Abstract
The development of pharmacokinetics led this science to achieve a relevant role in the investigation of new chemical entities for therapeutic application, and has allowed a series of new useful realizations of out of patent drugs like prolonged release and delayed release formulations, therapeutic delivery system (TDS) for drugs to be active in systemic circulation avoiding the first pass effect, orodispersible and effervescent formulations, intramuscular and subcutaneous depot formulations acting over a long period, oral inhalatory systems, and drug association at fixed dose. The above applications had pharmacokinetics as protagonist and have required the support from bioanalytical methods to assay drug concentrations, even in pg·mL−1 of plasma, that really have paralleled the synergic development of pharmacokinetics.
The complexity of the above realizations required specific guidelines from the regulatory authorities, mainly the US FDA and EU EMA, which have normalized and, in most cases, simplified the above applications admitting some waivers of in vivo bioequivalence.
However, this review highlights some critical points, not yet focused on by operating guidelines, which need to be clarified by regulatory authorities. One of the most relevant issues is about the planning and conducting bioavailability and bioequivalence trials with endogenous substances, that possess own homeostatic equilibria with fluctuations, in some cases with specific rhythms, like melatonin and female sex hormones. The baseline subtraction required by guidelines to define the net contribute to the exogenous absorbed drug in most cases is a non-solvable problem.
Keywords: analytical bioassay methods, bioavailability, bioequivalence, endogenous substances, pharmacokinetics
Introduction
The name pharmacokinetics was coined by Dost in 1953.1 This science initially developed slowly, and then quickly, paralleling the development of analytical bioassay methods that now are able to quantify plasma concentration of drugs possessing high distribution volume (Vd), reaching low limit of quantification (LLOQ) with values ranging from pg·mL−1 to ng·mL−1. This is now achievable with chromatographic methods, mainly with the tandem mass spectrometry (LC-MS-MS) that assures the highest specificity, sensitivity, and short-lasting analytic runs.2
The guidelines edited by the US FDA and EU EMA have progressively directed and clarified several aspects in planning and performing pharmacokinetic (PK), bioavailability (BA), and bioequivalence (BE) trials. Some other questionable aspects classified in literature as ‘open questions on BE’ still exist and need a definite clarification by regulatory authorities.3–5 The main open question not yet clarified is that related to the PKs and BE of endogenous substances that in most cases do not present well evident peak shapes after oral administration and thus do not allow the baseline subtraction required by guidelines.6
Development steps in pharmacokinetics as substantiated by analytic improvement
Radiotracing approach
This was the first pharmacokinetic approach and was characterized by the administration of drugs labeled with 14C or 3H. PKs were studied following the total radioactivity with the liquid scintillation technique. This technique is not specific as evaluates together parent drug and metabolite(s). The first attempt, that should be considered pioneering, was that of Okita et al. who developed Digitalis purpurea into an airtight growing chamber in the presence of 14CO2. After 2 years, the above investigators extracted and purified 14C-digitoxin from the leaves and administered this cardiac glycoside to dogs and humans, thus producing the first PK data on this drug.7–9 The further development was to randomly label drugs with 3H with a H/3H exchanging chromatographic process. Doherty et al. produced the first data on 3H-digoxin, another cardiac glycoside largely used in that period to treat congestive heart failure and other circulation disorders.10
The radiotracing technique was largely used to achieve PK data on several drugs,11,12 but was quickly neglected when more specific techniques were available. This technique, however, is still used in the first development steps of drugs to evaluate the ADME (absorption, distribution, metabolism, excretion), namely the whole amount of the given drug that is excreted via urine, via feces and is still present in the body. This trial is carried out in both animals and healthy volunteers.
Radioimmunoassay (RIA) approach
This method had its relevant promoter Lindenbaum who, using the RIA of digoxin just set up by Smith et al.,13 bioassayed plasma concentrations of this cardiac glycoside after administration of four different brands to four subjects following the cross-over design. Very relevant differences in digoxin profiles were observed comparing the four brands administered.14 This led the US FDA to prescribe a dissolution test for digoxin tablets that assured in further lots an acceptable uniformity. Lindenbaum used the term ‘biological availability’ that was quickly contracted in bioavailability. The experiment of Lindenbaum introduced not only the above term in the literature, but in particular the concept of bioavailability in the mind of scientific operators, that means the possibility to obtain plasma concentrations specific of a defined pharmaceutical formulation.
The RIA was applied only to a restricted number of drugs. It represented an improvement if compared to the previous radiotracing approach, but emerging chromatographic techniques quickly prevailed. Now RIA is still used to quantify some endogenous substances for PKs, BA, and BE.
Chromatographic approach
In comparison to radiotracing and RIA, chromatographic techniques allow a better specificity as the column involved can separate the peaks of the various analytic components, e.g. parent drug and metabolites or any interfering substance.
The first chromatographic technique used in PKs was gas chromatography (GC), which however needs the analyte to be heated in order to become volatile. All functional groups like hydroxyl (-OH), carboxyl (-COOH), and amino (-NH2, -NH-) must be derivatized to give esters, ethers, and amides that can become volatile. Detectors like flame ionization (FID), thermoionic specific (TSD), electron capture (ECD), and mass fragmentography (MS) allow a quantitative evaluation of the analyte eluted from the column.15,16 High performance liquid chromatography (HPLC) and capillary electrophoresis (CE) do not need any heating and use UV-Vis, or electrochemical or fluorimetric detectors. With both GC and HPLC the analytical run lasts several minutes, e.g. until 18 min, and the LLOQ that can be obtained is in the range of 20–100 ng·mL−1 (Tables 1 and 2). CE did not meet analytical development in bioassay techniques as, in spite of the very high sensitivity, it requires a very low volume of sample injected that vanishes its appeal of sensitivity.
Table 1.
Comparison of main analytical features between HPLC and LC-MS-MS bioassay of nimesulide and its active metabolite 4-hydroxy-nimesulide.
| HPLC | LC-MS-MS | |
|---|---|---|
| Drugs | Nimesulide and 4-hydroxy-nimesulide (4-OH-nimesulide) | |
| Detection | UV 330 nm | API 365 triple quadrupole mass spectrometry with turbo ion spray interface |
| Dynamic range; LOQ | 100–8000 ng·mL−1; LOQ 100 ng·mL−1, with both analytes | 0.5–80 ng·mL−1; LOQ 0.5 ng·mL−1, with both analytes |
| Retention times | Nimesulide 11.0 min, 4-OH-nimesulide 6.1 min, I.S. 14.2 min | Nimesulide 2.42 min, 4-OH-nimesulide 2.18 min, I.S. 2.66 min |
| Duration of the run | 18 min | 5.5 min |
| Sample size of the run | 50 | 150 |
| Time required for 1000 analyses by one operator | 20 days | 7 days |
Table 2.
Comparison of main analytical features between GC and LC-MS-MS bioassay of isosorbide-5-mononitrate.
| GC | LC-MS-MS | |
|---|---|---|
| Drug | Isosorbide-5-mononitrate | |
| Detection | ECD 63Ni | API 365 triple quadrupole mass spectrometry with turbo ion spray interface |
| Dynamic range; LOQ | 20–700 ng·mL−1; LOQ 20 ng·mL−1 | 5–80 ng·mL−1; LOQ 5 ng·mL−1 |
| Retention times | Isosorbide-5-mononitrate 8.9 min, I.S. 6.3 min | Isosorbide-5-mononitrate 3.5 min, I.S. 4.2 min |
| Duration of the run | 15 min | 5.5 min |
| Sample size of the run | 30 | 150 |
| Time required for 1000 analyses by one operator | days | 7 days |
Both HPLC and GC techniques were surpassed by LC-MS-MS and are still used for some applications, mainly in pharmaceutics.
Chromatographic techniques are still now preferentially useful with chiral columns when enantiospecific separation of enantiomers is requested, this both in pharmaceutics and in pharmacokinetics.17
Tandem mass spectrometry
The triple quadrupole analytical technique, currently called tandem mass spectrometry, in comparison to the previous analytical techniques, possesses the following advantages:
- the highest specificity
- a high sensitivity, in the range from pg·mL−1 to ng·mL−1
- the shortest run time, namely about 4–6 min
The most useful application of triple quadrupole is as detector of liquid chromatography (LC-MS-MS). This technique has allowed to bioassay a number of drugs in biological fluids, including drugs possessing high distribution volume that require high sensitivity to appreciate plasma concentrations of pg·mL−1.18–25). A less frequent application of mass spectrometry is as a detector of gas chromatographic eluate.26–28
Tables 1 and 2 show compared conditions to bioassay in human plasma nimesulide and its 4-hydroxy metabolite, and isosorbide-5-mononitrate with chromatographic techniques (HPLC and GC) and tandem mass spectrometry.2 The advantage of tandem mass spectrometry is evident from the run lasting that is 3–5 times shorter, from the sensitivity that expressed from LLOQ is 2–200 times better and from the time requested to bioassay 1000 analyses, that was 3–4 times shorter.
The above characteristics promoted this technique as the most used in pharmacokinetics, bioavailability, and bioequivalence.2
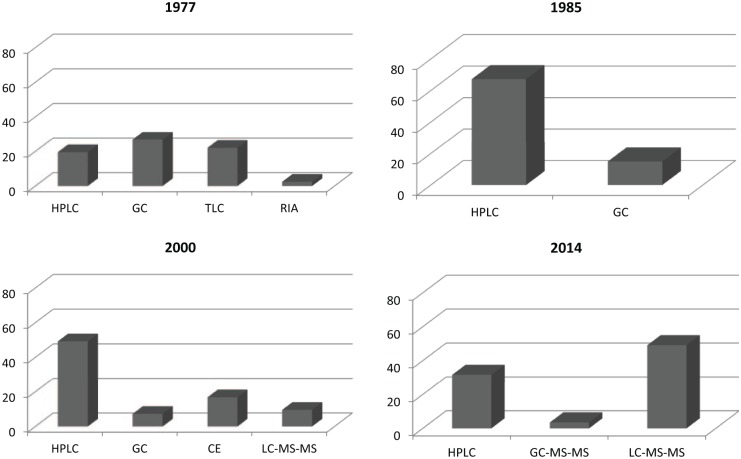
Figure 1 shows the development of the analytical techniques to bioassay drugs in biological fluids in the period 1977–2014. Data were drawn from a number of papers in the range of 41–57 per year published in 1977, 1985, 2000, and 2014 in the Journal of Chromatography, Biomedical Applications.
Figure 1.
Frequency in the use of analytical techniques for bioassay, as published in the Journal of Chromatography, Biomedical Applications in 4 different years.
EU guidelines on bioavailability and bioequivalence
The first EU guideline on BA and BE was edited in 1991.29 Ten years later EMA emitted a further guideline on BA and BE.30 A deeply revised guideline was edited by EMA in 2010.31 The last guideline was focused only on bioequivalence of immediate release formulations. The guideline on modified release formulations and transdermal dosage forms was edited by EMA in 199632 and updated in draft in 2013.33 The above guidelines have focused on most specific problems that occur in BA and mainly in BE trials.
However, some critical aspects now still need specific suggestions in order to plan and carry out some specific kind of trials. The main problems are definitely clarified and those not well-defined in the last guideline31 are discussed in the sections below.
Problems focused on by guidelines
Titer difference between test and reference
In bioequivalence trials, the titer of test and reference must differ from each other by not more than 5%. In the case of a higher difference, BE on Cmax and AUC must be checked on dose normalized parameters. Previous EMA guidelines did not allow the above dose-normalization. This possibility can be very useful in the case of drugs that are acceptable by pharmaceutics in a titer range larger than 95.0–105.0%.5
Carryover from the pre-dose to the second study period
In crossover trials the pre-dose baseline of the second study period in a given volunteer could contain a measurable drug concentration > LLOQ. If this value is <5% of Cmax, the volunteer can be statistically processed. If the baseline is >5% of Cmax, the given volunteer must be excluded from the statistical analysis.31 This statement is in line with US FDA guidelines,34 but was not considered in previous EU guidelines. The greater sensitivity achievable with LC-MS-MS has increased the frequency of the presence of the above carryover that can be avoided prolonging the wash out separating the two study periods.35
Enlargement of the 90% confidence interval for Cmax
The first EU guideline (EMA, 1991) allowed, in the case of Cmax, the 90% confidence interval (CI) for assessing BE to be enlarged from the stipulated 0.80–1.25 interval without, however, giving any limit. From publications the expanded limit was set to 0.70–1.43.36 The EU guideline edited on 200130 indicated how 90% CI of Cmax could be enlarged in the case of high variability as 0.75–1.33. The last EU guideline31 allowed 90% CI of Cmax to be enlarged only in the case of intrasubject coefficient of variation (CV) ⩾30% and if the replicate design was adopted and only for oral immediate release formulations as reported in Table 3.
Table 3.
Enlarged limit of 90% CI of Cmax for different values of CV %.
| Intrasubject CV % | Enlarged limit of 90% CI of Cmax |
|---|---|
| 30 | 0.8000–1.2500 |
| 35 | 0.7723–1.2948 |
| 40 | 0.7462–1.3402 |
| 45 | 0.7215–1.3859 |
| ⩾50 | 0.6984–1.4319 |
US FDA guideline on bioequivalence does not allow any enlargement of 90% CI of Cmax from the statistics 0.80–1.25 range.34
Drugs characterized as narrow therapeutic index (NTIDs) with Cmax of particular importance for safety, efficacy, or drug level monitoring must produce 0.90–1.11 as the acceptance interval for Cmax to be assessed as bioequivalent.31
Parent drug or active metabolite(s)
In the case of parent drug and active metabolite, the last EMA guideline31 has clarified to bioassay and assess BE only on parent drug. However, in some cases the parent drug disappears quickly so that it is not possible to follow its plasma concentration, whereas active metabolite produces a well evident shape of plasma concentration. Some parent drugs largely biotransformed in active metabolite are reported in Table 4.
Table 4.
Metabolite to parent drug AUC ratio of some largely biotransformed parent drugs.
| Active metabolite | Parent drug | Metabolite to parent drug AUC ratio |
|---|---|---|
| Enalaprilat | Enalapril | 2.575 |
| Zofenoprilat | Zofenopril | 6.775 |
| Hydroxyflutamide | Flutamide | 6076 |
| Hydroxypurinol | Allopurinol | 4477 |
| Acid metabolite of terfenadine | Terfenadine | ⩾10078 |
| Monohydroxycarbamazepine | Oxcarbazepine | ⩾10079 |
| Mycophenolic acid | Mycophenolatemofetil | ⩾2552,80 |
| Desmethylclozapine N-oxideclozapine and other metabolites | Clozapine | ~70% metabolized81 |
In the above and similar cases, the guideline accepts the assessment of bioequivalence on metabolite, even if it is not active, like in the case of betahistine.
Waivers for in vivo bioequivalence
EU and US guidelines accept in some specific cases the waiver of in vivo bioequivalence trial. Water solutions are exempt. With several doses of a given drug in most cases their approval can be authorized on the basis of a BE trial on only one dose, usually the highest, in other cases the lowest. Also intravenous (IV) injectable solutions are exempt from BE. However, the most interesting case is that arose from the Biopharmaceutical Classification System (BCS) published by Amidon et al.37 and focused on by guidelines.31,38 Amidon classified drugs into four groups according to their solubility and permeability (Table 5).
Table 5.
Four drug classes according to solubility and permeability, by Amidon et al.37
| Drug class | Solubility | Permeability |
|---|---|---|
| 1 | High | High |
| 2 | Low | High |
| 3 | High | Low |
| 4 | Low | Low |
US FDA and EU EMA guidelines31,38 exempt from in vivo BE drugs classified in groups 1 and 3 of BCS, if they however demonstrate to possess the requested solubility: dissolution >85% within 15 min or >85% within 30 min in the pH range of 1–6.8. The waiver is restricted to oral immediate release formulations and drugs not classified as narrow therapeutic index.
The number of drugs targeted as laying in groups 1 or 3 of the BCS increased in the last years and are listed in Table 6, in which the drugs are grouped in class 1 and class 3 of BCS and are susceptible to a BE waiver.39–41 The enlargement of the number of drugs classified in the two above classes has reduced the need of BE trials. Their approval is supported by studies in vitro on the solubility and the dissolution rate at the requested series of pH.
Table 6.
List of drugs classified in classes 1 and 3 of BCS.
| Class 1 | Class 3 |
|---|---|
| Acetylsalicylic acid | Abacavir |
| Allopurinol | acyclovir |
| Amiloride HCl | Alendronic acid |
| Amitriptyline HCl | Amodiaquine |
| Amlodipine | Anastrazole |
| Amoxicillin | Atenolol |
| Ascorbic acid | Benznidazole |
| Bisoprolol | Biperiden HCl |
| Calcium folinate | Captopril |
| Cetirizine | Carbidopa |
| Chloroquine sulfate | Cefaclor |
| Citalopram/escitalopram | Chlorambucil |
| Clindamycin | Chloramphenicol |
| Cyclophosphamide | Chlorphenamine hydrogen maleate |
| Diazepam | Chlorpromazine HCl |
| Diethylcarbamazine dihydrogen citrate | Cimetidine |
| Digoxin | Ciprofloxacin HCl |
| Donepezil | Clavulanic acid |
| Doxazosin | Clomifene citrate |
| Doxycycline HCl | Clomipramine HCl |
| Fluconazole | Cloxacillin sodium salt |
| Lamivudine | Codeine phosphate |
| Levodopa | Colchicine |
| Levofloxacin | Cycloserine |
| Levonorgestrel | Didanosine |
| Lithium carbamate | Enalapril |
| Loratadine | Ergocalciferol |
| DL-methionine | Ergotamine tartrate |
| Metronidazole | Ethambutol HCl |
| Mirtazapine | Ethinylestradiol |
| Nicotinamide | Ethionamide |
| Norethisterone | Ethosuximide |
| Ofloxacin | Ferrous salt |
| Ondaserton | Flucytosine |
| Paracetamol | Gabapentin |
| Phenobarbital | Glyceryl trinitrate |
| Phenoxymethyl penicillin potassium salt | Hydralazine HCl |
| Potassium iodide | Hydrochlorothiazide |
| Pravastatin | Isoniazid |
| Prednisolone | Isosorbide dinitrate |
| Primaquine diphosphate | Letrozole |
| Proguanil HCl | Levamisole HCl |
| Promethazine HCl | Levetiracetam |
| Propranolol HCl | Levothyroxine sodium salt |
| Propylthiouracil | Lisinpril |
| Pyridoxine HCl | Losartan |
| Quinapril | Medroxyprogesterone acetate |
| Quinine sulfate | Metformin HCl |
| Ramipril | Methotrexate sodium salt |
| Riboflavin | Methyldopa |
| Salbutamol sulfate | Metoclopramide HCl |
| Sertraline | Morphine sulfate |
| Sildenafil | Neostigmine bromide |
| Stavudine | Nifurtimox |
| Tamoxifen citrate | Penicillamine |
| Terbinafin | Pentamine |
| Theophylline | Procarbazine HCl |
| Tramadol | Pyrazinamide |
| Valproic acid | Pyridostigmine bromide |
| Venlafaxine | Quinidine sulfate |
| Warfarin sodium salt | Ranitidine HCl |
| Zidovudine | Risedronic acid |
| Zolpidem | Sulfadoxine |
| Terazosin | |
| Thiamine HCl | |
| Topiramate | |
| Zinc sulfate |
Problems that are still now neglected by guidelines
Multiple peak phenomenon
Some drugs are absorbed producing two or more peaks of plasma concentration.
Typical is the two-peak phenomenon of drugs that meet the enterohepatic circulation, that produce a first peak within 2 h, and a second peak within 6–12 h after administration. This occurs inter alia with piroxicam,4,42 glibenclamide,43,44 ursodeoxycholic acid,45 mycophenolic acid, and its parent drug mycophenolate mofetil.46
More complex and less predictable are other cases when the second peak or a series of peaks appears quicker, namely within 3–4 h after dosing. Among these cases what happens with diclofenac would be remembered. According to Bettini et al.47 the second peak of diclofenac found by various authors2,48–50 is the result of a hydratation process of the drug to tetrahydrodiclofenac, less soluble, occurring in the gut. A formulation of diclofenac able to be absorbed very fast proved to produce only one peak, namely the first one, of relevant entity.51
In these cases Marzo and Reiner3 suggested assessing bioequivalence only on the values of 90% CI of AUC, whereas Cmax should be managed checking individual values to lay in the range expected from previous literature for the given dose, thus excluding any problem of unexpected activity or tolerability. This suggestion was not considered by EMA neither in the guideline31 nor in the Questions and Answers.52
Ethics problems on bioequivalence
Some drugs can produce safety problems if administered in bioequivalence trials on healthy volunteers. Excluding the case of cytostatic agents that could not be given to healthy volunteers, some other cases should be considered, as follows. Carbamazepine in steady state that requires 30 days to be achievable;53 cyclosporine that can affect renal clearance;54 flutamide that can cause gynecomastia in men;55 morphine for several adverse events;56 warfarin that requires a long period of treatment to achieve steady state;57 and clozapine for relevant side effects.58,59
In the above cases and, of course, in other similar situations the administration in healthy volunteers, mainly in trials of repeated dose regimen to reach a steady state, should be carefully considered and possibly avoided.4 Indeed, operating guidelines require steady state to assess BE of extended-release oral formulations, for transdermal delivery systems and for immediate release formulations of drugs that are cleared from the body with long half-lives.31–33
Endogenous substances and their baseline
PKs, BA, and BE of endogenous substances in most cases are faced with very complex problems not considered by EU operating guideline. The difficulties in managing trials with these substances are the following:
(a) the presence of a baseline, that can fluctuate around an average level, and can have specific rhythms, as in the case with melatonin, cortisol, and female sexual hormones;6
(b) the request from the EU EMA to subtract baseline, that in the above situation (a) often presents relevant difficulties, mainly in the presence of endogenous rhythms;
(c) the homeostatic equilibria of the body that operate in order to avoid excessively high or low concentrations of the endogenous substances through strictly controlled mechanisms;
(d) the dilution effect of the low amount entering the systemic circulation with that more relevant contained in the body;
(e) the multicomponent reversible metabolism, common with endogenous substances;60
(f) the specific body storages, e.g. bones, fats, red blood cells;
(g) the renal threshold that is a mechanism able to assure constant systemic concentrations of several substances, as for instance L-carnitine, most ions, and some amino acids.6,60
The consequence of the above homeostatic mechanisms is that the absorption of endogenous substances administered exogenously in most cases does not produce a well-defined shape of plasma concentration.
In these cases, even when a peak shape is obtained, a serious problem of increased variability arises about the regulatory request to subtract baseline, as follows in the simulation reported as an example in the Scheme 1.
Scheme 1.
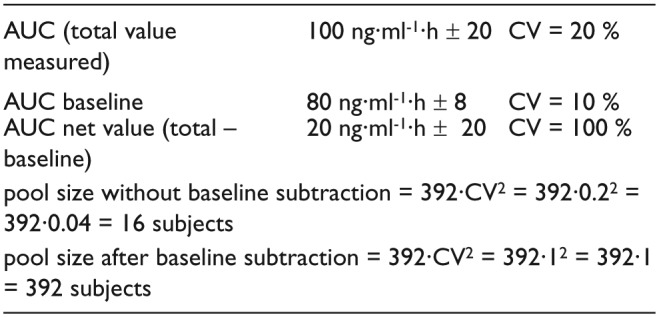
Pool size evaluation of an endogenous substance without and after baseline subtraction.61
A simulation case was described for cholecalciferol that, after an oral administration of 100,000 IU (2.50 mg), reaches the peak at 7 days whereas the baseline is restored at 100 days.62,63
The above considerations lead to conclude that the baseline subtraction should be avoided in most cases.
With drugs excreted via urine, like L-carnitine, most ions, and some amino acids, the bioavailability evaluation from cumulative urinary excretion is preferable to the profile of plasma concentrations.64,65 With endogenous substances biotransformed the solution is more complex. In certain cases the subtraction can be avoided administering high doses of the drugs, providing a peak shape well evident. In other cases, a repeated dose regimen for a relatively long period could be sufficient to consider also baseline as a product of the exogenous administration. Another solution is to approach the Test Vs. Reference comparison with a phase III trial on target population. This approach was followed by Cerutti et al.66 in assessing BE of levothyroxine.
Specific cases focused on by regulatory authorities are those of potassium, levothyroxine, and omega 3 derivatives. In the case of potassium, the US FDA guidance suggests assessing bioequivalence on the basis of urinary excretion of this ion and suggests neglecting the plasma concentration of this ion as it is not an expression of bioavailability.67 In the case of levothyroxine the specific guideline edited by the US FDA suggests administering a high oral dose that produces a relevant peak shape and to avoid baseline subtraction.68 The EU EMA on the Questions and Answers document has focused on the case of omega 3 derivates eicosapentaenoic acid and docosahexaenoic acid to assess bioequivalence according an exemption justified by the fact that the soft gelatin capsule contains only the above two drugs without any excipient.52
Preanalytical preparation of samples for chromato-graphic bioassay
All chromatographic techniques, namely HPLC, LC-MS-MS, and GC, require a previous extraction of the analytical compounds from the matrix that can be achieved with liquid-liquid and solid-liquid extraction, and the addition of internal standard (IS).15 In the case of LC-MS-MS the best IS should be the deuterated analyte, which possesses identical chemical characteristics of the true analyte, but can be identified and detected by the multiquadrupole analytical system.
The analyst must pay attention to some relevant aspects, as follows:
(a) the plasma-red cell distribution of the analyte. In the case of asymmetric distribution, the procedure must be focused on, e.g. bioassay the analyte on whole blood;
(b) if reversible metabolism is operating, both parent drug and metabolite must be assayed; alternatively the two or more compounds must be chemically transformed in one of them;
(c) in the presence of hydrolytic metabolism active on parent drug, the test tube containing blood before centrifugation must be put in one ice-bath and centrifuged at a planned time, the shorter possible, and then must be frozen in ice-dry and then put in freezer. This is inter alia the case of acetylsalicylic acid;
(d) the anticlothing agent must be selected at the validation of analytical procedure and maintained along the further bioassay of samples.
Guidelines on analytical validation
On 1992 Shah et al. published the first suggestions how to manage and validate a bioanalytical method.69 After a decade the same authors published an updated revision of their previous considerations.70 Specific guidelines on analytical validation were edited by the US FDA34 and by the EU.71,72
The above guidelines focused on detailed procedures for the pre-study validation that must be extensively described in the Validation Report and for study specific validation that must be documented in the Analytical Report. A specific section is devoted to incurred sample reanalysis, that is considered also in Questions and Answers.52
Adjunctive remarks
The following adjunctive remarks should be considered by regulatory agency, which would help the scientific operators in planning their pharmacokinetic research.
Drugs with narrow therapeutic index are excluded from several waivers, as noticed in the present review. However the lack of its univocal definition makes problematic its application.
In certain cases the repeated dose design, required by operating guidelines, can produce tolerability problems that would suggest avoiding this design, adopting only that in single dose. Ethics problems discussed in the present review are typical examples of drugs that should not be administered to healthy volunteers in repeated dose regimen. However, guidelines on bioequivalence do not focus on this aspect.
Another problem that is controversial is whether to use healthy volunteers with PK trial instead of a phase III clinical trial in same applications that do not involve bioavailability, but only local treatment for local activity. According to personal experience, the PK comparison of some test vs. reference, have produced extremely high coefficient of variation that would vanify any bioequivalence assessment. The responsibility of the above very high CV was considered difficult in calibrating the planned dose of test and reference. On the contrary, we have obtained satisfactory results in comparing these topical drugs, test vs. reference, for local and systemic tolerability in healthy volunteers treated for a complete therapeutic cycle. This kind of design in the past was accepted by AIFA in Italy to assess BE for a period of about 3 years. This approach could be considered by the EU at least for certain cases as an alternative to a Phase III trial in assessing bioequivalence of topical drugs for local activity.
Discussion
The updated research of new chemical entities for therapeutic use involves thousands of molecules that must be screened and investigated in order to find one or some interesting among them for new drug application (NDA). This compels scientific operators to design and follow a screening schedule that involves PKs just at the first steps to evaluate the couple of solubility and permeability, in order to classify the molecules in the four classes of BCS of Amidon.37 The scarcity of new chemical entities in the last two decades has opened an increased space for applied research evolving out of patent drugs addressed to their new applications, as follows. Prolonged release oral formulations that allow the once-a-day regimen, even with drugs possessing a short t1/2, like nifedipine, whose half-life is about 2 h. Delayed release formulations of drugs that must act on the colon and if administered orally must be formulated in order to release the active ingredient only after the ileo-cecal valve, in the colon, like mesalazine. Transdermal delivery systems (TDS) for systemic activity that can overcome the first pass effect that metabolizes presystemically a relevant part of the drug, around 99% in the case of nitroglycerin.73 Female sex hormones, clonidine, and nicotine are also formulated in TDS. Depot formulations of drugs injected i.m. or s.c. allow sustained pharmacological activity that persists over 1 month. Relevant applications are also orodispersible and effervescent formulations of various drugs, oral inhaler systems for treatment of asthma, and new fixed-dose combination products.74
The above applications in most cases were reached on research of BE assessment comparing the given drug on test vs. feference formulations according to the ANDA procedures that exempted out of patent drug from clinical trials on target population, considering already well-achieved data on their activity and safety. These applications have as primary protagonist pharmacokinetics that, however, needed the invaluable support of analytical procedures, the LC-MS-MS being the most relevant. All these realizations were normalized and governed by operating guidelines, both the US FDA and EU EMA, which allowed some waivers of in vivo bioequivalence in most cases supported by in vitro data on solubility, disgregation, and dissolution.
Some open questions still need to be considered and governed by guidelines, the most relevant being the puzzle of endogenous substances that are strictly controlled in the body by homeostatic equilibria, an obstacle in most cases of the evidence of well-defined peak shape after an exogenous administration, and to find net plasma concentrations resulting from the baseline subtraction.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the “G. d’Annunzio” University of Chieti-Pescara with funds and equipments to carry on the studies.
References
- 1. Gladtke E. (1988) History of pharmacokinetics. In: Pecile A, Rescigno A. (eds.) Pharmacokinetics. New York: Plenum Press, pp. 1–10. [Google Scholar]
- 2. Marzo A, Dal Bo L. (2007) Tandem mass spectrometry (LC-MS-MS): A predominant role in bioassays for pharmacokinetic studies. Arzneimittel Forschung 57: 122–128. [DOI] [PubMed] [Google Scholar]
- 3. Marzo A, Reiner V. (2004) Open questions on bioequivalence: The case of multiple peak phenomenon. Journal of Pharmacology and Pharmacotherapeutics 56: 281–282. [DOI] [PubMed] [Google Scholar]
- 4. Marzo A. (2007) Open questions on bioequivalence: An updated reappraisal. Current Clinical Pharmacology 2: 179–189. [DOI] [PubMed] [Google Scholar]
- 5. Marzo A. (1999) Open questions on bioequivalence: Some problems and some solutions. Pharmacological Research 40: 357–368. [DOI] [PubMed] [Google Scholar]
- 6. Marzo A, Rescigno A. (1993) Pharmacokinetics of endogenous substances: Some problems and some solutions. European Journal of Drug Metabolism and Pharmacokinetics 18: 77–88. [DOI] [PubMed] [Google Scholar]
- 7. Okita GT, Kalsley FE, Walaszek EJ, et al. (1954) Biosynthesis and isolation of carbon-14-labeled digitoxin. Journal of Pharmacology and Experimental Therapeutics 110: 244–250. [PubMed] [Google Scholar]
- 8. Okita GT, Talso PJ, Curry JH, Jr, et al. (1955) Metabolic fate of radioactive digitoxin in human subjects. Journal of Pharmacology and Experimental Therapeutics 115: 371–379. [PubMed] [Google Scholar]
- 9. Okita GT, Talso PJ, Curry JH, Jr, et al. (1955) Blood level studies of 14C-digitoxin in human subjects with cardiac failure. Journal of Pharmacology and Experimental Therapeutics 113: 376–382. [PubMed] [Google Scholar]
- 10. Doherty JE, Perkins WH. (1962) Studies with tritiated digoxin in human subjects after intravenous administration. American Heart Journal 63: 528–536. [DOI] [PubMed] [Google Scholar]
- 11. Marchetti GV, Marzo A, De Ponti C, et al. (1971) Blood levels and tissue distribution of 3H-ouabain administered per os. An experimental and clinical study. Arzneimittel Forschung 21: 1339–1403. [PubMed] [Google Scholar]
- 12. Marzo A, Ghirardi P, Riva O, et al. (1976) Plasma turnover and excretion of K-strophantoside-3H in human volunteers after parenteral administration. Naunyn-Schmiedeberg’s Archives of Pharmacology 94: 115–120. [DOI] [PubMed] [Google Scholar]
- 13. Smith TW, Butler VP, Haber E. (1969) Determination of the therapeutic and toxic serum digoxin concentrations by radioimmunoassay. New England Journal of Medicine 281: 1212–1216. [DOI] [PubMed] [Google Scholar]
- 14. Lindenbaum J, Mellow MH, Blackstone MO, et al. (1971) Variation in biological availability of digoxin from four preparations. New England Journal of Medicine 24: 1344–1347. [DOI] [PubMed] [Google Scholar]
- 15. Marzo A. (1989) Chromatographic methods and selective detectors in pharmacokinetics. Bollettino Chimico Farmaceutico 128: 45–53. [PubMed] [Google Scholar]
- 16. Bressolle F, Bromet-Petit M, Audran M. (1996) Validation of liquid chromatographic and gas chromatographic methods. Applications to pharmacokinetics. Journal of Chromatography B 86: 3–10. [DOI] [PubMed] [Google Scholar]
- 17. Marzo A, Heftmann E. (2002) Enantioselective analytical methods in pharmacokinetics with specific references to generic polymorphic metabolism. Journal of Biochemical and Biophysical Methods 54: 57–70. [DOI] [PubMed] [Google Scholar]
- 18. Dal Bo L, Mazzucchelli P, Marzo A. (2000) Assay of zofenopril and its active metabolite zofenoprilat by liquid chromatography coupled with tandem mass spectrometry. Journal of Chromatography B 749: 287–294. [DOI] [PubMed] [Google Scholar]
- 19. Dal Bo L, Mazzucchelli P, Marzo A. (1999) A high sensitive bioassay of lidocaine in human plasma by LC-MS-MS. Journal of Chromatography A 854: 3–11. [DOI] [PubMed] [Google Scholar]
- 20. Marzo A, Dal Bo L, Rusca A, et al. (2002) Bio-equivalence of ticlopidine hydrochloride administered in single dose to healthy volunteers. Pharmacological Research 46: 401–407. [DOI] [PubMed] [Google Scholar]
- 21. Marzo A, Dal Bo L, Ceppi Monti N, et al. (2004) Pharmacokinetics and pharmacodynamics of safinamide, a neuroprotectant with antiparkinsonian and anticonvulsant activity. Pharmacological Research 50: 77–85. [DOI] [PubMed] [Google Scholar]
- 22. Keller T, Cambon N, Genevray M, et al. (2005) Bioequivalence study of fluoxetine hydrochloride in healthy volunteers. Arzneimittel Forschung 55: 491–497. [DOI] [PubMed] [Google Scholar]
- 23. Marzo A, Zava D, Coa K, et al. (2009) Pharmacokinetics of isoxsuprine hydrochloride administered orally and intramuscularly to female healthy volunteers. Arzneimittel Forschung 59: 455–460. [DOI] [PubMed] [Google Scholar]
- 24. Marzo A, Coa K, Fontana E, et al. (2010) Pharmacokinetics and pharmacodynamics of ritodrine hydrochloride administered orally and intramuscularly to female healthy volunteers. Arzneimittel Forschung 60: 510–518. [DOI] [PubMed] [Google Scholar]
- 25. Dal Bo L, Mazzucchelli P, Fibbioli M, et al. (2006) Bioassay of safinamide in biological fluids of humans and various animal species. Arzneimittel Forschung 56: 814–819. [DOI] [PubMed] [Google Scholar]
- 26. Endele R, Senn M. (1983) MS/MS in pharmacokinetic studies. International Journal of Mass Spectrometry and Ion Physics 48: 81–84. [Google Scholar]
- 27. Settlage JA, Geilsdorf W, Jaeger H. (1983) Femtogram level quantitative determination of nitroglycerin and metabolites in human plasma by GC-MS negative ion chemical ionization, single ion monitoring. J High Res. Chromatography 6: 68–71. [Google Scholar]
- 28. McAllister A, Mosberg H, Settlage JA, et al. (1986) Plasma levels of nitroglycerin generated by three nitroglycerin patch preparations, Nitradisc, Transderm-Nitro and Nitro-Dur and one ointment formulation, Nitrobid. British Journal of Clinical Pharmacology 21: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. EMA (1991) Note for guidance: Investigation of bioavailability and bioequivalence. III/54/89-EN Final. London: EMA. [Google Scholar]
- 30. EMA (2001) Note for guidance: Investigation of bioavailability and bioequivalence. CPMP/EWP/QWP/1401/98. London: EMA. [Google Scholar]
- 31. EMA (2010) Guidance on the investigation of bioequivalence, CPMP/QWP/EWP/1401/98 Rev. 1. London: EMA. [Google Scholar]
- 32. EMA (1999) Note for guidance on modified release oral and transdermal dosage forms. Section II, pharmacokinetic and clinical evaluation. CPMP/EWP/280/1996. London: EMA. [Google Scholar]
- 33. EMA (2013) Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms, EMA/CPMP/EWP/280/96 Corr. 1, Draft XXIII. London: EMA. [Google Scholar]
- 34. US FDA (2003) Bioavailability and bioequivalence studies for orally administered drug products: general considerations. Silver Spring, MD: US FDA. [Google Scholar]
- 35. Marzo A, Fontana E. (2009) How improved sensitivity of bioassays and terminal half-life of drugs impact on bioequivalence trials. Arzneimittel Forschung 59: 55–58. [Google Scholar]
- 36. Diletti E, Hauschke D, Steinijans VW. (1992) Sample size determination: Extended tables for the multiplicative model and bioequivalence ranges of 0.9 to 1.11 and 0.7 to 1.43. Clinical Pharmacology, Therapy and Toxicology 30: 559–592. [PubMed] [Google Scholar]
- 37. Amidon GL, Lennemas H, Shah VP, et al. (1995) A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharmaceutical Research 12: 413–420. [DOI] [PubMed] [Google Scholar]
- 38. US FDA (2000) Guidance for industry. Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms containing certain active moieties/active ingredients based on a biopharmaceutics classification system. Silver Spring, MD: US FDA. [Google Scholar]
- 39. Lindenberg M, Kopp S, Dressman JB. (2004) Classification of orally administered drugs on the World Health Organization Model list of essential medicines according to the biopharmaceutics classification system. European Journal of Pharmaceutics and Biopharmaceutics 58: 265–278. [DOI] [PubMed] [Google Scholar]
- 40. Ramirez E, Laosa O, Guerra P, et al. (2010) Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the biopharmaceutic classification system. British Journal of Clinical Pharmacology 70: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Health Organization (2006) Technical Report Series Annex 8. Proposal to waive in vivo bioequivalence, requirements for WHO model list of essential medicines immediate-release, solid oral dosage forms. No. 937. Geneva: WHO, pp. 391–437. [Google Scholar]
- 42. Fourtillan JB, Bubourg D. (1983) Pharmacokinetic study of piroxicam in healthy man after administration of a single dose equal to 20 mg, by the oral route. Therapie 38: 163–170. [PubMed] [Google Scholar]
- 43. Ikegami H, Shima K, Tanaka A, et al. (1986) Interindividual variation in the absorption of glibenclamide in man. Acta Endocrinologica 111: 528–532. [DOI] [PubMed] [Google Scholar]
- 44. Balan G, Timmins P, Greene DS, et al. (2000) In-vitro in-vivo correlation models for glibenclamide after administration of metformin/glibenclamide tablets to healthy human volunteers. Journal of Pharmacology and Pharmacotherapeutics 52: 831–838. [DOI] [PubMed] [Google Scholar]
- 45. Parquet M, Metman EH, Raizman A, et al. (1985) Bioavailability, gastrointestinal transit, solubilization and faecal excretion of ursodeoxycholic acid in man. European Journal of Clinical Investigation 15: 171–178. [DOI] [PubMed] [Google Scholar]
- 46. Jiao Z, Ding J, Shen J, et al. (2008) Population pharmacokinetic modelling for enterohepatic circulation of mycophenolic acid in healthy Chinese and the influence of polymorphism in UGT19A. British Journal of Clinical Pharmacology 65: 893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bettini R, Giordano F, Donini C, et al. (2000) Swelling force development as a result of hydrate formation in diclofenac sodium or nitrofurantoin in tablets. STP Pharma Sciences 10: 335–339. [Google Scholar]
- 48. Chan KH, Mojaverian P, Ziehmer BA, et al. (1990) Application of radiotelemetric technique in evaluating diclofenac sodium absorption after oral administration of various dosage forms in healthy volunteers. Pharmaceutical Research 7: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 49. Terhaag B, Gramatte T, Hrdlcka P, et al. (1991) The influence of food on the absorption of diclofenac as a pure substance. International Journal of Clinical Pharmacology, Therapy and Toxicology 29: 418–421. [PubMed] [Google Scholar]
- 50. Macia MA, Frias J, Carcas AJ, et al. (1995) Comparative bioavailability of a dispersible formulation of diclofenac and finding of double plasma peaks. International Journal of Clinical Pharmacology and Therapeutics 33: 333–339. [PubMed] [Google Scholar]
- 51. Reiner V, Reiner A, Reiner G, et al. (2011) Increased absorption rate of diclofenac from fast acting formulations containing its potassium salt. Arzneimittel Forschung 11: 885–890. [DOI] [PubMed] [Google Scholar]
- 52. EMA (2013) Questions and Answers: Positions on specific questions addressed to the pharmacokinetics working party, EMA/618604/2008 Rev. 8. London: EMA. [Google Scholar]
- 53. Bertlisson L, Tomson T. (1986) Clinical pharmacokinetics and pharmacological effects of carbamazepine 10,11-epoxide. Clinical Pharmacokinetics 11: 177–198. [DOI] [PubMed] [Google Scholar]
- 54. Bennet WM, Pulliam JP. (1983) Cyclosporine nephrotoxicity. Annals of Internal Medicine 99: 851–854. [DOI] [PubMed] [Google Scholar]
- 55. Jacobo E, Schmidt JD, Weinstein SH, et al. (1976) Comparison of flutamide (SCH-13521) and diethylstilbestrol in untreated advanced prostatic cancer. Urology 8: 231–233. [DOI] [PubMed] [Google Scholar]
- 56. Osborne R, Joel S, Trew D, et al. (1990) Morphine and matebolite behaviour after different routes of morphone administration: Demonstration of the importance of the active metabolite morphine-6-glucuronide. Clinical Pharmacology & Therapeutics 47: 12–19. [DOI] [PubMed] [Google Scholar]
- 57. Palareti G, Legnani C. (1996) Warfarin withdrawal: Pharmacokinetic-pharmacodinamic considerations. Clinical Pharmacokinetics 30: 300–313. [DOI] [PubMed] [Google Scholar]
- 58. Pokorny R, Finkel MJ, Robinson WT. (1994) Normal volunteers should not be used for bioavailability or bioequivalence studies of clozapine. Pharmacological Research 11: 1221. [DOI] [PubMed] [Google Scholar]
- 59. Meyer MC. (2001) United States food and drug administration requirements for approval of generic drug products. Journal of Clinical Psychiatry 62 (Suppl. 5): 4–9. [PubMed] [Google Scholar]
- 60. Marzo A. (1996) Toxicokinetics of endogenous substances: A neglected issue. Arzneimittel Forschung 46: 1–10. [PubMed] [Google Scholar]
- 61. Marzo A, Balant LP. (1995) Bioequivalence. An updated reappraisal addressed to applications of interchangeable multi-source pharmaceutical products. Arzneimittel Forschung 45: 109–115. [PubMed] [Google Scholar]
- 62. Marzo A, Barassi A, Porro E. (2013) Open question on bioequivalence: The case of cholecalciferol. Italian Journal of Medicine 7: 156–159. [Google Scholar]
- 63. Ilahi M, Armas LAG, Heaney RP. (2008) Pharmacokinetics of a single, large dose of cholecalciferol. American Journal of Clinical Nutrition 87: 688–691. [DOI] [PubMed] [Google Scholar]
- 64. Marzo A, Vuksic D, Crivelli F. (2000) Bioequivalence of endogenous substances facing homeostatic equilibria: An example with potassium. Pharmacological Research 42: 523–525. [DOI] [PubMed] [Google Scholar]
- 65. Scotti A, Bianchini C, Abbiati G, et al. Absorption of calcium administered alone or in a fixed combination with vitamin D to healthy volunteers. Arzneimittel Forschung 51: 493–500. [DOI] [PubMed] [Google Scholar]
- 66. Cerutti R, Rivolta G, Cavalieri L, et al. (1999) Bioequivalence of levothyroxine tables administered to a target population in steady state. Pharmacological Research 39: 193–201. [DOI] [PubMed] [Google Scholar]
- 67. Cerny I, Dighe SV. (1987) Guidance for in vivo bioequivalence study for slow-release potassium chloride tablets/capsules. Silver Spring, MD: US FDA. [Google Scholar]
- 68. US FDA (2001) Guidance for Industry. Levothyroxine sodium products. Enforcement of August 14, 2001 compliance date and submission of new applications. Silver Spring, MD: US FDA. [Google Scholar]
- 69. Shah VP, Midha KK, Dighe SV. (1992) Analytical method validation: Bioavailability, bioequivalence and pharmacokinetic studies. Pharmacology Research 9: 558–592. [DOI] [PubMed] [Google Scholar]
- 70. Shah VP, Midha KK, Findlay JW, et al. (2000) Bioanalytical method validation – a revisit with a decade of progress. Pharmacology Research 17: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 71. CPMP Working Party on Quality of Medicinal Products (1989) Note for guidance on analytical validation. CPMP 111/844/87-EN Final. London: EMA. [Google Scholar]
- 72. EMA (2011) Guideline on bioanalytical method validation EMA/CHMP/EWP/192217/2009. London: EMA. [Google Scholar]
- 73. Lalka D, Griffith RK, Cronenberger CL. (1993) The hepatic first-pass metabolism of problematic drugs. Journal of Clinical Pharmacology 33: 657–669. [DOI] [PubMed] [Google Scholar]
- 74. Marzo A. (2012) Development steps of pharmacokinetics: A perspective on bioanalytical method and bioequivalence. Current Clinical Pharmacology 7: 328–332. [DOI] [PubMed] [Google Scholar]
- 75. Marzo A, Dal Bo L, Mazzucchelli P, et al. (2002) Pharmacokinetic and pharmacodynamic comparative study of zofenopril and enalapril in healthy volunteers. Arzneimittel Forschung 52: 233–242. [DOI] [PubMed] [Google Scholar]
- 76. Schulz M, Schmoldt A, Donn F, et al. (1998) The pharmacokinetics of flutamide and its major metabolites after a single oral dose and during chronic treatment. European Journal of Clinical Pharmacology 34: 633–636. [DOI] [PubMed] [Google Scholar]
- 77. Walter-Sack I, de Vries JX, Kreiner C, et al. (1993) Bioequivalence of allopurinol preparations: To be assayed by parent drug or the active metabolite? Journal of Clinical Investigation 71: 240–246. [DOI] [PubMed] [Google Scholar]
- 78. Gasteiz DA, Hook RH, Walker BJ, et al. (1982) Pharmacokinetics and biotransformation studies of terfenadine in man. Arzneimittel Forschung 32: 1185–1190. [PubMed] [Google Scholar]
- 79. Kaiser E, Prasse C, Wagner M, et al. (2004) Transformation of oxocarbazepine and human metabolites of carbamazepine in waste water treatment and sand filters. Environmental Science & Technology 48: 10208–10216. [DOI] [PubMed] [Google Scholar]
- 80. Tartara A, Galimberti CA, Manni R, et al. (1993) The pharmacokinetics of oxocarbazepine and its active metabolite 10-hydroxy-carbazepine in healthy subjects and in epileptic patients treated with phenobarbitone or valproic acid. British Journal of Clinical Pharmacology 36: 336–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pirmohamed M, Williams D, Madden S, et al. (1995) Metabolism and bioactivation of clozapine by human liver in vitro. Journal of Pharmacology and Experimental Therapeutics 272:984–990. [PubMed] [Google Scholar]