until death or December 31, 2011 (study completion). Therefore, the entire study took place prior to the 2013 ACC/AHA guidelines and while ATP III was in effect.
The cohort was constructed using de-identified data from the VA National Patient Care Database, the VA Decision Support System Pharmacy Datasets, and the Centers for Medicare and Medicaid Services Medicare claims files, including Part D Event files. The Edward Hines, Jr. Veterans Affairs Hospital IRB approved this study.
2.2 Outcome
Our primary outcome was the prevalence of statin use over time, among the entire cohort. Statin use was defined as receipt of at least a 30-day supply during a 90-day period. This definition provided a lenient estimate of statin use because 1) possessing a medication does not necessarily indicate taking it, and 2) one pill every three days is a modest criterion for use. The definition was purposefully constructed this way so that estimates of suboptimal use were conservative. The nine-year study was divided into 90-day periods, with the baseline period defined as January 1, 2003 through March 30, 2003. If a patient filled a prescription at the end of a 90-day period, the remaining days’ supply was rolled over to the next period. As secondary analyses, we also examined statin use over time among: 1) patients not receiving cholesterol lowering medications in the baseline period, and 2) patients with comorbid cardiovascular disease, defined as myocardial infarction, heart failure, stroke, or peripheral arterial disease diagnosed prior to or during the study. These two are not mutually exclusive subgroups; a patient could be included in neither, one, or both of these subgroup analyses.
2.3 Explanatory Variables
The following variables were assessed at baseline and modeled as time-independent: age at study start date, sex, race/ethnicity, marital status, and whether diabetes was diagnosed more than five years prior to the study start date. Co-existing conditions, HbA1c, body mass index (BMI), LDL-c, diabetes medication use, and use of non-statin cholesterol lowering medication were all assessed at baseline and for each period as time-varying covariates. All records for fiscal year 2002 were used to obtain values for the baseline period. If individuals had multiple measurements of HbA1c, body mass index (BMI), and LDL-c taken within the baseline period or during a follow-up period, we calculated an average value across time points within the same period. When there were no measures of an independent variable during a follow-up period, we used the last observation carried forward (LOCF) method to impute the value for the current period. Missing values for the baseline period were not imputed. Persons whose values were not measured or missing at baseline for any other reason were classified as unknown. Of the 25 million person-periods, 49% of A1c values and 52% of LDL-c were imputed with the LOCF method, while 19% of A1c values and 22% of LDL-c were classified as missing.
Comorbidities were identified using the Elixhauser algorithm for the baseline period using all records from the past one year and for each follow-up period.14 The subset of patients with cardiovascular disease was identified using this algorithm’s definitions for heart failure, stroke, and peripheral vascular disease, with the addition of myocardial infarction, which was identified by ICD-9 code 410. Anti-diabetic medications were also identified at baseline and during each follow-up period. Possession of 30-day supply or more of insulin and oral agents during the last year before the baseline or during each follow-up period was used to identify whether a patient used insulin, or oral agents, or both. Similar to our definition of statin use, use of non-statin cholesterol-lowering medications was identified for each period separately. A patient who filled a non-statin cholesterol-lowering medication for 30 days or longer during a period was defined as a user of non-statin cholesterol medications. Non-statin cholesterol-lowering medications included fibrates, bile acid sequestrants, niacin, or ezetimibe.
2.4 Statistical Analysis
Descriptive statistics were computed for the entire cohort, the subset of the sample not receiving cholesterol-lowering medications during the baseline period, and the subset with cardiovascular disease. Statin use for the overall cohort as well as the two subgroups were identified for each of the 36 follow-up periods and plotted for trends over time. Patients taking a statin may be more likely to survive, such that an increase in the proportion of the cohort taking a statin over time may reflect the death of patients not on a statin, rather than initiation or re-introduction of this drug class (survival bias). To assess for this potential bias, we also analyzed the trend in statin use among patients surviving to the end of the nine-year study.
We examined factors affecting statin initiation among patients not receiving cholesterol-lowering medications during the baseline period in multivariable analyses. Statin use was modeled as time-varying to best reflect the complicated nature of discontinuations and/or switches to other classes of cholesterol-lowering medications. Each patient can potentially be represented in our data for up to 36 periods. To account for the repeated nature of our data and correct for clustering in estimating standard errors, we modeled statin use as a function of the explanatory variables using a two-level, random-intercept logistic regression. Stata SE v14 was used for all statistical analysis (StataCorp, College Station, TX).
3. RESULTS
The total cohort consisted of 899,664 patients with diabetes aged 40 years or older who were treated in the VA healthcare system. Of those, 480,111 (53.4%) did not receive any cholesterol-lowering medications during the baseline period and 516,407 (57.4%) did not receive a statin. The majority (89.1%) of patients on a cholesterol-lowering medication with a cholesterol measurement at baseline had an LDL-c at or under the ATP III goal of 130 mg/dL. Of the total cohort, 494,274 (54.9%) had baseline comorbid cardiovascular disease; and 520,726 (57.9% of the total cohort) survived to the end of the nine-year study. The average length of follow-up was 28 ± 11 periods (82 ± 34 months).
The total cohort was predominantly male (98%), with 57% at least 65 years old and 77% non-Hispanic white (Table 1). Eighteen percent had diabetes for five years or longer prior to the study start date. The majority (82%) were not taking insulin. The proportion of the total cohort with HbA1c <7% (53 mmol/mol) was 35.4%; 27.3% had HbA1c values between 7 and 8.9% (53 – 74 mmol/mol).
Table 1.
Patient characteristics of the total cohort, stratified by cholesterol drug use and cardiovascular disease, all assessed during the baseline period
| Characteristic | Total cohort | Cholesterol drug use | Cardiovascular disease | ||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| N | 899,664 | 419,553 | 480,111 | 494,274 | 405,390 |
| Age, mean (SD) | 67.1 (10.6) | 67.5 (9.7) | 66.7 (11.3) | 68.8 (10.2) | 65.0 (10.7) |
| Male* | 97.9 | 98.3 | 97.6 | 98.4 | 97.4 |
| Race/ethnicity | |||||
| NH White | 76.7 | 82.1 | 71.9 | 80.2 | 72.5 |
| NH Black | 14.3 | 10.6 | 17.6 | 12.1 | 17.0 |
| Hispanic | 6.7 | 5.9 | 7.4 | 5.5 | 8.2 |
| Other | 2.3 | 1.4 | 3.1 | 2.3 | 2.3 |
| Married | 65.7 | 69.7 | 62.2 | 67.2 | 63.8 |
| Diabetes duration ≥5 years | 18.1 | 19.3 | 17.0 | 18.8 | 17.2 |
| HbA1c, % (mmol/mol) | |||||
| <7 (53) | 35.4 | 38.2 | 32.9 | 32.6 | 38.7 |
| 7 – 8.9 (53 – 74) | 27.3 | 32.3 | 23.0 | 26.4 | 28.4 |
| ≥ 9 (75) | 9.5 | 9.9 | 9.2 | 9.0 | 10.2 |
| Unknown | 27.8 | 19.7 | 34.9 | 32.0 | 22.6 |
| Diabetes medications | |||||
| None | 35.8 | 21.5 | 48.4 | 38.6 | 32.4 |
| Oral only | 46.2 | 56.5 | 37.1 | 42.3 | 50.9 |
| Oral and insulin | 8.0 | 11.2 | 5.2 | 8.0 | 8.0 |
| Insulin only | 10.0 | 10.8 | 9.3 | 11.1 | 8.7 |
| LDL cholesterol, mg/dL | |||||
| <70 | 7.5 | 10.0 | 5.3 | 7.7 | 7.2 |
| 70 – 99.9 | 20.7 | 26.5 | 15.6 | 20.2 | 21.3 |
| 100 – 129.9 | 19.4 | 20.8 | 18.1 | 17.2 | 22.0 |
| ≥ 130 | 11.1 | 10.9 | 11.2 | 9.5 | 13.0 |
| Unknown | 41.4 | 31.8 | 49.8 | 45.4 | 36.5 |
| Cholesterol medications | |||||
| None | 53.4 | 0 | 100 | 52.6 | 54.5 |
| Statins | 39.4 | 84.6 | 0 | 40.5 | 38.1 |
| Non-statins | 4.0 | 8.5 | 0 | 3.6 | 4.4 |
| Both | 3.2 | 6.8 | 0 | 3.3 | |
| BMI, kg/m2 | |||||
| <25 | 14.2 | 11.2 | 16.7 | 14.8 | 13.4 |
| 25 – 29.9 | 34.1 | 35.4 | 33.0 | 34.0 | 34.3 |
| ≥30 | 46.0 | 51.1 | 41.6 | 43.6 | 49.0 |
| Unknown | 5.7 | 2.3 | 8.7 | 7.7 | 3.3 |
| Elixhauser comorbidity count, mean (SD) | 2.5 (2.1) | 2.5 (1.9) | 2.5 (2.2) | 3.0 (2.4) | 1.8 (1.4) |
| Cardiovascular comorbidity | |||||
| Congestive heart failure | 15.7 | 16.8 | 14.8 | 28.6 | 0.0 |
| Valvular disease | 8.9 | 9.6 | 8.3 | 13.2 | 3.5 |
| Pulmonary/circulation disease | 1.9 | 1.8 | 2.0 | 3.0 | 0.6 |
| PVD | 16.3 | 17.9 | 15.0 | 29.7 | 0.0 |
| Hypertension | 73.8 | 80.3 | 68.0 | 76.5 | 70.4 |
Values represent percentages of the respective cohorts unless otherwise stated; Cholesterol drug use indicates use of any class of cholesterol-lowering medications for 30 days or longer in the baseline period (January 1, 2013 – March 31, 2013); Cardiovascular disease indicate presence of myocardial infarction, heart failure, stroke, or PVD. NH = non-Hispanic; LDL = low density lipoprotein; BMI = body mass index; PVD = peripheral vascular disease
3.1 Longitudinal Trends in Statin Use
Statin use among the total cohort increased from 42.6% at baseline to 59.2% toward the end of the nine-year follow-up period (Figure 1). Patients were 1.8% more likely to use statins in each succeeding 90-day period (unadjusted p-value for trend <0.001). Our sensitivity analysis, which restricted the cohort to those who survived the entire study period to account for survival bias, demonstrated a similar trend to the total cohort. Statin use among the surviving cohort increased from 44% to 60%. Among those with cardiovascular disease at baseline, statin use increased from 43% to 58%. Among those not taking a cholesterol-lowering medication during the baseline period, the shift towards statin use was slow. After the first year (at the conclusion of period 4), 15% were taking a statin. This proportion reached 46% towards the end of the nine-year study.
Figure 1.
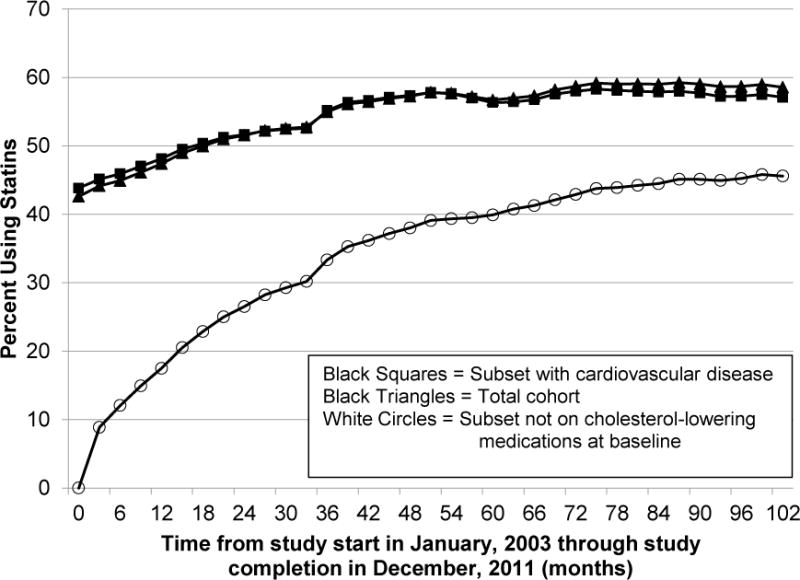
Longitudinal trends in statin use among the total cohort, the subset not on cholesterol-lowering medications at baseline, and the subset with cardiovascular disease. One period was three months in length.
Statin use increased for patients of all races and ethnicities, although the amount of improvement differed (Figure 2). Use among Hispanic patients increased the most (36% to 61%), reaching parity with non-Hispanic white patients toward the end of the study. Use among non-Hispanic black patients started out considerably lower than that for non-Hispanic white patients (32% vs 46%) and reached a high of 54%. In contrast, patients in the Other group (non-Hispanic, non-black minorities, or those whose race/ethnicity is unknown) started with the lowest statin use and experienced the slowest gains in uptake (26% to 38%).
Figure 2.
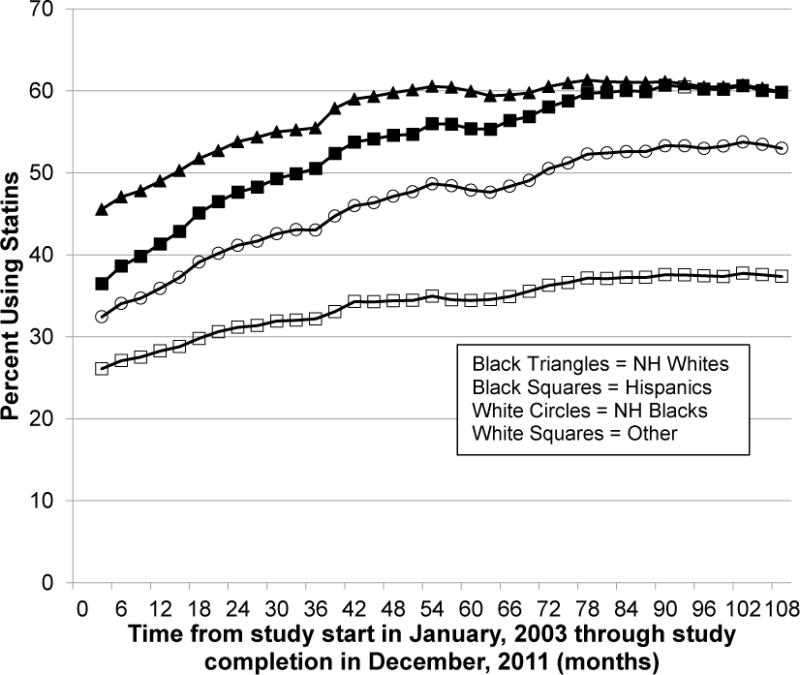
Longitudinal trends in statin use within the total cohort, stratified by race/ethnicity. One period was three months in length. NH= Non-Hispanic.
3.2 Factors Associated with Statin Uptake among Patients Not Taking Cholesterol Lowering Medications at Baseline
Among patients who were not taking a cholesterol-lowering medication during the baseline period, clinically overt diabetes– i.e. necessitating hypoglycemic agents or evidenced by elevated HbA1c levels– was associated with increased odds of statin uptake. Specifically, as the intensity of diabetic medication regimens increased from diet-control to oral hypoglycemic agents, and the introduction of insulin, the odds of statin use increased. Compared to patients who were not taking any hypoglycemic medications, patients taking both oral agents and insulin experienced a 19-fold increase in the likelihood of starting a statin (p <0.001, Table 2). Compared to patients with HbA1c levels below 7% (53 mmol/mol), those with HbA1c values between 7% and 8.9% (53 – 74 mmol/mol) were 10% more likely (Adjusted Odds Ratios [AOR] = 1.10; 95% CI, 1.09 – 1.10) to start a statin, whereas those with unmeasured HbA1c were less likely to start a statin (AOR = 0.47; 95% CI, 0.47 – 0.48; p <0.001). After adjustment, patients diagnosed with diabetes more than five years prior to the baseline were less likely to initiate a statin (AOR= 0.61; 95% CI, 0.59 – 0.63; p <0.001). Comorbid cardiovascular disease was associated with increased odds of statin uptake. Specifically, AORs for those with hypertension, chronic heart failure, or peripheral vascular disorders were 2.78 (95% CI, 2.73 – 2.79), 1.09 (95% CI, 1.08 – 1.10), and 1.18 (95% CI, 1.17 – 1.19), respectively (all p-values <0.001, Table 2).
Table 2.
Odds ratios and 95% confidence intervals of statin uptake among the subset of patients not taking cholesterol lowering medications at baseline (n= 459,601)*
| Variable | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Age | 0.957 (0.956–0.958) | < 0.001 | 0.958 (0.957 – 0.959) | < 0.001 |
| Male | 1.20 (1.13 – 1.23) | < 0.001 | 0.89 (0.83 – 0.95) | 0.001 |
| Race/ethnicity [NH White]* | ||||
| NH Black | 0.95 (0.92 – 0.97) | < 0.001 | 0.76 (0.72 – 0.77) | < 0.001 |
| Hispanic | 1.61 (1.55 – 1.67) | < 0.001 | 1.11 (1.07 – 1.17) | < 0.001 |
| Other | 0.20 (0.19 – 0.21) | < 0.001 | 0.62 (0.58 – 0.66) | < 0.001 |
| Married [not married]† | 1.26 (1.24 – 1.29) | < 0.001 | 1.30 (1.27 – 1.33) | < 0.001 |
| Diabetes duration ≥5 years | 1.06 (1.03 – 1.09) | < 0.001 | 0.61 (0.60 – 0.63) | < 0.001 |
| HbA1c, % (mmol/mol) [<7% (53)] | ||||
| 7 – 8.9 (53 – 74) | 1.29 (1.28 – 1.29) | < 0.001 | 1.10 (1.09 – 1.10) | < 0.001 |
| ≥ 9 (75) | 1.11 (1.10 – 1.12) | < 0.001 | 1.02 (1.01 – 1.03) | < 0.001 |
| Unknown | 0.068 (0.067 – 0.069) | < 0.001 | 0.47 (0.47 – 0.48) | < 0.001 |
| Diabetes medications [None] | ||||
| Oral only | 8.67 (8.62 – 8.73) | < 0.001 | 9.21 (9.15 – 9.28) | < 0.001 |
| Oral and insulin | 28.89 (28.62 – 29.16) | < 0.001 | 19.38 (19.19 – 19.57) | < 0.001 |
| Insulin only | 16.64 (16.48 – 16.80) | < 0.001 | 9.31 (9.22 – 9.41) | < 0.001 |
| LDL cholesterol, mg/dL [<70] | ||||
| 70 – 99.9 | 0.35 (0.35 – 0.36) | < 0.001 | 0.39 (0.38 – 0.40) | < 0.001 |
| 100 – 129.9 | 0.138 (0.137 – 0.139) | < 0.001 | 0.17 (0.17 – 0.18) | < 0.001 |
| ≥ 130 | 0.098 (0.097 – 0.099) | < 0.001 | 0.137 (0.136 – 0.138) | < 0.001 |
| Unknown | 0.019 (0.019 – 0.019) | < 0.001 | 0.062 (0.062 – 0.063) | < 0.001 |
| Non-statin cholesterol medications‡ | – | 1.37 (1.36 – 1.38) | < 0.001 | |
| BMI, kg/m2[<25] | ||||
| 25 – 29.9 | 1.08 (1.08 – 1.09) | < 0.001 | 1.28 (1.27 – 1.29) | < 0.001 |
| ≥30 | 1.29 (1.28 – 1.31) | < 0.001 | 1.48 (1.46 – 1.50) | < 0.001 |
| Unknown | 0.034 (0.033 – 0.035) | < 0.001 | 0.39 (0.37 – 0.41) | < 0.001 |
| Elixhauser comorbidity count | 1.391 (1.390 – 1.393) | < 0.001 | 1.18 (1.18 – 1.19) | < 0.001 |
| Cardiovascular comorbidity | ||||
| Congestive heart failure | 3.68 (3.65 – 3.71) | < 0.001 | 1.09 (1.08 – 1.10) | < 0.001 |
| Valvular disease | 3.56 (3.53 – 3.59) | < 0.001 | 1.07 (1.06 – 1.08) | < 0.001 |
| Pulmonary/circulatory disease | 2.96 (2.92 – 3.00) | < 0.001 | 0.81 (0.79 – 0.82) | < 0.001 |
| PVD | 3.74 (3.72 – 3.77) | < 0.001 | 1.18 (1.17 – 1.19) | < 0.001 |
| Hypertension | 10.63 (10.52 – 10.73) | < 0.001 | 2.76 (2.73 – 2.79) | < 0.001 |
Reference categories are inside square brackets; NH = non-Hispanic; HbA1c = glycosylated hemoglobin; LDL = low density lipoprotein; BMI = body mass index; PVD = peripheral vascular disease.
The reference category for the variable “married” is all patients who did not identify themselves as married, including those who are single, divorced, or widowed and those whose marital status is unknown.
The unadjusted model did not converge.
Hispanic patients were most likely to be started on a statin (AOR 1.11; 95% CI, 1.07 – 1.16; p <0.001, Table 2). Compared to non-Hispanic white patients, non-Hispanic black patients were 26% less likely (AOR = 0.74; 95% CI, 0.72 – 0.76; p < 0.001) to be started on a statin, and patients in the Other group were 56% less likely (AOR = 0.62; 95% CI, 0.58 – 0.66; p <0.001).
4. DISCUSSION
Statin use in the 2003 cohort of VA patients with diabetes aged 40 years or older increased from ~43% to just under 60% during the nine-year follow-up period. This increase most likely reflected statin initiation rather than survival bias. Our proportions at the end of the study date are similar to that reported for the general U.S. population. According to 2011 – 2012 National Health and Nutrition Examination Survey (NHANES) data, an estimated 58.8% of Americans with diabetes between the ages of 40 and 75 years are taking a statin.11 An estimated 5 to 15% of patients do not tolerate statins.15,16 Another 10 to 15% of patients experience no substantial reduction in their LDL-cholesterol, although this is not a clear indication to stop therapy because patients with diabetes may still experience cardio-protective benefits.1,17 Assuming between 5 and 30% of our cohort was not taking a statin due to intolerance or limited effect on their LDL-cholesterol levels, this leaves 10 to 35% of the cohort (or between 89,966 and 314,882 patients) who were not receiving a statin despite their elevated cardiovascular risk.
Diabetes has been recognized as a coronary heart disease (CHD) risk equivalent since 2002.8 However, our study suggests that not all diagnoses of diabetes are given equal weight when considering statin use. Patients with HbA1c values >7% (53 mmol/mol) or who were treated with hypoglycemic medications were more likely to receive a statin. Co-existing cardiovascular disease was also associated with an increased likelihood of statin use. This association has been demonstrated previously in a large U.S. and Canadian surveys, as well as in a U.S. managed care system.11,18,19
The findings that more clinically apparent diabetes and cardiovascular disease may trigger statin initiation suggests that clinicians may prioritize statin use among patients with suboptimal control of diabetes and other cardiovascular domains. This potential correlation between statin use and poorly controlled diabetes and/or cardiovascular disease was not detected in an earlier study within the VA healthcare system.20 One potential explanation for this emerging pattern is that diabetes was not widely perceived as a CHD risk equivalent at the time of the Jackson et al. study, but it has subsequently gained recognition as such. Recent data identify cardiovascular disease and diabetes as concordant comorbidities that now share similar clinical management strategies and goals.21,22 This increases the odds of achieving shared goals, including LDL-cholesterol level targets.23 Our study findings extend these results by suggesting that diabetes with HbA1c values >7, compared to more mild disease, is a stronger clinical trigger for achieving goals shared with cardiovascular disease.
Our study also tracks patterns in racial and ethnic disparities regarding statin use for patients with diabetes in an equal-access healthcare system. Prior VA studies have demonstrated that black and Hispanic patients with diabetes are less likely to receive LDL-c testing or attain LDL-c level goals than their white counterparts.24,25 A decade later, our study documents the persistence of healthcare disparities for management of hyperlipidemia among black patients with diabetes. It is somewhat encouraging that the gap in statin uptake closed during the study period for Hispanic patients. Understanding how this was achieved may provide useful insights into addressing remaining disparities and preserving gains toward equity in the VA system.
Our study raises an important policy issue: should efforts to promote statin use focus on subgroups with low statin use but also relatively low cardiovascular risk (i.e. patients with diet-controlled diabetes and no cardiovascular comorbidities), or should they focus on groups with higher statin use but also higher cardiovascular risk (i.e. patients with diabetes and a prior myocardial infarct). If a healthcare goal is to ensure all patients with diabetes receive statins, and increase compliance with current guidelines, protocol-driven health service interventions may be needed to overcome current difference based on diabetes severity, additional cardiovascular risks, and race/ethnicity. Such protocols have successfully increased statin use for patients with diabetes in different healthcare systems.26,27 Healthcare system interventions aimed at improving diabetes management have the potential to decrease racial and ethnic disparities, regardless of whether or not they specifically focus on improving care for minority patients.28,29
While protocol-driven initiatives may be beneficial in increasing the use of statins, we recognize that clinical discretion is still required to determine appropriate deviations. For instance, physicians tend not to prescribe statins for patients with a short life expectancy.30 Increasing age and comorbid cancer were associated with lower odds of statin prescription in our cohort, which may be entirely appropriate.
The strengths of this study include its large sample size, longitudinal data, the availability of laboratory values and medication use, and use of merged information from the VA and Medicare systems. Nevertheless, the study does have limitations. We were unable to control for smoking, an important cardiovascular risk factor. The subgroup of patients we classified as having cardiovascular disease excluded those with coronary artery disease but without ischemic cardiac injury. While this definition is highly specific, it is likely to have misclassified patients with milder cardiovascular disease and resulted in an underestimate of statin use among those with cardiovascular disease and diabetes. We also did not query clinicians to understand the reasoning behind our observed practice patterns. The main limitation of our study may be that the entire study period took place when the ATP III guidelines were in effect.8 While this avoids the need to account for historical trends in guideline recommendations, it introduces concern that our findings may no longer be generalizable to current practice patterns under the 2013 ACC/AHA guidelines.10 ATP III guidelines recommended statin use for adult patients with diabetes whose LDL cholesterol level was > 130 mg/dL; it suggested considering statin use for patients with LDL cholesterol levels between 100 and 130 mg/dL. The 2013 ACC/AHA guidelines recommend statin use for all adults with diabetes, regardless of LDL cholesterol levels, with the exception that the level should not drop below 40 mg/dL.10
We believe our findings remain generalizable to contemporary practice under the ACC/AHA guidelines for the following reasons. (1) Higher LDL cholesterol levels were associated with lower statin use in our population. If providers were refraining from prescribing statins for diabetic patients because their cholesterol levels were below ATP III guideline thresholds, we would have expected to observe the opposite trend. (2) We controlled for LDL levels when assessing the impact of diabetic variables and cardiovascular disease on statin use. LDL-independent estimates should remain germane to clinical practice patterns since statin use is now recommended regardless of LDL levels. (3) Although ATP III guidelines did impose LDL targets, they also recommended statin use for patients with two or more cardiovascular risk factors. Since most patients with diabetes have additional cardiovascular risk factors, both ATP III and ACC/AHA guidelines are fairly well aligned.9
4.1 Conclusion
This study found that HbA1c values >7 and comorbid cardiovascular disease were associated with increased odds of statin use among a national cohort of VA patients with diabetes. While Hispanic patients achieved parity with their white counterparts, disparities among other minorities persist. Future studies should focus on understanding why these patterns exist to help optimize statin use moving forward. Protocol-driven health systems interventions may be useful in minimizing these differences, but clinicians must retain the ability to deviate from them when clinically appropriate. Resources are limited. To best focus future interventions that improve compliance with ADA guidelines on statin use, we pose the following question: should we target the relatively low-risk group of patients with diabetes, where the number of patients not on a statin is the greatest, but the individual risk-reduction may be the least? Alternatively, should we aim to increase statin use among the higher-risk group of patients, where the number of patients who could benefit may be smaller, but the individual risk-reduction would be greater?
Highlights.
Less than 60% of patients with diabetes are using a statin
Among those not using a cholesterol medication at baseline, uptake was slow
Comorbid cardiovascular disease, HbA1c >7% (53 mmol/mol), and Hispanic ethnicity were associated with an increased odds of statin uptake
Acknowledgments
FUNDING
The authors thank the personnel of the Center of Innovation for Complex Chronic Healthcare (CINCCH) at the Edward Hines, Jr. VA Hospital in Hines, IL for generous computer and data support. We thank Dr. Laura Hogan, University of Wisconsin, for her editorial assistance. This work was supported by the Agency for Healthcare Research and Quality (AHRQ) [grant number R01HS018542]; and National Institutes of Health (NIH) [grant numbers UL1TR000427 and KL2TR000428]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, AHRQ, or VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicenter randomized placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 2.Beishuizen ED, van de Ree MA, Jukema JW, et al. Two-year statin therapy does not alter the progression of intima-media thickness in patients with type 2 diabetes without manifest cardiovascular disease. Diabetes Care. 2004;27:2887–2892. doi: 10.2337/diacare.27.12.2887. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomized controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 4.Sohn MW, Meadows JL, Oh EH, et al. Statin use and lower extremity amputation risk in nonelderly diabetic patients. J Vasc Surg. 2013;58:1578–1585. doi: 10.1016/j.jvs.2013.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen OE, Birkeland KI, Jørgensen AP, et al. Diabetic foot ulcer burden may be modified by high-dose atorvastatin: a 6-month randomized controlled pilot trial. J Diabetes. 2009;1:182–187. doi: 10.1111/j.1753-0407.2009.00031.x. [DOI] [PubMed] [Google Scholar]
- 6.Fox JD, Baquerizo-Nole KL, Macquhae F, et al. Comment on Yang et al. association of statin use and reduced risk of lower-extremity amputation among patients with diabetes: a nationwide population-based cohort observation. Diabetes Care. 2016;39:159–160. doi: 10.2337/dc15-2376. [DOI] [PubMed] [Google Scholar]
- 7.Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventative Services Task Force. JAMA. 2016;316:2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 8.NCEP. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 9.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 10.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Adedinsewo D, Taka N, Agasthi P, et al. Prevalence and factors associated with statin use among a nationally representative sample of U.S. Adults: National Health and Nutrition Examination Survey, 2011 – 2012. Clin Cardiol. 2016;39:491–496. doi: 10.1002/clc.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus- mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 14.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Bays H. Statin safety: an overview and assessment of the data—2005. Am J Cardiol. 2006;97:6C–26C. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Banach M, Rizzo M, Toth PP, et al. Statin intolerance – an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Mora S, Rose L, JUPITER Trial Study Group Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016;37:1373–1379. doi: 10.1093/eurheartj/ehw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steen DL, Kahn I, Becker L, et al. Patterns and predictors of lipid-lower therapy in patients with atherosclerotic cardiovascular disease and/or diabetes mellitus in 2014: insights from a large US managed-care population. Clin Cardiol. 2016 doi: 10.1002/clc.22641. https://doi.org/10.1002/clc.22641. [DOI] [PMC free article] [PubMed]
- 19.Bai JW, Boulet G, Halpern EM, et al. Cardiovascular disease guideline adherence and self-reported statin use in longstanding type 1 diabetes: results from the Canadian study of longevity in diabetes cohort. Cardiovasc Diabetol. 2016;15:14–24. doi: 10.1186/s12933-015-0318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson GL, Edelman D, Weinberger M. Simultaneous control of intermediate diabetes outcomes among Veterans Affairs primary care patients. J Gen Intern Med. 2006;21:1050–1056. doi: 10.1111/j.1525-1497.2006.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnan EM, Palta M, Johnson HM, et al. The impact of a patient’s concordant and discordant chronic conditions on diabetes care quality measures. J Diabetes Complications. 2015;29:288–294. doi: 10.1016/j.jdiacomp.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnan EM, Gittelson R, Bartels CM, et al. Establishing chronic condition concordance and discordance with diabetes: a Delphi study. BMC Fam Pract. 2015;16:42–52. doi: 10.1186/s12875-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnan EM, Bolt DM, Greenlee RT, et al. Stratifying patients with diabetes into clinically relevant groups by combination of chronic conditions to identify gaps in quality care. Health Serv Res. 2016 doi: 10.1111/1475-6773.12607. https://doi.org/10.1111/1475-6773.12607. [DOI] [PMC free article] [PubMed]
- 24.Heisler M, Smith DM, Hayward RA, et al. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care. 2003;41:1221–1232. doi: 10.1097/01.MLR.0000093421.64618.9C. [DOI] [PubMed] [Google Scholar]
- 25.Halanych JH, Wang F, Miller DR, et al. Racial/ethnic differences in diabetes care for older veterans: accounting for dual health system use changes conclusions. Med Care. 2006;44:439–445. doi: 10.1097/01.mlr.0000207433.70159.23. [DOI] [PubMed] [Google Scholar]
- 26.Dudl RJ, Wang MC, Wong M, Bellows J. Preventing myocardial infarction and stroke with a simplified bundle of cardioprotective medications. Am J Manag Care. 2009;15:e88–94. [PubMed] [Google Scholar]
- 27.Gold R, Nelson C, Cowburn S, et al. Feasibility and impact of implementing a private care system’s diabetes quality improvement intervention in the safety net: a cluster-randomized trial. Implement Sci. 2015 doi: 10.1186/s13012-015-0259-4. https://doi.org/10.1186/s13012-015-0259-4. [DOI] [PMC free article] [PubMed]
- 28.Sequist TD, Adams A, Zhang F, et al. Effect of quality improvement on racial disparities in diabetes care. Arch Intern Med. 2006;166:675–681. doi: 10.1001/archinte.166.6.675. [DOI] [PubMed] [Google Scholar]
- 29.Fayfman M, Haw S. Diabetes in racial and ethnic minorities in the United States: individualizing approaches to diagnosis and management. Curr Diabetes Rev. 2016 doi: 10.2174/1573399812666160926142036. https://doi.org/10.1210/jc.2016-3197. [DOI] [PubMed]
- 30.AB E, Denig P, van Vliet Ton, Dekker JH. Reasons of general practitioners for not prescribing lipid-lowering medication to patients with diabetes: a qualitative study. BMC Fam Pract. 2009;10:24–31. doi: 10.1186/1471-2296-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]


