Abstract
The chief manifestations of scabies are mediated through hypersensitivity-like reactions and immune responses which are so far not well understood and remain poorly characterized. The aim of this study is to investigate the role of inflammatory cytokines in relation to humoral immunity in patients with scabies. Serum levels of total IgE, specific IgG, IL-10, IL-6, INF-γ, and TNF-α were investigated in a cross-sectional study including 37 patients with manifestations suggestive of scabies and serologically positive for anti-Sarcoptes IgG, in addition to 20 healthy controls. The median value of total IgE was 209 (range, 17–1219 IU/mL), reflecting its wide range within cases. IL-10 showed significant higher levels (287 ± 139) in cases than in controls (17.4 ± 11.32). A positive correlation was reported between total IgE and severity of manifestations (r = 0.429, P <0.005). A significant positive correlation was observed between total IgE and both IgG and IL-6. On the contrary, a negative correlation was recorded between IL-6 and TNF-α which makes us suggested anti-inflammatory rather than pro-inflammatory effect of IL-6. Moreover, a negative correlation was noticed between the anti-inflammatory cytokine IL-10 and severity of manifestations, specific IgG, total IgE, and INF-γ. Therefore, the current study theorized a regulatory role of IL-10 in inflammatory responses of scabietic patients suggesting further future analysis of its therapeutic potential.
Keywords: immune response, inflammatory cytokines, interferon, interleukin, scabies
Introduction
Scabies is a major global health problem caused by the mite Sarcoptes scabiei. It is transmitted by skin-to-skin contact, as established in classical studies by Mellanby.1 Scabies affects any sex, all ages as well as all racial groups and socioeconomic levels. The main disease manifestations are mediated through inflammatory and hypersensitivity-like reactions to mite products leading to different pruritic lesions depending on host and parasite factors.2 Unfortunately, there are no accurate estimates of the prevalence of Sarcoptic infestation in most of the populations affected worldwide, including Egypt. However, there are several examples of how S. scabiei can lead to devastating morbidity which is still underestimated.3 The clinical appearance of scabies is reported to be of wide range from mild to severe destructive type. Spontaneous recovery of scabies in humans has been described to occur only with subsequent re-infestation.4 Despite the economic and health significance of Sarcoptic infestation in both human and animal populations, the pathogenesis and immune responses to this disease are not yet well understood and remain poorly characterized.5
Studies of the symptoms and signs of scabies identified the development of host immunity to Sarcoptes antigens. Immunological memory to mite antigens has been demonstrated with an induction time of only 24 h for hypersensitivity in patients infested for a second time. Additionally, parasite numbers were significantly reduced with approximately 60% failure of re-infestation of sensitized hosts.4 However, studies concerning immune response to specific Sarcoptes antigens are very few because of the difficulty in acquiring sufficient numbers of the organism. Therefore, most of the scientific studies were done using antigens extracted from mites of canine origin or from house dust mites.2 On the other hand, studies on cytokine production during scabies infestation revealed variable results and hence further studies are suggested to elucidate immunological aspects during scabies infestation. Our study aims to investigate the level of two anti-inflammatory cytokines (IL-10 and IL-6) and two pro-inflammatory cytokines (IFN-γ and TNF-α) in relation to humoral immunity (IgG and IgE) in patients suffering from symptoms suggestive of scabies.
Materials and methods
Study design and population
This cross-sectional study was conducted at the Dermatology Outpatient Clinic, Dermatology Department at Kasr Al-Ainy Teaching Hospital, Faculty of Medicine, Cairo University between November 2013 and June 2014. The study started after being approved by the Ethical Committee, Deanship of Higher Education and Scientific Research, and Dermatology Department Council, Faculty of Medicine, Cairo University. The study population was recruited from patients with manifestations suggestive of scabies. The study included 50 cases in addition to 20 healthy control subjects. All cases, after giving an informed consent, were subjected to history-taking, full clinical examination, and skin scraping that was performed only for those taking part in the procedure. In addition, serum samples were collected for immunological analysis; enzyme-linked immunosorbent assay (ELISA) for detection of the levels of IFN-γ, TNF-α, IL-10, and IL-6; specific IgG against Sarcoptes scabiei antigen; and automated chemiluminescence immunoassay for detection of total IgE level. Cases receiving anti-scabies treatment, suffering from chronic diseases or acute infections (systemic or cutaneous), suffering from any other skin diseases in addition to feverish cases were all excluded from the study.
Clinical examination
Distribution and extent of the cutaneous lesions were investigated to determine the topographic distribution of the lesions and the extent of skin affection. The body surface was divided into 16 areas according to the method of Jackson et al.6 as follows: interdigital spaces; hand; wrist; arm; elbow; axilla; leg; foot; abdomen; thorax; mammilla/perimammillar area; back; buttocks; genitals; inguinal area/medial area of the thigh; and scalp/neck/face. Therefore, total surface body area (TSBA) can be calculated. Moreover, primary lesions were differentiated into macular, papular, papulo-crusted, and vesicular besides the presence of scratch marks.
Skin scraping and microscopic examination
For each patient, skin was examined with a magnifying lens for lesions suggestive of scabies infestation and mineral oil was then applied. The non-inflamed areas were scraped with a scalpel blade or glass slide held at a 90° angle to the skin. A cover slip was placed over each slide and examined microscopically.
Immunological analysis
Enzyme linked immunosorbent assay (ELISA) for specific IgG
This assay was used to detect IgG antibodies against Sarcoptes scabiei (var. Canis) in serum samples in micro-titer plate format (ELISA, Afosa GmbH, Dahlewitz/Berlin, Germany). The micro-titer plate was coated with a Sarcoptes antigen. During serum incubation specific anti-Sarcoptes antibodies were bound to the immobilized antigen. Unbound materials as well as unbound enzyme-conjugate were removed by washing. Newly formed immune complexes were incubated with TMB substrate solution leading to the development of a blue color that correlates with the amount of Sarcoptes antibodies in the serum sample. The (OD) values were measured using an ELISA reader (ELISA reader, Dynex Dynatech) at 450 nm and within 10 min after adding the stop solution.
Enzyme linked immunosorbent assay (ELISA) for specific cytokines
The test was performed according to the manufacturer’s instructions (Assaypro LLC −30 Triad South Drive, St. Charles, MO, USA). This assay employs a quantitative sandwich enzyme immunoassay technique that measures different human cytokines: IFN- γ, TNF-α, IL-6, and IL-10. A polyclonal antibody specific for each cytokine has been pre-coated onto a 96-well microplate. Each cytokine was sandwiched by the immobilized antibody and biotinylated polyclonal specific antibody and was recognized by a streptavidin-peroxidase conjugate. Any unbound material was then washed away and a peroxidase enzyme substrate was added. The intensity of the color was measured and OD values were calculated as previously mentioned above.
Automated chemiluminescence immunoassay for total IgE
Total IgE detection was done at Clinical Pathology Department, Faculty of Medicine, Cairo University by automated chemiluminescence immunoassay technique using ADVIA Centaur® ReadyPack™ machine. The ADVIA Centaur assay is a two-site sandwich immunoassay using direct chemiluminometric technology which uses constant amounts of two antibodies. The first antibody, in the Lite Reagent, is an affinity purified polyclonal rabbit anti-specific target antibody labeled with acridinium ester. The second antibody, in the Solid Phase, is a monoclonal mouse anti-specific target (IgE) antibody covalently coupled to paramagnetic particles. All steps were automatically performed by the system.
Results
This cross-sectional study was initially carried out on 50 patients with manifestations suggestive of scabies, in addition to 20 healthy controls. The clinical manifestations of different cases were suggestive of ordinary scabies and crusted scabies was not reported among cases of the current study. Anti-Sarcoptes specific IgG was investigated for both cases and controls using ELISA technique. The cutoff value, calculated as the mean of positive control −2SD, was 0.485. ELISA OD values for the control group were below the cutoff value while 37 out of the 50 suspected cases recorded positive readings of mean 0.88 ± 0.21. The 13 cases who obtained negative results were excluded from the study. Subsequently, the number of cases was reported to be 37 scabietic cases and 20 healthy controls. Only 20 skin scraping samples were examined microscopically for the presence of Sarcoptes mites, eggs, or fecal pellets; however, no parasitological evidence was demonstrated in the skin scraping samples. On the other hand, and according to Jackson et al.’s6 classification, cases included in the current study were classified as follows: (1) Severe extended lesions; erythema and/or papules with intense scratch marks, extending over >30% of TSBA (about five areas or more); (2) Severe less extended lesions; erythema and/or papules with intense scratch marks in 10–30% TSBA (two to four areas); (3) Moderate lesions; no scratch marks in one or two areas. In the current study severe extended lesions were observed in 17 cases (46%), while in 15 cases (40.5%) the lesions were severe less extended. Moderate itchy lesions were noticed in a significant lower number: five cases (13.5%).
Total IgE was measured in both cases and controls. The median value was 209 (range, 17–1219 IU/mL). A geometric mean value up to150 lU/mL was considered normal. The difference between total IgE for cases and control was statistically significant (P <0.0001) (Figure 1).
Figure 1.
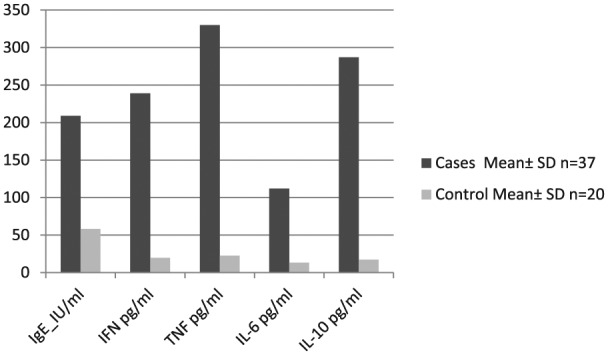
Bar chart representing mean level of different cytokines in cases and control groups.
The OD readings of IFN, TNF, IL-6, and IL-10 were 0.239 ± 0.188, 0.330 ± 0.087, 0.112 ± 0.028 and 0.287 ± 0.139, respectively. Using the standard curves, these values were equivalent to 243 ± 169, 326 ± 94, 123 ± 32, and 269 ± 112 pg/mL, respectively, which showed significant higher levels in cases than in controls (P <0.0001 in all cytokines except in IL-6 where P <0.001).
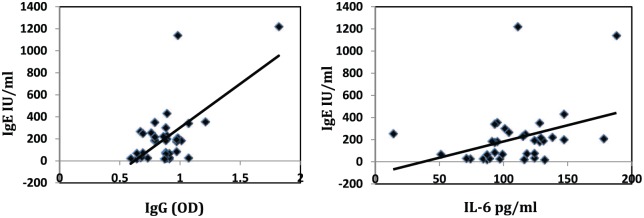
A significant positive correlation was observed between the total IgE and both specific IgG and IL-6 (r = 0.641 and 0.429, P value <0.001 and = 0.008, respectively) (Figure 2).
Figure 2.
Positive correlation between total IgE and each of specific IgG (left) and IL-6 (right).
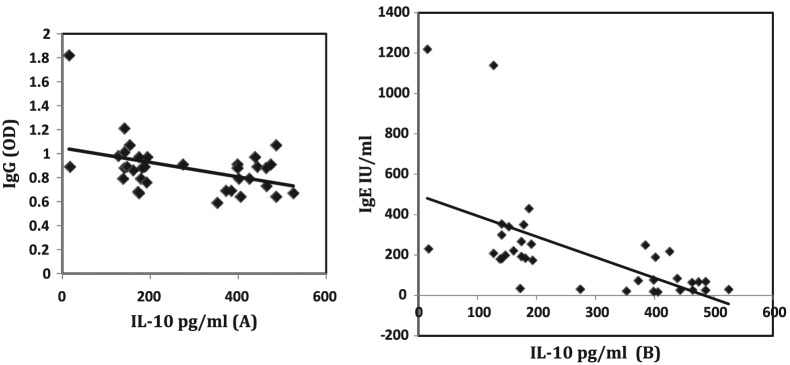
On the contrary, a significant negative correlation was recorded between IL-10 and each of IgG (Figure 3, left), total IgE (Figure 3, right), and INF-γ (Figure 4) (r = −0.340, −0.527, and −0.395, P = 0.040, 0.001, and 0.016, respectively).
Figure 3.
Negative correlation between total IL-10 and each of specific IgG (left) and IgE (right).
Figure 4.
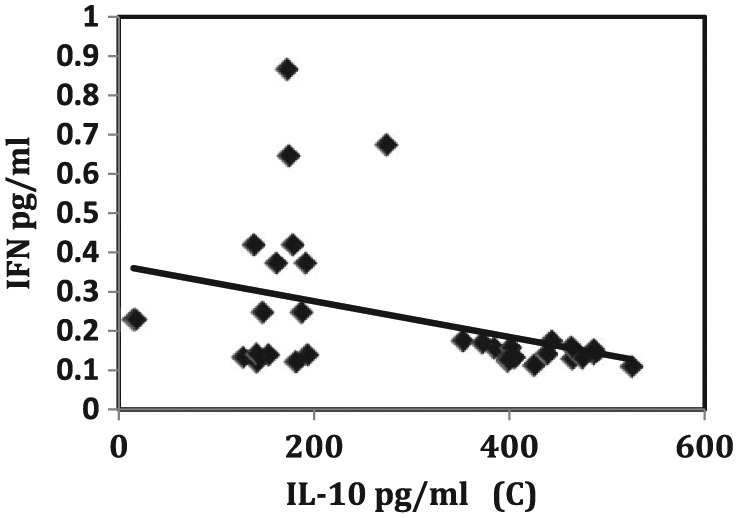
Negative correlation between IL-10 and INF-γ.
On the other hand, an insignificant correlation was noticed between IgE and both TNF-α and IFN-γ (r = −0.256, −0.033 and P = 0.126 and 0.848, respectively). Moreover, a negative correlation was recorded between IL-6 and TNF-α. Other correlations were found to be insignificant. Concerning correlation with the severity of clinical manifestations, a positive correlation was reported between each of total IgE and specific IgG and severity of manifestations (r = 0.436, P <0.005). On the contrary a negative correlation was noticed between IL-10 and severity of manifestations.
Discussion
The current study was carried out on 37 ordinary scabietic patients serologically positive for specific anti-Sarcoptes IgG. In general, scabies is a challenging infection and a negative result, even from an expert, does not exclude such infestation.2,7 Diagnosis of our cases was based not only on clinical manifestations, but also on serological findings to relatively assure actual diagnosis of scabietic cases. In the present study, the median value of total IgE was 209 (range, 17–1219 IU/mL), reflecting a wide variant range. The difference between total IgE for cases and control was statistically significant P <0.0001. A wide variation in serum IgE levels has also been documented by Nye et al.8 and Barbee et al.9 who have attempted to define supposed levels in healthy non-allergic adult populations. In general, investigations of humoral immunity in scabietic patients have shown contradictory results.4 Concerning total IgE, elevated levels were reported in ordinary scabies by a number of researchers10–13 while others reported its decrease after treatment14–16 or no differences.17,18 On the contrary, dramatic increases in total IgE levels have been documented in crusted scabies.13,19,20 This disparity could be attributed to lack of sensitivity, timing of blood collection in relation to disease progression, or any other unknown cause of an immune response in the host. The results of IgE in the present work seem to contradict that documented by Walton et al.21 who stated that total serum IgE appears to be of no benefit in the preliminary clinical investigation into a suspected host. But our results showed positive correlation between total IgE and severity of cutaneous lesions. On the other hand, this study showed a positive correlation between IL-6 and total IgE. This was in agreement with the work of Martino et al.22 who suggested that the mechanisms of immunoglobulin overproduction in some cases might be through the unbalanced interleukin network and elevated IL-6 synthesis. Potential evidence related to such correlation was reported by Gagari et al.,23 who stated that activation of mouse mast cells via stem cell factor (SCF) can induce the release of IL-6. The authors reported that mast cells that produce IgE represented a potential source of IL-6 as well as other cytokines that have been implicated in host defense, tissue maintenance, remodeling, immunoregulation, and other biological responses. Therefore, the increased level of IL-6 in some of our cases seemed to be a response to IgE production by the mast cells. Moreover, released IL-6 resulted in little or no detectable release of TNF-α, histamine, or serotonin as reported by Gagari et al.23 This is also in agreement with our results, in which negative correlation was recorded between IL-6 and TNF-α. IL-6 is an interleukin that acts as both a pro- and anti-inflammatory cytokine and its role as an anti-inflammatory cytokine is mediated through its inhibitory effects on TNF-α.24 Therefore, the results of the current study suggested that IL-6 acts as anti-inflammatory cytokine in scabietic cases rather than a pro-inflammatory cytokine due to its inhibition of TNF-α.
Roberts et al.25 concluded that the occurrence of increased serum levels of total IgE and IgG might be related to an inappropriate anti-inflammatory T-helper-2 (Th2) type immune response. This observation might be verified by the observation in the present study, as a significant negative correlation was recorded between IL-10 (anti-inflammatory Th2 cytokine) and both parameters of humoral immunity measured in the current work: specific IgG and IgE (r = −0.340 and −0.527 and P = 0.040 and 0.001, respectively). However, this humoral immune response seems non-protective, as shown by high rates of re-infestation reported by Roberts et al.25 despite increased antibody levels. Such observation may be clarified once more by the results of the present work concerning the significant high levels of IgE and low levels of IL-10 that was noticed with cases suffering from severe extended manifestations. Therefore, the present study may theorize that anti-inflammatory Th2 cytokines such as IL-10 and not the humoral immunity may play a protective role to guard against severe manifestations in human scabies. The significant negative correlation recorded in the current study between INF-γ (Th-1 inflammatory cytokine) and IL-10 (Th-2 anti-inflammatory cytokine) (r = −0.395 and P = 0.016) may support such hypothesis. Kennedy26 suggested a regulatory role of IL-10 in inflammatory responses in scabietic patients. The authors concluded that IL-10 may be a natural regulator of mast cell activator that reduces inflammation and irritability related to allergic reactions. The authors added that IL-10 polymorphism is associated with increased IgE levels and incidence of allergic reaction disease; hence IL-10 dys-regulation may affect the course of the allergic condition. Cases with high IgE level may interfere with anti-inflammatory reaction of IL-10 and may suffer from extensive allergic reaction. Therefore, IL-10 may be considered as a possible treatment for these allergic conditions.
In general, hypersensitivity is understood to be an imbalance between the Th2 and Th1 immune responses. Th1 type cytokines (IFN-γ) have been found to exacerbate the inflammatory process and not to improve the allergic immune response.26 This may explain the negative correlation between INF-γ and IL-10 recorded in the current work and the resulting decrease in manifestations in severely manifested cases where elevated IL-10 resulted in decreased IFN-γ level. This comes in accordance with Bijjiga and Martino27 who stated that minimizing the development of Th1responses is done either by decreasing Th1-related cytokines or encouraging Th2 responses by increasing levels of Th2-related cytokines. IL-10 cytokine is therefore capable of inhibiting synthesis of the pro-inflammatory cytokines such as IFN-γ and TNF-α and is also stimulatory to certain T cells and mast cells. In addition, it stimulates B-cell maturation and antibody production, as suggested by Bijjiga and Martino.27 These results suggest a critical role of IL-10 in human delayed immune response in reducing the inflammatory and allergic responses. Generally, a balanced immune response and interleukin network seem to play a critical role during scabies infection.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Mellanby K. (1941) The transmission of scabies. British Medical Journal 2: 405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walton SF, Currie BJ. (2007) Problems in diagnosing scabies, a global disease in human and animal populations. Clinical Microbiology Reviews 20: 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mounsey KE, Holt DC, McCarthy JS. (2009) Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Archives of Dermatology 145: 840–841. [DOI] [PubMed] [Google Scholar]
- 4. Walton SF. (2010) The immunology of susceptibility and resistance to scabies. Parasite Immunology 32: 532–540. [DOI] [PubMed] [Google Scholar]
- 5. Mounsey K, Ho MF, Kelly A, et al. (2010) A tractable experimental model for study of human and animal scabies. PLoS Neglected Tropical Diseases 4: e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson A, Heukelbach J, Filho AF, et al. (2007) Scabies in Brazil. Tropical Medicine & International Health 12: 493–502. [DOI] [PubMed] [Google Scholar]
- 7. Chosidow O. (2006) Clinical practices. Scabies. New England Journal of Medicine 354: 1718–1727. [DOI] [PubMed] [Google Scholar]
- 8. Nye L, Merrett TG, Landon S, et al. (1975) A detailed investigation of circulating IgE levels in a normal population. Clinical Allergy 5: 13–24. [DOI] [PubMed] [Google Scholar]
- 9. Barbee RA, Brown WG, Kaltenborn W, et al. (1981) Allergen skin test reactivity in a community population sample: Correlation with age, histamin skin reactions and total serum immunoglobulin E. Journal of Allergy and Clinical Immunology 68: l5–19. [DOI] [PubMed] [Google Scholar]
- 10. Falk ES. (1980) Serum immunoglobulin values in patients with scabies. British Journal of Dermatology 102: 57–61. [DOI] [PubMed] [Google Scholar]
- 11. Reunala T, Ranki A, Rantanen T, et al. (1984) Inflammatory cells in skin lesions of scabies. Clinical and Experimental Dermatology 9: 70–77. [DOI] [PubMed] [Google Scholar]
- 12. Senol M, Ozerol I, Ozerol E, et al. (1997) Serum immunoglobulin and complement levels in scabies. Journal of Turgut Ozal Medical Centre 4: 37–39. [Google Scholar]
- 13. Walton SF, Beroukas D, Roberts-Thomson P, et al. (2008) New insights into disease pathogenesis in crusted (Norwegian) scabies: The skin immune response in crusted scabies. British Journal of Dermatology 158: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 14. Falk ES, Eide TJ. (1981) Histologic and clinical findings in human scabies. International Journal of Dermatology 20: 600–605. [DOI] [PubMed] [Google Scholar]
- 15. Falk ES. (1984) Scabies and giardiasis. Increased serum IgE due to scabies infestation in 2 children with scabies and giardiasis. Dermatologica 168: 253–254. [PubMed] [Google Scholar]
- 16. Morsy TA, Kenawi MZ, Zohdy HA, et al. (1993) Serum immunoglobulin and complement values in scabietic patients. Journal of the Egyptian Society of Parasitology 23: 221–229. [PubMed] [Google Scholar]
- 17. Hancock BW, Ward AM. (1974) Serum immunoglobulin in scabies. Journal of Investigative Dermatology 63: 482–484. [DOI] [PubMed] [Google Scholar]
- 18. Nassef NE, Makled KM, Elzayat EA, et al. (1991) Humoral and cell mediated immune responses in scabietic patients. Journal of the Egyptian Society of Parasitology 21: 765–770. [PubMed] [Google Scholar]
- 19. Morsy TA, Romia SA, Al-Ganayni GA, et al. (1990) Histocompatibility (HLA) antigens in Egyptians with two parasitic skin diseases (scabies and leishmaniasis). Journal of the Egyptian Society of Parasitology 20: 565–572. [PubMed] [Google Scholar]
- 20. Liu HN, Sheu WJ, Chu TL. (1992) Scabietic nodules: A dermatopathologic and immunofluorescent study. Journal of Cutaneous Pathology 19: 124–127. [DOI] [PubMed] [Google Scholar]
- 21. Walton SF, Pizzutto S, Slender A, et al. (2010) Increased allergic immune response to Sarcoptes scabiei antigens in crusted versus ordinary scabies. Clinical and Vaccine Immunology 17: 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martino M, Rossi ME, Azzari C, et al. (1999) Interleukin-6 synthesis and IgE overproduction in children with perinatal human immunodeficiency virus-type 1 infection. Annals of Allergy Asthma & Immunology 82: 212–216. [DOI] [PubMed] [Google Scholar]
- 23. Gagari E, Tsai M, Lantz C, et al. (1997) Differential release of mast cell interleukin-6 via c-kit. Blood 89: 2654–2663. [PubMed] [Google Scholar]
- 24. Shin HD, Park BL, Kim LH, et al. (2005) Interleukin-10 haplotype associated with total serum IgE in atopic dermatitis patients. Allergy 60: 1146–1151. [DOI] [PubMed] [Google Scholar]
- 25. Roberts LJ, Huffam SE, Walton SF, et al. (2005) Crusted scabies: Clinical and immunological findings in seventy-eight patients and a review of the literature. Journal of Infection 50: 375–381. [DOI] [PubMed] [Google Scholar]
- 26. Kennedy SB. (2007) Interleukin-10 Suppresses Mast Cell IgE Receptor Expression And Signaling In Vitro And In Vivo. MS Thesis. Paper 1415. Richmond, VA: Virginia Commonwealth University. [Google Scholar]
- 27. Bijjiga E, Martino AT. (2013) Interleukin 10 (IL-10) regulatory cytokine and its clinical consequences. Journal of Clinical & Cellular Immunology S1: 007. [Google Scholar]