Abstract
Controversy surrounds the role of dental infection/inflammation in the oral cavity in chronic spontaneous urticaria (CSU) and atrial fibrillation (AF), which is mainly due to scarce literature in this area. Therefore, this case report and review of literature illustrate a possible association between the acute-phase response (APR) and clinical conditions, such as CSU and dental infection/inflammation of oral cavity and AF.
We describe a 36-year-old man with an 8-year history of difficult-to-treat, uncontrolled CSU, co-existent with dental infection/inflammatory processes of oral cavity and permanent atrial fibrillation (AF). In the presented case, the most likely triggering or aggravating/maintaining factor of the symptoms was the inflammation/dental infection of the oral cavity because of rapid reduction of the urticarial symptoms, drug doses, and serum CRP levels after the dental therapy. Dental treatment may have a beneficial effect on the systemic inflammatory response, reducing/normalizing the circulating levels of APR markers. APR activation appears to worsen CSU course, early identification and treatment of infectious/inflammatory foci in the oral cavity would form the mainstay of supportive therapy for CU probably through reduction of the systemic inflammatory burden. APR associated with infectious/inflammatory foci in the oral cavity could be taken into account as a predisposing agents to AF.
Keywords: atrial fibrillation, chronic spontaneous urticaria, C-reactive protein, acute phase response, dental infection, inflammation of oral cavity
Background
Acute-phase response (APR) is a local and systemic coordinated reaction following different inflammatory states associated with numerous changes in levels of circulating proteins, including C-reactive protein (CRP) and IL-6.1,2 It has been established that low-grade systemic inflammation, characterized by increased levels of biomarkers is associated with the increased risk of the cardiovascular diseases (CVD).3,4 Therefore, the associations between cardiovascular disorders and different inflammatory states have been reported.1,2 Little is known, however, about the systemic consequences of chronic urticaria (CSU), an immune-inflammatory mast cells-dependent disease. Urticaria is a group of frequent diseases that can show a distinct skin/mucosal reaction profile and a wide range of etiologies. Among these, CSU is accompanied by APR and coagulation/fibrinolysis activation, as well as neuroimmunoendocrine dysfunction.5–11
Interestingly, it has been suggested that CRP and IL-6 may appear as markers for the inflammatory states which may play a role in pathogenesis of the atrial fibrillation (AF).12,13 On the other hand, CSU is associated with the increased serum levels of the APR biomarkers, parallel to the disease severity/activity. CRP is not only a very sensitive marker underlying the systemic inflammation, but may exert direct pro-inflammatory effects.1,2,3
Therefore, the presented case report analyzed a possible association between the APR and clinical conditions, such as CSU and dental infection/inflammation of oral cavity and AF.
Case presentation
A 36-year-old man with an 8-year history of severe difficult-to-treat chronic urticaria/angioedema was referred to the divisions of allergology and dermatology (Department of Internal Diseases, Dermatology and Allergology) for therapy. So far the patient was treated by dermatologists and a GP. His symptoms were poorly controlled despite continuous therapy with oral prednisone (mean dose, 20 mg/day; range, 15–40 mg) for 8 years and higher doses of various antihistamines (to 3× standard doses). Any attempt to reduce the oral prednisone dose brought aggravation of the symptoms. Upon exacerbation of the disease symptoms the intravenous steroids were also administered. A successful H. pylori eradication was performed some years before, that however had no influence on the course of CSU.
The diagnosis of CSU was based on typical urticarial lesions and recurrent angioedema without features of vasculitis in the skin biopsy findings (Figure 1).
Figure 1.
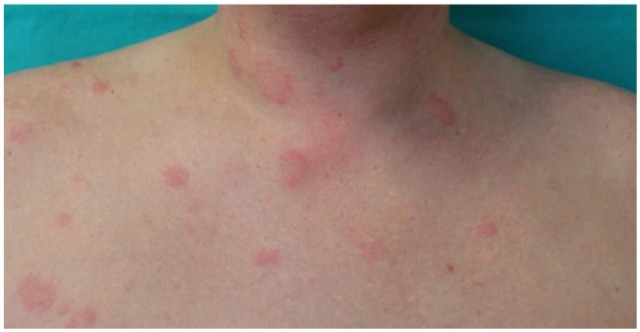
Typical urticarial lesions.
The patient did not give any history of other symptoms or diseases neither he take any other medications. His family history was insignificant.
Physical examination
Skin examination revealed intensely pruritic, recurrent urticarial lesions and steroid acne. In addition, the patient had an irregular pulse rate, suggesting arrhythmia. Electrocardiography (ECG) revealed AF. AF was found incidentally, the patient reported no clinical symptoms suggesting arrhythmia or other cardiovascular disorders. The onset of the symptoms was difficult to define for the lack of records from his GP which would include ECG results and description of physical examination of the heart. Blood pressure was normal, BMI: 29.4.
Laboratory findings showed: hemoglobin, 15.0 g/dL; hematocrit, 45%; full blood count: white cells, 12.2 × 109/L and 9.9/remission (normal range, 4–11 × 109/L), red cells, 5.16 × 1012/L; platelets, 291 × 109/L; differential blood count: neutrophils, 63%; lymphocytes, 30%; eosinophils, 3%; monocytes, 4%; erythrocyte sedimentation rate (I, 20 mm/h; II, 10 mm/h – after dental treatment), CRP (I, 12.4; II, 9.0; III, 5.0 mg/L – 6 months later in remission; normal range, the elevated serum CRP was defined as higher than 5.0 mg/L) (Table 1). During dental treatment elevation of CRP up to 38.5 mg/L was observed.
Table 1.
Main laboratory findings and therapy.
| Parameters | Before dental treatment | Two months after beginning of dental treatment | Six months after termination of dental treatment |
|---|---|---|---|
| CRP (mg/L) | 12.4 | 9 | 5 |
| OB (mm/h) | 20 | NA | 10 |
| WBC (× 109/L) | 12.2 | NA | 9 |
| Therapy | Prednisone | Prednisone | Without |
| 20 mg/day | 5–10 mg/day | regular treatment | |
| antihistamines (3× standard doses) | antihistamine (standard dose) |
CRP, C-reactive protein; NA, data not available; OB, erythrocyte sedimentation rate; WBC, white blood cells.
Serum biochemical examinations: glucose, creatinine, liver enzymes, sodium, and potassium ion were within the normal range. Thyroid function tests (TSH, fT4), complement, and urine analysis were normal. Lipid profile: total cholesterol, 214 mg/dL (normal range, <200 mg/dL); LDL, 138 mg/dL (normal range, <129 mg/dL); HDL, 42.3 mg/dL (normal range, >55 mg/dL); triglycerides, 171 mg/dl (normal range, <200 mg/dL).
Immunoglobulins: A, 289 mg/dL and 442 (normal range, 70–400 mg/dL); G, 1229 mg/dL (normal range, 700–1600 mg/dL); M, 39 mg/dL and 47 mg/dL (normal range, 40–230 mg/dL); specific IgE to a panel of common inhalant and the main food allergens were normal.
Antibodies: anti-nuclear profile, anti-dsDNA, anti-cardiolipin (IgM, IgG), anti-neutrophil (cytoplasmic and perinuclear pattern), anti-transglutaminase (IgA, IgG), anti-thyroid in serum were all negative.
Testing for antistreptolysin-O, syphilis, rheumatoid factor, Borrelia burgdorferi (IgM, IgG), Ascaris lumbricoides (IgG), Toxocara canis (IgG), and hepatitis B and C viruses were all negative.
Coagulogram: the prothrombin time, international normalized ratio (INR), and activated partial thromboplastin time all were within the normal range.
Stool examinations for ova, parasites, and Helicobacter pylori monoclonal antigen all proved negative.
Histological examination of the involved skin
The skin biopsy was performed twice. Histopathologically, there was mild lymphocytic infiltration without deposition of immunoglobulins or complement on direct immunofluorescence (11707/2013).
Other investigations: chest radiography and abdominal ultrasonography were normal, autologous serum skin test (ASST) was negative.
ENT consultation: physical examination and culture of nasopharyngeal samples were normal.
Dental consultation
On oral examination six decayed teeth were found in the maxilla and mandible (caries dentes 11, 23, 26, 35, 32, 46), decayed root of tooth 24 left in upper jaw, and dental plaque especially in the lower jaw (Figure 2).
Figure 2.
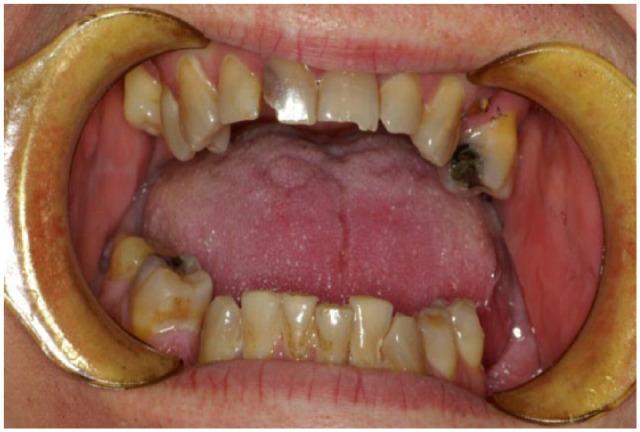
Picture presentation of decayed teeth, root of tooth 24, and dental plaque.
In addition, dental X-ray confirmed decayed teeth (caries dentes 11, 23, 26, 35, 32, 46); and decayed root of tooth 24. The X-ray also revealed: two molars in the mandible (retentio dentes 38 and 48); periapical abscesses in teeth 23 and 26; periodontium extension of teeth 18, 21, and 22; pathological root canal treatment in tooth 47 also with decay (caries atypical) (Figure 3).
Figure 3.

Panoramic X-ray presentation of decayed teeth, pathological root canal treatment, abscesses periapical, and molar teeth in the lower jaw.
The diagnosis of inflammation of oral cavity/dental infection was based on clinical examination. The patient was treated to eliminate the inflammatory process in the oral cavity by: surgical extraction of teeth 38 and 48, extraction of the decayed root of tooth 24, scaling and polishing of the upper and lower jaws, and removing caries from the decayed teeth. In a tooth 11 caries media and in teeth 26, 46, and 35 profound caries were removed and restored with light cured materials.
Cardiac consultation
The exercise test performed according to Bruce protocol was negative. Echo study revealed: normal left ventricle contractility with ejection fraction of 64%, left ventricle hypertrophy with increased thickness of interventricular septum and posterior wall of left ventricle, left atrial enlargement. Cardiac magnetic resonance imaging confirmed atrial enlargement and good left ventricle contractility without any signs of inflammation or other myocardial disease. Considering asymptomatic AF, probably longstanding persistent AF or even permanent with atrial enlargement, a strategy of rhythm control was adopted with bisoprolol 2.5 mg per day.
Follow-up
Eventually, during 2 months following the dental treatment, the urticaria symptoms subsided significantly, controlled by 5–10 mg/day of prednisone in combination with antihistamine drug (standard dose). Taking into account long history of the corticosteroid therapy, it was recommended to reduce dosage of the medication very slowly.
Next, during the following 3 months the CSU symptoms were controlled with antihistamine drugs (up to 2× standard dose administered only temporarily at the beginning of therapy upon intensified symptoms). At present the patient reports only some mild symptoms (during infection) and demand no therapy. Throughout the 8-year history of urticarial symptoms no such clinical improvement or reduction in treatment as observed after dental treatment was achieved. The main laboratory findings and therapy are presented in Table 1.
Association between APR and CSU and infectious/inflammatory foci in the oral cavity
APR reflects a systemic response to the localized inflammatory processes. The acute-phase proteins are not only the markers of the systemic inflammatory response, but may also play active role in initiation, progression, or severity/exacerbation of different systemic diseases.1,2 As mentioned above, the urticarial inflammation is accompanied by activation of APR14,15 with increased circulating levels of CRP and IL-6 corresponding to the disease severity/activity.6,7
It seems, therefore, that significantly increased serum CRP level in our patient reflects non-specific systemic inflammatory response in the course of CSU, resulting from the disease activity/severity; it might, however, be associated with infectious/inflammatory foci in the oral cavity. Despite extensive investigations performed, we were unable to identify any other causes which could be responsible for the increased level. Controversy surrounds the role of ENT or dental infection/inflammation in the oral cavity in CSU, which is mainly due to scarce literature in this area.16 The associations between diverse infections/inflammation in the oral cavity and pharynx (including occult dental and tonsil infection) and the origin or maintenance/amplification of the chronic urticarial processes have been proposed.16
Nevertheless, in many cases the relationship is difficult to prove and the mechanism itself remains unclear.16 Despite the controversy, in some cases dental treatment17–20 or tonsillectomy result in resolution or reduction of CSU symptoms,16 which is in line with our unpublished clinical observations. The main studies on dental infection and chronic urticaria are presented in Table 2. In this description a role of H. pylori infection and other infections in CSU are beyond the scope of this paper.
Table 2.
Main studies on dental infection and chronic urticarial.
| Authors (year) | Clinical response | Ref./Journal |
|---|---|---|
| Resch and Evans (1958) | (+) | Cleve Clin Q |
| Unger (1960) | (+) | South Med J |
| Shelley (1969) | (+) | Arch Dermatol |
| Tanphaichitr (1981) | (+) | Reference 18 |
| Thyagarajan and Kamalam (1982) | (+) | Reference 19 |
| Goga et al. (1988) | (−) | Rev Stomatol Chir Maxillofac |
| Sonoda et al. (2001) | (+) | Reference 17 |
| Büchter et al. (2003) | (−) | Mund Kiefer Gesichtschir |
| Brzewski et al. (2013) | (+) | Reference 20 |
| Kasperska-Zajac et al. (2015) | (+) | Int J Immunopathol Pharmacol |
(+), remission/improvement after dental treatment; (−), not improved after dental treatment.
In the presented case the most likely triggering or aggravating/maintaining factor of the CSU was the inflammation/dental infection of the oral cavity because of rapid reduction of the urticarial symptoms, doses of corticosteroids used, and serum CRP levels after the dental therapy. The decrease in serum CRP level may result from both the therapy of dental infection/inflammatory conditions as well as secondary reduction of CSU activity/severity itself, which is known to correlate with CRP level. It should be emphasized that despite healing of the dental infectious/inflammatory foci, initially the CRP values remained elevated, though lower than prior to treatment, which may result from urticarial inflammation associated with the residual symptoms of CSU. The residual inflammation might have still induced the release of a sufficient amount of pro-inflammatory mediators, including IL-6, to maintain low level of the marker. It should be emphasized that after recovery from the infectious/inflammatory processes, gradual subsidence of the symptoms, sometimes during several months, is observed. Similarly, in our case, gradual remission of skin lesions occurred after a few months, which was associated with normalization of CRP level.
It seems that concurrent infectious/inflammatory processes may trigger or aggravate the CSU symptoms by stimulating: (1) autoimmune/autoreactive reactions; (2) the ongoing urticarial inflammation; and (3) mast cells hyperactivity.16–18 In this regard different immunological and non-immunological factors (e.g. bacteriaemia, toxins, anaphylatoxins) have been proposed.16–18 We suggest that CRP and other APR biomarkers may also stimulate severity of the urticarial inflammation; it is known that CRP may directly and via a classic complement-dependent mechanism contribute to the pro-inflammatory state.3,4
Taken together, our case confirms that dental treatment may have a beneficial effect on the systemic inflammatory response, reducing/normalizing the circulating levels of APR markers. In addition, APR activation appears to worse CSU course, early identification and treatment of infectious/inflammatory foci in the oral cavity would form the mainstay of supportive therapy for CSU probably through reduction of the systemic inflammatory burden.
The association between APR and AF in CSU
Little information is available on cardiac involvement in different types of urticaria.
Cardiac problems in CSU are considered to be very rare, but much underestimation may be due to subclinical involvement. In some cases, patients with spontaneous urticaria/angioedema, especially during exacerbation, may complain of symptoms suggesting cardiac disorders. Such clinical features appear difficult to interpret, basically because scarce studies to confirm their nature with the use of different diagnostic methods. The symptoms are uncharacteristic and unpredictable, they may vary from individual to individual and change over time. However, the mechanism and extent of the cardiac involvement is unclear. Therefore, in clinical practice, cardiac consultations have never been recommended on routine basis.
Apart from urticarial vasculits there have been no studies carried out to assess the cardiac functions in that group of diseases. It has been documented that patients with hypocomplementic urticarial vasculitis syndrome and associated Jaccoud’s arthropathy should be evaluated for the presence of valvular heart disease, probably resulting from immune complexes and T cell inflammation.21 However, data regarding systemic consequences of other forms of longlasting, severe urticarial inflammation are limited. It has been suggested that patients with uncontrolled CSU should be evaluated for metabolic syndrome in order to reduce the cardiovascular risk and to improve the CSU outcomes.22
On the other hand, the urticarial inflammation does not seem to be explicitly related to AF; however, there might be a causal relation if longlasting uncontrolled CSU is accompanied by higher levels of APR markers assumed as a risk factor of cardiac complications. In addition, it is postulated that oral inflammatory/infectious processes are contributors to and/or triggers for the systemic inflammatory responses associated with activation of APR proteins, which influence the initiation and/or progression of several systemic disease processes, including cardiovascular disorders.23–25
Therefore, we suggest that in some individuals APR associated with uncontrolled longlasting CSU might be a risk factor itself, but there is no definite evidence to confirm this. Moreover, it cannot be excluded that combination of different factors, such as urticarial inflammation and infectious/inflammatory processes in the oral cavity acting together play a role in AF development/progression in the presented case. To our knowledge, there have been no earlier records of such association in the literature.
AF is a common heterogeneous disorder of multifactorial and incompletely understood pathophysiology, with severe implications for the patients.12,26,27
There are many known risk factors associated with AF, including ageing, thyroid dysfunction, diabetes, hypertension, obesity, as well as underlying functional and structural cardiac diseases, which may stimulate the development or progression of such arrhythmia.26
However, those risk factors do not explain all cases of AF, there are many potentially treatable conditions, including local and/or systemic inflammation, which should therefore be considered in some individuals. It seems that the inflammatory response may contribute to promoted progression rather than initiation of AF.12,13 CRP elevation was greatest in patients with more persistent AF, suggesting that CRP may be a marker for the inflammatory states that may increase propensity for AF progression to persistent or permanent forms, potentially by inducing inflammatory infiltrates and oxidative damage leading to structural and/or electrical remodeling of the atria.12,13 Interestingly, the APR proteins, such as TNF-alpha, IL-1-beta, and IL-6 were also elevated in lone AF. However, a causal versus secondary role for inflammation remains unclear. It is unclear whether reduction of CRP levels by CRP-lowering drugs, including corticosteroid therapy, would have any beneficial effect on the clinical incidence or persistence of AF.28,29
In addition, it has been suggested that lipid lowering drugs (statins, ezetimibe, fenofibrate, niacin, diets), angiotensin converting enzyme inhibitors, angiotensin receptor blockers, antidiabetic drugs, anti-inflammatory (cyclooxygenase inhibitors) and antiplatelet drugs, vitamin E, and beta-adrenoreceptor antagonists reduce the levels of CRP.30
In our case the underlying cause of the AF is not apparent. All known potential risk factors and secondary causes to prevalent cardiovascular disease mentioned above have been excluded by appropriate investigation. AF seems not to be related to ischemia caused by coronary vasculitis and abnormal microcirculation because the inflammatory process of small vessel is not a feature of CSU. In addition, this arrhythmia was not associated with myocarditis.
Our observations and the literature available indicate that the association between the systemic inflammatory state and AF origin, and its persistence in particular, are considered most probable in the discussed case.
The optimal management of all AF cases requires early diagnosis and effective therapy to maintain the sinus rhythm, as well as treatment of the underlying conditions and/or processes which can independently enhance vulnerability to AF.
The success rates for pharmacotherapy to maintain the sinus rhythm are still disappointingly low. In contrast to pharmacological therapy, catheter or surgical ablation of arrhythmogenic foci are rational targeted treatments, which can be considered as a potentially disease-modifying intervention for AF with the long-lasting freedom from the arrhythmia or even curing it.27,31 Unfortunately, in our patient with chronic permanent AF and secondary advanced structural changes in the left atrium the chance to restore and maintain the sinus rhythm was very low. Therefore, only anticoagulation and the heart rate control therapies have been used.
Of course, it could be argued that the occurrence of permanent AF in our severe longlasting CSU patient was unnecessarily due to part of the systemic manifestations and this association may be merely coincidental. However, one could hardly ignore the fact that apart from the systemic inflammation no heart diseases or other likely precipitating cause of AF was found; there is also increasing evidence of the effect of low grade-systemic inflammatory response upon the cardiovascular disorders.
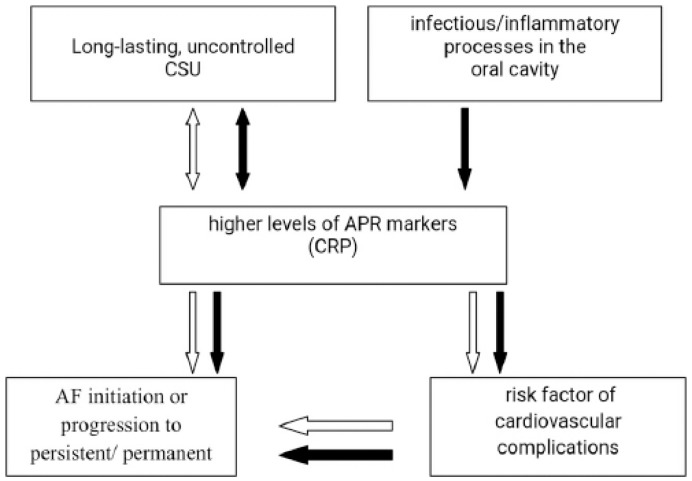
Having taken together the available data, we suggest that in the presented case the systemic inflammatory state associated with concomitant medical conditions, such as uncontrolled CSU and/or infectious/inflammatory state in the oral cavity, showed at least the additive effect on the progression of paroxysmal AF to the permanent form. It is difficult to determine whether such disorder is the result of primary myocardial involvement or rather the secondary to milieu changes associated with the systemic immune-inflammatory response. It remains unclear, however, why some individuals are more susceptible to development of AF following the low-grade systemic inflammatory response. It seems that underlying the genetic mechanisms or other risk factors may influence the process.27 The probable associations between CSU and APR and AF are illustrated in Figure 4.
Figure 4.
Probable association between CSU and APR and AF. Concomitant medical conditions, such as uncontrolled CSU and/or infectious/inflammatory state in the oral cavity, may have at least the additive effect on the progression of paroxysmal AF to the permanent form.
1. White arrow. Longlasting, uncontrolled CSU is associated with activation of APR, manifested by low-grade systemic inflammation. Reflexively, a direct or indirect response (secondary to activation of coagulation-firbrynolysis system) may lead to activation cells associated with urticarial inflammation. CRP may be a marker for the inflammatory states that may increase propensity for AF progression to persistent/permanent forms or its origin, potentially by inducing inflammatory infiltrates and oxidative damage leading to structural and/or electrical remodeling of the atria. In addition, APR is a known risk factor of cardiovascular complications, which may secondarily lead to the AF processes, mentioned above.
2. Black arrow. Oral inflammatory/infectious processes are contributors to and/or triggers for activation of APR proteins, which may trigger or aggravate the CSU symptoms and AF progression.
CSU, chronic spontaneous urticaria; AF, atrial fibrillation; APR, acute phase response; CRP, C-reactive protein.
Conclusions
We believe that difficult-to-treat and poorly controlled symptoms of CSU in our patient were related to the systemic inflammatory state, due to dental infection/inflammation in the oral cavity, basically in view of the arguments indicated, mainly the fact that skin lesions and drug doses were significantly reduced shortly after the dental treatment; other probable reasons were excluded.
We conclude that for CSU patients, careful clinical assessment and simple investigation of the APR markers, such as CRP could be easily included in the routine repeated assessment of their clinical state. In individuals with no evidence of infection, higher serum levels of CRP would identify most patients with more severe the CSU activity, who are more likely to benefit from more intensive treatment, including corticosteroids, which are known to reduce the APR proteins.
Apart from the commonly known risk factors for AF, other rarely recognized processes, such as systemic inflammatory response associated with urticarial inflammation or infectious/inflammatory foci in the oral cavity, could be taken into account as a predisposing agents to arrhythmia and other cardiovascular complications. In our CSU patient AF was diagnosed too late. It is essential for the GPs, the allergologists and the dermatologists to be aware that urticaria is not always a standalone disease and as such may be associated with other disorders, including AF. This arrhythmia in many patients is completely asymptomatic and may be detectable upon physical examination. AF is associated with high morbidity and mortality risk from the heart failure and thromboembolic complications. Prolonged episodes of AF can cause irreversible remodeling of the left atrium, which reduces the chance to eliminate arrhythmia. Restoration and maintenance of the sinus rhythm is more likely if arrhythmia is of the recent onset and the left atrium shows only little or no enlargement. As early diagnosis brings better outcome, routine physical examination should be regularly performed in CSU patients and not be restricted to the skin only.
It is important for the physicians to keep a high index of suspicion for similar cases of long-lasting poorly controlled CSU with increased CRP levels which may reflect an accompanying inflammatory/infectious reactions and may be associated with the systemic complications. Such patients should be evaluated for the presence and/or development of cardiovascular compli-cations2 and the metabolic syndrome.22 Moreover, there is no doubt that, if infectious/inflammatory foci are identified in the oral cavity, they should be successfully treated.
Management of CSU patients must be individualized as based on the patient’s clinical status. The APR markers levels clearly need to be taken into account when considering the diagnostic and treatment options.
The observation suggests a need for enhanced understanding of the pathophysiology and predictors of severe difficult-to-treat CSU. In addition, better identification of reasons and possible mechanisms underlying the early stages of AF may allow for the reduction in the rate of onset, progression, and redevelopment of the disease.
Acknowledgments
We would like to express our warm gratitude to all those who, at different stages of the project, participated in the diagnostic procedures, treatment of the patient throughout the years, including skin biopsy, histopathological evaluation, imaging, dental, dermatological and gastrological consultation, photo documenting, and other. In particular, we wish to express our gratitude to Z Brzoza.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Gabay C, Kushner I. (1999) Acute-phase proteins and other systemic responses to inflammation. New England Journal of Medicine 340: 448–454. [DOI] [PubMed] [Google Scholar]
- 2. Kasperska-Zajac A. (2012) Acute-phase response in chronic urticaria. Journal of the European Academy of Dermatology and Venereology 26: 665–672. [DOI] [PubMed] [Google Scholar]
- 3. Blake GJ, Ridker PM. (2001) Novel clinical markers of vascular wall inflammation. Circulation Research 89: 763–71. [DOI] [PubMed] [Google Scholar]
- 4. Griselli M, Herbert J, Hutchinson WL, et al. (1999) C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. Journal of Experimental Medicine 190: 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabroe RA, Grattan CEH, Francis DM, et al. (1999) The autologous serum skin test: A screening test for autoantibodies in chronic idiopathic urticatia. British Journal of Dermatology 140: 446–453. [DOI] [PubMed] [Google Scholar]
- 6. Takahagi S, Mihara S, Iwamoto K, et al. (2010) Coagulation/fibrinolysis and inflammation markers are associated with disease activity in patients with chronic urticaria. Allergy 65: 649–656. [DOI] [PubMed] [Google Scholar]
- 7. Kasperska-Zajac A, Grzanka A, Misiolek M, et al. (2015) Pentraxin-3 as a local inflammatory marker in chronic spontaneous urticaria. Cytokine 76: 566–568. [DOI] [PubMed] [Google Scholar]
- 8. Kasperska-Zając A, Grzanka A, Czecior E, et al. (2013) Acute phase inflammatory markers in patients with non-steroidal anti-inflammatory drugs (NSAIDs)-induced acute urticaria/angioedema and after aspirin challenge. Journal of the European Academy of Dermatology and Venereology 27: 1048–1052. [DOI] [PubMed] [Google Scholar]
- 9. Asero R, Cugno M, Tedeschi A. (2011) Activation of blood coagulation in plasma from chronic urticaria patients with negative autologous plasma skin test. Journal of the European Academy of Dermatology and Venereology 25: 201–205. [DOI] [PubMed] [Google Scholar]
- 10. Kasperska-Zajac A. (2011) Does dehydroepiandrosterone influence the expression of urticaria? A mini review. Inflammation 34: 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grzanka A, Machura E, Misiolek M, et al. (2014) Relationship between vitamin D status and the inflammatory state in patients with chronic spontaneous urticaria. Journal of Inflammation (London) 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brugts JJ, Akin S, Helming AM, et al. (2011) The predictive value of cardiac biomarkers in prognosis and risk stratification of patients with atrial fibrillation. Current Opinion in Cardiology 26: 449–456. [DOI] [PubMed] [Google Scholar]
- 13. Chung MK, Martin DO, Sprecher D, et al. (2001) C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 104: 2886–2891. [DOI] [PubMed] [Google Scholar]
- 14. Kasperska-Zajac A, Grzanka A, Machura E, et al. (2013) Increased serum complement C3 and C4 concentrations and their relation to severity of chronic spontaneous urticaria and CRP concentration. Journal of Inflammation (London) 24: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kasperska-Zajac A, Grzanka A, Machura E, et al. (2013) Analysis of procalcitonin and CRP concentrations in serum of patients with chronic spontaneous urticaria. Inflammation Research 62: 309–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wedi B, Raap U, Wieczorek D, et al. (2009) Urticaria and infections. Allergy Asthma and Clinical Immunology 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sonoda T, Anan T, Ono K, et al. (2001) Chronic urticaria associated with dental infection. British Journal of Dermatology 145: 516–518. [DOI] [PubMed] [Google Scholar]
- 18. Tanphaichitr K. (1981) Chronic urticaria associated with bacterial infection. A case of dental infection. Cutis 27: 653–656. [PubMed] [Google Scholar]
- 19. Thyagarajan K, Kamalam A. (1982) Chronic urticaria due to abscessed teeth roots. International Journal of Dermatology 21: 606. [DOI] [PubMed] [Google Scholar]
- 20. Brzewski PŁ, Spałkowska M, Podbielska M, et al. (2013) The role of focal infections in the pathogenesis of psoriasis and chronic urticaria. Postepy Dermatologii Alergologii 30: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Houser SL, Askenase PW, Palazzo E, et al. (2002) Valvular heart disease in patients with hypocomplementemic urticarial vasculitis syndrome associated with Jaccoud’s arthropathy. Cardiovascular Pathology 11: 210–216. [DOI] [PubMed] [Google Scholar]
- 22. Ye YM, Jin HJ, Hwang EK, et al. (2013) Co-existence of chronic urticaria and metabolic syndrome: Clinical implications. Acta Dermato-Venereologica 93: 156–160. [DOI] [PubMed] [Google Scholar]
- 23. Ebersole JL, Cappelli D, Mathys EC, et al. (2002) Periodontitis in humans and non-human primates: Oral-systemic linkage inducing acute phase proteins. Annals of Periodontology 7: 102–111. [DOI] [PubMed] [Google Scholar]
- 24. Taylor B, Tofler G, Morel-Kopp MC, et al. (2010) The effect of initial treatment of periodontitis on systemic markers of inflammation and cardiovascular risk: A randomized controlled trial. European Journal of Oral Science 118: 350–356. [DOI] [PubMed] [Google Scholar]
- 25. Taylor BA, Tofler GH, Carey HM, et al. (2006) Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. Journal of Dental Research 85: 74–78. [DOI] [PubMed] [Google Scholar]
- 26. Schnabel RB, Sullivan LM, Levy D, et al. (2009) Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 373: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, et al. (2010) Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). European Heart Journal 31: 2369–2429. [DOI] [PubMed] [Google Scholar]
- 28. Aviles RJ, Martin DO, Apperson-Hansen C, et al. (2003) Inflammation as a risk factor for atrial fibrillation. Circulation 108: 3006–3010. [DOI] [PubMed] [Google Scholar]
- 29. Psychari SN, Apostolou TS, Sinos L, et al. (2005) Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. American Journal of Cardiology 95: 764–767. [DOI] [PubMed] [Google Scholar]
- 30. Prasad K. (2006) C-reactive protein (CRP)-lowering agents. Cardiovascular Drug Reviews 24: 33–50. [DOI] [PubMed] [Google Scholar]
- 31. Santangeli P, Di Biase L, Burkhardt DJ, et al. (2012) Catheter ablation of atrial fibrillation: State-of-the-art techniques and future perspectives. Journal of Cardiovascular Medicine (Hagerstown) 13: 108–124. [DOI] [PubMed] [Google Scholar]