Abstract
Significant losses of synapses have been demonstrated in studies of Alzheimer’s disease (AD), but structural and functional changes in synapses that depend on alterations of the postsynaptic density (PSD) area occur prior to synaptic loss and play a crucial role in the pathology of AD. Evidence suggests that curcumin can ameliorate the learning and memory deficits of AD. To investigate the effects of curcumin on synapses, APPswe/PS1dE9 double transgenic mice (an AD model) were used, and the ultra-structures of synapses and synapse-associated proteins were observed. Six months after administration, few abnormal synapses were observed upon electron microscopy in the hippocampal CA1 areas of the APPswe/PS1dE9 double transgenic mice. The treatment of the mice with curcumin resulted in improvements in the quantity and structure of the synapses. Immunohistochemistry and western blot analyses revealed that the expressions of PSD95 and Shank1 were reduced in the hippocampal CA1 areas of the APPswe/PS1dE9 double transgenic mice, but curcumin treatment increased the expressions of these proteins. Our findings suggest that curcumin improved the structure and function of the synapses by regulating the synapse-related proteins PSD95 and Shank1.
Keywords: APPswe/PS1dE9 double transgenic mice, curcumin, synapse, PSD95, Shank1
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that is characterized by cognitive decline and brain atrophy and involves three neuropathological lesions that have been described as synaptic and neuronal loss, abundant amyloid plaques, and intracellular aggregates of hyper phosphorylated tau protein.1
Significant losses of synapses in many areas of the neocortex and hippocampus have been demonstrated in morphologic studies of the neuropathology in the middle and late stages of AD,2 but many patients in the early stages do not exhibit significant declines in the numbers of synapses.3 These patients only exhibit synaptic changes and dysfunction.4 Changes in synaptic efficacy may occur prior to synapse loss, and such changes primarily depend on alterations in the quantity of postsynaptic glutamate receptors that are accompanied by the enlargement or shrinkage of the dendritic spine and postsynaptic density (PSD) area.5 The PSD refers to the electron-dense thickenings that primarily contain NMDA- and AMPA-type glutamate receptors, PSD proteins such as PSD95, Shanks, and so on. The number of glutamate receptors on a synapse is one of the main determinants of synaptic efficacy, estimating the amplitude of the excitatory postsynaptic current in synapses. Moreover, PSD proteins could help explain the functional and regional specialization of the synapses and their possible roles in synaptic plasticity.5
Shank proteins are the organizers of glutamate receptors in the PSD and are encoded by three genes: Shank1, Shank2, and Shank3.6,7 Shank proteins play important roles in the structural and functional organizations of the dendritic spines and synaptic junctions.8 The application of soluble amyloid β-protein (Aβ) decreases the ProSAP2/Shank3 levels in the PSD, and this change in turn leads to reductions in Shank1 protein and synapse density in hippocampal neurons, whereas the level of the Shank2 protein is increased.9,10 In AD, the process of synaptic damage could involve a beginning with dysregulation of synapse-related proteins such as PSD95 and Shank1.11 AD patients with MCI demonstrated loss of postsynaptic markers such as PSD95 and Shank1.12
Curcumin is a polyphenolic compound that is extracted from turmeric, which is a spice that is commonly used in Asian cuisine. Curcumin is well known for its anti-inflammatory and antioxidant properties and is a preventive and chemotherapeutic agent for many types of disease.13 As a traditional herbal medicine, curcumin exhibits great promise for modern clinical applications and is currently in clinical trials for a variety of diseases, including AD.14,15 Recently, curcumin has been studied for the treatment of AD. Rosiglitazone maleate (RSG) is an antidiabetic drug that works as an insulin sensitizer by binding to the peroxisome proliferator-activated gamma receptor. Studies have shown that RSG therapy improves cognitive function in AD model mice.16,17 A phase II study of rosiglitazone extended release (RSG XR) in mild-to-moderate AD detected a treatment benefit to cognition in apolipoprotein E (APOE)-4-negative patients.18 Though the phase III trial showed that RSG was ineffective in all patients, many factors will affect the results and RSG which modify insulin sensitivity, remain as therapeutic options worthy of further inquiry.18,19 Therefore, we used RSG as the positive control in this study.
APPswe/PS1dE9 double transgenic mice expressing mutant amyloid precursor protein (APPswe) and mutant presenilin1 (PS1dE9) were used in our study. These mice exhibit increases in Aβ42 and Aβ-derived diffusible ligands (ADDLs) and model the pathological changes of AD.20,21 So APPswe/PS1dE9 double transgenic mice as mouse model are well-accepted in AD studies. Curcumin treatment attenuates cognitive impairment in APPswe/PS1dE9 double transgenic mice.22 We have reported that curcumin reduces Aβ-induced toxicity and the aggregation of Aβ into fibrils potentially by inhibiting presenilin2 and/or increasing degrading enzymes such as insulin-degrading enzyme and neprilysin.23
These findings prompted us to investigate the mechanism by which curcumin exerts its effect on synapses in AD. In the present study, we used APPswe/PS1dE9 double transgenic mice to investigate the effects of curcumin on synapses by observing the ultra-structures of synapses in the hippocampal CA1 area and the expressions of the synapse-associated proteins PSD95 and Shank1.
Materials and methods
Materials
Curcumin (cat. no. C1386) was obtained from Sigma-Aldrich. RSG (cat. no. 09060108) was purchased from GlaxoSmithKline Ltd. Co. (Tianjin, PR China). The primary antibody for Shank1 (mouse anti-mouse, cat. no. ab94576, diluted 1:100 for the immunohistochemical staining and 1:500 for the western blot analysis) was purchased from Lifespan. The PSD95 antibody (rabbit anti-mouse, cat. no. ab18258, diluted 1:50 for the immunohistochemical staining and 1:500 for the western blot analysis) and β-actin antibody (mouse monoclonal, cat. no. ab6276, diluted 1:5000 for the western blot analysis) were obtained from Abcam (Hong Kong, PR China). The Strept Actividin-Biotin Complex (SABC) immunohistochemical and 3,3’-diaminobenzidine (DAB) development kits were purchased from ZSGQ-BIO (Beijing, PR China). The Mini-Protean 3 gel electrophoresis instrument was purchased from Bio-Rad (Hercules, CA, USA). The images were analyzed with a Motic Digital Medical Image Analysis System 6.0. The numbers and structures of the synapses were observed using a transmission electron microscope (HITACHI, H-7650).
Animals
We used APPswe/PS1dE9 double transgenic mice and C57/BL6J wild-type (WT) littermates of the double transgene mice from the same colony as the controls. Both groups of mice were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (SCXK, Beijing, PR China) and raised in the Barrier Environment Animal Lab in the Key Laboratory of Pharmacology of Dongzhimen Hospital, which is affiliated with the Beijing University of Chinese Medicine (BUCM; SYXK). When the mice were aged exactly 3 months, the experiment was initiated. The mice were randomly divided into six groups of 15 mice each. C57/BL6J WT littermates were used as the control group (Wild). APPswe/PS1dE9 double transgenic mice were used in the other five groups. Only vehicle was used in the model group (Model). For the positive control group (RSG), 10 mg/kg/day of rosiglitazone maleate was administered for treatment. The following doses were used in the curcumin treatment groups: the low-dose curcumin group (LDC) received 100 mg/kg/day, the medium-dose curcumin group (MDC) received 200 mg/kg/day, and the high-dose curcumin group (HDC) received 400 mg/kg/day.23
Gavage
Curcumin and RSG were dissolved in 0.5% sodium carboxymethyl cellulose (CMC) and administered to the mice via gavages of 0.1 mL/10 g body weight/day for 6 months. An equivalent amount of 0.5% CMC was administered via gavage to the mice of in the Wild and Model groups.
Transmission electron microscopy (TEM)
Three mice in each group were used for electron microscopy. Briefly, the mice were sacrificed by cervical dislocation and were then decapitated. The hippocampus was immediately dissociated in an ice bath. The hippocampal CA1 area was quickly removed and placed in 2.5% glutaraldehyde liquid at 4°C for 2 h. All of the tissues were then postfixed with 1% osmium tetroxide for 2 h. After four washes with buffer, the samples were dehydrated in a graded series of acetone (50, 70, 90, and 100%) and embedded in Epon812 resin at room temperature for 24 h.24 The polymerization was performed at 60°C for 48 h.
Samples were cut into 1-µm-thick sections prior to staining. The sections were stained with azure-methylene blue, and locations rich in neurons were identified under light microscopy. Ultra-thin sections (50 nm) were cut from these thick sections for electron microscopy. The slices were double-stained with uranyl acetate and lead citrate and subsequently analyzed with a HITACHI H-7650 transmission electron microscope (HITACHI, Japan).
Immunohistochemical staining and quantification
Six mice from each group were anesthetized with 4% chloral hydrate. The thorax and abdomen were opened, and the heart and liver were exposed. The left ventricle was cut at the apex, and a catheter was inserted while the right auricle was simultaneously cut. Normal saline (NS) was injected directly into the heart until clear fluid flowed from the right ventricle. Next, 4% paraformaldehyde (PFA) was injected into the heart until the liver and limbs hardened.
The fixed tissues were sectioned at a thickness of 4 μm after the hippocampi of the mice were observable. Briefly, the slices were baked in an oven heated to 56°C for 1 h and then dewaxed and dehydrated. Subsequently, the sections were incubated in 3% H2O2 in absolute methanol for 15 min and subsequently treated with 5% goat serum for 30 min to block non-specific antibody binding. Overnight incubation with the primary antibody was performed in humidified boxes at 4°C. The following day, the slices were incubated with biotin-conjugated secondary antibodies. DAB staining was performed to detect the expressions of the PSD95 and Shank1 proteins in the hippocampal CA1 areas. In the negative controls, rabbit serum was included in place of the primary antibodies to verify the specificities of the antibodies.
Three slices per mouse were used for the analyses; thus, a total of 18 sections from each group were observed. The numbers of positively stained neurons in the hippocampal CA1 areas were counted at ×20 magnification. Photographs were taken and analyzed with the Motic Med 6.0 Image software.
Western blot analysis
Six mice from each group were used for the western blotting. The western blots were performed as described elsewhere.23 The proteins in the hippocampal tissues were quantified with bicinchoninic acid assays. Samples containing protein were denatured by boiling for 5 min and then separated by SDS-PAGE at 120 V. The proteins were transferred to polyvinylidene fluoride membranes by applying a 200-mA current at 4°C for 2 h. After blocking with 5% skimmed milk, the membranes were probed separately with PSD95 and Shank1 antibodies overnight at 4°C. The blots were washed in PBS-Tween20 and incubated with horseradish peroxidase (HRP)-conjugated IgG secondary antibody (Jackson Immuno-Research, Beijing, PR China; dilution 1:5000). Enhanced chemiluminescence was performed and followed by exposure of the X-ray films. After stripping, the same membrane was used to detect β-actin. The protein bands were quantified with NIH ImageJ software, and β-actin was used as the internal control.
Statistical analysis
The analyses were performed with SPSS 15.0. All data are presented as mean ± SD. For the immunohistochemical staining and western blot analyses, the data from more than two groups were analyzed with one-way ANOVA tests, and post-hoc comparisons were performed with the least significant difference (LSD) test. The differences were considered statistically significant at P <0.05. First, data normality tests were performed to verify that all of the data were normally distributed (P >0.05). Next, one-way ANOVAs were performed with descriptive and homogeneity of variance tests. If the variances were not equal, the Brown-Forsythe and Welch tests were performed. The LSD test was then chosen for the post hoc multiple comparisons.
Results
TEM
Fifteen fields of the CA1 areas of the mice from the Wild, Model, RSG, and each of the curcumin groups (five fields per mouse from a total of three mice from each group) were chosen randomly and examined under an electron microscope at ×20,000 magnification. Five representative fields from each group were chosen, and the normal synapses were examined and averaged. The numbers of synapses in the curcumin groups (i.e. the LDC, MDC, and HDC groups) were higher than that in the Model group (Table 1). Additionally, the ultra-structures of the synapses were observed.
Table 1.
Synapse counts for the different groups based on transmission electron microscopy.
| Group | Field of vision |
Mean | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Wild | 17 | 18 | 20 | 16 | 19 | 18 |
| Model | 7 | 6 | 7 | 10 | 7 | 7.4 |
| RSG | 18 | 20 | 15 | 12 | 16 | 16.2 |
| HDC | 18 | 16 | 17 | 17 | 20 | 17.6 |
| MDC | 14 | 16 | 18 | 17 | 16 | 16.2 |
| LDC | 14 | 13 | 12 | 15 | 11 | 13 |
Clear and intact neurons were observed in the Wild group. These neurons had normal nuclei, evenly distributed chromatin, and abundant organelles (Figure 1a). The ultra-structures of the synapses appeared normal and exhibited clear presynaptic and postsynaptic membranes and synaptic clefts (Figure 1a). The synaptic vesicles were amassed inside the presynaptic membrane close to the synaptic cleft. Thick postsynaptic membranes with uniform postsynaptic densities were observed. (Figure 1a).
Figure 1.
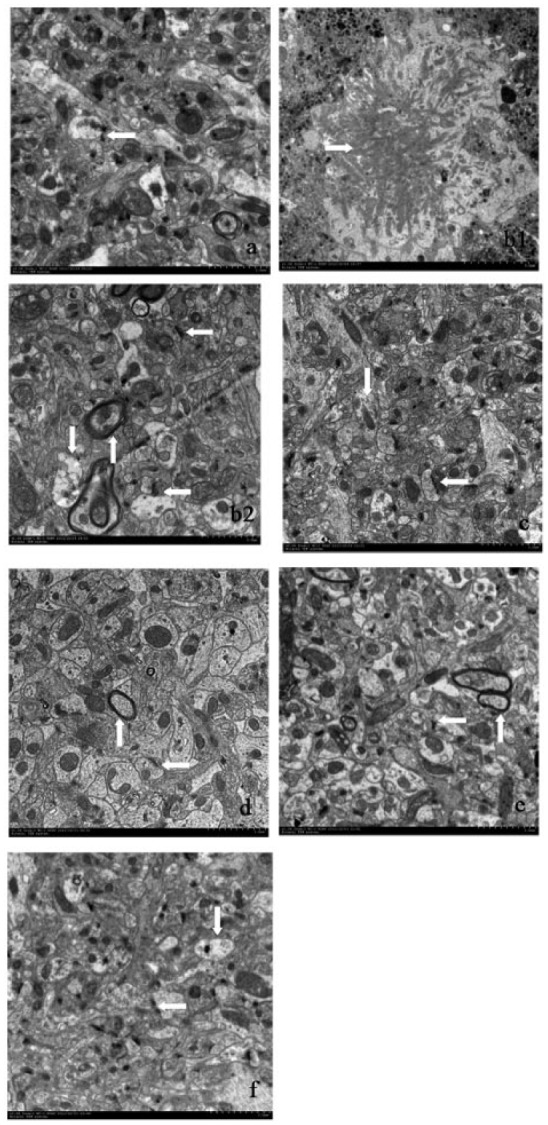
Ultra-structures of the synapses in the hippocampal CA1 area by TEM. (a) Wild group; (b) Model group; (c) RSG group; (d) HDC group; (e) MDC group; (f) LDC group. Note: → senile plaques; ↓ mitochondria; ← synapses; ↑ myelin sheaths. (a) The ultra-structures of the synapses exhibited clear presynaptic and postsynaptic membranes and synaptic clefts. The synaptic vesicles were amassed inside the presynaptic membrane close to the synaptic cleft (scale bars = 2.0 μm). (b1) The neurons exhibited different degrees of degeneration. Senile plaques can be observed around the neurons (scale bars = 5.0 μm). (b2) There were few synapses, and the synapse structures were abnormal. The mitochondrial membranes were obscured, and the cristae were distorted. The structures of the myelin sheaths were loose and exhibited missing microtubules and microfilaments (scale bars = 2.0 μm). (c) The shapes of the synapses and mitochondria were normal (scale bars = 2.0 μm). (d, e, f) The structures of the synapses were nearly normal and included clear synaptic clefts and synaptic vesicles that were in good order. The mitochondria inside the axons appeared clearly, and the structures were complete. There was normal myelin, and inside the sheaths, the microtubule and microfilament structures appeared clearly (scale bars = 2.0 μm).
The hippocampal CA1 neurons of the Model group exhibited different degrees of degeneration. Senile plaques were deposited extracellularly around neurons (Figure 1b1). Inside the neuropil, there were fewer synapses, and the synapse structures were abnormal. The mitochondrial membranes were obscured and the cristae were distorted. The myelin sheaths structures were loose and missing microtubules and microfilaments (Figure 1b2).
The shapes of neurons and mitochondria in the RSG group were normal. The myelin sheaths were intact and exhibited normal microtubules and microfilaments inside (Figure 1 c).
In the curcumin groups (LDC, MDC, and HDC), the neuron structures were improved compared with the Model group. The structures of the synapses were nearly normal, clear synaptic clefts were present, and the synaptic vesicles were in good order. The mitochondria inside the axons appeared clearly, and their structures were complete. There was normal myelin and inside the sheaths, and the microtubule and microfilament structures were clearly present (Figure 1d–f).
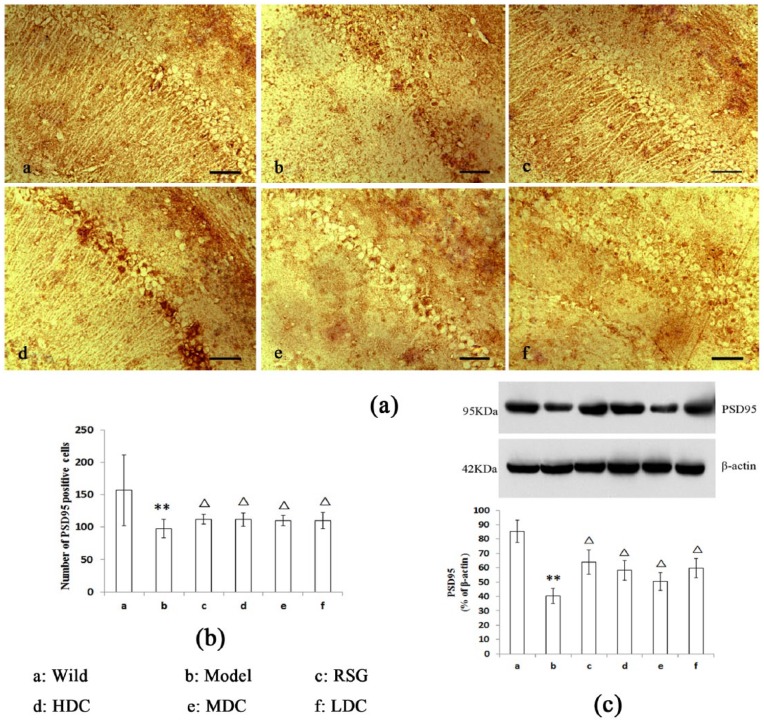
Effects of curcumin on hippocampal PSD95 expression in the APPswe/PS1dE9 mice
Immunostaining of the CA1 region revealed PSD95 protein expression. In the figures, the brown color indicates positive staining for PSD95 in the neurons. In the Model group, there were slightly stained and sparse neurons in the hippocampus (Figure 2a). Compared with the Model group, the positive hippocampal neurons in the RSG and curcumin groups (LDC, MDC, and HDC) were densely distributed (Figure 2a). Quantification revealed decreased PSD95 expression in the cells of the Model group compared with the Wild group (P <0.01, Figure 2b). Greater numbers of PSD95-stained cells were present in the RSG and curcumin groups (LDC, MDC, and HDC) than in the Model group (P <0.05, Figure 2b).
Figure 2.
Effect of curcumin on PSD95 expression in the hippocampal CA1 region (scale bars = 50 μm). (a, b) The number of PSD95-stained cells in the Model group was lower than that in the Wild group (P <0.01). Compared with the Model group, there were greater numbers of PSD95-stained cells in all of the other treatment groups (P <0.05). (c) The western blot results: PSD95 expression in the Model group was decreased compared with the Wild group. There PSD95 protein levels in the treatment groups were increased compared with the Model group (P <0.05). **P <0.01 vs. the Wild group. ∆P <0.05 vs. the Model group.
The results of the PSD95 western blot analyses are also shown in Figure 2C. Compared with the Wild mice, the Model group exhibited a significant decrease in PSD95 expression (P <0.01, Figure 2C). The RSG, HDC, MDC, and LDC groups exhibited significantly increased PSD95 expression compared with the Model group (P <0.05, Figure 2c).
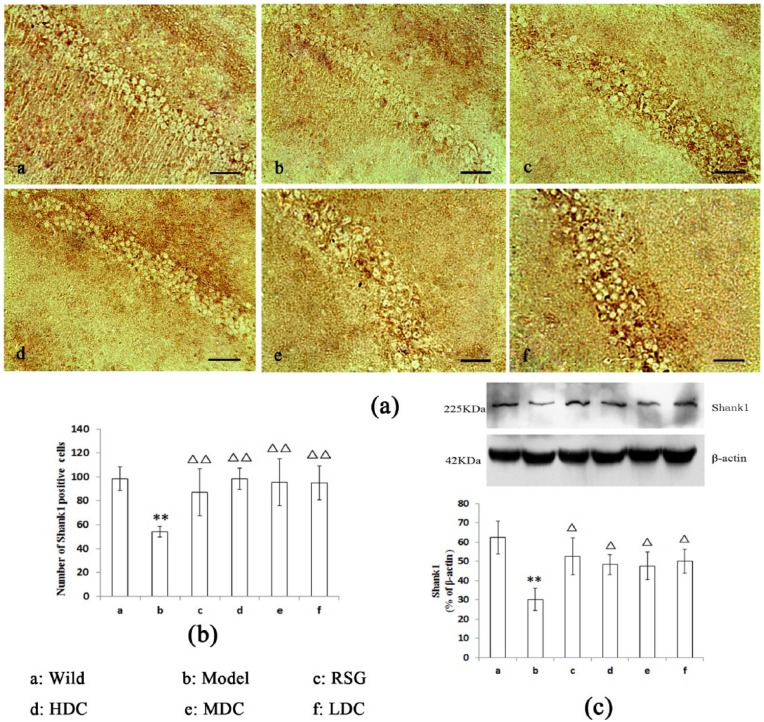
Effect of curcumin on hippocampal Shank1 expression in APPswe/PS1dE9 mice
As shown in Figure 3a and 3b, there were large numbers of brown neurons in the CA1 regions of the wild-type mice. Fewer brown neurons were observed in the Model group than in the Wild group (P <0.01). Compared with the Model group, greater numbers of brown neurons were densely distributed in the hippocampi of the treatment groups (RSG, LDC, MDC, and HDC) (P <0.01).
Figure 3.
Effect of curcumin on Shank1 expression in the hippocampal CA1 region (scale bars = 50 μm). (a, b) The number of Shank1-stained cells in the Model group was lower than that in the Wild group (P <0.01). Compared with the Model group, there were increased numbers of Shank1-stained cells in all of the other treatment groups (P <0.01). (c) The western blot results: The Shank1 expression level in the Model group was decreased compared with that in the Wild group. There were increased Shank1 protein levels in all of the treatment groups compared with the Model group (P <0.05). **P <0.01 vs. the Wild group. ∆∆P <0.01 vs. the Model group. ∆P <0.05 vs. the Model group.
The western blot analyses indicated that the Model group exhibited a significant decrease in Shank1 expression (P <0.01, Figure 3c). The RSG and curcumin treatments increased the expression of Shank1 (P <0.05, Figure 3c).
Discussion
In studies of AD, synapses are considered sites of the earliest pathology, and synaptic changes and loss are the best pathological correlates of cognitive impairment.25,26 Previous studies have demonstrated that curcumin can ameliorate the patho-physiological changes of AD.27,28
Curcuminoids can restore the capacity for plastic changes in CA1 excitability that is impaired by exposure to the Aβ peptide and rescue the reduction in long-term potentiation (LTP) in Aβ-peptide-exposed hippocampal CA1 neurons.29 We have previously reported that curcumin improves the spatial learning and memory abilities of APPswe/PS1dE9 double transgenic mice.23 Therefore, we sought to determine whether curcumin affects the pathology of AD via the synapses. Specifically, we sought to determine whether curcumin influences the structures and functions of the synapses of APPswe/PS1dE9 double transgenic mice.
Many patients in the early stages of AD exhibit only synaptic changes and dysfunction.4 Changes in synaptic efficacy may occur prior to synapse loss, and these changes primarily depend on the PSD area.5 To investigate the structural changes in the synapses of APPswe/PS1dE9 double transgenic mice that had been treated with curcumin, we observed the ultra-structures of synapses in the hippocampal CA1 area with electron microscopy. The results revealed that the numbers of synapses in the curcumin groups were greater than that in the Model group, that the synapse structures were improved and exhibited clear synaptic clefts, and that the synaptic vesicles were in good order. These results indicate that curcumin improved the structures of the synapses in the hippocampal CA1 area of the APPswe/PS1dE9 double transgenic mice.
The PSD refers to the electron-dense thickenings in the postsynaptic membranes of excitatory synapses that primarily contain NMDA- and AMPA-type glutamate receptors and other PSD proteins. The functions of shank proteins, along with other proteins, include the positioning of signaling molecules for the induction of synaptic LTP and long-term depression (LTD).30,31 These processes are thought to be the bases of learning and memory formation. Early memory loss derives from synapse failure prior to neuron death, and synapse failure stems from ADDLs rather than fibrils. These oligomer clusters bind to neuronal surfaces, and experimental data have established that >90% of the binding sites co-localize with the synaptic marker PSD95.32 Aβ42 is toxic to neurons and induces a downregulation of the expression of PSD95, which is associated with synaptic plasticity; this downregulation causes impairments in learning and memory in rats.33 To investigate the functional changes in the synapses of the APPswe/PS1dE9 double transgenic mice that had been treated with curcumin, we examined the synapse-related protein PSD95 in the present study. Immunohistochemistry revealed significant increases in the numbers of PSD95-positive cells in the curcumin groups. Western blotting revealed that the PSD95 expression levels were greater in the curcumin groups than in the Model group. These results indicate that curcumin affected synaptic function by protecting PSD95.
Shank proteins are multidomain scaffolding proteins of the PSD that interact with NMDA receptor and metabotropic glutamate receptor complexes and play central roles in the structural and functional organizations of the dendritic spines and synaptic junctions.8 Decreases in the synaptic levels of Shank1 and Shank3 have been observed in the brains of APP transgenic mice.34 Some authors have provided evidence supporting decreases in Shank1 levels in the frontal cortices of patients with AD.35 In the present study, we examined the effects of curcumin on the synapse-related Shank1 protein in APPswe/PS1dE9 double transgenic mice. Immunohistochemical and western blot analyses revealed that there were greater numbers of Shank1-positive hippocampal neurons in the curcumin treatment groups than in the Model group. These results indicate that curcumin affected the function of the synapses by protecting Shank1.
Taken together, these results indicate that structural and functional changes in the synapses are crucial in the pathology of AD and that the dysregulations of PSD95 and Shank1 are part of the molecular pathology of AD. Using APPswe/PS1dE9 double transgenic mice, we found that curcumin affected the structure and function of synapses in the hippocampus by increasing the levels of the synapse-related proteins PSD95 and Shank1. However, additional studies are needed to confirm our hypothesis. There are other synapse-related proteins that are important in the pathology of AD, and we intend to further our investigations of the changes in synapse-related proteins in relation to AD using APPswe/PS1dE9 double transgenic mice. Furthermore, curcumin at high doses is safe though vigilance is suggested for potential hepatic, gastric, and thyroid toxicity. We should evaluate those potential effects in future studies.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by the 111 Project (B08006), the National Natural Science Foundation of China (No. 81073076 and No. 81573927).
References
- 1. Goedert M, Spillantini MG. (2006) A century of Alzheimer’s disease. Science 314: 777–781. [DOI] [PubMed] [Google Scholar]
- 2. Scheff SW, Price DA. (2003) Synaptic pathology in Alzheimer’s disease: A review of ultrastructural studies. Neurobiology of Aging 24: 1029–1046. [DOI] [PubMed] [Google Scholar]
- 3. Spires-Jones T, Knafo S. (2012) Spines, plasticity and cognition in Alzheimer’s model mice. Neural Plasticity 2012: 319836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nava-Mesa MO, Jimenez-Diaz L, Navarro-Lopez JD, et al. (2014) GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer’s disease. Frontiers in Cellular Neuroscience 8: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shinohara Y. (2012) Quantification of postsynaptic density proteins: Glutamate receptor subunits and scaffolding proteins. Hippocampus 22: 942–953. [DOI] [PubMed] [Google Scholar]
- 6. Sheng M, Kim E. (2000) The Shank family of scaffold proteins. Journal of Cell Science 113: 1851–1856. [DOI] [PubMed] [Google Scholar]
- 7. Bockers TM, Bockmann J, Kreutz MR, et al. (2002) ProSAP/Shank proteins—a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. Journal of Neurochemistry 81: 903–910. [DOI] [PubMed] [Google Scholar]
- 8. Kreienkamp HJ. (2008) Scaffolding proteins at the postsynaptic density: Shank as the architectural framework. Handbook of Experimental Pharmacology 186:365–380. [DOI] [PubMed] [Google Scholar]
- 9. Grabrucker AM, Schmeisser MJ, Udvardi PT, et al. (2011) Amyloid beta protein-induced zinc sequestration leads to synaptic loss via dysregulationof the ProSAP2/Shank3 scaffold. Molecular Neurodegeneration 6: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong YS, Lippa CF, Zhu JH, et al. (2009) Disruption of glutamate receptors at Shank-postsynaptic platform in Alzheimer‘s disease. Brain Research 1292: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Overk CR, Masliah E. (2014) Pathogenesis of synaptic degeneration in Alzheimer’s disease and Lewy body disease. Biochemical Pharmacology 88: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pham E, Crews L, Ubhi K, et al. (2010) Progressive accumulation of amyloid-beta oligomers in Alzheimer’s disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS Journal 277: 3051–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marchiani A, Rozzo C, Fadda A, et al. (2014) Curcumin and curcumin-like molecules: From spice to drugs. Current Medicinal Chemistry 21: 204–222. [DOI] [PubMed] [Google Scholar]
- 14. Ringman JM, Frautschy SA, Teng E, et al. (2012) Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Research & Therapy 4: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang C, Su X, Liu A, et al. (2013) Advances in clinical study of curcumin. Current Pharmaceutical Design 19: 1966–1973. [PubMed] [Google Scholar]
- 16. Escribano L, Simon AM, Perez-Mediavilla A, et al. (2009) Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer’s disease mouse model. Biochemical and Biophysical Research Communications 379: 406–410. [DOI] [PubMed] [Google Scholar]
- 17. O’Reilly JA, Lynch M. (2012) Rosiglitazone improves spatial memory and decreases insoluble Abeta1–42 in APP/PS1 mice. Journal of Neuroimmune Pharmacology 7: 140–144. [DOI] [PubMed] [Google Scholar]
- 18. Risner ME, Saunders AM, Altman JF, et al. (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics Journal 6: 246–254. [DOI] [PubMed] [Google Scholar]
- 19. Gold M, Alderton C, Zvartau-Hind M, et al. (2010) Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: Results from a randomized, double-blind, placebo-controlled phase III study. Dementia and Geriatric Cognitive Disorders 30: 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Savonenko A, Xu GM, Melnikova T, et al. (2005) Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: Relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiology of Disease 18: 602–617. [DOI] [PubMed] [Google Scholar]
- 21. Xiong HQ, Callaghan D, Wodzinska J, et al. (2011) Biochemical and behavioral characterization of the double transgenic mouse model (APPswe/PS1dE9) of Alzheimer’s disease. Neuroscience Bulletin 27: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang C, Zhang X, Teng Z, et al. (2014) Downregulation of PI3K/ Akt / mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. European Journal of Pharmacology 740: 312–320. [DOI] [PubMed] [Google Scholar]
- 23. Wang PW, Shu CX, Li RS, et al. (2014) Mechanisms and effects of curcumin on spatial learning and memory improvement in APPswe/PS1dE9 mice. Journal of Neuroscience Research 92: 218–231. [DOI] [PubMed] [Google Scholar]
- 24. Gutiérreza L, Quintanab C, Patiñoc C, et al. (2009) Iron speciation study in Hfe knockout mice tissues: Magnetic and ultrastructural characterization. Biochimica et Biophysica Acta 1792: 541–547. [DOI] [PubMed] [Google Scholar]
- 25. Coleman PD, Yao PJ. (2003) Synaptic slaughter in Alzheimer’s disease. Neurobiology of Aging 24: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 26. Tampellini D, Gouras GK. (2010) Synapses, synaptic activity and intraneuronal Aβ in Alzheimer’s disease. Frontiers in Aging Neuroscience 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calcul L, Zhang B, Jinwal UK, et al. (2012) Natural products as a rich source of tau-targeting drugs for Alzheimer’s disease. Future Medicinal Chemistry 4: 1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Narasingappa RB, Javagal MR, Pullabhatla S, et al. (2012) Activation of α-secretase by curcumin-aminoacid conjugates. Biochem Biophys Res Commun 424:691–696. [DOI] [PubMed] [Google Scholar]
- 29. Ahmed T, Gilani AH, Hosseinmardi N, et al. (2011) Curcuminoids rescue long- term potentiation impaired by amyloid peptide in rat hippocampal slices. Synapse 65: 572–582. [DOI] [PubMed] [Google Scholar]
- 30. Chen XB, Winters C, Azzam R, et al. (2008) Organization of the core structure of the postsynaptic density. Proceedings of the National Academy of Sciences of the United States of America 105: 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gold MG. (2012) A frontier in the understanding of synaptic plasticity: Solving the structure of the postsynaptic density. BioEssays 34: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lacor PN, Buniel MC, Chang L, et al. (2004) Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. Journal of Neuroscience 24: 10191–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ding J, Xi YD, Zhang DD, et al. (2013) Soybean isoflavone ameliorates β-amyloid 1-42-Induced learning and memory deficit in rats by protecting synaptic structure and function. Synapse 67: 856–864. [DOI] [PubMed] [Google Scholar]
- 34. Pham E, Crews L, Ubhi K, et al. (2010) Progressive accumulation of amyloid-beta oligomers in Alzheimer’s disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS Journal 277: 3051–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guilmatre A, Huguet G, Delorme R, et al. (2014) The emerging role of Shank genes in neuropsychiatric disorders. Developmental Neurobiology 174: 113–122. [DOI] [PubMed] [Google Scholar]