Abstract
The aim of this study was to assess safety and efficacy of ultrasonography (US)-guided intra-articular injections using tumor necrosis factor (TNF) blockers compared to corticosteroids in rheumatoid arthritis (RA) or psoriatic arthritis (PsA) patients, experiencing refractory monoarthritis despite the current systemic therapy. Eighty-two patients were randomized to receive three intra-articular injections monthly of either corticosteroid or TNF blockers. Primary endpoints were the safety and an improvement greater than 20% for visual analogic scales of involved joint pain in patients injected with anti-TNFα. Further clinical, US, and magnetic resonance imaging (MRI) evaluations were considered secondary endpoints. Intra-articular TNF blockers are a safe strategy, determining a significant reduction of patient and physician reported clinical outcomes and US/MRI scores, in RA and PsA patients, when compared to intra-articular injections of corticosteroids. US guidance excluded the possibility to inject the drug in the wrong site, maximizing local effects, reducing systemic effects, and increasing the safety of the procedure. Patients with inflammatory monoarthritis could be successfully treated with US-guided intra-articular TNF blockers that are a safe and well tolerated procedure, to achieve a longstanding clinical and radiological good clinical response and/or disease remission.
Keywords: anti-tumor necrosis factor α agent, intra-articular injection, magnetic resonance imaging (MRI), psoriatic arthritis, rheumatoid arthritis, treat-to-target strategy, ultrasonography
Introduction
Despite growing progress in the treatment of articular chronic inflammatory disorders, refractory mono-oligo-arthritis is still a common clinical problem. In this case, therapeutic approach is rather complex, often requiring the combination of systemic non-steroid anti-inflammatory drugs (NSAIDs) and local corticosteroid administration. Unfortunately, the outcome of pharmacological treatment is often poor and weighted by adverse events, therefore surgical procedures including synoviectomy and joint replacement might be required.1 Indeed, persistent monoarthritis may be a debilitating and destructive condition as a consequence of both inflammatory-related joint destruction and secondary osteoarthritis. Tumor necrosis factor α (TNFα) emerged as a potent pro-inflammatory mediator in the pathogenesis of inflammatory arthritides. TNFα is was found in the synovial tissue and cartilage-pannus junctions in patients with rheumatoid arthritis (RA) or psoriatic arthritis (PsA). TNFα is involved in cartilage and bone tissue degradation and in the modulation of joint pain.2–4 Moreover, TNFα in synovium correlates with disease activity in patients with RA5 and may directly regulate angiogenetic mechanisms.6 Overexpression of TNFα is believed to play a key role in the pathogenic mechanisms linking psoriasis and arthritis, mediating a number of biological processes that can result in joint damage characterized by stimulation of bone resorption and inhibition of bone formation and of synthesis of proteoglycans. TNFα may also contribute to vascular proliferation, which is probably one of the key and earlier observable changes in psoriasis and PsA.7
On this background, TNFα antagonists currently represent a widely and effective employed therapeutic approach in chronic arthritides.8,9 Although systemic anti-TNFα treatment offers considerable improvement in systemic inflammatory joint diseases, many patients still experience persistent symptoms in a single or few joints. An insufficient level of drug in the inflamed joints has been suggested as possible explanation and intra-articular injections of corticosteroid is a well-established treatment to overcome this problem.10 On this basis, the association of systemic and intra-articular TNFα blockers could allow to achieve a better clinical response. In recent years several studies were published concerning the use of intra-articular anti-TNFα agents to treat refractory monoarthritis. However only conflicting results are available in literature.11–22 At present, both magnetic resonance imaging (MRI) and ultrasonography (US) are established methods to evaluate the severity of monoarthtitis flares in these conditions;23–26 furthermore, US improves the performance of intra-articular injections overcoming technical problems such as the correct position of the needle and the aspiration of joint effusion.27 In fact it is well know that only in 50% of intra-articular injections is the drug placed in the right place and this strongly decreases the efficacy of these procedures.28
The aim of this randomized, controlled, single-blinded study was to assess the safety and efficacy of US-guided intra-articular injection using TNF blockers compared to corticosteroids in patients with RA or PsA, experiencing refractory monoarthritis. US scan was employed in the study both to guide intra-articular injections assessing the joint clinical response by validated methods, where possible, and observing the structural modification in the joint where we do not still have validated measures. MRI was employed in addition to US in patients with RA to evaluate the clinical response by validated measures in wrist and metacarpophalangeal joints during the follow-up.23,29–31
Materials and methods
Study groups and US-guided injections
Eighty-two consecutive patients referring to the Rheumatology Unit, University of L’Aquila, Italy were included in the study; seven patients, assessed for eligibility, declined to participate. Of these, 41 patients were affected by RA and 41 by PsA. All patients were aged over 18 years, fulfilled the criteria for RA32 or for PsA33 and were receiving stable doses of an anti-TNFα agent (infliximab, etanercept, adalimumab) in combination with one or more disease modifying anti-rheumatic drugs (DMARDs) for at least 3 months. The inclusion criteria for the study was the clinical evidence of a persistent arthritis despite the aforementioned therapy or a new single joint arthritis (shoulder, elbow, wrist, metacarpophalangeal joint, proximal interphalangeal joint, hip, or knee). We did not consider distal interphalangeal joints eligible for local treatment, due to the lack of synovial tissue.
After informed consent in accordance with the Declaration of Helsinki, patients were randomized to receive one intra-articular injection each month for 3 consecutive months of either corticosteroid (triamcinolone 40 mg) or an anti-TNFα agent (infliximab 100 mg; etanercept 50 mg; adalimumab 40 mg). In the latter cases, patients were injected with the same anti-TNFα agent that they were receiving systemically. During the study period, systemic therapy with anti-TNFα agents and DMARDs was maintained stable. Patients were not allowed to increase their oral prednisolone therapy. The whole study was approved by the local Ethics Committee and performed according to Good Clinical Practice guidelines. The study was randomized, stratified by the underlying disease and the type of joint with a pre-given code of randomization from an external observer. The allocation to one of two treatment groups was based on a permuted block randomization list. A study clinician, collaborated with the external observer, performed the randomization, and prepared a syringe. All calculations of data were performed before breaking the code. US-guided intra-articular injections were performed at baseline, week 4, and week 8. US was used to determine the placement of the syringe prior to and during aspiration and injection into the joint cavity. The measure of needle (from 20G for shoulder to 27G for proximal interphalangeal joint) and the amount of injected drug was chosen on the basis of joint type. In particular, intra-articular infliximab was injected at the following dosages: 100 mg/10 mL for the knee, shoulder, and hip; 50 mg/5 mL for elbow and wrist; and 10 mg/1 mL for metacarpophalangeal joint. Intra-articular etanercept was injected at a dosage of 50 mg/1 mL for all the treated joints; intra-articular adalimumab 40 mg/0.8 mL was injected at a dosage of 50 mg/1 mL for all the treated joints; intra-articular triamcinolone was injected at a dosage of 40 mg/1 mL for all the treated joints. A 10 cc syringe, prefilled with the drug, was covered with an obscuring sleeve so that the patient could not detect which was being administered.
Primary endpoints
The primary endpoints of this study were: (1) the evaluation of intra-articular anti-TNFα injection safety compared to corticosteroid; (2) a 20% or greater improvement on visual analogic scales for involved joint pain (jVAS) in patients injected with anti-TNFα compared to patients injected with corticosteroid. Patients were evaluated at the time of the first injection and then at weeks 4, 8, 12, and 24. For safety evaluation, the follow-up period was extended to week 52. Patients were asked about systemic or local adverse event occurrence at each visit that could be related to the injective procedure. Adverse events included local or systemic infection, allergy (cutaneous or respiratory), hyperglycemia, arterial hypertension, and headache. Adverse events were judged as serious if they resulted in death, were life-threatening according to the investigator’s own judgment, caused hospital admission, resulted in birth defect (from unplanned pregnancies) or disability, or were important medical events that could have jeopardized the patient or needed intervention to prevent another serious adverse event, or both.
Clinical evaluation and secondary endpoints
Patients were evaluated at baseline, at weeks 4, 8, and 12, and after 12 weeks from the last injection (week 24) for the evaluation of secondary endpoints, chosen among validated clinical and instrumental measures. The following self-reported outcomes on visual analogic scales (VAS) were employed both in RA and PsA patients: global pain (gVAS), Patient Global Assessment (GA) of Disease Activity, Global Health (GH) Status; Health Assessment Questionnaire (HAQ) was also completed at each time point. An independent clinician, provided the degree of Physician-GA by VAS. Tender and swollen joints (Tj and Sj) were graded in the range of 0–3, with 0 = no activity and 3 = most prominent activity. Erythrocyte sedimentation rate (ESR, mm/h) and C-reactive protein (CRP, mg/L) were also recorded. Disease Activity Score on 28 joints (DAS28), using ESR, and Clinical Disease Activity Index (CDAI) were also calculated, both in RA patients and PsA patients, following the report that DAS28 has been validated for use in PsA.34,35
Clinical follow up at week 52 was performed in order to identify diseases flares in the injected joint. To this aim, we reported only new-onset arthritis in the joint treated with intra-articular anti-TNFα or corticosteroid. In order to avoid any biases, we did not assess all other clinical parameters employed at baseline to week 24, because of these data could be affected by other conditions such as arthritis involving different joints, extra-articular manifestations (enthesitis, dactylitis), infectious diseases, and increase of ERS and CRP not related to rheumatic flares.
Magnetic resonance imaging
Contrast-enhanced MRI was performed, for technical reasons, at baseline and at week 12 only for RA patients with metacarpophalangeal joint (13 patients) or wrist (11 patients) monoarthritis. All MRI examinations were performed using a 0.25 T musculoskeletal dedicated scanner (G-scan, Esaote Biomedica, Genoa, Italy). Application of MRI sequences and use of intravenous injection of gadolinium-DTPA (Magnevist, Schering AG, Berlin, Germany) was performed as described elsewhere.23 Synovitis, bone erosion, and bone edema were assessed according the EULAR–OMERACT RA MRI reference image atlas29,30 and disease activity was scored according the OMERACT RA MRI score (OMERACT RAMRIS).23,31
Ultrasonography
US examination was performed at baseline and 4 weeks after each intra-articular injection (at weeks 4, 8, and 12). Grayscale, B-mode, and power Doppler (PD) evaluation were performed with a ESAOTE MyLab70 (Esaote, Genova, Italy) with a linear transducer at 7–13 MHz. For B-Mode a frequency of 11 MHz was used. PD settings were as follows: frequency, 7 MHz; repetition frequency, 1 kHz at low filter wall (150 Hz). The color gain was set at the level at which noise artifacts appeared and gradually reduced, until only a flow signal, if present, was left. Synovial hypertrophy and synovial effusion were evaluated on two axes, longitudinal and transverse; the color-power Doppler was used to demonstrate, by evaluating the vascularity, the entity of synovial inflammation. US scan of the inflamed joint was performed by the same specialist, an arbitrary scoring system for assessment of inflamed joint was applied considering synovial hypertrophy (0–3), joint effusion (0–3), and PD evaluation (0–3).24–26,36–42
Statistical analysis
Our study was powered to detect a 20% difference in efficacy, regarding jVAS, in favor of anti-TNFα injection versus corticosteroid injection (α = 0.05, ß = 0.2) and SD calculated based on literature data,13,18,21 with 20 patients in each group. All data were analyzed by intention-to-treat analysis. Statistical analysis was performed with Graph-Pad 5.0 software. One-way analysis of variance and multiple comparison post hoc tests were employed to calculate differences between baseline and following time points. Differences between the two treatment arms, small and large joints involvement, in both intra-articular corticosteroid and intra-articular anti-TNFα group were tested with non-parametric Mann-Whitney U test. P values less than 0.05 were considered significant.
Results
Study groups, baseline demographic, and clinical characteristics
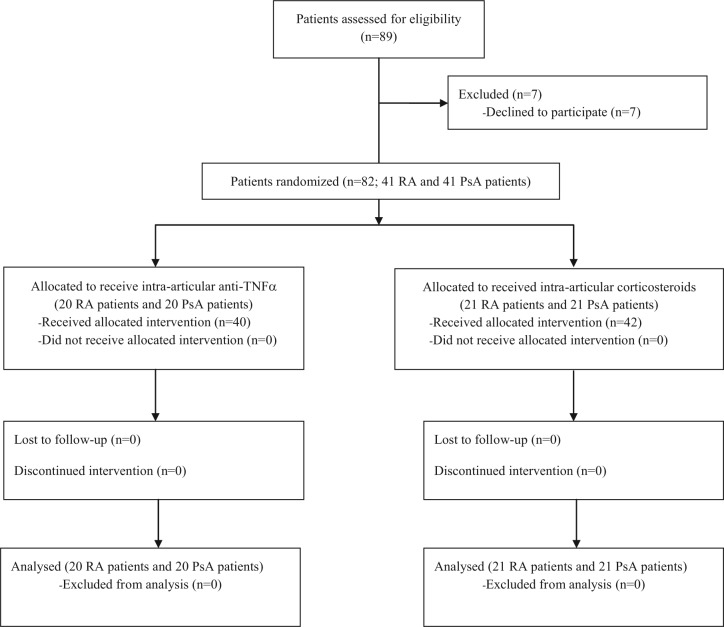
Demographic characteristics of patients are summarized in Table 1. Eighty-two patients completed the observation period of 52 weeks (Figure 1). The study was approved in 2009 and recruitment ended in 2013.
Table 1.
Demographic and clinical characteristics of patient cohort.
| Treatment group | RA |
PsA |
||
|---|---|---|---|---|
| Intra-articular corticosteroid | Intra-articular anti-TNFα | Intra-articular corticosteroid | Intra-articular anti-TNFα | |
| Age, median (range) (years) | 48.2 (34–73) | 49.6 (38–71) | 44.3 (31–68) | 41.6 (33–62) |
| Patients (female/male) | 21 (15/6) | 20 (15/5) | 21 (11/10) | 20 (13/7) |
| Disease duration, median (range) (years) | 7.5 (0.8–19) | 8.3 (0.9–21) | 8.2 (0.9–17) | 8.8 (1.3–19) |
| Joint, n (INF/ETA/ADA) | ||||
| Shoulder | 1 | 1 (1/0/0) | 2 | 2 (1/0/1) |
| Elbow | 1 | 1 (0/1/0) | 3 | 4 (1/2/1) |
| Wrist | 6 | 5 (2/1/2) | 2 | 2 (0/1/1) |
| MCP | 6 | 7 (1/4/2) | 4 | 3 (0/1/2) |
| PIP | 2 | 1 (0/0/1) | 0 | 0 (0/0/0) |
| Hip | 1 | 1 (1/0/0) | 4 | 3 (1/0/2) |
| Knee | 4 | 4 (2/1/1) | 6 | 6 (3/2/1) |
| Systemic anti-TNFα, number of patients (INF/ETA/ADA) | 21 (8/6/7) | 20 (7/7/6) | 21 (7/6/8) | 20 (6/6/8) |
| Single DMARD, number of patients (MTX/LEF/CsA/SSZ/HCQ) | 8 (5/3/0/0/0) | 9 (7/2/0/0/) | 9 (6/0/3/0/0) | 8 (7/0/1/0/0) |
| Combined DMARDs, number of patients (MTX/LEF/CsA/SSZ/HCQ) | 13 (7/4/6/2/8) | 11 (7/4/3/0/9) | 12 (8/3/5/8/0) | 12 (9/2/6/7/0) |
| Systemic corticosteroids, number of patients | 18 | 17 | 14 | 12 |
ADA, adalimumab; CsA, ciclosporin; DMARDs, disease-modifyingantirheumaticdrugs; ETA, etanercept; HCQ, hydroxychloroquine; INF, infliximab; LEF, leflunomide; MTX, methotrexate; MCP, metacarpophalangeal joint; PIP, proximalinterphalangeal joint; SSZ, sulfasalazine.
Figure 1.
Flow-chart of patients in this study.
Primary endpoints
In both treatment groups, no adverse events, with the exception of temporary local soreness after intra-articular injection, were reported during the study. Intra-articular anti-TNFα injections were generally well-tolerated and no increased local pain, signs of infection or any other local or systemic reaction were observed. No adverse events judged by the investigator to be possibly, probably, or definitely related to intra-articular anti-TNFα injection were observed in the 52-week follow-up period.
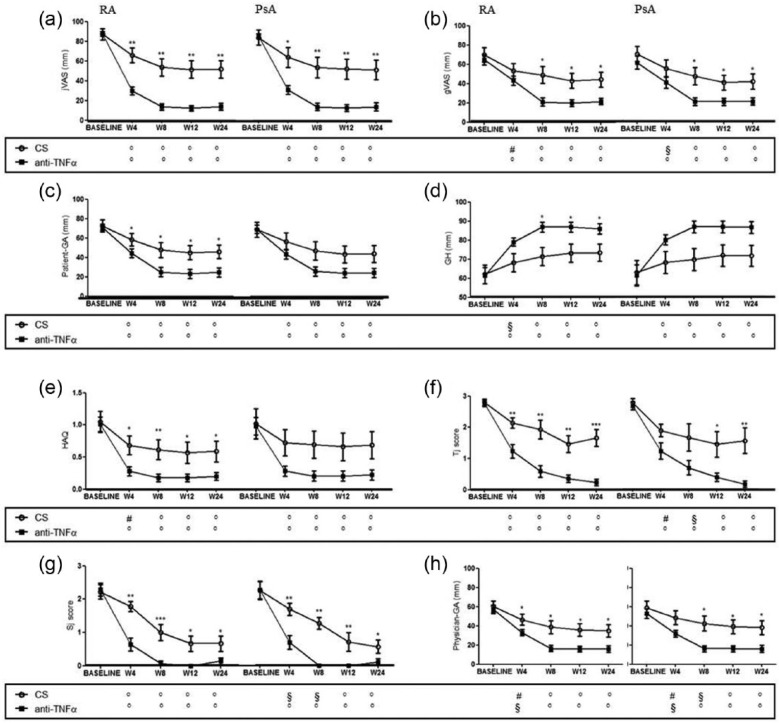
The results at each time point, inside the four experimental groups, of visual analogic scales for involved joint pain (jVAS) are shown in Figure 2a. RA patients treated with intra-articular anti-TNFa agent reported much lower values of jVAS with respect to those treated with intra-articular corticosteroid at any time point expressed. This significant reduction of jVAS, starting from week 4, was maintained over time (percentage of jVAS reduction, compared to baseline ± SEM, in anti-TNFα vs. corticosteroid group at week 4: 57.6 ± 3.93 vs. 21.2 ± 7.59, P <0.001; at week 8: 73.9 ± 3.30 vs. 33.5 ± 8.97, P <0.0001; at week 12: 75.4 ± 2.93 vs. 35.6 ± 8.86, P <0.0001; at week 24: 73.8 ± 3.43 vs. 35.5 ± 8.54, P <0.001, respectively). Similar results were observed in PsA patients treated with intra-articular anti-TNFα agent, when compared with PsA patients treated with with intra-articular corticosteroid (percentage of jVAS reduction, compared to baseline ± SEM, in anti-TNFα vs. corticosteroid group at week 4: 55.9 ± 4.68 vs. 20.3 ± 10.20, P <0.05; at week 8: 73.9 ± 3.81 vs. 30.5 ± 10.53, P <0.001; at week 12: 74.5 ± 3.51 vs. 32.4 ± 10.26, P <0.001; at week 24: 73.1 ± 4.34 vs. 33.2 ± 9.84, P <0.01, respectively).
Figure 2.
The results of primary endpoint and clinical outcomes, in RA and PsA groups, are shown. The primary endpoint of the study was (a) Visual Analogic Scale for involved joint pain (jVAS); (b) gVAS, Visual Analogic Scale for global pain; (c) Patient-GA, Patient Global Assessment; (d) GH, Global Health Status; (e) HAQ, Health Assessment Questionnaire; (f) Tj, Tender joint score; (g) Sj, Swollen joint score; (h) Physician-GA, Physician Global Assessment. Bars in the line charts indicate mean ± SEM. Inside the box below the chart symbols refer to the comparison between each time point and the baseline according to the treatment arm (° p<0.001; § p<0.01; # p<0.05). ***p<0.001; **p<0.01 and *p<0.05 inside the chart refer to the comparison between the two treatment arms at the corresponding time point. CS, corticosteroids; TNFα, tumor necrosis factor α.
Secondary clinical outcomes
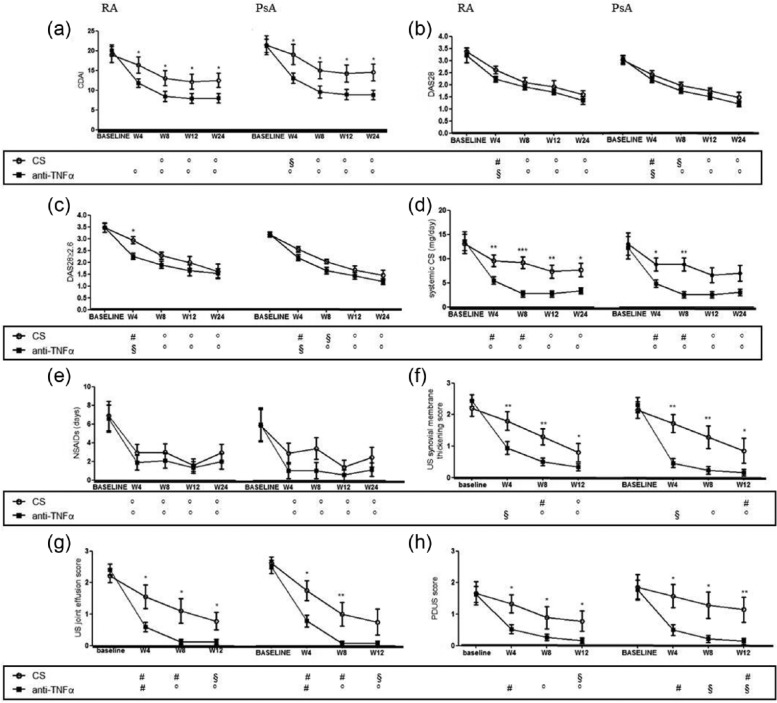
The modification at each time point, inside the four experimental groups, of the selected outcome measures is shown in Figures 2 and 3. A reduction of global pain was reported by all patients. Interestingly, while in patients treated with corticosteroids, such a decrease was stable over time with no further reduction after week 4, in anti-TNFα-treated patients we observed a stronger improvement, over time, of global pain. This result was confirmed by the evidence of a statistically significant difference in the two treatment groups starting from week 8 (Figure 2b). In anti-TNFα-treated patients, affected by RA, we observed a significant improvement of global outcomes, including Patient-GA and HAQ, and such a reduction was more pronounced with respect to that observed in patients treated with corticosteroids. Furthermore, no difference in Patient-GA and HAQ reduction according to intra-articular treatment was observed in patients with PsA (Figure 2c, e). Concerning GH, a significant improvement was observed in all patients starting from week 4 but no further increase could be detected overtime (Figure 2d). In RA patients, GH was significantly higher after intra-articular anti-TNFα treatment compared to patients treated with corticosteroids starting from week 8. Conversely, in PsA patients, no difference in GH assessment was observed in the two treatment groups. In our study, an overall improvement of Tj score and Sj score was observed in RA patients treated with anti-TNFα, starting from week 4, and such improvements were more pronounced when compared to patients treated with corticosteroids at each time point. Conversely in PsA both anti-TNFα and corticosteroid treatment were comparable in the first 4 weeks (Figure 2f, g). Similar data were obtained regarding Physician-GA (Figure 2h). As expected, we failed to observe any difference in ESR and CRP values following intra-articular anti-TNFα or corticosteroid treatment for both RA and PsA patients (data not shown). Finally, a reduction of DAS28 was also found in all patients and this reduction was confirmed at 24 weeks of follow-up, in both treatment groups. Of note, all RA patients with DAS28 ⩾2.6, and treated with intra-articular anti-TNFα agent, quickly achieved remission (DAS28 <2.6) at week 4. On the contrary, this was not observed in the intra-articular corticosteroid group which needed at least 8 weeks to achieve clinical remission. Regarding clinical remission in PsA patients, we failed to observe any significant difference in the two treatment arms (Figure 3b, c). In order to identify whether the changes in DAS28 were independent on changes in the acute phase response, CDAI was also performed. A significant reduction of CDAI was observed in any experimental groups independently on the treatment and the underlying disease, but this improvement was more significant in patients treated with intra-articular anti-TNF agents (Figure 3a). Interestingly in RA patients who received intra-articular anti-TNFα agents, we observed a consistent decrease in the need of systemic corticosteroids that was significantly more pronounced compared to those patients receiving intra-articular corticosteroids at any time. Concerning PsA patients such difference was evident only at weeks 4 and 8 (Figure 3d). In this setting, a consistent reduction of NSAIDs intake was also reported from all patients independently on intra-articular treatment and underlying disease (Figure 3e).
Figure 3.
The results of clinical outcomes and US scores, in RA and PsA groups, are shown. (a) CDAI, Clinical Disease Activity Index; (b) DAS28, Disease Activity Score on 28 joints; (c) DAS28 ⩾2.6; (d) the mean dosage of systemic corticosteroids; (e) the daily use of NSAIDs, Non-Steroid Anti-Inflammatory Drugs; (f) the US synovial membrane thickening score; (g) the US joint effusion score; (h) the power Doppler US score (PDUS). Bars in the line charts indicate mean ± SEM. Inside the box below the chart symbols refer to the comparison between each time point and the baseline according to the treatment arm (° P <0.001; § P <0.01; # P <0.05). ***P <0.001; **P <0.01; and *P <0.05 inside the chart refer to the comparison between the two treatment arms at the corresponding time point. CS, corticosteroids; TNFα, tumor necrosis factor α; US, ultrasonography.
To note, in the 52 weeks follow-up, no new flare of disease in the injected joint was observed in RA or PsA patients treated with intra-articular anti-TNFα. On the contrary, in the RA and PsA group treated with intra-articular corticosteroids, five (24%) and seven (33%) new flares were recorded, respectively.
Ultrasonography
In the course of the study, US scan was performed both to assess peculiar features of either rheumatoid or seronegative synovitis and to guide intra-articular injections. In this setting, semi-quantitative scores were calculated by an expert radiologist at baseline, weeks 4, 8, and 12. A global reduction of both US synovial membrane thickening score, US joint effusion score, and PDUS score were observed in all treatment groups. Interestingly, such a decrease was much evident in anti-TNFα-treated patients when compared to patients treated with corticosteroids of both disease groups at each time point (Figures 3f–h and 4).
Figure 4.
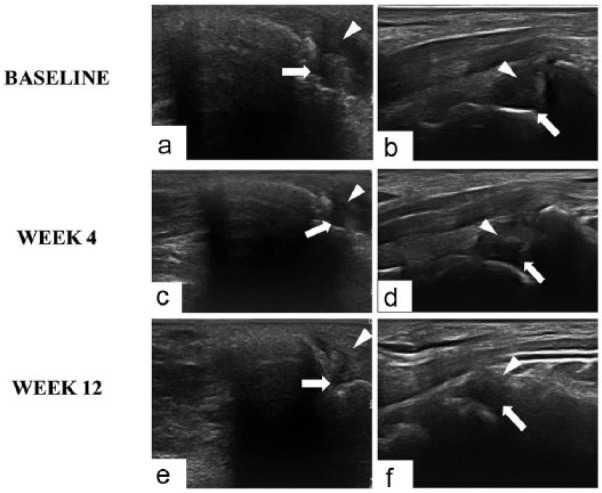
Ultrasonography imaging of wrist joints of patients with RA injected with intra-articular anti-TNFα agent (a, c, e) and intra-articular corticosteroid (b, d, f). Both anti-TNFα and corticosteroid treatment determine a progressive reduction of joint effusion (arrow) but reduction of synovial membrane thickness (arrowhead) is more expressed in intra-articular anti-TNFα group.
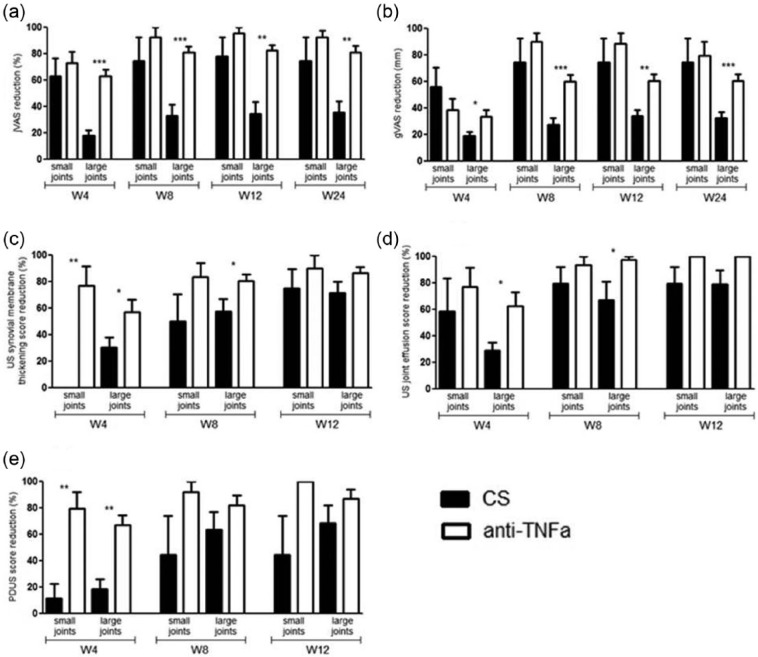
Comparison between small and large joints in RA patients
We subdivided RA patients into two groups according to the injected joint (small vs. large), in order to identify any differences in clinical and US scores. Concerning clinical outcomes (jVAS, gVAS), the percentage of improvement at each time point compared to baseline, in the anti-TNFα-injected RA patients, was statistically significant in large joints but not in small joints (Figure 5a, b). Moreover, a significant improvement was observed in US joint effusion score only in large joints injected with anti-TNFα at weeks 4 and 8 in RA patients (Figure 5c–e). To note a similar trend was observed in the large joints of PsA patients but no statistical analysis was performed due to the low number of patients with small joints involvement (data not shown).
Figure 5.
Comparison between small and large joints at each time point according to the treatment arm for RA patients: (a) clinical outcomes, jVAS reduction; (b) clinical outcome, gVAS reduction; (c) US synovial membrane thickening score; (d) US joint effusion score; (e) PDUS score. Bars indicate mean ± SEM. ***P <0.001; **P <0.01; *P <0.05. CS, corticosteroids; gVAS, Visual Analogic Scale for global pain; jVAS, Visual Analogic Scale for involved joint pain; PD, power Doppler; TNFα, tumor necrosis factor α; US, ultrasonography.
Magnetic resonance imaging
In selected RA patients, MRI scan was also performed at baseline and week 12 to calculate validated scores for specific joints (wrist and MCP), including the MRI synovitis score, the MRI bone erosion score, and the MRI bone edema score. We observed a statistically significant reduction of both MRI synovitis and bone edema scores in RA patients injected with either an anti-TNF agent or with corticosteroids. Interestingly, the reduction of MRI synovitis score in patients injected with anti-TNFα was significantly more evident when compared with the corticosteroid injected group. Conversely, no differences in MRI bone erosion score were observed probably due to the short time between the observations (Figure 6).
Figure 6.
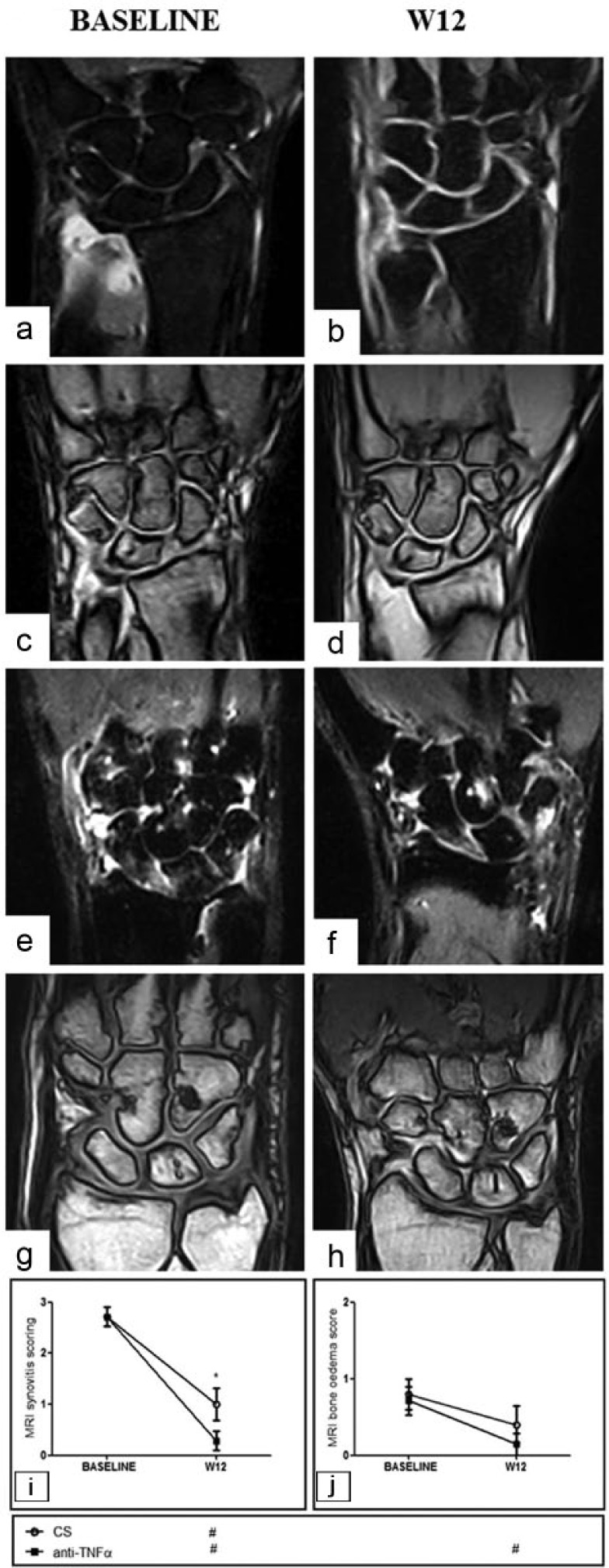
MRI of wrist joints of patients with RA injected with intra-articular anti-TNFα agent (a–d) or intra-articular corticosteroids (e–h). A reduction of synovitis is more pronounced in intra-articular anti-TNFα group (a, b, e, f: gradient echo STIR sequences without gadolinium contrast; c, d, g, h: gradient echo sequences with gadolinium contrast). RA MRI scores concerning synovitis (i) and bone edema (j). Bars in the line charts indicate mean ± SEM. Inside the box below the chart symbols refer to the comparison between each time point and the baseline according to the treatment arm (# P <0.05). *P <0.05 inside the chart refer to the comparison between the two treatment arms at the corresponding time point. CS, corticosteroids; MRI, magnetic resonance imaging; TNFα, tumor necrosis factor α.
Discussion
To our knowledge, this is the first randomized, controlled, single-blinded study performed in a large cohort of patients with RA and PsA suffering with monoarthritis despite systemic therapy with anti-TNFα agent and DMARDs. We performed a 52-week follow-up to address the safety and efficacy of US-guided joint injections of anti-TNFα agents compared with corticosteroids to treat inflammatory monoarthritis by using both clinical and instrumental validated measures.
TNFα is a significant cytokine that mediates inflammation in RA and PsA. Elevation of TNFα levels have been observed in synovial fluid and the synovium of patients with RA or PsA, playing a central role in driving inflammation and bone degradation.43 Due to its influence on various cells in synovial membrane, such as synoviocytes, macrophages, chondrocytes, and osteoclasts, which are able to produce metalloproteinases, collagenase, and stromelysin, TNFα induces local inflammation and pannus formation, eventually leading to further erosion of cartilage and bone destruction.44 Introduction of TNFα inhibitors has revolutionized RA and PsA treatment options resulting in the development of further biologic DMARDs.8,45 The effects of a TNFα blockade are partially dependent on synovial TNFα expression and infiltration by TNFα-producing inflammatory cells.5,46
In chronic and recurrent arthritis, inflammation and synovial tissue hyperplasia lead to joint damage, pain, and loss of function. Currently, intra-articular corticosteroids are the first line therapy;10,47 however, their efficacy is rather varying. More than 50% of patients experience relapses within 6 months after injection.48,49 An alternative local therapy, such as radiation synovectomy, did not show better results when compared to intra-articular corticosteroids in patients with recurrent knee arthritis.50 Several studies concerning the use of intra-articular anti-TNFα agents have been published so far but results are not conclusive, with a wide range of clinical response and differences in the study design.11–22
In our study, intra-articular anti-TNFα injections were generally well-tolerated and no local or systemic reaction were observed. Furthermore, US guidance excluded the possibility to inject the drug in the wrong site, maximizing local effects, reducing systemic effects, and increasing the safety of the procedure.27,28 Intra-articular injections of anti-TNFα agents determined a significant reduction of jVAS in both RA and PsA patients, achieving a 20% difference in efficacy versus corticosteroid injection strategy, starting from week 4 and this effect was maintained overtime.
As far as the other clinical outcomes in RA patients are concerned, we demonstrated a statistically significant improvement of gVAS, Patient-GA, HAQ, GH, Physician-GA, Tj, and Sj scores in the anti-TNFα-injected group when compared to the corticosteroid-injected group. Of note, good clinical response was achieved rapidly (week 4) and maintained over time. A similar trend was observed in PsA patients, although we failed to reach significance between anti-TNFα- and corticosteroid-injected groups for gVAS, Patient-GA, HAQ, and GH, probably because inflammatory back pain, enthesitis, or skin psoriasis might affect global subjective clinical outcomes.51 However, the reduction of Physician-GA, was statistically significant in the anti-TNF group compared to the corticosteroid group.
Another pivotal point of this study is the reduction of DAS28 and CDAI in RA and PsA groups. After the first injections we observed a significant reduction of DAS28 and CDAI, independent on the treatment. As far as CDAI was concerned, this reduction was even more evident. Indeed different from DAS28, CDAI does not consider acute phase reactants which are more strictly correlated to systemic involvement rather than with monoarthritis. Moreover, a sub-analysis of RA patients with DAS28 ⩾2.6 showed that at week 4 anti-TNFα injection was able to significantly reduce DAS28 values when compared to corticosteroid injected group. Conversely, no differences in DAS28 were observed in PsA group. All these data confirm that a tight control of the disease is important to achieve clinical remission (DAS28 <2.6) and we could speculate that intra-articular anti-TNFα injections represent a powerful tool in a “treat-to-target” strategy. To note, in the 52-week follow-up period, no new flare of disease in the injected joint was observed in RA or PsA patients treated with intra-articular anti-TNFα. On the contrary, in the RA and PsA group treated with intra-articular corticosteroids, five (24%) and seven (33%) new flares were recorded, respectively.
US and MRI score analysis further supported this hypothesis. In fact, a significant reduction of US synovial membrane thickening, joint effusion, and PDUS scores was achieved in RA and PsA patients treated with intra-articular anti-TNFα compared to patients treated with intra-articular corticosteroids. Of note, this significant reduction was more pronounced at week 4 and such improvement was stable at each time point to week 12. In addition, an overall significant reduction of synovitis score in RA patients treated with intra-articular anti-TNFα was found by MRI evaluation at week 12, mirroring the trend observed by US throughout the study. On this basis, we could postulate that intra-articular anti-TNF agents are more effective and faster to achieve a clinical and imaging improvement in patients with RA and PsA.
Intra-articular anti-TNFα treatment displayed a systemic corticosteroid sparing activity within few weeks, thereby confirming the effectiveness of this approach. On the contrary, we were unable to demonstrate any differences in NSAIDs patient self-administration, probably because of other conditions or underlying articular disorders.52 Finally we did not observe any relevant modification in acute phase reactants, probably due to the administration route, suggesting that the effect of the therapies might be limited into the intra-articular space without any systemic effect. The intra-articular half-life of corticosteroids and anti-TNFα agents is not known but we assume that anti-TNFα agents have a predominant local effect by binding a large amount of cytokines in the joint cavity before entering the bloodstream.53
Some authors demonstrated the improvement in both synovial thickness and expression of biological markers in synovial tissue and synovial fluid,5,22,54,55 showing an effective biological role of intra-articular TNFα blockade. No data have been reported to elucidate the pharmacokinetics of anti-TNFα in the joint cavity; however, a more concentrated action of this drug may be assumed when delivered directly into the joint space. Moreover the different joints treated in this study display different anatomical and physiological features that might influence the pharmacokinetics of the injected compounds. For example, after intra-articular therapy in the knee, the drug rapidly diffuses throughout the joint space while in the wrist, the drug might be compartmentalized.56 Although the number of recruited patients was limited, we tried to perform a subgroup analysis, according to the type of involved joint. Interestingly, in large joints the improvement of several outcomes was more evident than in small joints when treated with intra-articular anti-TNFα agents. We might suggest that anatomical differences, a major amount of drug injected, or a different subjective perception of pain and discomfort in large joints, might explain these results.57
On these bases, many clinical and pharmacodynamic variables might explain the discrepancy in the results observed in literature and mainly: (1) the amount of injected antibodies was not sufficient to bind the majority of TNFα molecules present in the synovial cavity; (2) antibodies injected into the joint neutralized only TNFα present in the synovial fluid, but did not penetrate into the synovial tissue; (3) local processes supporting inflammation within the joints were obviously not restricted to TNFα; (4) previous lack of response to steroid injection may be a predictor of lack of response to anti-TNFα factor injection; and (5) the IA injection not performed by US guidance minimized the beneficial local effect of anti-TNFα agent.11,27,48,53 Larger studies specifically designed need to clarify these still open questions. We are aware that human anti-mouse antibodies and other neutralizing antibodies may cause acquired drug resistance to local anti-TNF agents as well as it was already described to systemic therapy. Similarly, the measurement of TNFα levels throughout the study period would help to better understand the disease course. Therefore larger studies including such evaluations would shed some light on different clinical outcomes.
The improvement in both articular and systemic outcomes, observed in our study, was independent by the type of anti-TNFα molecules used, although the small number of enrolled patients do not permit any definitive conclusions and analyses of larger cohort of patients need to clarify this therapeutic topic. We acknowledge that this study displays some limitations including the heterogeneity of involved joints and systemic treatment, the small number of patients studied in subgroup analyses, and MRI scans performed only in RA patients with a wrist or metacarpophalangeal joint arthritis. Moreover, we performed a 52-week follow-up to address safety and efficacy issues; however a longer follow-up period might be required to confirm our data.
During the past two decades, early and aggressive treatment of RA and PsA with DMARDs has significantly reduced the effects of the disease in terms of joint damage and disability. Today, remission is an achievable goal in many patients, irrespective of the type of DMARD used, whether synthetic or biological. Our data provided the evidence that US guided injection of intra-articular anti-TNFα agent injection is a safe and well tolerated procedure in both RA and PsA patients with monoarthritis, refractory to the treatment with systemic anti-TNFα agent, combining the local and systemic effects of this class of molecules, to achieve a good clinical and radiological response and/or a DAS28 remission.
Footnotes
Abbreviations: CRP, C-reactive protein; DAS disease activity score; DMARDs, disease-modifying anti-rheumatic drugs; ESR, erythrocyte sedimentation rate; G, gauge; GA, global assessment; GH, global health; HAQ, health assessment questionnaire; MRI, magnetic resonance imaging; NSAIDs, non-steroidal anti-inflammatory drugs; PD, power Doppler; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SD, standard deviation; Sj swollen joint; Tj, tender joint; TNF, tumor necrosis factor; US, ultrasonography; VAS, visual analogic scale.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Fisher BAC, Keat A. (2006) Should we be using intraarticular tumor necrosis factor blockade in inflammatory monoarthritis? Journal of Rheumatology 33: 1934–1935. [PubMed] [Google Scholar]
- 2. Deleuran BW, Chu CQ, Field M, et al. (1992) Localization of tumor necrosis factor receptors in the synovial tissue and cartilage-pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis & Rheumatology 35: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 3. Ritchlin C, Haas-Smith SA, Hicks D, et al. (1998) Patterns of cytokine production in psoriatic synovium. Journal of Rheumatology 25: 1544–1552. [PubMed] [Google Scholar]
- 4. van Kuijk AW, Reinders-Blankert P, Smeets TJ, et al. (2006) Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: Implications for treatment. Annals of the Rheumatic Diseases 65: 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kanbe K, Hara R, Chiba J, et al. (2014) Application of a new immunohistology scoring system (IH score): Analysis of TNF-α in synovium related to disease activity score in infliximab-treated patients with rheumatoid arthritis. Modern Rheumatology 24: 910–914. [DOI] [PubMed] [Google Scholar]
- 6. Marrelli A, Cipriani P, Liakouli V, et al. (2011) Angiogenesis in rheumatoid arthritis: A disease specific process or a common response to chronic inflammation? Autoimmunity Reviews 10: 595–598. [DOI] [PubMed] [Google Scholar]
- 7. Chimenti MS, Ballanti E, Perricone C, et al. (2013) Immunomodulation in psoriatic arthritis: Focus on cellular and molecular pathways. Autoimmunity Reviews 12: 599–606. [DOI] [PubMed] [Google Scholar]
- 8. Smolen JS, Landewé R, Breedveld FC, et al. (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Annals of the Rheumatic Diseases 73: 492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gladman DD. (2003) Effectiveness of psoriatic arthritis therapies. Seminars in Arthritis and Rheumatism 33: 29–37. [DOI] [PubMed] [Google Scholar]
- 10. Hollander JL, Brown EM, Jr, Jessar RA, et al. (1951) Hydrocortisone and cortisone injected into arthritic joints: Comparative effects of and use of hydrocortisone as a local antiarthritic agent. Journal of the American Medical Association 147: 1629–1635. [DOI] [PubMed] [Google Scholar]
- 11. Bokarewa M, Tarkowski A. (2003) Local infusion of infliximab for the treatment of acute joint inflammation. Annals of the Rheumatic Diseases 62: 783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nikas SN, Temekonidis TI, Zikou AK, et al. (2004) Treatment of resistant rheumatoid arthritis by intra-articular infliximab injections: A pilot study. Annals of the Rheumatic Diseases 63: 102–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bliddal H, Terslev L, Qvistgaard E, et al. (2006) A randomized, controlled study of a single intra-articular injection of etanercept or glucocorticosteroids in patients with rheumatoid arthritis. Scandinavian Journal of Rheumatology 35: 341–345. [DOI] [PubMed] [Google Scholar]
- 14. Bliddal H, Terslev L, Qvistgaard E, et al. (2006) Safety of intra-articular injection of etanercept in small-joint arthritis: An uncontrolled, pilot-study with independent imaging assessment. Joint Bone Spine 73: 714–717. [DOI] [PubMed] [Google Scholar]
- 15. Schatteman L, Gyselbrecht L, De Clercq L, et al. (2006) Treatment of refractory inflammatory monoarthritis in ankylosing spondylitis by intraarticular injection of infliximab. Journal of Rheumatology 33: 82–85. [PubMed] [Google Scholar]
- 16. Boesen M, Boesen L, Jensen KE, et al. (2008) Clinical outcome and imaging changes after intraarticular (IA) application of etanercept or methylprednisolone in rheumatoid arthritis: Magnetic resonance imaging and ultrasound-Doppler show no effect of IA injections in the wrist after 4 weeks. Journal of Rheumatology 35: 584–591. [PubMed] [Google Scholar]
- 17. Conti F, Ceccarelli F, Priori R, et al. (2008) Intra-articular infliximab in patients with rheumatoid arthritis and psoriatic arthritis with monoarthritis resistant to local glucocorticoids. Clinical efficacy extended to patients on systemic anti-tumour necrosis factor alpha. Annals of the Rheumatic Diseases 67: 1787–1790. [DOI] [PubMed] [Google Scholar]
- 18. van der Bijl AE, Teng YK, van Oosterhout M, et al. (2009) Efficacy of intraarticular infliximab in patients with chronic or recurrent gonarthritis: A clinical randomized trial. Arthritis & Rheumatology 61: 974–978. [DOI] [PubMed] [Google Scholar]
- 19. Haroon M, O’Gradaigh D. (2010. Efficacy and safety of combining intra-articular methylprednisolone and anti-TNF agent to achieve prolonged remission in patients with recurrent inflammatory monoarthritis. Joint Bone Spine 77: 232–234. [DOI] [PubMed] [Google Scholar]
- 20. Bello S, Bonali C, Serafino L, et al. (2010) Intra-articular therapy with infliximab in psoriatic arthritis: Efficacy and safety in refractory monoarthritis. Reumatismo 62: 46–50. [DOI] [PubMed] [Google Scholar]
- 21. Roux CH, Breuil V, Valerio L, et al. (2011) Etanercept compared to intraarticular corticosteroid injection in rheumatoid arthritis: Double-blind, randomized pilot study. Journal of Rheumatology 38: 1009–1011. [DOI] [PubMed] [Google Scholar]
- 22. Fiocco U, Sfriso P, Oliviero F, et al. (2013) Blockade of intra-articular TNF in peripheral spondyloarthritis: Its relevance to clinical scores, quantitative imaging and synovial fluid and synovial tissue biomarkers. Joint Bone Spine 80: 165–170. [DOI] [PubMed] [Google Scholar]
- 23. Østergaard M, Peterfy C, Conaghan P, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. Journal of Rheumatology 30: 1385–1386. [PubMed] [Google Scholar]
- 24. Mandl P, Naredo E, Wakefield RJ, et al. (2011) OMERACT ultrasound task Force. A systematic literature review analysis of ultrasound joint count and scoring systems to assess synovitis in rheumatoid arthritis according to the OMERACT filter. Journal of Rheumatology 38: 2055–2062. [DOI] [PubMed] [Google Scholar]
- 25. Scheel AK, Hermann KG, Kahler E, et al. (2005) A novel ultrasonographicsynovitis scoring system suitable for analyzing finger joint inflammation in rheumatoid arthritis. Arthritis & Rheumatology 52: 733–743. [DOI] [PubMed] [Google Scholar]
- 26. Coates LC, Hodgson R, Conaghan PG, et al. (2012) MRI and ultrasonography for diagnosis and monitoring of psoriatic arthritis. Best Practice & Research Clinical Rheumatology 26: 805–822. [DOI] [PubMed] [Google Scholar]
- 27. Bliddal H, Torp-Pedersen S. (2000) Use of small amounts of ultrasound guided air for injections. Annals of the Rheumatic Diseases 59: 926–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones A, Regan M, Ledingham J, et al. (1993) Importance of placement of intra-articular steroid injections. British Medical Journal 307: 1329–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conaghan P, Bird P, Ejbjerg B, et al. (2005) The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: The metacarpophalangeal joints. Annals of the Rheumatic Diseases 64 (Suppl. 1): i11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ejbjerg B, McQueen F, Lassere M, et al. (2005) The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: The wrist joint. Annals of the Rheumatic Diseases 64 (Suppl. 1): i23–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Østergaard M, Edmonds J, McQueen F, et al. (2005) An introduction to the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Annals of the Rheumatic Diseases 64 (Suppl. 1): i3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aletaha D, Neogi T, Silman AJ, et al. (2010) 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & Rheumatology 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 33. Taylor W, Gladman D, Helliwell P, et al. (2006) Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis & Rheumatology 54: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 34. Vander Cruyssen B, De Keyser F, Kruithof E, et al. (2007) Comparison of different outcome measures for psoriatic arthritis in patients treated with infliximab or placebo. Annals of the Rheumatic Diseases 66: 138–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saber TP, Ng CT, Renard G, et al. (2010) Remission in psoriatic arthritis: Is it possible and how can it be predicted? Arthritis Research & Therapy 12: R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Backhaus M, Burmester GR, Gerber T, et al. (2001) Working Group for Musculoskeletal Ultrasound in the EULAR Standing Committee on International Clinical Studies including Therapeutic Trials. Guidelines for musculoskeletal ultrasound in rheumatology. Annals of the Rheumatic Diseases 60: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Szkudlarek M, Court-Payen M, Strandberg C, et al. (2001) Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: A comparison with dynamic magnetic resonance imaging. Arthritis & Rheumatology 44: 2018–2223. [DOI] [PubMed] [Google Scholar]
- 38. Ribbens C, André B, Marcelis S, et al. (2003) Rheumatoid hand joint synovitis: Gray-scale and power Doppler US quantifications following anti-tumor necrosis factor-alpha treatment: Pilot study. Radiology 229: 562–569. [DOI] [PubMed] [Google Scholar]
- 39. Iagnocco A, Filippucci E, Perella C, et al. (2008) Clinical and ultrasonographic monitoring of response to adalimumab treatment in rheumatoid arthritis. Journal of Rheumatology 35: 35–40. [PubMed] [Google Scholar]
- 40. Naredo E, Wakefield RJ, Iagnocco A, et al. (2011) The OMERACT ultrasound task force-status and perspectives. Journal of Rheumatology 38: 2063–2067. [DOI] [PubMed] [Google Scholar]
- 41. Ohrndorf S, Backhaus M. (2013) Advances in sonographic scoring of rheumatoid arthritis. Annals of the Rheumatic Diseases 72 (Suppl. 2): ii69–75. [DOI] [PubMed] [Google Scholar]
- 42. Dejaco C, Duftner C, Wipfler-Freissmuth E, et al. (2012) Ultrasound-defined remission and active disease in rheumatoid arthritis: Association with clinical and serologic parameters. Seminars in Arthritis and Rheumatism 41: 761–767. [DOI] [PubMed] [Google Scholar]
- 43. Moelants EA, Mortier A, Van Damme J, et al. (2013) Regulation of TNF-α with a focus on rheumatoid arthritis. Immunology & Cell Biology 91: 393–401. [DOI] [PubMed] [Google Scholar]
- 44. Osta B, Benedetti G, Miossec P. (2014) Classical and paradoxical effects of TNF-α on bone homeostasis. Frontiers in Immunology 5: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spadaro A, Lubrano E. (2013) Beyond anti-TNF-α agents in psoriatic arthritis. Expert Review of Clinical Immunology 9: 507–509. [DOI] [PubMed] [Google Scholar]
- 46. Wijbrandts CA, Dijkgraaf MG, Kraan MC, et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Annals of the Rheumatic Diseases 67: 1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hetland ML, Østergaard M, Ejbjerg B, et al. (2012) CIMESTRA study group. Short- and long-term efficacy of intra-articular injections with betamethasone as part of a treat-to-target strategy in early rheumatoid arthritis: Impact of joint area, repeated injections, MRI findings, anti-CCP, IgM-RF and CRP. Annals of the Rheumatic Diseases 71: 851–856. [DOI] [PubMed] [Google Scholar]
- 48. Weitoft T, Uddenfeldt P. (2000) Importance of synovial fluid aspiration when injecting intra-articular corticosteroids. Annals of the Rheumatic Diseases 59: 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schumacher HR, Chen LX. (2005) Injectable corticosteroids in treatment of arthritis of the knee. American Journal of Medicine 118: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 50. Jahangier ZN, Jacobs JW, Lafeber FP, et al. (2005) Is radiation synovectomy for arthritis of the knee more effective than intraarticular treatment with glucocorticoids? Results of an eighteen-month, randomized, double-blind, placebo-controlled, crossover trial. Arthritis & Rheumatology 52: 3391–3402. [DOI] [PubMed] [Google Scholar]
- 51. Coates LC, Mumtaz A, Helliwell PS, et al. (2011) Development of a disease severity and responder index for psoriatic arthritis (PsA)-report of the OMERACT 10 PsA special interest group. Journal of Rheumatology 38: 1496–1501. [DOI] [PubMed] [Google Scholar]
- 52. Cavagna L, Caporali R, Trifiro’ G, et al. (2013) Overuse of prescription and otc non-steroidal anti-inflammatory drugs in patients with rheumatoid arthritis and osteoarthritis. International Journal of Immunopathology and Pharmacology 26: 279–281. [DOI] [PubMed] [Google Scholar]
- 53. Conti F, Priori R, Chimenti MS, et al. (2005) Successful treatment with intraarticular infliximab for resistant knee monoarthritis in a patient with spondylarthropathy: A role for scintigraphy with 99mTc-infliximab. Arthritis & Rheumatology 52: 1224–1226. [DOI] [PubMed] [Google Scholar]
- 54. Ahern MJ, Campbell DG, Weedon H, et al. (2008) Effect of intra-articular infliximab on synovial membrane pathology in a patient with a seronegative spondyloarthropathy. Annals of the Rheumatic Diseases 67: 1339–1342. [DOI] [PubMed] [Google Scholar]
- 55. Fiocco U, Sfriso P, Oliviero F, et al. (2010) Synovial effusion and synovial fluid biomarkers in psoriatic arthritis to assess intraarticular tumor necrosis factor-α blockade in the knee joint. Arthritis Research & Therapy 12: R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Metz VM, Mann FA, Gilula LA. (1993) Lack of correlation between site of wrist pain and location of noncommunicating defects shown by three-compartment wrist arthrography. American Journal of Roentgenology 160: 1239–1243. [DOI] [PubMed] [Google Scholar]
- 57. Scott DL, Smith C, Kingsley G. (2003) Joint damage and disability in rheumatoid arthritis: An updated systematic review. Clinical and Experimental Rheumatology 5 (Suppl. 31): S20–27. [PubMed] [Google Scholar]