Abstract
Activated microglia, especially polarized M1 cells, produce pro-inflammatory cytokines and free radicals, thereby contributing directly to neuroinflammation and various brain disorders. Given that excessive or chronic neuroinflammation within the central nervous system (CNS) exacerbates neuronal damage, molecules that modulate neuroinflammation are candidates as neuroprotective agents. In this study, we provide evidence that Safflor yellow (SY), the main active component in the traditional Chinese medicine safflower, modulates inflammatory responses by acting directly on BV2 microglia. LPS stimulated BV2 cells to upregulate expression of TLR4-Myd88 and MAPK-NF-κB signaling pathways and to release IL-1β, IL-6, TNF-α, and COX-2. However, SY treatment inhibited expression of TLR4-Myd88 and p-38/p-JNK-NF-κB, downregulated expression of iNOS, CD16/32, and IL-12, and upregulated CD206 and IL-10. In conclusion, our results demonstrate that SY exerts an anti-inflammatory effect on BV2 microglia, possibly through TLR-4/p-38/p-JNK/NF-κB signaling pathways and the conversion of microglia from inflammatory M1 to an anti-inflammatory M2 phenotype.
Keywords: BV2 cells, microglia polarization, neuroinflammation, safflower yellow
Introduction
Microglia are resident innate immune cells in the central nervous system (CNS), with similar functional and phenotypic characteristics as macrophages. They participate in innate immune responses in the brain and play an important role in the incidence and development of CNS diseases.1 In the injured CNS, microglial activation plays a critical and dual role in the CNS microenvironment.2 In the immune response of diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and cerebral ischemia, activated microglia can secrete both beneficial and harmful molecules, including neurotrophic cytokines and inflammatory factors.3–6
Like peripheral macrophages, microglia can be polarized to distinct functional phenotypes: namely, the classic activation type (M1 microglia) and the selective activation type (M2 microglia). Microglia respond to many stimuli such as LPS, IFN-γ, TNF-α, hypoxia, and β-amyloid, and are a major source of inflammatory cytokines and oxidative metabolites such as IL-1β, IL-6, TNF-α, iNOS, and COX-2, thereby contributing to neuron damage and death. M2 microglia, however, are activated by Th2 cell factors like IL-4, IL-10, and IL-13, which can inhibit immuno-inflammatory responses by secreting anti-inflammatory factors and increasing neurotrophic factors such as glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF), thus promoting nerve repair and regeneration.7 Priming of microglial polarization towards M2 phenotype by drug intervention should therefore provide novel strategies for the treatment of neurological diseases with neuroinflammation.
Although multiple cell lines, such as astrocytes and neurons, express toll-like receptor (TLR)-4, and TLR-4 concentration is highest on microglia,8 it is clear that microglial activation occurs through a TLR-4–mediated pathway. Functional TLR-4 of microglia is known to have deleterious effects in stroke and neurodegenerative models. Previous studies indicated that TLR-4 was upregulated in microglia after oxygen-glucose deprivation (OGD), that microglia with intact TLR-4 expressed a significantly higher amount of inflammatory cytokines, and that the absence of a functional TLR-4 is associated with a decreased production of inflammatory cytokines.9,10 In stroke models, TLR-4 has been associated with larger infarct volumes and more severe functional deficits.11 In addition, ischemic spinal cord also demonstrated a significant increase in microglia with intact TLR-4, suggesting that TLR-4 signaling has an effect on reperfusion injury in the spinal cord.12 Taken together, these results indicate the TLR-4 on microglia plays an important role in condition-induced CNS injury.
Safflor yellow (SY), the main active component of the traditional Chinese medicine safflower, is a mixture that contains water soluble chalcone, which includes hydroxyl safflor yellow A (HSYA), hydroxylsafflor yellow B (HSYB), and others. HSYA, molecular formula C27H32O16, with a molecular weight of 612, is the main component of SY.13 SY has been used for many years in traditional Chinese medicine as a purgative, analgesic, antipyretic, and poison antidote. SY has multiple pharmacological actions such as inhibiting platelet aggregation, anticoagulation, anti-oxidation, and anti-inflammation.14–17 It has been reported that HSYA inhibits the phosphorylation of NF-κB and p38 MAPK in BV2 cells in OGD/reoxygeneration (OGD/R) conditions, accompanied by the downregulation of inflammatory IL-1β, IL-6, and TNF-α.18 Human epithelial cell strain A549 has been stimulated by LPS to activate the TLR4/Myd88/NF-κB inflammatory pathway, while HYSA also reduces TLR4 expression on mRNA and protein levels.19 In many CNS disorders, microglia are the cells principally responsible for the development of a neuroinflammatory microenvironment. Inhibition of the microglial inflammatory pathway is therefore of primary importance for improving the neuroinflammatory microenvironment, reducing neuronal injury, and promoting tissue regeneration and repair in the CNS.
To better understand the molecular basis of SY’s anti-inflammatory capacity in the CNS, we observed its ability to inhibit TLR expression while simultaneously suppressing inflammatory responses. We found that SY inhibited TLR4-Myd88- P38 MAPK-NF-κB signaling pathways, accompanied by the suppression of inflammatory TNF-α, IL-1β, IL-6, IL-12, NO, COX-2, and iNOS, promoting the depolarization of inflammatory M1 toward anti-inflammatory M2 microglia.
Materials and methods
BV2 microglia culture and treatments
The BV-2 immortalized microglial cell line was obtained from ShenKe Biological Technology Co., Ltd., Shanghai, PR China, and cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco) at 37°C in a humidified cell incubator with a 95%/5% (v/v) mixture of air and CO2. HSYA is major active component of SY that is obtained from traditional Chinese medicine safflower carthamus by Shanxi HuaHuiKaiDe Pharmaceutical Co., Ltd., PR China. A control group, LPS group, and LPS+SY group were set for the experiments. After microglial cells had been attached to the culture dish for 2 h, LPS (Sigma, St. Louis, MO, USA) at a final concentration of 0.5 μg/mL was added to the LPS group, and LPS plus SY at a final concentration of 80 μg/mL was added to the LPS+SY group. BV-2 cells in all groups were then cultured for 48 h.
Cell viability
Cell viability of BV2 microglia was measured by MTT assay. Briefly, BV-2 microglia (2×104 / mL) were inoculated on 96-well plates, and cultured with LPS (100 ng/mL, 200 ng/mL, 500 ng/mL, 1 µg/mL, 2 µg/mL) and/or SY (20 µg/mL, 40 µg/mL, 80 µg/mL, 100 µg/mL) for 48 h. A total of 100 µl of MTT solution (0.5 mg/mL in the medium) was added to each well, and the plates were incubated at 37°C for an additional 4 h. Plates were then centrifuged to remove the supernatants, and the crystals were dissolved in 150 μL of DMSO. Cell viability was determined by measuring the optical density (OD) at 490 nm using a quantified microplate reader (Multiscan Ascent, Labsystem, Finland). All determinations were confirmed by replication in at least three independent experiments. Cell viability (%) = (OD value of experimental well – OD value of zero-setting well) / (OD value of control well – OD value of zero-setting well) × 100%.
Cytokine ELISA assay
Collected supernatants were put into a −80°C refrigerator and the concentrations of IL-1β, IL-6, TNF-α, and IL-10 were measured with the commercial ELISA kit (PeproTech Inc., USA) following the manufacturer’s instructions. All determinations were confirmed by replication in at least three independent experiments. Concentrations of cytokine are expressed as pg/mL.
Nitrite assay
Nitric oxide (NO) was assayed by measuring the end product nitrite, which was determined by a colorimeter assay based on the Griess reaction. Supernatants (100 μL) of cultured cells were mixed with 100 μL of Griess reagent for 10 min at RT. Absorbance was measured at 510 nm in an automated plate reader. Concentrations of nitrite were determined by reference to a standard curve of sodium nitrite (Sigma). Determinations were performed in duplicate and repeated in three independent experiments. Results are expressed as micromolar. In order to remove the possible effect of SY’s color on background absorbance, an SY control was used in addition to the control group.
Western blotting
Cells were homogenized with a glass homogenizer using RIPA Lysis Buffer (Beyotime Institute of Biotechnology, PR China) supplemented with protease inhibitors. Protein concentration was measured by BCA (Beyotime Institute of Biotechnology, PR China). Cell extracts (30 μg) were loaded onto SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane (Millipore). After blocking with 5% milk was at room temperature for 1 h, membranes were incubated at 4°C overnight with primary antibodies against TLR4, TLR2, p-NF-κB(p65), p-JNK (1:1000, Cell Signaling Technology, USA), Myd88 (1:1000, Abcam, USA), p-P38, p-ERK (1:1000, Santa Cruz Biotechnology, USA), iNOS (1:1000, Enzo Life Sciences, USA), Arg (1:1000, BD Biosciences, USA), COX-2 (1:1000, Epitomics, USA), GAPDH, β-actin(1:10000, Cell Signaling Technology, USA). The following day, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:10,000, Earthox LLC, USA) for 1 h. Immunoblots were developed with an enhanced chemiluminescence system (GE Healthcare Life Sciences) and measured using Quantity Software (Bio-Rad, Hercules, CA, USA). GAPDH or β-actin was used as the optical density of internal reference.
Real-time PCR
Total RNA was isolated from cells using RNAiso Plus Reagent (Takara, Dalian, PR China), and reverse-transcribed by using PrimeScriptTMRT Reagent Kit (Takara, Dalian, PR China) to produce cDNA. Quantitative PCR (ABI StepOne Plus, USA) was performed with SYBR® Premix Ex Taq™ (Tli RNase Plus) (Takara, Japan). Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control. PCR primers were as follows: TLR4: F:5′-TTCAGAACTTCAGTGGCTGGATTTA-3′,R:5′-GTCTCCACAGCCACCAGATTCTC-3′; TLR2: F: 5′-GTCTCCACAGCCACCAGATTCTC-3′, R: 5′-AGAGTCAGGTGATGGATGTCG-3′;GAPDH:F:5′-TGTGTCCGTCGTGGATCTGA-3′, R: 5′-TTGCTGTTGAAGTCGCAGGAG-3′.
The GenBank accession numbers of the primers are TLR4: (NM-021297.2 http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&id=118130391), TLR2: (NM-011905.3), GAPDH: (NM-144900.2 http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&id=340007435), species: Mus musculus (house mouse). Differences in expression of TLR-4 and TLR-2 between the control and treated cells were calculated by normalizing with GAPDH gene expression according to the following formula: 2–ΔΔCt = ((Ct gene of interest-Ct internal control) sample A-(Ct gene of interest-Ct internal control) sample B).
Flow cytometry analysis
BV2 microglia were stimulated with LPS (0.5 μg/mL) in the presence or absence of SY (80 μg/mL) for 48 h, and stained for 20 min at room RT in 1% BSA-PBS buffer with the following antibodies: PE-CD206 (eBioscience, USA), PE-CD16/32 (eBioscience, USA), TLR-2 (Cell Signaling Technology, USA), and TLR-4 (Cell Signaling Technology). For intracellular staining, BV2 microglia were stained for 20 min at RT in 0.3% saponin/1% BSA-PBS buffer with the following antibodies: PE-IL-10 and PE-IL-12 (eBioscience, USA), Arginase-1 (Arg-1) (BD Biosciences, America), iNOS (Enzo Life Sciences, USA). For TLR-2, TLR-4, Arg-1, and iNOS staining, we adopted indirect fluorescence to label Arg-1 and iNOS with the fluorescence second antibodies Alexa Fluor 488 (Invitrogen Corporation, USA) and Alexa Fluor 555 (Invitrogen Corporation, USA). Cells were gated by using forward and sideward scatter characteristics for monocytes/macrophages, and at least 10,000 gated events were collected using a flow cytometer (BD Biosciences, USA). Data were analyzed with CellQuest software. Results are expressed as mean fluorescence intensity.
Immunofluorescent staining
A slide coated with Poly L-lysine (0.1 mg/mL) was put in a 24-well plate. After BV2 microglia were cultured and treated based on experimental requirements, BV2 microglia were fixed with 4% paraformaldehyde for 30 min and stained with the following antibodies: anti-CD16/32 (1:1000), anti-CD206 (1:1000), anti-iNOS (1:1000), anti-Arg-1 (1:1000), anti-IL-12 (1:1000), and anti-IL-10 (1:1000), and kept overnight at 4°C. The next day, Alexa Fluor 488-conjugated secondary antibodies (1:1000; Invitrogen) or Alexa Fluor 555-conjugated secondary antibodies (1:1000; Invitrogen) were added at RT for 2 h. As a negative control, additional slides were treated similarly, but primary antibodies were omitted. The stained slides were examined with a fluorescence microscope (Olympus, Japan).
Statistical analyses
The data were statistically analyzed with statistical software SPSS13.0 by one-way ANOVA followed by post-hoc Tukey’s test. A statistically significant difference was assumed at P <0.05.
Result
Effect of SY on cell viability stimulated with LPS
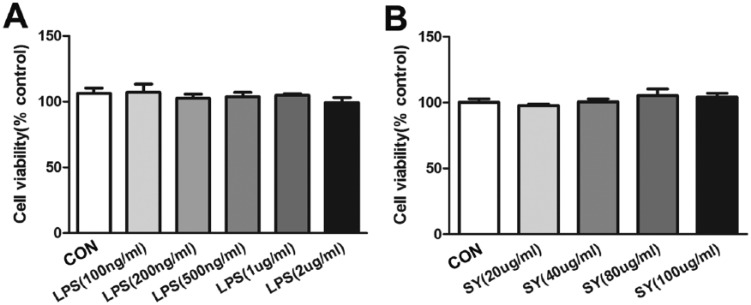
The viability of BV2 microglia under different LPS and SY conditions was tested using the MTT method. Based on our pilot results, LPS (0.5 μg/mL) and SY (80 μg/mL) were used in the following experiments. As shown in Figure 1a, the viability of BV2 microglia did not exhibit statistical difference (P >0.05) when stimulated with LPS at different concentrations (100, 200, 500, 1000, and 2000 ng/mL), indicating that LPS stimulation alone had no significant influence on the viability of BV2 microglia. The viability of BV2 microglia was not affected by the addition of different SY concentrations (20, 40, 80, and 100 µg/mL) either (Figure 1b), suggesting that SY also did not induce BV2 microglial cell death.
Figure 1.
Effect of SY on cell viability stimulated with LPS. BV2 microglia were treated with different concentrations of LPS (a) and SY (b), and cell viability was measured by MTT assay.
Effect of SY on LPS-induced TLRs/ Myd88 signaling in BV2 microglia
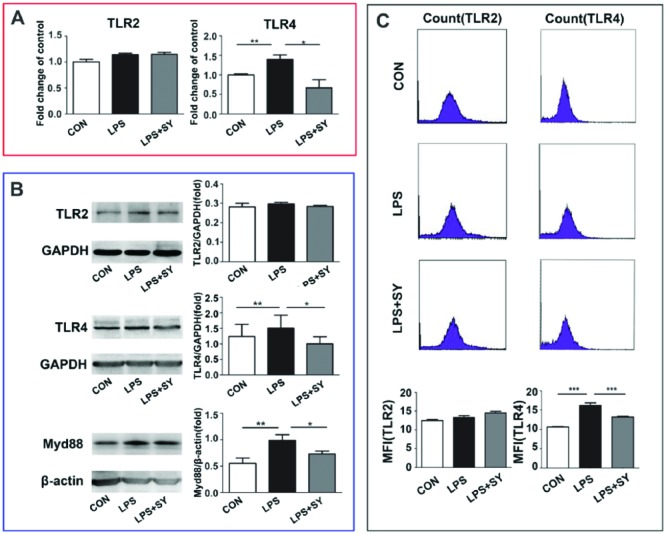
TLRs play key roles in the innate defense mechanism against pathogens and the development of adaptive immunity, and are possibly the major determinants of the susceptibility to inflammation and infection, through MyD88-dependent and independent signaling pathways. Compared with control group, LPS stimulation resulted in upregulation of TLR-4 at both mRNA and protein levels, as determined by RT-PCR, western blot, and flow cytometry assays (P <0.01–0.001, Figure 2) and increase of MyD88 at protein by western blot(P <0.01, Figure 2), suggesting LPS-induced TLR-4-MyD88 activation. On the contrary, SY treatment effectively inhibited the TLR-4 and MyD88 expression at levels of mRNA and/or protein (P <0.05–0.001), revealing a suppressive role of SY in TLR-4-MyD88 signaling in BV2 microglia. However, no influence was observed by LPS stimulation or SY treatment on TLR-2 expression (Figure 2).
Figure 2.
Effect of SY on LPS-induced TLRs/ Myd88 signaling in BV2 microglia. BV2 microglia were treated with LPS (0.5 μg/mL) or LPS (0.5 μg/mL) plus SY (80 μg/mL) for 48 h. Levels of TLR2, TLR4, and Myd88 were measured by RT-PCR (a), western blot (b), and flow cytometry (c). Quantitative results are expressed as mean ± SEM from three independent experiments with similar results (in triplicate/each experiment). *P <0.05, **P <0.01, ***P <0.001.
Influence of SY on LPS-induced NF-κB/MAPK activation in BV2 microglia
Given that MyD88 signaling mediates NF-κB and MAPK pathway, we next determined the activation of NF-κB/MAPK pathway by western blot. As shown in Figure 3, LPS stimulation resulted in the activation of NF-κB (P <0.01) and the phosphorization of P-38 (P <0.05) and JNK (P <0.001), and SY treatment effectively inhibited the expression of p-NF-κB(P65) (Figure 3a, P <0.05), and P-38 (Figure 3b, P <0.05). These results indicate that SY treatment inhibited the LPS-induced activation of NF-κB/P-38 signaling pathway in BV2 microglia in vitro. Simultaneously, LPS stimulation or SY treatment did not influence ERK activity, and SY did not suppress LPS-induced JNK activity (Figure 3b).
Figure 3.
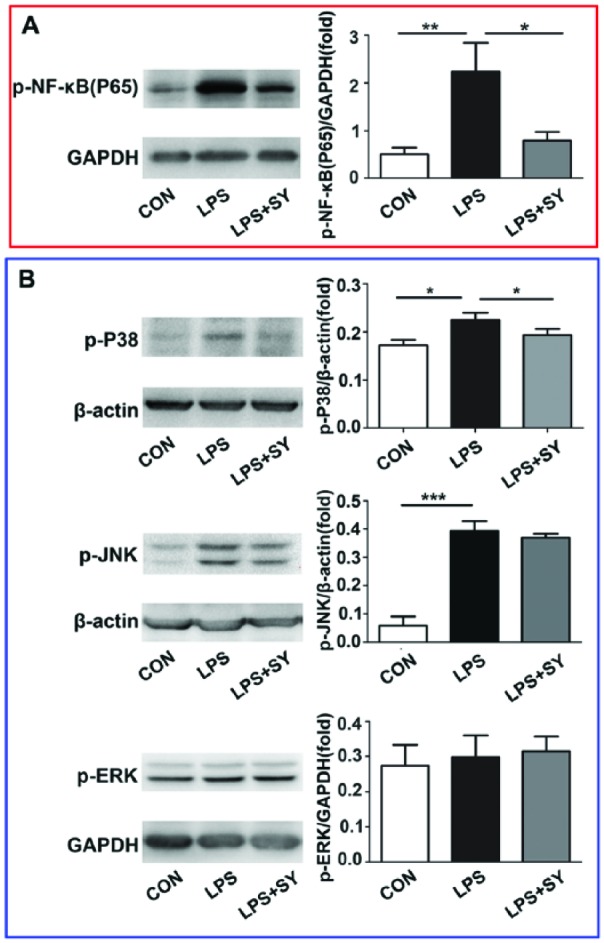
Influence of SY on LPS-induced NF-κB/MAPK activation in BV2 microglia. BV2 microglia were treated with LPS (0.5 μg/mL) or LPS (0.5 μg/mL) plus SY (80 μg/mL) for 48 h. Protein levels of p-NF-κB/65 (a), p-P38 (b), p-JNK (b), and p-ERK (b) were measured by western blot assay. Quantitative results are expressed as mean ± SEM from three independent experiments with similar results (in triplicate/each experiment). *P <0.05, **P <0.01, ***P <0.001.
Influence of SY on LPS-induced BV2 microglial polarization
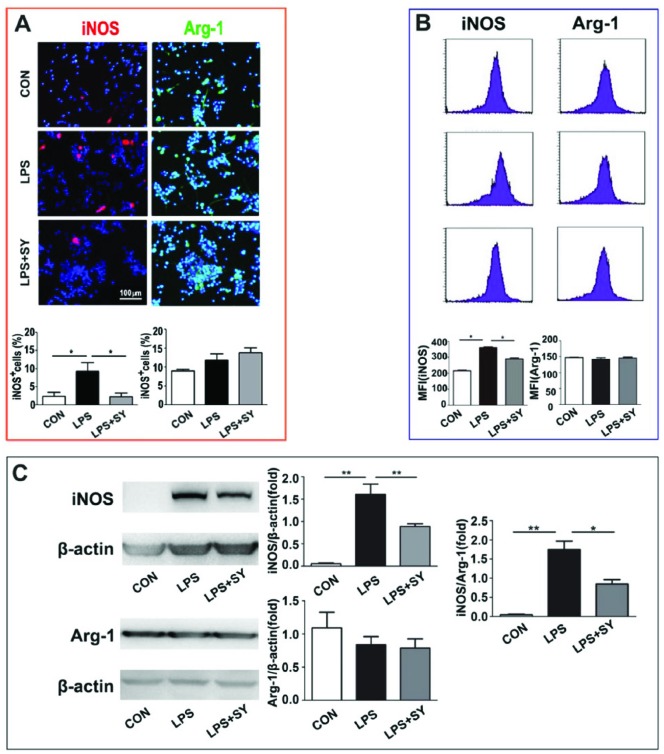
To further ascertain whether SY can trigger the polarization of inflammatory BV2 microglia toward anti-inflammatory M2 cells under LPS stimulation, we measured the expression of iNOS, CD16/32, IL-12, and Arg-1, CD206, IL-10, which are representatives of M1 and M2 microglia/macrophage. LPS induced iNOS expression in BV2 cells as shown by immunocytochemistry staining (Figure 4a, P <0.05), flow cytometry (Figure 4b, P <0.05), and western blot (Figure 4c, P <0.01), but did not significantly affect Arg-1 expression (Figure 4). However, the treatment of SY effectively inhibited the expression of iNOS (Figure 4; P <0.05) and the ratio of iNOS/Arg-1 (Figure 4c, P <0.01).
Figure 4.
Influence of SY on LPS-induced iNOS and Arg-1 expression in BV2 microglia. BV2 microglia were treated with LPS (0.5 μg/mL) or LPS (0.5 μg/mL) plus SY (80 μg/mL) for 48 h. Protein levels of iNOS (M1) and Arg-1 (M2) were measured by immunocytochemistry staining (a), flow cytometry (b), and western blot assay (c). Quantitative results are expressed as mean ± SEM from three independent experiments with similar results (in triplicate/each experiment). *P <0.05, **P <0.01.
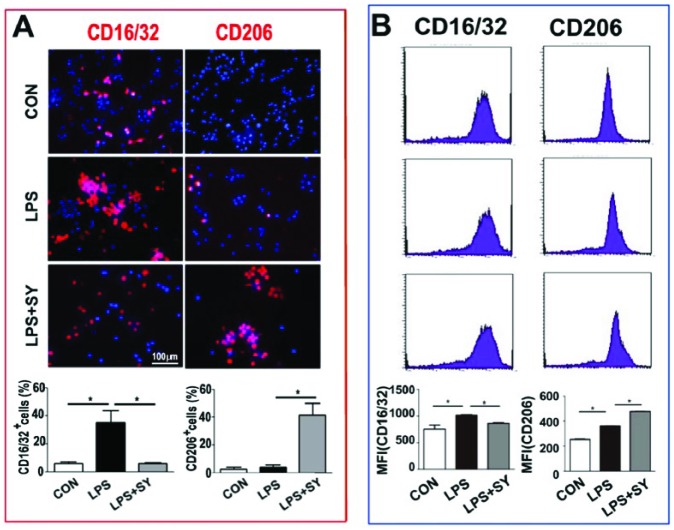
Similarly, LPS stimulated CD16/32 expression in BV2 cells (P <0.05), but slightly enhanced CD206 expression (P <0.05). SY treatment effectively inhibited CD16/32 expression, and further increased LPS-induced CD206 expression in BV2 cells (Figure 5a, b, both P <0.05).
Figure 5.
Influence of SY on LPS-induced the expression of CD16/32 and CD206 protein on BV2 microglia treated by LPS. BV2 microglia were treated with LPS (0.5 μg/mL) or LPS (0.5 μg/mL) plus SY (80 μg/mL) for 48 h. Protein levels of CD16/32 (M1) and CD206 (M2) were measured by immunocytochemistry staining (a) and flow cytometry (b). Quantitative results are expressed as mean ± SEM from three independent experiments with similar results (in triplicate/each experiment). *P <0.05.
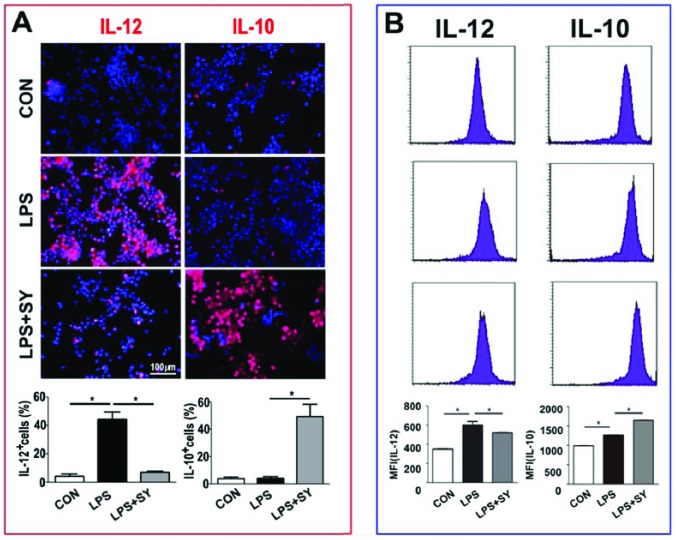
Further, while LPS induced obviously IL-12 production of BV2 cells (P <0.05), SY treatment effectively suppressed its production but elevated the production of IL-10 (Figure 6, both P <0.05). Taken together, these results clearly demonstrate that SY treatment converted LPS-induced inflammatory M1 microglia toward anti-inflammatory M2 cells.
Figure 6.
Influence of SY on LPS-induced IL-12 and IL-10 production in BV2 microglia. BV2 microglia were treated with LPS (0.5 μg/mL) or LPS (0.5 μg/mL) plus SY (80 μg/mL) for 48 h. Production of IL-12 (M1) and IL-10 (M2) was measured by immunocytochemistry staining (a) and flow cytometry (b). Quantitative results are expressed as mean ± SEM from three independent experiments with similar results (in triplicate/each experiment). *P <0.05.
Influence of SY on LPS-induced production of inflammatory factors and NO by BV2 microglia
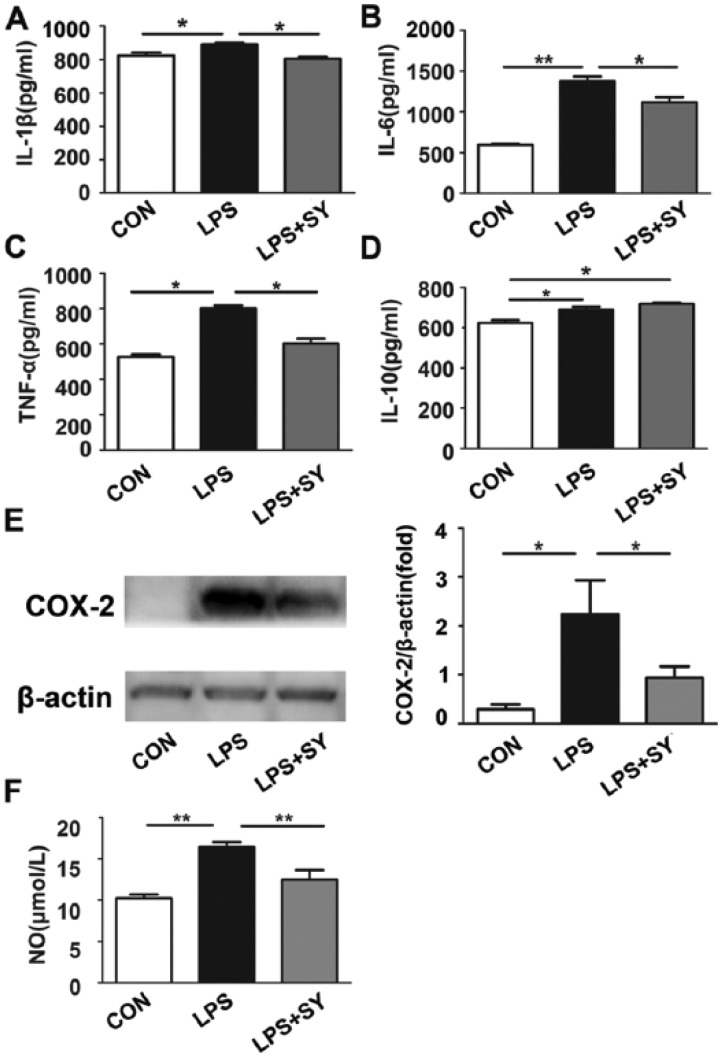
TLR-activated signaling pathway on microglia has been linked to neuroinflammation that is known to increase the risk for the development and promotion of neurodegeneration and neuron damage. We thus further measured the production levels of inflammatory factors IL-1, IL-6, TNF-α, COX-2, and NO in BV2 microglia. As shown in Figure 7a–e, LPS stimulated BV2 cells to produce IL-1β(P <0.05), IL-6 (P <0.01), TNF-α (P <0.05), and COX-2 (P <0.05), and the production of these molecules was significantly inhibited by treatment (all P <0.05). Further, LPS stimulated BV-2 cells to produce NO, while SY significantly inhibited this production (Figure 7f; P <0.01).
Figure 7.
Influence of SY on LPS-induced inflammatory factors and NO release by BV2 microglia. BV2 microglia were treated with LPS (0.5 μg/mL) or LPS (0.5 μg/mL) plus SY (80 μg/mL) for 48 h. The levels of IL-1β, IL-6, TNF-α, and IL-10 in culture supernatants were measured by ELISA assay (a–d), COX-2 expression was determined by western blot assay (e), and NO generation was evaluated by the Griess reaction (f). Quantitative results are expressed as mean ± SEM from three independent experiments with similar results (in triplicate/each experiment). *P <0.05, **P <0.01, ***P <0.001.
Discussion
Accumulated evidence shows that neuroinflammation is involved in the pathogenesis of a number of neurodegenerative diseases and cerebrovascular diseases including PD, AD, MS, and stroke.20,21 In the process of inflammatory response, M1 and M2 macrophages/microglia dynamically regulate the immune response and inflammatory microenvironment. M1 microglia can secrete a large number of inflammatory mediators and ingest dead cells and other debris. When the production of inflammatory mediators is not well controlled, however, excessive inflammatory response will result in tissue damage. On the other hand, M2 microglia can inhibit the inflammatory response in damaged area and reduce the release of inflammatory factors, possessing the function of neuroprotection and restoration.22–24
Defining the molecular mechanism for macrophage/microglial polarization could result in the identification of novel opportunities for manipulating immune and inflammatory responses. Many factors contribute to the diversity of macrophage/microglial functions, including the synergistic or antagonistic effects of different cells, cytokines, chemokines, protein kinases, hormones, TLR ligands, complements, and other endogenous molecules.6,25,26 In this study, we observed that SY, the main active component of the traditional Chinese medicine safflower, effectively inhibited LPS-mediated inflammatory signaling and factors in microglia, including TLR-4/NF-κB, NF-κB/MAPK pathways and COX-2, IL-1β, IL-6, and TNF-α. At the same time, HSYA treatment is able to induce the conversion of microglia from inflammatory M1 to an anti-inflammatory M2 phenotype.
LPS, an inducer of M1 microglia,27 especially acts on TLRs of cells, which is able to stimulate the activation of NF-κB/MAPK pathway by their downstream molecule MyD88. This signaling pathway is also required for immune protection and inflammatory responses during many infections, which might be lethal in the absence of MyD88.28 In seven randomized controlled trials with 762 participants, SY seems to be effective and safe in the treatment of acute ischemic stroke.29 In a series of experimental studies, SY enhanced Bcl-2 expression and inhibited Bax and caspase-3 activation, thus attenuating neuronal apoptosis and spinal cord ischemia reperfusion injury16 and protected PC12 cells from H2O2-induced injury and apoptosis.30 SY also inhibited neuroinflammation which was mediated by Aβ1-4214 and oxygen-glucose deprivation in BV-2 cells.18 The mechanisms of SY action include the phosphorylation of JAK2/STAT3 pathway,14 the upregulation of Bcl-2/p22 (phox) expression15 as well as the inhibition of oxidative stress/apoptosis and NF-κB signaling pathway.18,31 On the other hand, MAPKs comprise a group of serine and threonine signaling kinases that transduce a variety of extracellular stimuli through a cascade of protein phosphorylation, leading to activation of transcription factors. Among MAPKs, ERK pathway is linked to cellular proliferation and differentiation while p-JNK and p-P38 pathways are linked to inflammation and apoptosis.32 In the current study, we observed the SY significantly reduced p-38, but not p-ERK, expression, indicating a p-38/p-JNK-dependent and ERK-independent signaling pathway for NF-κB inhibition by SY.
In conclusion, our results demonstrate that SY exerts an anti-inflammatory effect on BV2 microglia, possibly through TLR-4/p-38/p-JNK/NF-κB signaling pathways and the conversion of microglia from inflammatory M1 to an anti-inflammatory M2 phenotype.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by the 2011 Cultivation Project of Shanxi University of Traditional Chinese Medicine (No. 2011PY-1), Research Project Supported by Shanxi Scholarship Council of China (2014-7) and Doctoral Scientific Research Funds of Shanxi Datong University (No. 2008-b-21). We thank Dr. GX Zhang and Ms. Katherine Regan at Department of Neurology, Thomas Jefferson University, Philadelphia, PA, USA, for their editorial assistance.
References
- 1. Perry VH, Nicoll JA, Holmes C. (2010) Microglia in neurodegenerative disease. Nature Reviews Neurology 6: 193–201. [DOI] [PubMed] [Google Scholar]
- 2. Polazzi E, Monti B. (2010) Microglia and neuroprotection: From in vitro studies to therapeutic applications. Progress in Neurobiology 92: 293–315. [DOI] [PubMed] [Google Scholar]
- 3. Sudduth TL, Schmitt FA, Nelson PT, et al. (2013) Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiology of Aging 34: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blandini F. (2013) Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. Journal of Neuroimmune Pharmacology 8: 189–201. [DOI] [PubMed] [Google Scholar]
- 5. Cao L, He C. (2013) Polarization of macrophages and microglia in inflammatory demyelination. Neuroscience Bulletin 29: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin Q, Cheng J, Liu Y, et al. (2014) Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain, Behavior, and Immunity 40: 131–142. [DOI] [PubMed] [Google Scholar]
- 7. Zhang HF, Li YH, Yu JZ, et al. (2013) Rho kinase inhibitor fasudil regulates microglia polarization and function. Neuroimmunomodulation 20: 313–322. [DOI] [PubMed] [Google Scholar]
- 8. Lehnardt S, Massillon L, Follett P, et al. (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proceedings of the National Academy of Sciences of the United States of America 100: 8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao L, Kan EM, Lu J, et al. (2013) Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: Role of TLR4 in hypoxic microglia. Journal of Neuroinflammation 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pradillo JM, Fernández-López D, García-Yébenes I, et al. (2009) Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. Journal of Neurochemistry 109: 287–294. [DOI] [PubMed] [Google Scholar]
- 11. Kilic U, Kilic E, Matter CM, et al. (2008) TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiology of Disease 31: 33–40. [DOI] [PubMed] [Google Scholar]
- 12. Li XQ, Wang J, Fang B, et al. (2014) Intrathecal antagonism of microglial TLR4 reduces inflammatory damage to blood-spinal cord barrier following ischemia/reperfusion injury in rats. Molecular Brain 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asgarpanah J, Kazemivash N. (2013) Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chinese Journal of Integrative Medicine 19: 153–159. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Z, Wu Z, Zhu X, et al. (2014) Hydroxy-safflor yellow A inhibits neuroinflammation mediated by Aβ1–42 in BV-2 cells. Neuroscience Letters 562: 39–44. [DOI] [PubMed] [Google Scholar]
- 15. Wang C, He Y, Yang M, et al. (2013) Safflor yellow B suppresses angiotensin II-mediated human umbilical vein cell injury via regulation of Bcl-2/p22(phox) expression. Toxicology and Applied Pharmacology 273: 59–67. [DOI] [PubMed] [Google Scholar]
- 16. Zhou D, Liu B, Xiao X, et al. (2013) The effect of safflower yellow on spinal cord ischemia reperfusion injury in rabbits. Oxidative Medicine and Cellular Longevity 2013: 692302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang C, Zhang D, Li G, et al. (2007) Neuroprotective effects of safflor yellow B on brain ischemic injury. Experimental Brain Research 177: 533–539. [DOI] [PubMed] [Google Scholar]
- 18. Li J, Zhang S, Lu M, et al. (2013) Hydroxysafflor yellow A suppresses inflammatory responses of BV2 microglia after oxygen-glucose deprivation. Neuroscience Letters 535: 51–56. [DOI] [PubMed] [Google Scholar]
- 19. Song L, Zhu Y, Jin M, et al. (2013) Hydroxysafflor yellow a inhibits lipopolysaccharide-induced inflammatory signal transduction in human alveolar epithelial A549 cells. Fitoterapia 84: 107–114. [DOI] [PubMed] [Google Scholar]
- 20. Jha MK, Jeon S, Suk K. (2012) Glia as a link between neuroinflammation and neuropathic pain. Immune Network 12: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Polazzi E, Contestabile A. (2002) Reciprocal interactions between microglia and neurons: From survival to neuropathology. Reviews in the Neurosciences 13: 221–242. [DOI] [PubMed] [Google Scholar]
- 22. Hu X, Li P, Guo Y, et al. (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43: 3063–3070. [DOI] [PubMed] [Google Scholar]
- 23. Wang G, Zhang J, Hu X. (2013) Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism 33: 1864–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boche D, Perry VH, Nicoll JA. (2013) Review: Activation patterns of microglia and their identification in the human brain. Neuropathology and Applied Neurobiology 39: 3–18. [DOI] [PubMed] [Google Scholar]
- 25. Pisanu A, Lecca D, Mulas G, et al. (2014) Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiology of Disease 71: 280–291. [DOI] [PubMed] [Google Scholar]
- 26. Liu CY, Li YH, Yu JZ, et al. (2013) Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS One 8: e54841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fenn AM, Henry CJ, Huang Y, et al. (2012) Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain, Behavior, and Immunity 26: 766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold-Schrauf C, Berod L, Sparwasser T. (2015) Dendritic cell specific targeting of MyD88 signalling pathways in vivo. European Journal of Immunology 45: 32–39. [DOI] [PubMed] [Google Scholar]
- 29. Fan S, Lin N, Shan G, et al. (2014) Safflower yellow for acute ischemic stroke: A systematic review of randomized controlled trials. Complementary Therapies in Medicine 22: 354–361. [DOI] [PubMed] [Google Scholar]
- 30. Wang C, Ma H, Zhang S, et al. (2009) Safflor yellow B suppresses pheochromocytoma cell (PC12) injury induced by oxidative stress via antioxidant system and Bcl-2 /Bax pathway. Naunyn-Schmiedeberg’s Archives of Pharmacology 380: 135–142. [DOI] [PubMed] [Google Scholar]
- 31. Duan JL, Wang JW, Guan Y, et al. (2013) Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacologica Sinica 34: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seo JH, Lim JW, Kim H. (2013) Differential role of ERK and p38 on NF- κ B activation in Helicobacter pylori-infected gastric epithelial cells. Journal of Cancer Prevention 18: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]