Abstract
This study evaluated the serum concentrations of the main sex hormones in selected patients with non-bacterial male accessory gland infection (MAGI). The results suggest that the mean serum concentrations of 17β-estradiol (method : chemiluminescence) in these patients are significantly higher compared to the controls (55.0 ± 15.0 vs. 26.5 ± 12.0 pg/mL; P <0.05) and the percentage of patients with MAGI and associated hyperestrogenism (according to the laboratory range used in this study) was significantly higher (25.00% vs. 3.00%; P <0.05). Moreover, the percentage of patients with non-bacterial MAGI and associated testosterone deficiency (serum total testosterone <2.49 ng/mL) was significantly higher (18.00% vs. 2.00%; P <0.05). Finally, patients with non-bacterial MAGI showed a significantly lower total testosterone-17β-estradiol ratio compared to the controls (72.7 vs. 173.0; P <0.05). The results of this study, with some limitations (in particular the method applied for the determination of serum concentrations of 17β-estradiol) represent in our opinion, a topic worthy of further investigation for a correct endocrinological characterization of these patients, useful for clinical practice.
Keywords: 17β-estradiol, male accessory gland infection (MAGI), total testosterone
Introduction
Male accessory gland infection (MAGI) represents a pathological inflammatory condition of the prostate, seminal vesicles, and epididymis with possible negative consequences on the quality of sperm parameters.1 The prevalence of this condition varies in the different published studies, probably for the failure to apply the correct diagnostic algorithm proposed by the World Health Organization (WHO) in 1993.2–4 The potential mechanisms associated with the alteration of the sperm parameters are different: anatomical obstruction of the sperm ducts, oxidative stress, the presence of microorganisms.5,6 From an ultrasonographic point of view, we reported an important classification between uncomplicated (prostatitis) and complicated forms (prostato vesiculitis and prostato vesciculo epididymitis) with different consequences on the quality of the sperm parameters (lower in the second group).7 Another aspect of this condition scarcely evaluated is represented by the hormonal characterization of these patients. This topic is worthy of investigation for different reasons: (1) patients with testosterone (T) deficiency have a higher extension of inflammatory alterations;8 (2) the higher prevalence of MAGI regards men in fertile age, a time of life that requires a normal hormonal balance for reproductive aims;2–4 (3) experimental evidence of prostatic inflammation associated with alteration of the serum concentrations of circulating androgens and estrogens.9 Based on these premises, this study evaluated the hormonal characterization of patients with MAGI in fertile age, assessing in particular the frequency of conditions of excess or deficiency of the main sex hormones according to laboratory range used in the our clinical practice.
Materials and Methods
The protocol was approved by the internal Institutional Review Board and informed written consent was obtained from each patient.
Patients
Among the 1302 patients consecutively evaluated during 2013–2015 (3 years) for male infertility at the Andrology Center of the University of Catania (http://www.andrologyacademy.net), 100 patients with MAGI2 were enrolled in the study. Healthy age-matched fertile men represent the control group (n = 20).
Exclusion criteria
Exclusion criteria included: obesity (body mass index >30 kg/m2), cryptorchidism, orchitis, primary tumor of the testis, varicocele, adrenal disease, liver disease, hyperthyroidism, regular use of soy with the diet, habitual consumption of alcohol,10 severe oligozoospermia (<5 million/mL), bacterial form of MAGI (negative microbiological assessment for Chlamydia trachomatis, Mycoplasma hominis, Ureaplasma urealyticum, Candida albicans, Trichomonas vaginalis, gram negative bacteria, and papillomavirus),11 and pharmacological treatment 6 months before enrollment in the study.
Hormonal measurements
Blood sampling was performed at 08:00, after at least 8 h of sleep. Determination of prolactin was repeated at a distance of 30 min. Luteinizing hormone (LH), follicle stimulating hormone (FSH), 17β-estradiol (E2), total testosterone (TT), and prolactin (PRL) evaluation was performed by electrochemiluminescence immunoassay (ECLIA) with Cobas equipment. Normal values were: LH, 1.7–8.6 mIU/mL (coefficient of variability (CV)% = 8); FSH, 1.5–12.4 mIU/mL (CV% = 6); E2, 25.8–60.7 pg/mL (CV% = 6); TT, 2.49–8.36 ng/mL (CV% = 7); PRL, 4.04–15.2 ng/mL (CV% = 8). Testosterone deficiency was defined as a serum TT 2.49 ng/mL, according to the range of laboratory used in this study. Serum TT:E2 ratio was calculated as testosterone in ng/mL divided by estradiol in ng/mL, although otherwise the estradiol level was expressed in pg/mL.
Sperm analysis
Semen samples were collected by masturbation into a sterile container after 2–7 days of sexual abstinence and were transported to the laboratory within 30 min after ejaculation. Each sample was evaluated according to the WHO criteria.12
Ultrasound evaluation
The ultrasound evaluation of the patients was performed according to the criteria previously published.7 The evaluation of testicular volume was calculated automatically by the ultrasound machine (GX Megas Esaote SpA, Genova, Italy) using the ellipsoid formula (length × width × thickness × 0.52).
Statistical analysis
Continuous variables are presented as means ± standard deviations and differences between groups were tested by Student’s independent t-test or Mann–Whitney U-test according to their normal or not-normal distribution, respectively (normality of variables’ distribution was tested by Kolmogorov–Smirnov test). Kruskal–Wallis H-test was performed to compare differences between more than two groups. Accordingly, Pearson’s or Spearman’s correlation coefficients were used in order to test the associations between the different variables. Multivariate linear regression models were performed for factors significantly correlated at Spearman’s analysis. All tests were completed using SPSS v.19 software (SPSS Inc., IBM Corp, Somers, NY, USA). For all statistical comparisons significance was considered as P <0.05.
Results
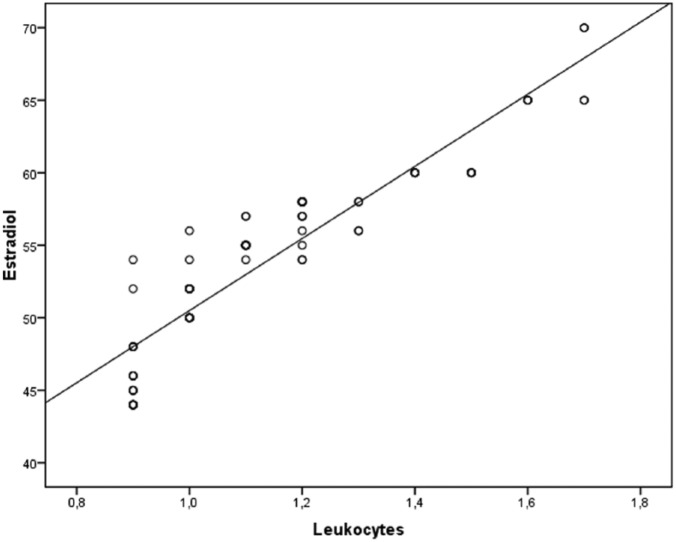
No significant differences among patients and controls regarding age (30.0 ± 4.0 vs. 34.0 ± 4.0 years), body mass index (22.0 ± 3.0 vs. 24.5 ± 5.0 kg/m2), and testicular volume (18.0 ± 3.0 vs. 20.0 ± 4.0 mL) were detected. Patients with MAGI showed serum concentrations of E2 significantly higher than the controls. The percentage of men with high serum concentrations of E2, according to our laboratory range was significantly higher among patients with MAGI. Moreover, the percentage of men with T deficiency was significantly higher among patients with MAGI (Table 1). Patients with MAGI showed a significant reduction of the main sperm parameters (concentration, morphology, progressive motility) compared to the controls; moreover, the concentration of leukocytes was significantly higher compared to the controls (Table 1). Patients with MAGI showed significantly lower value of TT:E2 ratio compared to the controls (Table 1). Patients with MAGI and concomitant excess of serum concentrations of E2 as well as patients with MAGI and T deficiency showed a significant reduction of the main sperm parameters compared to the patients with MAGI and normal serum concentrations of these two hormones. Moreover, these patients showed concentration of leukocytes significantly higher (Table 2). At linear regression analysis adjusted for age and BMI (Figure 1), increase of leucocytes was significantly associated with high 2-estradiol (r = 0.925, P <0.01) but not with TT (r = −0.006; P = 0.95).
Table 1.
Table 1 shows the main hormonal and sperm parameters of the patients and controls.
| MAGI | Controls | |
|---|---|---|
| Hormonal evaluation | ||
| Testosterone (ng/mL) | 4.0 ± 2.0 | 4.5 ± 2.1 |
| 17β-estradiol (pg/mL) | 55.0 ± 15.0* | 26.5 ± 12.0 |
| Testosterone-17β-estradiol ratio | 72.7* | 173.0 |
| Follicle stimulating hormone (mIU/mL) | 3.0 ± 0.6 | 2.5 ± 0.4 |
| Luteinizing hormone (mIU/mL) | 2.5 ± 0.4 | 2.2 ± 0.8 |
| Prolactin (ng/mL) | 8.0 ± 6.0 | 9.5 ± 8.0 |
| Testosterone deficiency (%) | 18.00* | 2.00 |
| 17β-estradiol excess (%) | 25.00* | 3.00 |
| Sperm parameters | ||
| Sperm concentrations (mil/mL) | 25.0 ± 11.0* | 50.0 ± 20.0 |
| Normal morphology (%) | 8.0 ± 4.0* | 14.0 ± 3.0 |
| Progressive motility (%) | 23.0 ± 6.0* | 39.0 ± 5.0 |
| Leukocytes (mil/mL) | 1.3 ± 0.4* | 0.6 ± 0.3 |
P <0.05.
Table 2.
Table 2 shows the main sperm parameters of patients with higher serum concentrations of E2 or lower serum concentrations of TT compared to the laboratory range used in this study.
| MAGI (with E2 excess) | MAGI (with normal E2 levels) | |
|---|---|---|
| Sperm parameters | ||
| Sperm concentrations (mil/mL) | 15.0 ± 2.0* | 29.0 ± 4.0 |
| Normal morphology (%) | 4.0 ± 2.0* | 8.0 ± 2.0 |
| Progressive motility (%) | 13.0 ± 2.0* | 27.0 ± 4.0 |
| Leukocytes (mil/mL) | 1.2 ± 0.4* | 0.7 ± 0.2 |
| MAGI (with T deficiency) | MAGI (with normal T levels) | |
| Sperm concentrations (mil/mL) | 18.0 ± 3.0* | 26.0 ± 4.0 |
| Normal morphology (%) | 7.0 ± 2.0* | 12.0 ± 2.0 |
| Progressive motility (%) | 17.0 ± 2.0* | 29.0 ± 3.0 |
| Leukocytes (mil/mL) | 1.1 ± 0.3* | 0.8 ± 0.3 |
P <0.05.
Figure 1.
Relationship between leukocytes (R2 = 0.925; P < 0.01) and 2-estradiol as derived from age and BMI adjusted regression analysis.
Discussion
The results of the study suggest that selected patients with non-bacterial MAGI, an important cause of male infertility,5 have serum E2 concentrations significantly higher than age-matched controls, and a significantly higher percentage of them have E2 values higher than the reference range used. Moreover, these patients have a serum TT:E2 ratio significantly lower than the controls, as well as, a significantly greater percentage of them have TT values lower than the reference range used. These results, although preliminary and affected by several limitations, represent in our opinion a topic which deserves further evaluation for potential important consequences on the clinical management of this category of infertile patients. The main limitations of the study are represented by the E2 assay method carried out through chemiluminescence that is not, as is well known, the reference method for this assessment, although it is the most used in the clinical practice.13 Several studies and the recent Endocrine Society Position Statement have addressed the deficiencies in current immunoassays used for the quantitation of estradiol.14 Direct immunoassays lack appropriate sensitivity at low estradiol levels (<40 pg/mL). Although a liquid chromatography tandem mass spectrometry estradiol assay is considered the gold standard, it is complex to use in a routine clinical laboratory and is not amenable to high test through put. Generally, serum concentrations of E2 in men are lower than detection limit of the method, although little information is available regarding the different aromatization of T to E2 in different tissues.14 Another limitation of the study is the lack of evaluation of the levels of sex hormone binding globulin (SHBG) that is a protein that binds tightly to the hormones T, dihydrotestosterone, and E2. In this bound state, SHBG transports these hormones in the blood as biologically inactive forms. Changes in SHBG levels can affect the amount of hormone that is available to be used by the body’s tissues.15
In our opinion, the main issues to be clarified are represented by the causes of the hyperestrogenism and the possible practical implications of this apparent hormonal feature. The increase of E2 in men can be caused and/or associated with different factors: testicular tumors, adrenal disease, hyperthyroidism, liver disease, intake of anti-androgen drugs, obesity, and hypogonadism. All these causes have been excluded at enrollment, among the exclusion criteria, with the exception of hypogonadism. As mentioned before, among the examined patients a significantly higher percentage had serum TT concentrations lower than the reference range used in the present study. These patients in particular show a reduction in the serum TT:E2 ratio than the controls. We previously showed that among patients with lower serum TT concentrations a higher frequency of MAGI and a greater anatomic extension of ultrasound signs of the inflammatory process with the involvement of the prostate, seminal vesicles, and epididymis are seen.8 The hypothesis is that the reduction of the serum TT:E2 ratio could be a risk factor for chronic inflammation, as demonstrated by previous experimental evidences. In particular, estrogens regulate their effects at the level of the target cells through interaction with estrogen receptors (ER), ER-α and ER-β, that like the AR are transcription factors. In general, activation of ER-α in the prostate is associated with hyperplasia and inflammation. ER-β is located in the epithelial cells and ER-α is primarily found in the stromal cells. ER-β is associated with antiproliferative activity; indeed, ER-β knockout mice develop hyperplasia of the stromal cells during aging.16 Naslund et al. showed that exogenous administration of E2 worsens the severity and incidence of non-bacterial prostatitis in Wistar rats (100% of the treated animals).17 Bernoulli et al. showed that the reduction of the serum TT:E2 ratio is associated with onset of non-bacterial prostatitis without urodynamic alterations in the animal model with hypoandrogenism combined with hyperestrogenism, while obstructive disorders appeared in the hyperandrogenic animals with low serum TT:E2 ratio.18 In another experimental model, chronic treatment with estrogens results in the activation of metalloproteinases 2,7,9 in the lateral lobe of the prostate and increase of leukocyte infiltrate.19
Other conceivable causes could be represented by the functional alterations of aromatase, the enzyme responsible for the conversion of T into E2, which are more frequent in infertile patients,20 or the TT reduction associated with the psychological stress which is another important characteristic of patients with chronic prostate inflammation.21,22
In clinical practice, for infertile patients with a reduction of T:E2 ratio (threshold value <10), it may be suggested that the pharmacological therapy with aromatase inhibitors (testolactone, anastrozole, letrozole) which represent a class of drugs used off label,23 but for which there is no documented evidence in the literature regarding the clinical model of chronic prostatitis or MAGI. In the study by Gregoriou et al. in 2012,24 patients (with idiopathic infertility) treated with letrozole 2.5 mg/d for 6 months had a statistically significant increase of T:E2 ratio (9 ± 0.2 vs. 36 ± 4.5) after treatment, as well as the treated group with anastrozole 1 mg/d for 6 months (8 ± 0.4 vs. 34 ± 5.9).
In conclusion, further studies on a larger number of patients with MAGI will serve to confirm these preliminary data, confirming them with the use of most appropriate methods for the quantification of estrogens and finally possible therapeutic trials with aromatase inhibitors in these patients may be suggested.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Krause W. (2008) Male accessory gland infection. Andrologia 40: 113–116. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (1993) Manual for the Standardized Investigation and Diagnosis of the infertile couple. Cambridge: Cambridge University Press. [Google Scholar]
- 3. Bezold G, Politch JA, Kiviat NB, et al. (2007) Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertility and Sterility 87: 1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vicari E, Calogero AE, Condorelli RA, et al. (2012) Male accessory gland infection frequency in infertile patients with chronic microbial prostatitis and irritable bowel syndrome. International Journal of Andrology 35: 183–189. [DOI] [PubMed] [Google Scholar]
- 5. La Vignera S, Vicari E, Condorelli RA, et al. (2011) Male accessory gland infection and sperm parameters (review). International Journal of Andrology 34: 330–347. [DOI] [PubMed] [Google Scholar]
- 6. Comhaire FH, Mahmoud AM, Depuydt CE, et al. (1999) Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: The andrologist’s viewpoint. Human Reproduction Update 5: 393–398. [DOI] [PubMed] [Google Scholar]
- 7. La Vignera S, Calogero AE, Condorelli RA, et al. (2012) Ultrasonographic evaluation of patients with male accessory gland infection. Andrologia 44: 26–31. [DOI] [PubMed] [Google Scholar]
- 8. Condorelli RA, Calogero AE, Vicari E, et al. (2014) Male accessory gland infection: Relevance of serum total testosterone levels. International Journal of Endocrinology 2014: 915752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jia YL, Liu X, Yan JY, et al. (2015) The alteration of inflammatory markers and apoptosis on chronic prostatitis induced by estrogen and androgen. International Urology and Nephrology 47: 39–46. [DOI] [PubMed] [Google Scholar]
- 10. La Vignera S, Condorelli RA, Balercia G, et al. (2013) Does alcohol have any effect on male reproductive function? A review of literature. Asian Journal of Andrology 15: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. La Vignera S, Condorelli RA, Vicari E, et al. (2014) Microbiological investigation in male infertility: A practical overview. Journal of Medical Microbiology 63: 1–14. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization (2010) WHO Laboratory Manual for the Examination and processing of human semen. 5th ed. Cambridge: Cambridge University Press. [Google Scholar]
- 13. Ketha H, Girtman A, Singh RJ. (2015) Estradiol assays–The path ahead. Steroids 99: 39–44. [DOI] [PubMed] [Google Scholar]
- 14. Rosner W, Hankinson SE, Sluss PM, et al. (2013) Challenges to the measurement of estradiol: An endocrine society position statement. Journal of Clinical Endocrinology and Metabolism 98: 1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Ronde W, van der Schouw YT, Muller M, et al. (2005) Associations of sex-hormone-binding globulin (SHBG) with non-SHBG-bound levels of testosterone and estradiol in independently living men. Journal of Clinical Endocrinology and Metabolism 90: 157–162. [DOI] [PubMed] [Google Scholar]
- 16. Yang GQ, Li SW, Zheng H, et al. (2009) Expression of estrogen receptor beta in benign prostatic hyperplasia complicated by chronic prostatitis. Zhonghua Nan Ke Xue 15: 314–317. [PubMed] [Google Scholar]
- 17. Naslund MJ, Strandberg JD, Coffey DS. (1988) The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. Journal of Urology 140: 1049–1053. [DOI] [PubMed] [Google Scholar]
- 18. Bernoulli J, Yatkin E, Konkol Y, et al. (2008) Prostatic inflammation and obstructive voiding in the adult Noble rat: Impact of the testosterone to estradiol ratio in serum. Prostate 68: 1296–1306. [DOI] [PubMed] [Google Scholar]
- 19. Wilson MJ, Woodson M, Wiehr C, et al. (2004) Matrix metalloproteinases in the pathogenesis of estradiol-induced nonbacterial prostatitis in the lateral prostate lobe of the Wistar rat. Experimental and Molecular Pathology 77: 7–17. [DOI] [PubMed] [Google Scholar]
- 20. Lazaros L, Xita N, Kaponis A, et al. (2011) The association of aromatase (CYP19) gene variants with sperm concentration and motility. Asian Journal of Andrology 13: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riegel B, Bruenahl CA, Ahyai S, et al. (2014) Assessing psychological factors, social aspects and psychiatric co-morbidity associated with Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) in men – a systematic review. Journal of Psychosomatic Research 77: 333–350. [DOI] [PubMed] [Google Scholar]
- 22. Nargund VH. (2015) Effects of psychological stress on male fertility. Nature Reviews Urology 12: 373–382. [DOI] [PubMed] [Google Scholar]
- 23. Schlegel PN. (2012) Aromatase inhibitors for male infertility. Fertility and Sterility 98: 1359–1362. [DOI] [PubMed] [Google Scholar]
- 24. Gregoriou O, Bakas P, Grigoriadis C, et al. (2012) Changes in hormonal profile and seminal parameters with use of aromatase inhibitors in management of infertile men with low testosterone to estradiol ratios. Fertility and Sterility 98: 48–51. [DOI] [PubMed] [Google Scholar]