Abstract
Background:
Inflammation, which is a hallmark of asthma, is one of the main sources of oxidative stress in the human body. Thiols are powerful antioxidants that protect cells against the consequences of oxidative stress. We aimed to investigate whether asthma and montelukast monotherapy affect the total plasma thiol pool in children.
Methods:
A total of 60 children with asthma and 35 healthy controls participated in the study. Group I consisted of newly diagnosed asthmatics who did not have regular anti-asthmatic therapy previously. Group II consisted of patients who had been undertaking montelukast monotherapy regularly for at least 4 months. Plasma total antioxidant status (TAS) and plasma total thiol (PTT) were measured using spectrophotometric methods.
Results:
Bronchial asthma patients in both groups I and II had decreased median TAS levels compared with the control group (1.59 [interquartile range, 1.04–1.70] and 1.67 [1.50–1.75] vs. 2.98 [2.76–3.16] Trolox equiv./L, respectively;P <0.001). Group I had decreased PTT concentrations compared with the control group (0.18 [0.16–0.20] vs. 0.21 [0.19–0.22] mmol/L; P <0.001), and group II had similar PTT levels to the control group (0.20 [0.17–0.22] mmol/L; P >0.05). In addition, the median TAS and PTT levels for groups I and II were not statistically different (P >0.05). There was a positive correlation between TAS and PTT levels (rho = 0.38, P <0.05) in group I.
Conclusion:
In order to balance the oxidative stress, both TAS and PTT which are markers of the antioxidant system are reduced in children with asthma. Montelukast monotherapy can limit oxidative stress and thus restore PTT levels but not TAS levels in asthmatic children.
Keywords: asthma, children, montelukast, plasma, TAS, thiol, treatment
Introduction
Asthma is a common inflammatory disease affecting millions of people worldwide.1 The hallmark of asthma is airway inflammation, which is quite complex and involves many different cell types, different cytokines, and chemokines.2 Free radicals are important mediators of airway tissue damage that increase in people with asthma. Disturbances in oxidation/reduction reactions and impaired antioxidant defences are considered risk factors for asthma development and asthma severity.3
Thiol compounds are powerful antioxidants that are present in high concentrations in intracellular and extracellular fluids and they react with almost all physiological oxidants.4,5 They can also function as electron or hydrogen donors to scavenge free radicals and repair damaged molecules.4–6 Myeloperoxidase and eosinophil peroxidase-derived free radicals (e.g. hypochlorous acid and hypobromous acid) all react principally with thiol compounds compared with other antioxidants.5 This evidence highlights the importance of thiol compounds in asthma pathogenesis, taking into account that eosinophilic and neutrophilic inflammation are hallmarks of asthmatic inflammation.
Although we focus on the antioxidant properties of thiol compounds in this study, there is growing evidence that thiol homeostasis status has a critical role in the detoxification, signal transduction, apoptosis, and enzymatic regulation mechanisms.7,8 In particular, there has not been sufficient research focusing on plasma total thiol (PTT) levels in children with asthma. There is also no study in the literature about the effects of montelukast therapy on PTT levels in children with asthma. Therefore, the aim of this paper is to address these topics.
Materials and methods
Participants and samples
A total of 60 children with asthma and 35 healthy controls were enrolled in the study, which was conducted between August 2014 and December 2014 at the Pediatric Allergy and Immunology Department of Bezmialem Vakif University. The clinical diagnosis, asthma control status, and severity of asthma were determined using the criteria defined in the 2014 Global Initiative for Asthma guidelines.9 Group I consisted of newly diagnosed persistent asthmatics who had not had regular anti-asthmatic therapy previously; group II consisted of patients who had been taking montelukast monotherapy regularly for at least 4 months. All group II children had mild, well-controlled asthma based on the GINA guidelines, and none had experienced an asthma attack in the last month. These patients attended the same outpatient clinic regularly every 2 months. All group II children (study group) were taking 4 mg and 5 mg oral tablets montelukast sodium (Singulair®, Merck Sharp & Dohme, NJ, USA) in accordance with the manufacturer’s recommendations for at least 4 months. Patients’ drug compliance was checked every month by observing the drug package and by signing a diary card that indicates the medicine was taken.
Participants with acute or chronic diseases and a history of in-home smoking and those who were taking any medications or were overweight or obese (body mass index [BMI] >85th percentile) were excluded from the study. The control group (group III) consisted of 35 healthy age- and sex-matched children who were periodically attending pediatric clinics at the same hospital for regular developmental check-ups. Children were included in the control group if they had no history of any allergic disease or parental smoking and were not taking any medication.
The study was performed in accordance with the Declaration of Helsinki’s Good Clinical Practice guidelines and was approved by the Bezmialem Vakif University Ethical Committee (71306642-050.01.04/1562). All participants were given information about the study and signed consent was obtained from the parents of all study participants.
Skin prick tests
Skin prick tests were performed using Stallerpoint lancets and allergen solutions manufactured by Stallergenes (Stallergenes, Paris, France). A total of 10 different aeroallergens consisting of house dust mites, grass, tree pollens, fungi, and animal dander were tested. Skin prick tests were considered positive if the presence of at least a wheal of a maximum diameter of 3 mm remained once the negative value had been subtracted.
Lung function measurements
The rates of forced expiratory volume in 1 s (FEV1) were measured using dynamic spirometry (Vitalograph®; Buckingham, UK). The best of three successful manoeuvers was recorded.
Blood sample collection
After the participants fasted overnight, peripheral blood samples (total, 4 mL) were collected from an antecubital vein into heparinized tubes and were stored at 2–4°C. The blood was centrifuged at 1500 g for 10 min to obtain the plasma. The separated plasma was then stored at −80°C until further analysis of the TAS and PTT levels.
Measurement of total antioxidant status (TAS) and PTT
Plasma TAS was measured using a method developed by Erel.10 The results are expressed as mmol Trolox equiv./L. PTT levels were measured using the classical Ellman reagent, 5,5-dithiobis-(2-nitrobenzoic) acid. This compound is reduced by free thiols in an exchange reaction, forming a mixed disulphide and releasing one molecule of 5-thionitrobenzoic acid, which can be measured at 412 nm.7,11 The results are expressed as mmol/L.
Statistical analyses
A statistical analysis was performed using IBM SPSS 19 (IBM, Armonk, NY, USA). A Shapiro–Wilk test was used to test distributions for normality. Parametric data were expressed as the mean ± standard deviation (SD), and non-parametric data were expressed as the median, interquartile range (IQR). The Kruskal–Wallis test was used to compare more than two independent parameters and a Mann–Whitney U test was used to calculate the difference between two parameters in groups. The correlation between two variables was assessed by using the Spearman rank correlation coefficient. Categorical data were evaluated using the chi square test and P <0.05 was considered statistically significant.
Results
Demographic and clinical data for the study and control groups are shown in Table 1. The mean ages of patients (groups I and II) and controls are 8.0 ± 3.2, 7.9 ± 2.9, and 8.4 ± 3.1 years, respectively. There were no significant differences between the three groups with respect to age, gender, and BMI (P >0.05; Table 1). Group III had decreased total Ig-E and aeroallergen sensitivity compared to the other groups (P <0.05), but there were no statistically significant differences in groups I and II (P >0.05, Table 1).
Table 1.
Some demographic and clinical data for the study and control groups.
| Group I (Newly diagnosed asthmatics) (n = 30) | Group II (Montelukast-treated asthmatics) (n = 30) | Group III (Healthy controls) (n = 35) | P value | |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 8.0 ± 3.2 | 7.9 ± 2.9 | 8.4 ± 3.1 | >0.05 |
| Gender (M/F) | 15/15 | 14/16 | 16/19 | >0.05 |
| Body mass index (kg/m2±SD) | 17.8 ± 2.1 | 17 ± 1.4 | 17.8 ± 2.1 | >0.05 |
| Symptom duration (years) (mean ± SD) | 1.6 ± 0.9 | 1.9 ± 0.9 | – | >0.05 |
| Skin prick test positivity to aeroallergens/ patients (n) | 17/30 | 21/30 | 1/35 | I vs. II >0.05 |
| I vs. III <0.05 | ||||
| II vs. III <0.05 | ||||
| FEV1 (%) (predicted ± SD ) | 87.2 ± 4.2 | 87.9 ± 7.1 | – | >0.05 |
| Ig E total (Iu/L) (median, IQR) | 74.0 (34.8–259.3) | 156.0 (56.8–640.5) | 42.0 (19.0–60.0) | I vs. II >0.05 |
| I vs. III <0.05 | ||||
| II vs. III <0.05 |
FEV1, forced expiratory volume in 1 s; IQR, interquartile range; SD, standard deviation.
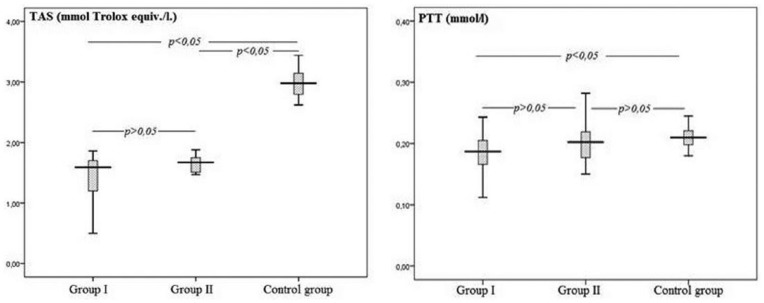
Group I and II patients had decreased median TAS levels compared with the control group (1.59 [1.04–1.70] and 1.67 [1.50–1.75] vs. 2.98 [2.76–3.16] mmol Trolox equiv./L, respectively; P <0.001, Figure 1), but there was no statistically significant difference between groups I and II (P >0.05). Group I had decreased median PTT concentrations compared with the control group (0.18 [0.16–0.20] vs. 0.21 [0.19–0.22] mmol/L; P <0.001, Figure 1). Group II had similar median PTT levels to the control group (0.20 [0.17–0.22] mmol/l; P >0.05). PTT levels between groups I and II were not statistically significant (P >0.05). There was a statistically significant positive correlation between TAS and PTT levels in group I (rho = 0.38, P = 0.04) but not for the other groups.
Figure 1.
Total antioxidant status (TAS) and plasma total thiol (PTT) levels of the study and control groups.
Discussion
This study shows that montelukast monotherapy can restore the depleted plasma thiol pool in children with mild asthma. Our study also shows that there is a positive correlation between plasma TAS and PTT levels in newly diagnosed asthmatic patients. Both these parameters may be useful biomarkers of oxidative stress, which is increased in asthma. Studies related to thiol compounds have mainly focused on glutathione synthesis, functions, and redox balance. Research about the role of plasma thiols in the pathogenesis of asthma and the effect of anti-asthmatic therapy on plasma thiol levels is insufficient. Thus, our study is the first study showing that anti-asthmatic therapy can restore the reduced plasma thiol pool in pediatric asthma.
While all aspects of asthma pathogenesis have not been revealed, there is ample evidence in the literature that reactive oxygen species (ROS) may play critical role. Several enzymatic and non-enzymatic antioxidants are available in the lungs and systemic circulation system to counteract ROS- mediated damage to various molecules, such as lipids, proteins, and DNA.1 Reduced glutathione, superoxide dismutase, catalase, vitamin E, vitamin C, and urate are some of the main antioxidant enzymes and molecules in the lungs.1 Similarly, blood contains an extensive range of antioxidants. There are many components of the oxidative and antioxidative systems, and the individual measurement of these molecules is time-consuming and costly and requires complicated techniques. Measuring TAS is a rapid, easy, reliable, sensitive, and inexpensive method of assessing antioxidative status.10,12
Studies show that inflammation and oxidative stress are quite prevalent not only in the lungs but also in the systemic circulation of asthmatics. Takamura et al. demonstrated that serum CRP levels correlated negatively with indices of lung function and positively with sputum eosinophil counts.13 Researchers also argue that lung inflammation may spill into systemic circulation due to the close proximity of the pulmonary vasculature to the blood capillary network. Therefore, blood is a convenient source of biomarkers for the analysis of pulmonary inflammation.1
Cysteinyl leukotrienes are potent bronchoconstrictors; they can promote inflammatory cell migration into airways and eosinophilopoiesis.14 Montelukast is a widely used agent to treat asthma in terms of both monotherapy and add-on therapy. The GINA guideline recommends montelukast at step 2 of treatment as an alternative drug to inhaled corticosteroids and one of several add-on therapy options for advanced treatment steps for all ages. Anti-asthmatic treatment may modify the oxidant–antioxidant balance in patients. There is good evidence that inhaled corticosteroids can decrease oxidative stress in asthma.15–17 Additionally, animal studies show that montelukast has a beneficial effect on oxidative stress-mediated conditions, such as ischemia-reperfusion injury, drug intoxication, and lipopolysaccharide-induced oxidative stress.18–21 Al Saadi reports that montelukast decreases ROS production in whole blood and isolates human polymorphonuclear neutrophils in asthmatic children.22 However, our recent study showed that montelukast monotherapy does not overcome the oxidative stress produced by asthma in children.23
In the study by Nadeem et al., plasma total antioxidant capacity and total protein sulfhydryls were found to be decreased in acute exacerbations of asthma compared to stable asthmatic patients. However, this work does not include a control group of healthy participants.24 In another study, Rahman et al. investigated systemic oxidative stress in patients with asthma, chronic obstructive pulmonary disease, and smokers. In this work, they found that clinically stable asthmatic patients have reduced Trolox equivalent antioxidant capacity (TEAC) compared to the control group. The TEAC was even lower in those who were studied during an exacerbation. There was a trend for TEAC values to improve by the time of discharge compared to the values upon admission.25 However, there were no differences in the thiol levels of patients with acute or stable asthma compared to the levels in the control group. However, only 20 asthmatic patients participated in this study, 11 of which had acute exacerbations of their condition. Perhaps the authors could not demonstrate a probable connection because of the small sample size.
In the study by Ahmad et al., the authors reveal that asthmatic patients have decreased plasma total protein sulfhydryl levels and total antioxidant capacity compared to the healthy controls. It was also noticed that these parameters were positively associated with FEV1. They therefore concluded that oxidative stress plays a significant role in the severity of disease.26 Jansen et al. conducted an extensive comparative analysis of different methods and assays to measure serum oxidative stress-related parameters. They report total thiol assays show a positive correlation with the TAS assay (r = 0.298).12 Our results are compatible with these findings, and our coefficient of correlation was rho = 0.38 in group I.
In our study, we found that montelukast monotherapy in asthmatic patients increases PTT levels to the level of healthy controls. However, we could not show a clear difference between groups 1 and 2 (P = 0.11). As previously mentioned, there is a relationship between PTT and the severity and acute exacerbation of asthma.24,26 Our patients were well-controlled mild asthmatics who had not had acute exacerbations in the last month. Perhaps that is why we did not clearly show the effectiveness of the treatment for groups I and II. Also, the small sample size makes it difficult to demonstrate the difference between the groups statistically.
We would have preferred to perform this study in a prospective manner. However, we were unable to overcome the financial and ethical problems associated with prospective drug studies. This is the main limitation of our study. We therefore used a cross-sectional study format. We studied the parameters in the closely matched newly diagnosed asthmatic group to compare it to the main study group who took montelukast for at least 4 months (group II). The data obtained from group I give sufficient information about the pre-treatment levels of group II.
In conclusion, plasma thiol pool is a critical part of the antioxidant system, and this is the first study that reveals that montelukast monotherapy can increase PTT levels to those of healthy controls in mild intermittent pediatric asthma. However, additional in vivo and in vitro studies are required to better understand the role of thiol compounds in the pathogenesis of asthma.
Acknowledgments
We thank Siddika Kesgin, Huri Dedeakayogullari, and Ersin Karatas for their assistance.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Nadeem A, Siddiqui N, Alharbi NO, et al. (2014) Airway and systemic oxidant-antioxidant dysregulation in asthma: A possible scenario of oxidants spill over from lung into blood. Pulmonary Pharmacology & Therapy 29: 31–40. [DOI] [PubMed] [Google Scholar]
- 2. Dozor AJ. (2010) The role of oxidative stress in the pathogenesis and treatment of asthma. Annals of the New York Academy of Sciences 1203: 133–137. [DOI] [PubMed] [Google Scholar]
- 3. Fitzpatrick AM, Jones DP, Brown LAS. (2012) Glutathione redox control of asthma: From molecular mechanisms to therapeutic opportunities. Antioxidants & Redox Signaling 17: 375–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takashima M, Shichiri M, Hagihara Y, et al. (2012) Reactivity toward oxygen radicals and antioxidant action of thiol compounds. BioFactors 38: 240–248. [DOI] [PubMed] [Google Scholar]
- 5. Winterbourn CC, Hampton MB. (2008) Thiol chemistry and specificity in redox signaling. Free Radical Biology & Medicine 45: 549–561. [DOI] [PubMed] [Google Scholar]
- 6. Włodek L. (2002) Beneficial and harmful effects of thiols. Polish Journal of Pharmacology 54: 215–223. [PubMed] [Google Scholar]
- 7. Erel O, Neselioglu S. (2014) A novel and automated assay for thiol/disulphide homeostasis. Clinical Biochemistry 18: 326–332. [DOI] [PubMed] [Google Scholar]
- 8. Biswas S, Chida AS, Rahman I. (2006) Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochemical Pharmacology 71: 551–564. [DOI] [PubMed] [Google Scholar]
- 9. Global Initiative for Asthma. Available at: http://www.ginasthma.org/.
- 10. Erel O. (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clinical Biochemistry 37: 277–285. [DOI] [PubMed] [Google Scholar]
- 11. Ellman G, Lysko H. (1979) A precise method for the determination of whole blood and plasma sulfhydryl groups. Analytical Biochemistry 93: 98–102. [PubMed] [Google Scholar]
- 12. Jansen EH, Ruskovska T. (2013) Comparative analysis of serum (anti)oxidative status parаmeters in healthy persons. International Journal of Molecular Science 14: 6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takemura M, Matsumoto H, Niimi A, et al. (2006) High sensitivity C-reactive protein in asthma. European Respiratory Journal 27: 908–912. [DOI] [PubMed] [Google Scholar]
- 14. O’Byrne PM, Gauvreau GM, Murphy DM. (2009) Efficacy of leukotriene receptor antagonists and synthesis inhibitors in asthma. Journal of Allergy and Clinical Immunology 124: 397–403. [DOI] [PubMed] [Google Scholar]
- 15. Antczak A, Kurmanowska Z, Kasielski M, et al. (2000) Inhaled glucocorticosteroids decrease hydrogen peroxide level in expired air condensate in asthmatic patients. Respiratory Medicine 94: 416–421. [DOI] [PubMed] [Google Scholar]
- 16. Ozaras R, Tahan V, Turkmen S, et al. (2000) Changes in malondialdehyde levels in bronchoalveolar fluid and serum by the treatment of asthma with inhaled steroid and beta2-agonist. Respirology 5: 289–292. [DOI] [PubMed] [Google Scholar]
- 17. Saleh D, Ernst P, Lim S, et al. (1998) Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: Effect of inhaled glucocorticoid. FASEB Journal 12: 929–937. [PubMed] [Google Scholar]
- 18. Duran A, Otiük H, Terzi EH, et al. (2013) Protective effect of montelukast, a cysteinyl leukotriene receptor-1 antagonist, against intestinal ischemia-reperfusion injury in the rat. Acta Chirurgica Belgica 113: 401–405. [PubMed] [Google Scholar]
- 19. Otunctemur A, Ozbek E, Cekmen M, et al. (2013) Protective effect of montelukast which is cysteinyl-leukotriene receptor antagonist on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Renal Failure 35: 403–410. [DOI] [PubMed] [Google Scholar]
- 20. Ahmed AA. (2009) Protective effect of montelukast on paraquat-induced lung toxicity in rats. Bioscience Trends 3: 63–72. [PubMed] [Google Scholar]
- 21. Mohamadin AM, Elberry AA, Elkablawy MA, et al. (2011) Montelukast, a leukotriene receptor antagonist abrogates lipopolysaccharide-induced toxicity and oxidative stress in rat liver. Pathophysiology 18: 235–242. [DOI] [PubMed] [Google Scholar]
- 22. Al Saadi MM, Meo SA, Mustafa A, et al. (2011) Effects of Montelukast on free radical production in whole blood and isolated human polymorphonuclear neutrophils (PMNs) in asthmatic children. Saudi Pharmaceutical Journal 19: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dilek F, Ozkaya E, Kocyigit A, et al. (2015) Effect of montelukast monotherapy on oxidative stress parameters and DNA damage in children with asthma. International Archives of Allergy and Immunology 167(2): 119–126. [DOI] [PubMed] [Google Scholar]
- 24. Nadeem A, Raj HG, Chhabra SK. (2005) Increased oxidative stress in acute exacerbations of asthma. Journal of Asthma 42: 45–50. [DOI] [PubMed] [Google Scholar]
- 25. Rahman I, Morrison D, Donaldson K, et al. (1996) Systemic oxidative stress in asthma, COPD, and smokers. American Journal of Respiratory and Critical Care Medicine 154: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 26. Ahmad A, Shameem M, Husain Q. (2012) Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Annals of Thoracic Medicine 7: 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]