Abstract
The aim of this study was to investigate the relationship between HIF-1α and SNAIL gene expression in the epithelial ovarian cancer (EOC) cell line. EOC cells were treated with hypoxia, hypoxia combined with rapamycin, and control. The expression of HIF-1α and E-cad were assessed by reverse transcription–polymerase chain reaction (RT-PCR) and western blotting. The gene expression of SNAIL was studied by RT-PCR and real-time PCR. RNA interference technology was used to determine the relationship between HIF-1α and SNAIL. The present study indicated that the HIF-1α protein was expressed and increased in EOC cell line. SNAIL mRNA was found to increase and E-cad expression decreased with the time of hypoxia prolonged. Hypoxia increased invasion abilities of EOC cell line, but compared with cells exposed to hypoxia, the change of invasive ability of cells with rapamycin had no effect. The expression of HIF-1α protein and SNAIL mRNA could be inhibited gradually by rapamycin. siRNA of HIF-1α could suppress the expression of SNAIL while siRNA of SNAIL had no influence on HIF-1α protein expression. HIF-1α may be the upstream of the SNAIL gene in EOC. Our data suggested that HIF-1α might be an upregulator of the SNAIL gene and HIF-1α-SNAIL-E-cad pathway may play an important role in EOC invasion and metastasis.
Keywords: epithelial ovarian cancer, hypoxia-inducible factor 1α, SNAIL gene
Ovarian cancer is the leading type of gynecological tumors. Despite reductive surgical and chemotherapeutic intervention, the recurrences followed by chemotherapy resistance lead to significant morbidity and mortality. The 5-year survival of patients with advanced ovarian cancer remains poor. The majority of ovarian cancers are epithelial ovarian cancer (EOC).
It has been validated that solid tumors are mostly located in a hypoxia environment. Intratumoral hypoxia is related to an increased risk of metastasis and invasion in human cancer. This has been shown in carcinoma of the breast, pancreas, cervix, and endometrial carcinomas.1–4 Hypoxia-inducible factor 1α (HIF-1α), a key regulator of the cellular response to hypoxia, can be detected in many malignant tumors.5 In normal ovarian tissue and benign tumors, the HIF-1α expression rate is notably lower than in borderline and invasive tumors.6 It can also be observed that overexpression of HIF-1α in ovarian carcinoma and the level of HIF-1α are correlated with the stage of tumor. There is a significant increase of HIF-1α in stage III and IV ovarian epithelial tumors.7,8 Increased levels of HIF-1α protein in cancer cell lines are correlated with increased metastasis potential.9
The SNAIL gene, a direct inhibitor of E-cadherin (E-cad), acted as a master regulator of the epithelial mesenchymal transition (EMT).10,11 Overexpression of the SNAIL gene has been studied in many tumors including hepatocellular carcinoma, squamous cell carcinoma, breast cancer, renal carcinoma, and ovarian cancer.12–17 All these works showed that the SNAIL gene is an important transcription factor in the process of metastasis and invasion. Hypoxia is an important factor that can induce SNAIL in many human cancers, as well as other EMT regulators (TWIST, slug, smads, ZEB) and also lead to the interaction among these regulators.18–21 Although it has been reported that hypoxia may reduce the expression of E-cad and upregulate the expression of the SNAIL gene in ovarian carcinoma and later initiate detachment of the ovarian cancer cells from the primary lesion,20 the signal transduction pathway of the SNAIL gene in ovarian carcinoma is still unclear. The relationship between HIF-1α and SNAIL are still to be explored.
In this study, we examined the expression of HIF-1α and SNAIL in SKOV3 and CAOV3, which cultured in ordinary condition or hypoxia with or without rapamycin. RNA interference was used to determine the relationship between HIF-1α and SNAIL.
Materials and methods
Materials
Rapamycin and cobalt chloride (CoCl2) was obtained from Sigma Chemical Co. (St. Louis, MO, USA). The human EOC cell line SKOV3 and CAOV3 used in this study was kindly provided by Dr. Yinhua Yu (Anderson Cancer Center, TX, USA). Cell culture supplies, RPMI-1640, were obtained from Gibco.
Cell culture
The human EOC cell lines SKOV3 and CAOV3 were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum under conditions at 37°C in a humidified atmosphere containing 5% CO2. For hypoxic treatment, cells were placed in a modular anaerobic chamber that was flushed for 15 min with a gas mixture consisting of 1% O2, 5% CO2, and 94% N2, sealed, and incubated at 37°C for 8 h, 16 h, or 24 h with or without 100 nM rapamycin.
Western blotting
Whole cell extracts were prepared as follows. Cells were washed and lysed in 8 M urea, 10% glycerol, 1% SDS, 10 mM Tris HCl (pH 6.8), protease inhibitors (F. Hoffmann-La Roche Ltd., Switzerland), followed by centrifugation 4°C for 30 min at 14,000 rpm in a micro centrifuge. The supernatants were collected and stored at −80°C until required. Equal amounts of protein (40 µg) were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes (Bio-Rad Laboratories). Immobilized proteins were blocked with 5% non-fat dry milk in phosphate-buffered saline (PBS) at 4°C overnight and then incubated for 2 h at room temperature with the anti-HIF-1α mouse monoclonal antibody Mab (1:500, Santa Cruz, CA, USA) or anti-E-cad mouse Mab (1:400, Zymed, South San Francisco, CA, USA) diluted in 5% milk/PBS. After incubation the membranes were washed three times with PBS/0.5% Tween 20 and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody diluted in 5% milk/PBS (1:2000). The immune complexes were visualized by enhanced chemiluminescence. Blots were stripped and incubated with mouse anti-human GAPDH Mab (1:5000, Kangchen Co. Ltd., PR China) to ensure equal amounts of protein in each lane.
Reverse transcription-polymerase chain reaction analyses (RT-PCR) and quantified real-time PCR
Total cellular RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized with RevertAidTM first strand cDNA synthesis kit (MBI Fermentas, Burlington, Canada). The primes for PCR of human HIF-1α, SNAIL, E-cad, GAPDH (SIBAS Biotech Development Ltd. Company, PR China) were as follows: E-cad forward primer 5’-GATGGGGTCTTGCTATGTTG-3’, reverse primer 5’-AACCACGGATCTTGTGTCAG-3’; HIF-1α forward primer 5’-TCCGATGGAAGCACTAGACA-3’, reverse primer 5’-TCAAAGCGACAGATAACACG-3’; SNAIL forward primer 5’-TCAGACGAGGACAGTGGGAAAG-3’, reverse primer 5’-GCTTGTGGAGCAGGGACATTC-3’; GAPDH forward primer 5’-ACCACAGTCCATGCCATCAC-3’, reverse primer 5’-CCACCACCCTGTTGCTGTAG-3’.
The PCR conditions for E-cad primer sets were as follows: hot start at 94°C for 5 min, 35 amplification cycles, each consisting of 94°C for 45 s, 58°C for 45 s, and 72°C for 55 s; and a final extension step at 72°C for 10 min. For HIF-1αand SNAIL primer sets were as follows: hot start at 94°C for 5 min, 30 amplification cycles, each consisting of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s; and a final extension step at 72°C for 10 min. The GAPDH mRNA was used as an internal standard. PCR products were separated on 2% agorose gel, stained with ethidium bromide, and visualized under UV light.
Real-time PCR was performed using DNA Engine OpticonTM sequence detector. The sequences of the forward primer, reverse primer, and TaqMan probe for human SNAIL were as follows: forward primer 5’-TCGGAAGCCTAACTACAGCG-3’, reverse primer 5’-GATGAGCATTGGCAGCGAG-3’, TaqMan probe 5’-TTACCTTCCAGCAGCCCTACGACC-3’.
Values were determined by reference to a standard curve generated by a serial dilution of cDNA and normalized by the levels of actin mRNA. Threshold cycle (CT) determination was automatically performed by the instrument for each reaction. The reaction conditions were 95°C for 5 min, and 40 complete cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s.
Transwell invasion assay
Cells cultured in medium with or without 100 nM rapamycin were harvested after 16 h and 24 h of being exposed to hypoxia. Cells cultured in normoxic were harvested after 16 h and 24 h and were used as the control group.
RNA interference
siRNA of HIF-1α was designed, in which target nucleotides 1521–1541 were synthesized and annealed (Genechem Co. Ltd., PR China). The siRNA of SNAIL duplex target nucleotides 1294–1312 of the SNAIL mRNA (NM005895) was designed and synthesized as follows. As a control for siRNA we used a corresponding random siRNA. siRNA of HIF-1α forward primer 5’-AGAGGUGGAUAUGUGUGGGdTdT-3’, reverse primer 5’-CCCACACAUAUCCACCUCUdTdT-3’; siRNA of SNAIL forward primer 5’-GGUGUGACUAACUAUGCAAdTdT-3’, reverse primer 5’-UUGCAUAGUUAGUCACACCdTdT-3’; siRNA of random forward primer 5’-UUCUCCGAACGUGUCACGUdTdT-3’, reverse primer 5’-ACGUGACACGUUCGGAGAAdTdT-3’.
siRNA was annealed at a final concentration of each 20 mM by heating at 90°C for 2 min in annealing buffer and transfection of siRNA was performed using Oligofectamin (Invitrogen).
Cells were plated onto 6-well plates. When grown to 50–60% confluence, they were washed with Opti-Mem I (Invitrogen, Carlsbad, CA, USA) to remove any residual serum and incubated with the oligonucleotide duplexes at a concentration of 100 nM in serum-free conditions for 4 h at 37°C. Serum was then added back to the wells, and cells were incubated for 24 h following an additional 24 h cultured hypoxia. Cells were then collected for western blotting to analyze the expression of HIF-1α and for real-time PCR to compare the expression of SNAIL mRNA.
Statistics
Each experiment was repeated three times. The results were presented as mean ± SD. Data were analyzed using SPSS 19.0 windows software. Differences were considered significant at P <0.05.
Results
HIF-1α related with increased expression of SNAIL and decreased of E-cad
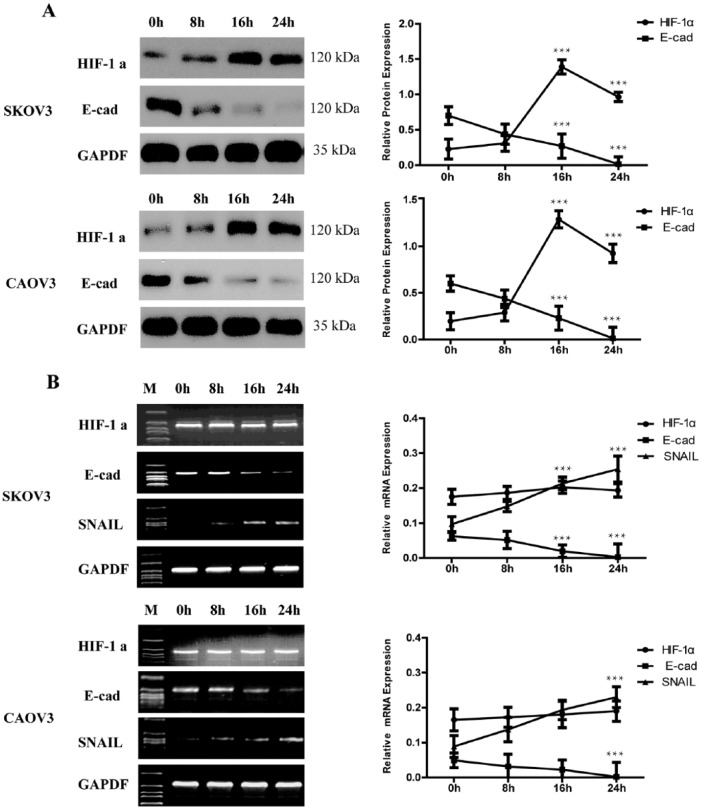
Cells cultured in medium were harvested after 8 h, 16 h, or 24 h exposed to hypoxia, and whole cell protein extracts or total RNA were prepared. Routine cultured cells were lysed at the same time and analyzed as control. Present study showed that routine cultured EOC cell line SKOV3 and CAOV3 have a relative low level of HIF-1α expression and increase in a significant level (more than six-fold, P <0.001) exposed to hypoxia for 16 h. After hypoxia 24 h, the expression of HIF-1α decreased a little compared with hypoxia 16 h, but were still more than four-fold to control (P <0.001) (Figure 1a). RT-PCR showed that there were no significant changes in HIF-1α mRNA expression levels (Figure 1b).
Figure 1.
HIF-1α related with increased expression of SNAIL and decreased of E-cad. (a) At different time after exposed to hypoxia, western blotting showed that the expression of HIF-1α protein can be detected in routine cultured SKOV3 and CAOV3 cells and the expression increased under hypoxia. While E-cad reduced accompany with the HIF-1α over express. GAPDH was used as the loading control. The errors reported represent the means ± SD. ***P <0.001. (b) The mRNA expression of HIF-1α, SNAIL, and E-cad by RT-PCR. During different hypoxia time, the level of HIF-1α showed no significant changes. SNAIL mRNA increased accompany with a decreased expression of E-cad with the hypoxia time prolonged. GAPDH was used as the loading control. The errors reported represent the means ± SD. ***P <0.001.
In addition, E-cad protein and mRNA expression was decreased gradually with time of hypoxia prolonged (Figure 1). It decreased 52% under hypoxia 16 h and 95% under hypoxia 24 h. The direct repressor of SNAIL mRNA was found to increase with the time of hypoxia prolonged by RT-PCR (Figure 1b) and real-time PCR (Tables 1 and 2). SNAIL mRNA copy is about three-fold to the control (normoxic cultured cells) (P <0.001). When cells cultured hypoxia 24 h, it reached about four-fold to the control (P <0.001) (Tables 1 and 2). As a direct repressor of E-cad, this model of expression is thought to be understandable. It suggested that SNAIL might be related to HIF-1α.
Table 1.
SNAIL mRNA was detected in SKOV3 without or with rapamycin by real-time PCR.
| Hypoxia | Copy of SNAIL |
|
|---|---|---|
| SKOV3 cultured without rapamycin | SKOV3 cultured with rapamycin | |
| 0 h | 1.350±0.107×106 | |
| 8 h | 1.831±0.121×106 | 1.812±0.378×106 |
| 16 h | 3.978±0.373×106 | 1.293±0.104×106 |
| 24 h | 5.681±0.65×106 *** | 0.607±0.107×106 ** |
At different times after being exposed to hypoxia, real-time PCR showed that the expression of SNAIL mRNA increased under hypoxia. Rapamycin could decrease the expression of SNAIL mRNA. The results were presented as mean ± SD. **P <0.01, ***P <0.001.
Table 2.
SNAIL mRNA was detected in CAOV3 without or with rapamycin by real-time PCR.
| Hypoxia | Copy of SNAIL |
|
|---|---|---|
| CAVO3 cultured without rapamycin | CAOV3 cultured with rapamycin | |
| 0 h | 1.220±0.107×106 | |
| 8 h | 1.771±0.121×106 | 1.635±0.378×106 |
| 16 h | 4.278±0.373×106 | 1.219±0.104×106 |
| 24 h | 5.289±0.65×106 *** | 0.591±0.107×106 ** |
At different times after being exposed to hypoxia, real-time PCR showed that the expression of SNAIL mRNA increased under hypoxia. Rapamycin could decrease the expression of SNAIL mRNA. The results were presented as mean ± SD. **P <0.01, ***P <0.001.
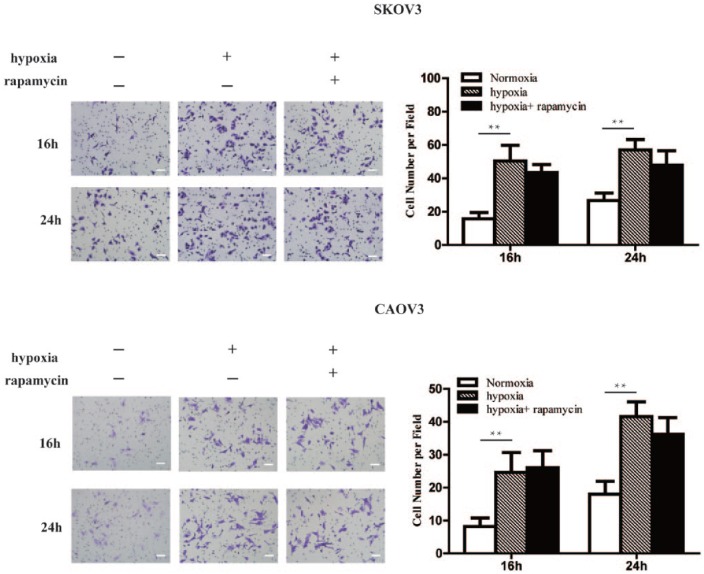
Hypoxia increased invasion abilities of EOC cell line
After transwell invasive cabin assay showed hypoxia, invasion abilities of SKOV3 and CAOV3 cells were significantly enhanced. Compared with the control group (normoxic cultured cells), the number of cells through the cell membrane increased significantly after 16 h and 24 h hypoxia. Compared with cells exposed to hypoxia, however, the change of invasive ability of cells with 16 h and 24 h rapamycin had no effect (Figure 2).
Figure 2.
Hypoxia increased invasion abilities of EOC cell line. Compared with the control group, the number of SKOV3 and CAOV3 cells through the cell membrane increased significantly after 16 h and 24 h hypoxia. But the change of invasive ability of cells with 16 h and 24 h rapamycin had no effect. Magnification, ×200. Scale bars, 100 μm. The errors reported represent the means ± SD. **P <0.01.
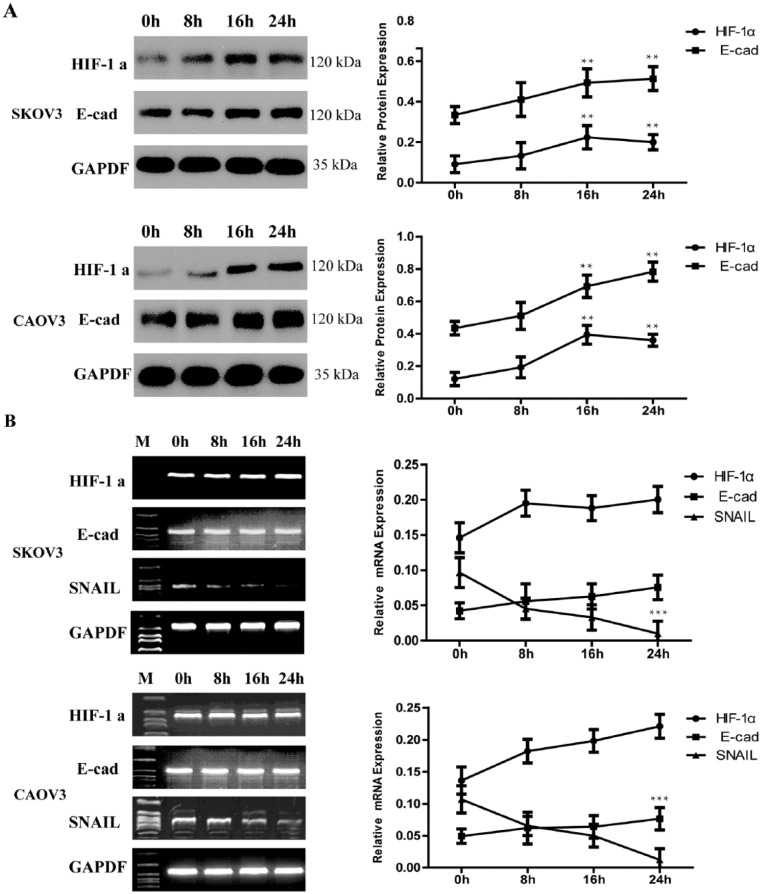
Rapamycin inhibits not only the expression of HIF-1α, but also the SNAIL gene
To elucidate the mechanism of the aberrant E-cad expression of EOC cells in hypoxia, rapamycin, which inhibits the expression of HIF-1α through target of the mammalian target of rapamycin (mTOR) and then interacts with the PI-3K pathway,22,23 was administrated to confirm if hypoxia induced E-cad was lost through the HIF-1α pathway. We found that rapamycin was able to blunt the upregulation effect of HIF-1α protein and maintain cells expressed E-cad in hypoxia. At the same time, we got another interesting finding that rapamycin had the ability to restrain SNAIL mRNA.
The protein expression of HIF-1α containing hypoxia 16 h and 24 h with rapamycin was about two-fold to control while those without rapamycin were about six-fold (P <0.001) and four-fold (P <0.001) each in EOC cells. E-cad protein was no more declined hypoxia with rapamycin in used (Figure 3a). Further analysis revealed that HIF-1α and E-cad mRNA expression in EOC cell with rapamycin have the same trend (Figure 3b). SNAIL mRNA was downregulated by rapamycin (Figure 3b).
Figure 3.
Rapamycin inhibits not only the expression of HIF-1α, but also the SNAIL gene. (a) Western blotting showed that rapamycin can inhibit the expression of HIF-1α and maintain the expression of E-cad hypoxia. GAPDH was used as the loading control. The errors reported represent the means ± SD. **P <0.01. (b) RT-PCR showed that rapamycin can inhibit the expression of HIF-1α and SNAIL, thus E-cad mRNA maintain a steady level. GAPDH was used as the loading control. The errors reported represent the means ± SD. ***P <0.001.
In order to examine the regulation of rapamycin on SNAIL, we determined mRNA levels by quantified real-time PCR. The expression of SNAIL mRNA was depressed significantly. As have been showed, the copy of SNAIL mRNA was decreased gradually with time of hypoxia prolonged and with rapamycin in used (P <0.01) (Tables 1 and 2). Thus it is suggested that the E-cad protein could be maintained by rapamycin through inhibit SNAIL pathway.
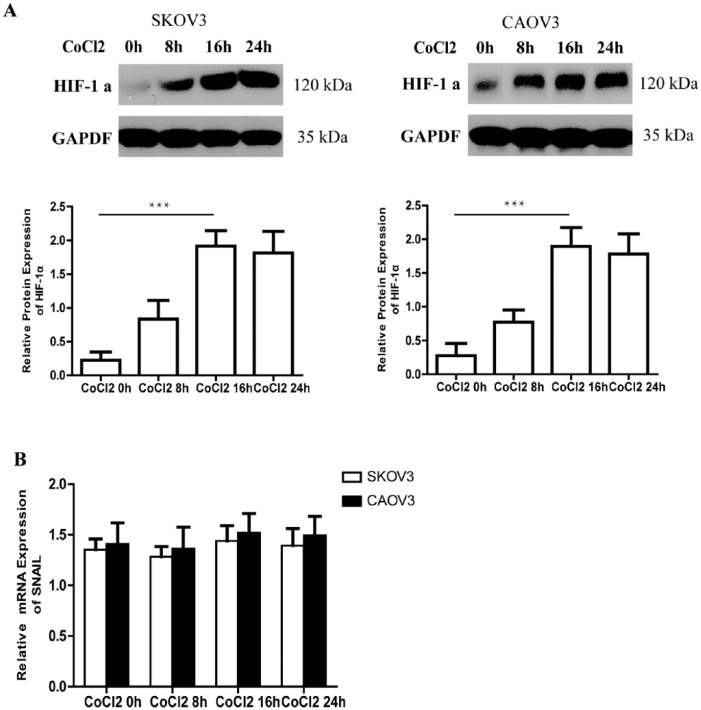
HIF-1α may be the upstream of SNAIL gene in EOC cell line
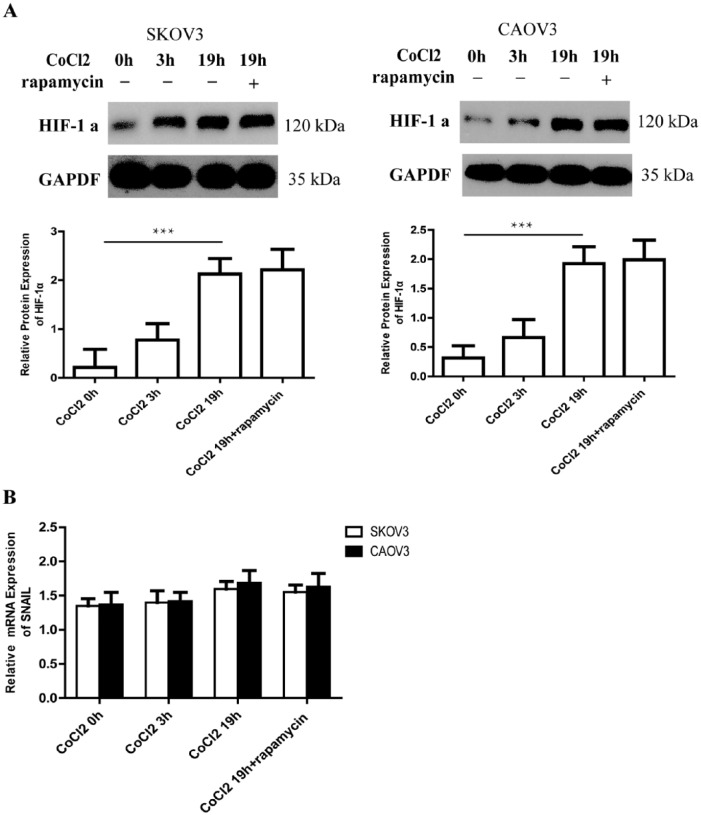
To investigate the relationship between HIF-1 α and SNAIL, SKOV3 and CAOV3 cells were exposed to 150 uM CoCl2 at different times. CoCl2 is a hypoxia mimic. We confirmed that CoCl2 might upregulate HIF-1α protein expression at a significant level after 0 h, 8 h, 16 h, and 24 h exposure (Figure 4a). But SNAIL mRNA expression had no change after exposed to CoCl2 at different times (Figure 4b). It seemed that CoCl2 has no effect on the expression of SNAIL.
Figure 4.
The expression of HIF-1α protein can upregulate and SNAIL mRNA expression had no change after exposed to CoCl2. (a) At different time after exposed to CoCl2, the expression of HIF-1α protein can be detected by western blotting in SKOV3 and CAOV3 cells. GAPDH was used as the loading control. The errors reported represent the means ± SD. ***P <0.001. (b) SNAIL mRNA expression is detected by real-time PCR after exposure to CoCl2 at different times. The errors reported represent the means ± SD. ***P <0.001.
We then overexpressed HIF-1α by having cells exposed to CoCl2 for 3 h before rapamycin was added and cultured for another 16 h. Western blotting proved that such overexpression of HIF-1α could not be inhibited by rapamycin (Figure 5a). Real-time PCR showed that the expression of SNAIL mRNA did not change after cells were exposed to CoCl2 for 3 h and 19 h and to CoCl2 for 3 h with rapamycin for another 16 h (Figure 5b). Our findings indicated that rapamycin lost the ability to restrain SNAIL mRNA in EOC cells with high levels of HIF-1α. Thus it was indicated that the inhibition of rapamycin on SNAIL might depend on HIF-1α.
Figure 5.
Overexpression of HIF-1α could not be inhibited by rapamycin and rapamycin lost the ability to restrain SNAIL mRNA with high level of HIF-1α. (a) Western blotting shows expression of HIF-1α culture in different medium. It upregulates the protein expression of HIF-1α after cells exposure to CoCl2 3 h and 19 h. A high level of protein expression is maintained despite rapamycin being added for another 16 h (CoCl2 3h+Rap16). GAPDH was used as the loading control. The errors reported represent the means ± SD. ***P <0.001. (b) SNAIL mRNA expression had no change after exposure to CoCl2 with or without rapamycin at different times. The errors reported represent the means ± SD. ***P <0.001.
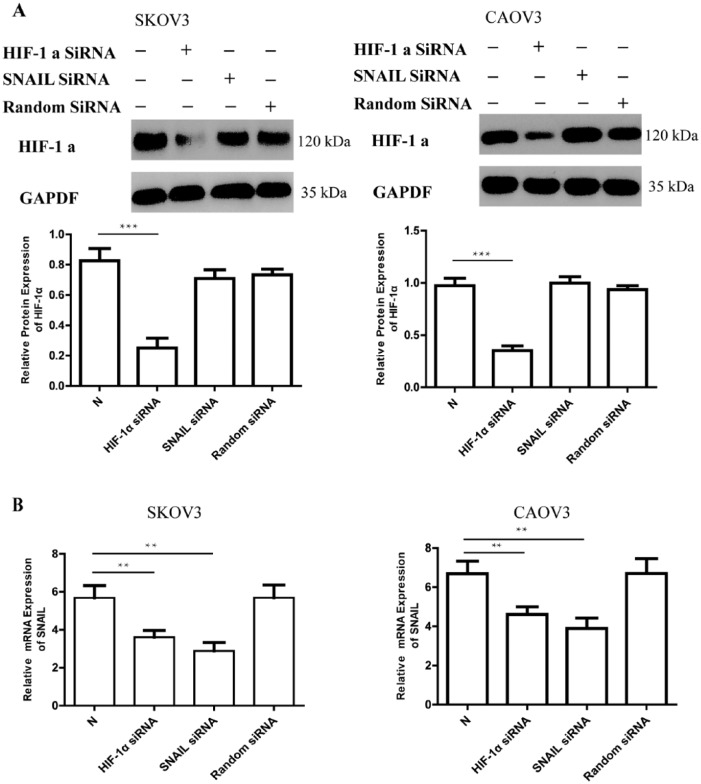
RNAi was used to confirm the above results. EOC cells were transfected with siRNA of HIF-1α or SNAIL gene for 24 h and then with cultured hypoxia for an additional 24 h. Protein and mRNA were prepared to detect the variety of HIF-1α and SNAIL, respectively. Compared with the control (hypoxia 24 h), HIF-1α siRNA can restrain about 65% of the increase expression of HIF-1α protein after hypoxia 24 h (Figure 6a). At the same time, SNAIL mRNA can be detected at a lower level than those of interference by HIF-1α siRNA (P <0.001) (Figure 6b).
Figure 6.
The expression of HIF-1α and SNAIL after RNA interference. (a) Western blotting shows the expression of HIF-1α after 48 h (20% O2 concentration 24 h and 1% O2 concentration 24 h). N: The expression of SNAIL in SKOV3 and CAOV3 with hypoxia 24 h. GAPDH was used as the loading control. The errors reported represent the means ± SD. ***P <0.001. (b) SNAIL mRNA expression can be detected by real-time PCR after different RNA interference (HIF-1α siRNA, SNAIL siRNA, and random siRNA). N: The expression of SNAIL in SKOV3 and CAOV3 with hypoxia 24 h. The errors reported represent the means ± SD. **P <0.01.
SNAIL siRNA could inhibit the expression of SNAIL mRNA. Compared with the Random siRNA, SNAIL mRNA expression in EOC interference by SNAIL siRNA was depressed after 24 h hypoxia cultured (P <0.01) (Figure 6b). Protein analysis showed that SNAIL siRNA had no influence on the expression of HIF-1α protein (Figure 6a).
Discussion
High expression of HIF-1α has been detected in many cancers. It can regulate the expression of more than 40 target genes.24 Such genes play important roles in angiogenesis, glycolysis, and erythropoiesis. They also play roles in tumor progression (cell survival, proliferation, invasion, and metastasis), thereby contributing to tumor aggressiveness, favor for further expansion, and metastasis.24,25 HIF-1α was found expressed in ovarian cancer and has tight connections to the tumor progression and prognosis.6–9 Previous studies have indicated that HIF-1α mRNA levels are constitutively expressed in a large number of human tissues human rodent cell lines. The expression of HIF-1α are thus unchanged upon treatment with hypoxia,26 suggesting that induction of functional HIF-1α activity could be regulated by a post-transcriptional mechanism. These results confirmed that the regulation of HIF-1α is through a protein degraded pathway, while HIF-1α mRNA are constitutively expressed and not significantly upregulated by hypoxia.27
Our study investigated for the first time that HIF-1α may be the regulator of the SNAIL gene in EOC. We have shown that rapamycin could inhibit not only the expression of HIF-1α protein, but also the expression of SNAIL mRNA. The data indicated that there might be some relationship between HIF-1α and SNAIL. The decrease of E-cad may result from the HIF-1α-SNAIL pathway. The expression of SNAIL mRNA could be blocked by siRNA of HIF-1α. This result could explain that epithelial ovarian cancer dissemination, progression, and metastasis might be promoted hypoxia by HIF-1α through the SNAIL pathway.
The SNAIL gene plays a critical role in the epithelial mesenchymal transition. Overexpression of the SNAIL gene resulted in the loss of E-cadherin expression, upregulation of vimentin gene expression, and change of their morphology to fibroblastic. SNAIL can also suppress desmoplakin, human mucin 1 (MUC1), cytokeratin 18, claudins and occludins, which are key proteins in the tight junctions, located at the most apical part of the epithelial junction complex.28–30 It has been reviewed that growth factors, such as fibroblast growth factor, epidermal growth factor, and TGF-beta, might induce SNAIL expression through different pathways.31 In our experiment, HIF-1α protein increased significantly under hypoxia accompany with SNAIL mRNA increasing. The expression of E-cad was decreased with the SNAIL gene overexpression. Such data indicated that the EMT induced by SNAIL might relate to the HIF-1α signal transduction pathway.
Aberrant expression of snail brings on changes in cells not only causing the loss of normal cell–cell contacts and acquisition of invasive growth inclination, but also enhancing the resistance to programmed cell death elicited by DNA damage in cancer cells.32 We had previously reported that rapamycin could repress the expression of HIF-1α associated with reduce ovarian tumor microvessel density, growth restraint, and promote tumor cell apoptosis.33 The present study showed that rapamycin, the inhibitor of HIF-1α, could also inhibit the upregulation of SNAIL mRNA. However, the inhibition of rapamycin on the SNAIL gene vanished when EOC cells expressed high levels of HIF-1α induced by CoCl2. The results suggested that the action of rapamycin on restraining SNAIL need the participation of HIF-1α. This was confirmed by RNAi. We observed that the expression of SNAIL mRNA was significant decline after treated with siRNA of HIF-1α, while the expression of HIF-1α could not be limited by siRNA of SNAIL. The expression of HIF-1α protein does not rely on the SNAIL gene. We confirmed that HIF-1α could act as a regulator of the expression of SNAIL. The inhibition of SNAIL expression by rapamycin was achieved through negative effect on HIF-1α. In another words, HIF-1α might be upstream of the SNAIL gene. Such results confirmed that the SNAIL gene might be a target gene of HIF-1α.
As we know, this is the first report about the relationship between the SNAIL gene and HIF-1α in EOC. It may explain that overexpression of HIF-1α might activate the SNAIL pathway and therefore inhibit the expression of adhesion proteins and trigger EMT.
In our experiment, CoCl2 alone has no influence on SNAIL expression although it is a stimulator of HIF-1α, giving us more space to discuss regulation of the SNAIL gene. Cell signal transduction exists as a model of network. The regulation of SNAIL through HIF-1α may require some other unknown factors and those factors are more like to be those sensitive to O2 concentration. Further research on the molecular mechanism should be done to develop novel molecular targeted therapies for EOC.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by the research fund of Shanghai Natural Science Foundation of China (15ZR1404700).
References
- 1. Vleugel MM, Greijer AE, Shvarts A, et al. (2005) Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. Journal of Clinical Pathology 58: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kitada T, Seki S, Sakaguchi H, et al. (2003) Clinicopathological significance of hypoxia-inducible factor-1alpha expression in human pancreatic carcinoma. Histopathology 43: 550–555. [DOI] [PubMed] [Google Scholar]
- 3. Birner P, Schindl M, Obermair A, et al. (2000) Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Research 60: 4693–4696. [PubMed] [Google Scholar]
- 4. Pansare V, Munkarah AR, Schimp V, et al. (2007) Increased expression of hypoxia-inducible factor 1alpha in type I and type II endometrial carcinomas. Modern Pathology 20: 35–43. [DOI] [PubMed] [Google Scholar]
- 5. Zhong H, De Marzo AM, Laughner E, et al. (1999) Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Research 59: 5830–5835. [PubMed] [Google Scholar]
- 6. Daponte A, Ioannou M, Mylonis I, et al. (2008) Prognostic significance of Hypoxia-Inducible Factor 1 alpha (HIF-1 alpha) expression in serous ovarian cancer: An immunohistochemical study. BMC Cancer 8: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyazawa M, Yasuda M, Fujita M, et al. (2009) Association of hypoxia-inducible factor-1 expression with histology in epithelial ovarian tumors: A quantitative analysis of HIF-1. Archives of Gynecology and Obstetrics 279: 789–796. [DOI] [PubMed] [Google Scholar]
- 8. Wong C, Wellman TL, Lounsbury KM. (2003) VEGF and HIF-1alpha expression are increased in advanced stages of epithelial ovarian cancer. Gynecologic Oncology 91: 513–517. [DOI] [PubMed] [Google Scholar]
- 9. Shimogai R, Kigawa J, Itamochi H, et al. (2008) Expression of hypoxia-inducible factor 1alpha gene affects the outcome in patients with ovarian cancer. International Journal of Gynecological Cancer 18: 499–505. [DOI] [PubMed] [Google Scholar]
- 10. Savagner P. (2001) Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays 23: 912–923. [DOI] [PubMed] [Google Scholar]
- 11. Peinado H, Ballestar E, Esteller M, et al. (2004) Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Molecular and Cellular Biology 24: 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanco MJ, Moreno-Bueno G, Sarrio D, et al. (2002) Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 21: 3241–3246. [DOI] [PubMed] [Google Scholar]
- 13. Kurrey NK, K A, Bapat SA. (2005) Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecologic Oncology 97: 155–165. [DOI] [PubMed] [Google Scholar]
- 14. Elloul S, Elstrand MB, Nesland JM, et al. (2005) Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 103: 1631–1643. [DOI] [PubMed] [Google Scholar]
- 15. Sugimachi K, Tanaka S, Kameyama T, et al. (2003) Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clinical Cancer Research 9: 2657–2664. [PubMed] [Google Scholar]
- 16. Grille SJ, Bellacosa A, Upson J, et al. (2003) The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Research 63: 2172–2178. [PubMed] [Google Scholar]
- 17. Peinado H, Olmeda D, Cano A. (2007) Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nature Reviews Cancer 7: 415–428. [DOI] [PubMed] [Google Scholar]
- 18. Bechtel W, Zeisberg M. (2009) Twist: a new link from hypoxia to fibrosis. Kidney International 75: 1255–1256. [DOI] [PubMed] [Google Scholar]
- 19. Katoh M, Katoh M. (2009) Integrative genomic analyses of ZEB2: Transcriptional regulation of ZEB2 based on SMADs, ETS1, HIF1alpha, POU/OCT, and NF-kappaB. International Journal of Oncology 34: 1737–1742. [DOI] [PubMed] [Google Scholar]
- 20. Imai T, Horiuchi A, Wang C, et al. (2003) Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. American Journal of Pathology 163: 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang MH, Wu KJ. (2008) TWIST activation by hypoxia inducible factor-1 (HIF-1): Implications in metastasis and development. Cell Cycle 7: 2090–2096. [DOI] [PubMed] [Google Scholar]
- 22. Sowter HM, Raval RR, Moore JW, et al. (2003) Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Research 63: 6130–6134. [PubMed] [Google Scholar]
- 23. Zhong H, Chiles K, Feldser D, et al. (2000) Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Research 60: 1541–1545. [PubMed] [Google Scholar]
- 24. Hudson CC, Liu M, Chiang GG, et al. (2002) Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Molecular and Cellular Biology 22: 7004–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeo EJ, Chun YS, Park JW. (2004) New anticancer strategies targeting HIF-1. Biochemical Pharmacology 68: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 26. Rankin EB, Giaccia AJ. (2008) The role of hypoxia-inducible factors in tumorigenesis. Cell Death and Differentiation 15: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gradin K, McGuire J, Wenger RH, et al. (1996) Functional interference between hypoxia and dioxin signal transduction pathways: Competition for recruitment of the Arnt transcription factor. Molecular and Cellular Biology 16: 5221–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang LE, Arany Z, Livingston DM, et al. (1996) Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. Journal of Biological Chemistry 271: 32253–32259. [DOI] [PubMed] [Google Scholar]
- 29. Guaita S, Puig I, Franci C, et al. (2002) Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. Journal of Biological Chemistry 277: 39209–39216. [DOI] [PubMed] [Google Scholar]
- 30. Ikenouchi J, Matsuda M, Furuse M, et al. (2003) Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. Journal of Cell Science 116: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 31. Cano A, Pérez-Moreno MA, Rodrigo I, et al. (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biology 2: 76–83. [DOI] [PubMed] [Google Scholar]
- 32. De Craene B, van Roy F, Berx G. (2005) Unraveling signalling cascades for the Snail family of transcription factors. Cellular Signalling 17: 535–547. [DOI] [PubMed] [Google Scholar]
- 33. Kajita M, McClinic KN, Wade PA. (2004) Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Molecular and Cellular Biology 24: 7559–7566. [DOI] [PMC free article] [PubMed] [Google Scholar]