Abstract
Introduction:
Intolerance to various foods, excluding bona fide coeliac disease and lactose intolerance, represents a growing cause of patient visits to allergy clinics.
Histamine intolerance is a long-known, multifaceted clinical condition triggered by histamine-rich foods and alcohol and/or by drugs that liberate histamine or block diamine oxidase (DAO), the main enzyme involved in the metabolism of ingested histamine. Histamine limitation diets impose complex, non-standardized restrictions that may severely impact the quality of life of patients.
Methods:
We retrospectively evaluated 14 patients who visited allergy outpatient facilities in northern Italy with a negative diagnosis for IgE-mediated food hypersensitivity, coeliac disease, conditions related to gastric hypersecretion, and systemic nickel hypersensitivity, and who previously underwent a histamine limitation diet with benefits for their main symptoms. Serum diamine oxidase levels and the clinical response to diamine oxidase supplementation were investigated.
Results:
We found that 10 out of 14 patients had serum DAO activity <10 U/mL, which was the threshold suggested as a cutoff for probable histamine intolerance. Moreover, 13 out of 14 patients subjectively reported a benefit in at least one of the disturbances related to food intolerances following diamine oxidase supplementation. The mean value (± SD) of diamine oxidase activity in the cohort of patients with histamine intolerance symptoms was 7.04 ± 6.90 U/mL compared to 39.50 ± 18.16 U/mL in 34 healthy controls (P = 0.0031).
Conclusion:
In patients with symptoms triggered by histamine-rich food, measuring the serum diamine oxidase activity can help identify subjects who can benefit from a histamine limitation diet and/or diamine oxidase supplementation.
Properly designed, controlled studies investigating histamine intolerance that include histamine provocation are indispensable for providing insights into the area of food intolerances, which are currently primarily managed with non-scientific approaches in Italy.
Keywords: diamine oxidase, food intolerance, histamine intolerance, low histamine diet
Introduction
Histamine intolerance is the disequilibrium between accumulated histamine and histamine degradation. The latter is mainly dependent on diamine oxidase (DAO)1 and to a lesser extent on histamine-N-methyltransferase (HNMT).2,3
Histamine is a biogenic amine that is present to varying degrees in many foods. The ingestion of histamine-rich food4 or alcohol4,5 may provoke heterogeneous symptoms and signs such as diarrhea, headache,5 rhinoconjunctivitis, asthma, hypotension,4,6,7 arrhythmia, urticaria, pruritus, and flushing.8
Additionally, several drugs have been suggested to induce histamine release or block DAO.9–12
DAO inhibits the trans-epithelial permeation of exogenous histamine,13 and impaired DAO activity results in increased enteral histamine uptake with consequent increased plasma histamine concentrations11,13 and corresponding symptoms.
Acquired histamine intolerance may be transient and therefore reversible after the elimination of causes (i.e. discontinuing DAO-blocking drugs).
There is a genetic background underlying reduced histamine metabolism. Various single nucleotide polymorphisms (SNPs) in the DAO gene have been identified14 and shown to be associated with DAO serum activity15 and with hypersensitivity to non-steroidal anti-inflammatory drugs (NSAIDs).16
Due to the multifaceted nature of histamine intolerance symptoms, the prevalence of this syndrome has long been underestimated and its symptoms misinterpreted. It was proposed that approximately 1% of the population had histamine intolerance and that 80% of affected patients were middle-aged.1
A few double-blind, placebo-controlled (DBPC) provocation studies using suspected foods have been performed to support this diagnosis, but it remains elusive.17
Altogether, several confounding factors surround this condition to the detriment of patients. While some patients are referred to allergists and gastroenterologists, patients are very often directed to non-conventional approaches, including non-scientific diagnostic assays.18
Here, we present our real-life experience in allergy outpatient clinics. We included only patients that were not affected by common clinical conditions with signs and symptoms that partly overlapped those of histamine intolerance in our retrospective study. To this end, we excluded patients with IgE-mediated food hypersensitivity, coeliac disease, systemic nickel allergy syndrome, and conditions related to gastric acid hypersecretion. Histamine intolerance was suspected in all patients by the allergist, and all patients underwent histamine limitation diets with benefits on food intolerance. Serum DAO levels and the clinical response to DAO supplements were investigated.
We discuss the relevance of histamine intolerance in daily clinical practice and highlight the need to increase controlled studies and the awareness of this condition between general practitioners and relevant specialists.
Materials and methods
Patient selection
Electronic records of consecutive patients who visited the allergy outpatient clinics between June 2014 and February 2015 were retrospectively analyzed for histamine intolerance.
Written informed consent was obtained from each participating subject according to the specific requirements of the Ethics Committee for studies that require blood sampling. Specifically, the protocol for the management of the Biological Bank for Immunological and Allergological studies were applied (Ethics Committee, protocol code BBI-ALL, 05052005).
In the first level screening, patients with symptoms associated with the intake of multiple foods not reducible to IgE-mediated sensitization to food or inhalant allergens and for which coeliac disease was excluded were considered for analysis.
To exclude IgE-mediated hypersensitivity reactions, patients with negative results of a prick test and/or specific IgE determination to most common food allergens were considered. The results from the prick test (ALK Abellò, Lainate, Italy) and/or a specific IgE (FEIA) (ImmunoCap systems. Phadia-Thermo Fisher, Uppsale, Sweden) response to extracts or to one or more allergen components from the following food allergen sources were available: milk, egg, fish, shrimp, soy, nuts, and dried fruits.
To exclude coeliac disease, serum IgA auto-antibodies to tissue transglutaminase were measured.19 Screening for celiac disease was performed by measuring total IgA and anti-transglutaminase IgA antibodies by ELISA (Eu-tTG® IgA. Eurospital. Trieste. Italy). Patients with neoplastic malignancies and autoimmune diseases were also excluded.
Subsequently, to eliminate any possible confounding factors, patients with the following morbidities (or with symptoms and signs suggestive of the following morbidities) were excluded from the analysis:
Chronic urticaria patients;20
Subjects with gastro-esophageal reflux disease,21 gastric ulcer, or patients who reported symptom relief following previous treatment with PPIs; and
Patients with a suspected systemic nickel allergy,22,23 including subjects who had a positive patch test for nickel and/or a positive anamnesis for contact allergy to metals.
In principle, these conditions may very well coexist or be worsened by histamine intolerance. However, this criterion was applied to ensure as much as possible that only subjects with bona fide histamine intolerance were included.
Patients with clinically and/or laboratory assessed (lactose hydrogen breath test)24 lactose intolerance were included only if they reported that the symptoms that brought them to the specialist partially or totally persisted after adopting a lactose avoidance diet (including hidden lactose sources).
Hypersensitivity to NSAIDs was evaluated as part of the conventional allergological anamnesis. All patients were evaluated at least twice at a 1-month interval.
Patients’ characteristics and treatment
Fourteen patients met the inclusion criteria and were considered for the analysis. The cohort was formed by 13 adults and one child (mean age, 38.6 years; age range, 10–67 years), and included 10 women.
Other specialists had previously prescribed a low histamine diet to 10 out of 14 patients, with a reported benefit on food intolerance. The low histamine diet was re-prescribed or prescribed for the first time in 10 patients (non-coincident with the above) at our allergy clinics. Serum DAO determination was performed in all patients. Additionally, all of the patients were prescribed DAO supplementation (Daosin, AET-Pharma, Italy, one capsule twice a day, 15 min before lunch and dinner) for at least 14 days. Daosin contains DAO isolated from pig kidneys. Patients were administered DAO supplementation and the histamine limitation diet in different regimens (i.e. simultaneously and systematically or on an “as needed” basis when the patient refused the histamine limitation diet).
Five patients had IgE-mediated sensitization to pollens and/or house dust mites, which correlated with signs and symptoms of rhinitis (with or without allergic asthma). In all cases, these signs and symptoms were not the reason for the visit of these five patients to the allergy clinics. Transient oral reactions were reported in three of these five patients upon intake of selected fruits that were suggestive of oral allergy symptoms and were not considered to be causally related to the symptoms relevant for the diagnosis of histamine intolerance.
DAO measurement
DAO activity was measured with a radio-extraction assay using a commercial kit (DAOREA, AET Pharma, Italy) according to manufacturer’s instructions. Briefly, DAO activity was determined by quantifying the reaction product. Radio-labeled putrescine-dihydrochloride was used as a substrate. The resulting Δ1pyrroline containing the radiolabel was selectively extracted from the matrix by a liquid extraction step. A non-toxic, chlorine-free solvent with a high capacity was used for the extraction. Finally, scintillation fluid was added to the organic phase containing the radiolabelled Δ1-pyrroline, and radioactivity was determined in a beta-counter (PerkinElmer, Waltham, MA, USA).
Histamine intolerance is highly likely in patients with DAO activity <3 U/mL, likely in patients with DAO activity <10 U/mL, and improbable in patients with DAO activity >10 U/mL.1,3
To compare DAO activity in patients with suspected histamine intolerance with the general population, sera from 34 healthy blood donors were independently analyzed.
Statistical analysis
Descriptive statistics were used, except for the comparison between the distribution of the values of serum DAO activity in patients with suspected histamine intolerance versus the control, which was performed with the Mann–Whitney two-sample statistic for non-parametric data. All statistical analyses were performed with the PRISM statistical software package (GraphPad Inc., San Diego, CA, USA). Statistical tests were two-sided with a significance level of 0.05.
Results
We observed that functional bloating25 and abdominal pain were prevalent in the selected cohort, although other typical histamine intolerance symptoms were also reported. By carefully collecting the 14 patients’ symptoms, the following typical histamine-intolerance disturbances were identified and grouped as follows: functional bloating (12 patients); abdominal pain (8 patients); palpitations/tachycardia (7 patients); diarrhea (6 patients); headache (5 patients); rash and/or pruritis (5 patients); flushing (4 patients); rhinorrhea (3 patients); and nausea and/or vomiting (2 patients).
Individual prevailing symptoms that could refer to histamine intolerance are listed in Table 1.
Table 1.
Symptoms of histamine intolerance.
| ID | Age (years) | Sex | DAO (U/mL) | Response to |
Abd. pain | Diarrhea | Nausea and/or vomiting | Bloating | Rash and/or pruritus | Palpitations tachycardia | Headache | Rhinorrea | Flushing | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prev. diet | Pres. diet | DAO suppl. | |||||||||||||
| 1 | 10 | F | 4.4 | N.D. | Y | Y | Y | Y | |||||||
| 2 | 34 | F | 7.5 | Y | N.D. | Y | Y | Y | Y | Y | Y | ||||
| 3 | 54 | F | 0.6 | Y | Y | Y | Y | Y | |||||||
| 4 | 22 | M | 8.2 | Y | Y | Y | Y | Y | Y | ||||||
| 5 | 34 | M | 0.4 | N.D. | Y | Y | Y | Y | Y | Y | |||||
| 6 | 67 | F | 11.3 | Y | Y | Y | Y | Y | Y | Y | |||||
| 7 | 54 | F | 7.6 | Y | Y | Y | Y | Y | Y | ||||||
| 8 | 32 | F | 12.4 | Y | Y | Y | Y | Y | Y | ||||||
| 9 | 34 | F | 5.5 | Y | Y | Y | Y | Y | Y | ||||||
| 10 | 23 | M | 4.7 | N.D. | Y | Y | Y | Y | Y | Y | Y | ||||
| 11 | 45 | M | 14.2 | Y | N.D. | N | Y | Y | Y | Y | Y | ||||
| 12 | 34 | F | 9.3 | N.D. | Y | Y | Y | Y | Y | Y | |||||
| 13 | 45 | F | 10.5 | Y | N.D. | Y | Y | Y | Y | ||||||
| 14 | 53 | F | 1.9 | Y | Y | Y | Y | Y | Y | Y | |||||
Demographic and clinical characteristics of patients with suspected histamine intolerance.
Abd.: abdominal, N: no, N.D.: not done, Pres.: present, Prev.: previous, Suppl.: supplementation, Y: yes.
Six patients also reported previous GI problems with the consumption of milk or dairy products that could be controlled by lactose avoidance and had no apparent correlation with the symptoms referenced at the time of the visit.
Nine individuals had a history of NSAID hypersensitivity that was not related to the symptoms that they reported at the time of the visit.
A low histamine diet was re-prescribed or prescribed for the first time in 10 patients, who subjectively reported a benefit in food intolerance symptoms.
Ten out of 14 patients were eligible to formalize the diagnosis of histamine intolerance according to previously proposed criteria1 (negative results for food allergen specific IgE, two typical symptoms of histamine intolerance associated with multiple foods for longer than 3 months, improvement with a histamine-free diet and reduced serum DAO levels).
All 14 patients reported that the previous and/or newly prescribed low histamine diet impaired their quality of life (to variable extents depending on the patient) and that it could not be maintained permanently. In most cases, a culprit food whose elimination from the diet was instrumental to radically prevent relapses of symptoms could not be identified.
DAO supplementation was associated with the reduction of at least one of the reported symptoms in 13 out of 14 patients. In patients where DAO supplementation was associated with a low histamine diet, the added value of the dietary supplement was reported compared to the diet alone due to the efficacy of DAO supplementation on at least one symptom and the favorable impact on the quality of life compared to the dietetic limitation.
Seven and three patients had DAO activity values <10 U/mL or <3 U/mL (i.e. the cutoff values of serum DAO activity below which the DAO deficit was indicated as likely or highly likely, respectively).1,3
The remaining four patients had DAO levels above 10 U/mL.
The mean value (± SD) of DAO activity in the cohort of patients with histamine intolerance symptoms was 7.04 ± 6.90 U/mL compared to 39.50 ± 18.16 U/mL in the 34 healthy controls (P = 0.003147).
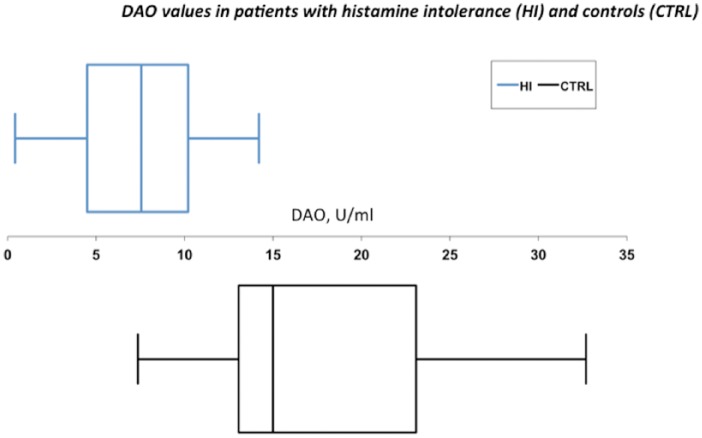
We used box-and-whisker plots to graphically express the data (Figure 1). The DAO activity values in patients were much closer to 10 (upper quartile: 10.20 U/mL) compared to a panel of 34 healthy control individuals (upper quartile: 54.00 U/mL).
Figure 1.
Whisker plot representation of the distribution of serum DAO values in U/mL (on the x axis) in patients with histamine intolerance (HI, top panel) and controls (CTRL, bottom panel). The box indicates the lower and upper quartiles and the central line represents the median. The points at the ends of the “whiskers” indicate extreme values.
Discussion
Histamine intolerance is defined as a condition whereby the ingestion of food containing high amounts of histamine by subjects with low intestinal histamine inactivation (or inhibition of this inactivation by other food constituents or drugs) leads to absorption of histamine in amounts sufficient to cause adverse reactions.1,26
Impaired degradation can follow genetic or acquired impairment of the enzymatic function of DAO or histamine-N-methyltransferase (HNMT).
Here, we investigated the possibility of making a formal diagnosis of histamine intolerance in a clinical practice setting by retrospectively evaluating patients who visited our allergy clinics. We took advantage of two recently introduced clinical tools: the in vitro measurement of serum DAO activity and DAO supplementation using capsules containing DAO isolated from pig kidneys (Daosin, AET Pharma, Italy).
To identify patients whose disturbances had the highest possibility of being due to bona fide histamine intolerances, we excluded common conditions that might present with overlapping symptoms and signs, including chronic urticaria, gastro-oesophageal reflux disease, gastric ulcer, or relief of symptoms following previous treatment with protein pump inhibitors or suspected systemic nickel allergy.
By applying this reductionist approach, we identified 14 patients and found that 10 of them had serum DAO activity below the threshold suggested as a cutoff for probable histamine intolerance. The mean DAO activity of this cohort was significantly lower than the activity detected in a group of healthy blood donors. Following DAO supplementation, 13 out of 14 patients subjectively reported a benefit on at least one of the disturbances related to food intolerances
There are a few limits in this study. First, a single DAO determination was performed. This may not be sufficient for the diagnosis of histamine intolerance due overlaps between different patients populations in relation to food intake and elimination diets.12,27 Second, the severity of symptoms was not assessed by a severity standard, as recommended by Komericki et al. by lack of reproducibility of single symptoms following oral provocation with histamine in a randomized, DBPC experimental setting.28
Taken together, our results suggest that histamine intolerance should be considered in patients visiting allergy clinics. Serum DAO activity measurements and DAO supplementation represent important tools to consolidate the diagnosis, reduce the level of uncertainty of this condition, and provide a causal treatment for the patients’ symptoms and signs.
Non-conventional testing for food intolerances (which had been prescribed for 9 out of 14 patients in our cohort before they came to our attention) is a challenge for evidence-based allopathic medicine.18 In this context, the study of histamine intolerance and its pathogenesis should allow conventional medicine to regain its role, particularly if proper care is maintained in establishing a therapeutic alliance and in the collection of the clinical history.29 Our results should raise interest in histamine intolerance and prompt studies with a randomized, placebo-controlled design to investigate histamine challenge and DAO supplementation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Maintz L, Novak N. (2007) Histamine and histamine intolerance. American Journal of Clinical Nutrition 85(5): 1185–1196. [DOI] [PubMed] [Google Scholar]
- 2. Brown DD, Tomchik R, Axelrod J. (1959) The distribution and properties of a histamine-methylating enzyme. Journal of Biological Chemistry 234: 2948–2950. [PubMed] [Google Scholar]
- 3. Klocker J, Mätzler SA, Huetz G-N, et al. (2005) Expression of histamine degrading enzymes in porcine tissues. Inflammation Research 54(S1): S54–57. [DOI] [PubMed] [Google Scholar]
- 4. Wantke F, Hemmer W, Haglmüller T, et al. (1996) Histamine in wine. Bronchoconstriction after a double-blind placebo-controlled red wine provocation test. International Archives of Allergy and Immunology 110(4): 397–400. [DOI] [PubMed] [Google Scholar]
- 5. Wantke F, Götz M, Jarisch R. (1994) The red wine provocation test: Intolerance to histamine as a model for food intolerance. Allergy Proceedings 15(1): 27–32. [DOI] [PubMed] [Google Scholar]
- 6. Sattler J, Häfner D, Klotter HJ, et al. (1988) Food-induced histaminosis as an epidemiological problem: plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO). Agents and Actions 23(3–4): 361–365. [DOI] [PubMed] [Google Scholar]
- 7. Wöhrl S, Hemmer W, Focke M, et al. (2004) Histamine intolerance-like symptoms in healthy volunteers after oral provocation with liquid histamine. Allergy and Asthma Proceedings 25(5): 305–311. [PubMed] [Google Scholar]
- 8. Pollock I, Murdoch RD, Lessof MH. (1991) Plasma histamine and clinical tolerance to infused histamine in normal, atopic and urticarial subjects. Agents and Actions 32(3–4): 359–365. [DOI] [PubMed] [Google Scholar]
- 9. Sattler J, Hesterberg R, Schmidt U, et al. (1987) Inhibition of intestinal diamine oxidase by detergents: A problem for drug formulations with water insoluble agents applied by the intravenous route? Agents and Actions 20(3–4): 270–273. [DOI] [PubMed] [Google Scholar]
- 10. Sattler J, Hesterberg R, Lorenz W, et al. (1985) Inhibition of human and canine diamine oxidase by drugs used in an intensive care unit: relevance for clinical side effects? Agents and Actions 16(3–4): 91–94. [DOI] [PubMed] [Google Scholar]
- 11. Sattler J, Lorenz W. (1990) Intestinal diamine oxidases and enteral-induced histaminosis: Studies on three prognostic variables in an epidemiological model. Journal of Neural Transmission Supplementum 32: 291–314. [DOI] [PubMed] [Google Scholar]
- 12. Wantke F, Proud D, Siekierski E, et al. (1998) Daily variations of serum diamine oxidase and the influence of H1 and H2 blockers: a critical approach to routine diamine oxidase assessment. Inflammation Research 47(10): 396–400. [DOI] [PubMed] [Google Scholar]
- 13. Ahrens F, Gäbel G, Garz B, et al. (2002) Release and permeation of histamine are affected by diamine oxidase in the pig large intestine. Inflammation Research 51 (Suppl. 1):S83–84. [DOI] [PubMed] [Google Scholar]
- 14. Petersen J, Raithel M, Schwelberger HG. (2005) Characterisation of functional polymorphisms of the human diamine oxidase gene. Inflammation Research 54(S1): S58–59. [DOI] [PubMed] [Google Scholar]
- 15. Maintz L, Yu C-F, Rodríguez E, et al. (2011) Association of single nucleotide polymorphisms in the diamine oxidase gene with diamine oxidase serum activities. Allergy 66(7): 893–902. [DOI] [PubMed] [Google Scholar]
- 16. Agúndez JAG, Ayuso P, Cornejo-García JA, et al. (2012) The diamine oxidase gene is associated with hypersensitivity response to non-steroidal anti-inflammatory drugs. PLoS One 7(11): e47571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jansen SC, van Dusseldorp M, Bottema KC, et al. (2003) Intolerance to dietary biogenic amines: A review. Annals of Allergy Asthma & Immunology 91(3): 233–240, quiz 241–2, 296. [DOI] [PubMed] [Google Scholar]
- 18. Mullin GE, Swift KM, Lipski L, et al. (2010) Testing for food reactions: The good, the bad, and the ugly. Nutrition in Clinical Practice 25(2): 192–198. [DOI] [PubMed] [Google Scholar]
- 19. Reese I. (2014) [Debating histamine intolerance: are adverse reactions to histamine-containing foods fact or fiction?]. Der Hautarzt 65(6): 559–566. [DOI] [PubMed] [Google Scholar]
- 20. Zuberbier T, Aberer W, Asero R, et al. (2014) The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: The 2013 revision and update. Allergy 69: 868–887. [DOI] [PubMed] [Google Scholar]
- 21. Bredenoord AJ, Pandolfino JE, Smout AJPM. (2013) Gastro-oesophageal reflux disease. Lancet 381(9881): 1933–1942. [DOI] [PubMed] [Google Scholar]
- 22. Braga M, Quecchia C, Perotta C, et al. (2013) Systemic nickel allergy syndrome: Nosologic framework and usefulness of diet regimen for diagnosis. International Journal of Immunopathology and Pharmacology 26(3): 707–716. [DOI] [PubMed] [Google Scholar]
- 23. Minelli M, Schiavino D, Musca F, et al. (2010) Oral hyposensitization to nickel induces clinical improvement and a decrease in TH1 and TH2 cytokines in patients with systemic nickel allergy syndrome. International Journal of Immunopathology and Pharmacology 23(1): 193–201. [DOI] [PubMed] [Google Scholar]
- 24. Ghoshal UC. (2011) How to interpret hydrogen breath tests. Journal of Neurogastroenterology and Motility 17(3): 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rome Foundation (2006) Guidelines—Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. Journal of Gastrointestinal and Liver Diseases 15: 307–312. [PubMed] [Google Scholar]
- 26. Schwelberger HG. (2009) Histamine intolerance: Overestimated or underestimated? Inflammation Research 58(S1):51–52. [DOI] [PubMed] [Google Scholar]
- 27. Töndury B, Wüthrich B, Schmid-Grendelmeier P, et al. (2008) Histamine intolerance: Is the determination of diamine oxidase activity in the serum useful in routine clinical practice? Allergologie 31(8): 350–356. [Google Scholar]
- 28. Komericki P, Klein G, Reider N, et al. (2011) Histamine intolerance: lack of reproducibility of single symptoms by oral provocation with histamine: A randomised, double-blind, placebo-controlled cross-over study. Wiener Klinische Wochenschrift 123(1–2): 15–20. [DOI] [PubMed] [Google Scholar]
- 29. Neumann M, Edelhäuser F, Kreps GL, et al. (2010) Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it?—an exploration on why and how to study the specific effect of the provider. Patient Education and Counseling 80(3): 307–314. [DOI] [PubMed] [Google Scholar]