Abstract
Serious multiple traumatic injuries may rapidly become fatal or be complicated by a life-threatening sequelae leading to a significant increase of the mortality rate. Trauma scoring systems are used to evaluate the critical status of the patient and recently many different biomarkers have been taken into account to better estimate the potential clinical outcome. The aim of the present study is to analyse the expression pattern of high-mobility group box-1 (HMGB1), oxidative stress markers and nuclear factor erythroid 2-related (Nrf2) in critically ill traumatic patients (at hospital admittance and after 6 and 24 h), in order to find out their potential role as early post-traumatic predictors markers. Forty-seven patients admitted for multiple trauma and 15 healthy participants were prospectively recruited. Eight patients (17%) died within 92 h of admission; this subgroup of patients presented the highest severity scores and their HMGB1 expression levels were significantly correlated with ISS, whereas patients with higher ISS exhibited higher levels of HMGB1 (P <0.001). Our study suggests the role of HMGB1 as a predictive biomarker of outcome in injured patients and hypothesizes the protective role of Nrf2 in bringing down the oxidative stress and HMGB1 release; measuring HMGB1 in combination with Nrf2 might represent a potentially useful tool in the early detection of post-trauma complications.
Keywords: high-mobility group box-1 (HMGB1), Injury Severity Score, multiple trauma, nuclear factor erythroid 2-related (Nrf2), outcome, oxidative stress markers
Introduction
Multiple traumatic injuries are among the main causes of death among young people. The most common primary injuries involve the brain, spinal cord, thorax and abdomen. Multiple trauma patients present with two or more injuries, one of which is likely to be life-threatening, often deserving a multidisciplinary approach. Moreover, these patients also present more complications, among which severe inflammation and infection leading to septic complications or to multi-organ dysfunction syndrome (MODS), significantly increasing the morbidity and mortality rate.1 All patients were recorded at the Emergency Department (ED) and then evaluated using the Injury Severity Score (ISS), considering moderate injuries to be between a score of 16–24 and severe injuries with a score of higher than 25.
Several molecules are being released after a tissue injury and among these high-mobility group box-1 (HMGB1), a non-histone DNA-binding protein, regarded as a potential biomarker of injury severity and survival in trauma. Recent findings demonstrated that HMGB1, first described as a late mediator of sepsis, could be also considered as an early mediator of sterile inflammation. HMGB1 is actively secreted from multiple cell types or passively released from dying cells into the extracellular milieu. HMGB1 activates immune cells and triggers an inflammatory response through the interaction with its receptors, namely the receptor for advanced glycation end products (RAGE), and toll like receptors (TLR)−2 and −4.2 The interaction of HMGB1 with RAGE, TLR2 or TLR4 induces activation of a downstream signalling pathway release of pro-inflammatory cytokines and chemokines, thus sustaining and amplifying the inflammatory state.3,4
Systemic inflammation is also triggered by oxidative stress, which contributes to the increase of the inflammation by means of some transcription factors, such as the nuclear factor-kappa B (NF-κB), signal transducer and activator of transcription 3 (STAT3) and the activator protein-1 (AP-1).5 The role of oxidative stress has been reported in patients after brain and pulmonary traumatic injuries.6 Moreover, the release of HMGB1 may also be modulated by oxidative stress signalling.7 Nevertheless, oxidative stress is counteracted through the activation of transcription factors binding the antioxidant response element (ARE), such as the nuclear factor erythroid 2-related (Nrf2), resulting in the synthesis of many antioxidant proteins.8 Nrf2-ARE induction shows a neuroprotective efficacy in experimental traumatic brain injury.9
The aim of the present study was to analyse the expression pattern of HMGB1, Nrf2 and oxidative stress markers in multiple trauma patients, at different time-points, in order to evaluate their potential role as predictors of clinical outcome in the early post-traumatic period.
Participants and methods
From January 2013 to September 2015, 47 patients out of 156 hospitalised for multiple trauma at the ED of the University Hospital of Messina were prospectively recruited. The local Ethics Committee approved the protocol, since it did not vary the clinical practice nor provide any administration of off-label molecules. Moreover, in all cases an informed written consent was given before the sample collection.
Inclusion criteria were: the presence of blunt multiple traumatic injuries with an ISS above or equal to 25; and an age range of 18–65 years. Exclusion criteria were: patients on medication (e.g. vasoactive drugs, anxiolytics, diuretics, vitamins); chronic tobacco smokers; those who had abused alcohol and participants with confounding co-morbidities, i.e. congestive heart failure, hypertension, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, chronic renal failure and peripheral vascular disease. All patients were assessed by means of contrast-enhanced whole body computed tomography, ultrasound and routine blood investigations. Such choice was made in order to obtain a homogeneous cohort of patients without possible influences on the marker values. Severity of injury was calculated at admission using the ISS and the Glasgow Coma Scale (GCS).
Blood sample collection
Blood samples were collected from patients at three successive times: at hospital admittance, and 6 h and 24 h later. Whole blood samples were collected in plasma EDTA tubes. Serum samples were collected in gel tubes, centrifuged at 3000 rpm for 10 min to remove the cellular components; then the serum obtained was stored at −80°C for long-term preservation.
HMGB1 serum levels
Total protein concentration of serum samples was evaluated using the Bradford assay (Bio-Rad, CA, USA). Equal amounts of diluted serum samples were subjected to sodium-dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). After transfer onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, CA, USA) they were subsequently blocked with 5% defatted dried milk in Tris buffered saline (TBS) containing 0.05% Tween-20 and probed with anti-HMGB1 monoclonal antibody (Abcam, Cambridge, UK). Bound antibodies were visualised with specific secondary antibody HRP-conjugated (Santa Cruz, CA, USA) and immunoreactivity assessed by chemiluminescence reaction using the ECL western blotting detection system (GE Healthcare Europe GmbH, Amersham, UK).
RNA extraction and real-time polymerase chain reaction (PCR) for HMGB1 and Nrf2
Total RNA was extracted from whole blood using Trizol Reagent (Invitrogen, Milan, Italy) according to the protocol provided by the manufacturer. RNA (5 µg) from each sample was reverse transcribed using High-Capacity cDNA Archive Kit according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). cDNA from each sample was amplified by real-time PCR (Applied Biosystems). β-actin was used as control, due to its constitutive expression as housekeeping gene. Each sample was analysed in duplicate using SDS 7300 (Applied Biosystems). Gene expression of HMGB1 and Nrf2 results were normalised with β-actin and relative fold change for each gene was calculated using the 2ΔΔCT method.
Oxidative stress
Oxidative stress and imbalance of antioxidant molecules are known to occur early after trauma. Since HMGB1 release might be modulated by oxidative stress in our cohort of patients, the levels of malondialdehyde (MDA) and hydrogen peroxide were investigated.
MDA measurement. Determination of MDA levels was carried out in blood samples. Blood was collected in polyethylene tubes to which a 10 µL of heparin solution was added. Plasma samples were obtained after centrifugation. Briefly, 0.65 mL of 10.3 mM N-methyl-2phenyl-indole in acetonitrile was added to 0.2 mL of samples. After vortexing and adding 0.15 mL of HCl 37%, samples were mixed well and closed with a tight stopper and incubated at 45°C for 60 min. All samples were then cooled on ice and their absorbance was measured spectrophotometrically at 586 nm.
Hydrogen peroxide. Hydrogen peroxide levels were determined using the Amplex Red kit according to the manufacturer’s instructions (Invitrogen, UK). Briefly, plasma samples were incubated with 50 µL of the Amplex Red reagent/HRP working solution for 30 min, then the spectrophotometric measure was reached after absorbance incubation at 560 nm.
Statistical analysis
All data are expressed as mean ± SD. A P value <0.05 was considered statistically significant. The correlation between HMGB1 and ISS was calculated by Pearson’s test. Statistical analysis was performed using GraphPad Prism software.
Results
Clinical outcome
Overall, among all patients (n = 47) observed in this study with an ISS above or equal to 25, eight patients (17.0%) died within 92 h after the hospital admittance. Their ISS mean values were the highest (42.9 ± 2.3; range, 41–48), and the body area most commonly affected by the prevalent trauma was the head and neck. Fifteen participants formed the control group. Therefore, data were processed considering the group of multiple trauma patients compared to both healthy controls and subgroup of patients who died as a consequence of severe injuries. Table 1 reported other clinical and demographic features of the patient cohort, whose most frequent cause of trauma was caused by motor vehicle accident.
Table 1.
Clinical and demographic features of the patient cohort.
| Patients | Overall mean age (years ± SD) |
Prevalent injury | Mean Injury Severity Score (ISS) | Mean systolic blood pressure (mmHg ± SD) | Mean heart rate (bpm ± SD) |
Mean SpO2 (%) | Mean Glasgow Coma Scale (GCS) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Multiple trauma group (n = 47) M:F ratio 38:9 (4.2:1) |
38.3 ± 16.5 (range, 18–63) |
Head and neck (n = 19) Chest (n = 12) Abdominal (n = 6) Extremity (n = 6) Face (n = 3) External (n = 1) |
32.8 ± 6.9 (range, 25–48) |
135.3 ± 21.1 | 90.4 ± 19.0 | 97.6 ± 2.7 | 12.4 ± 3.5 | Discharged n = 39 Deceased n = 8 |
| Control group (n = 15) M:F ratio 9:6 (1.5:1) |
39.9 ± 13.0 (range, 21–62) |
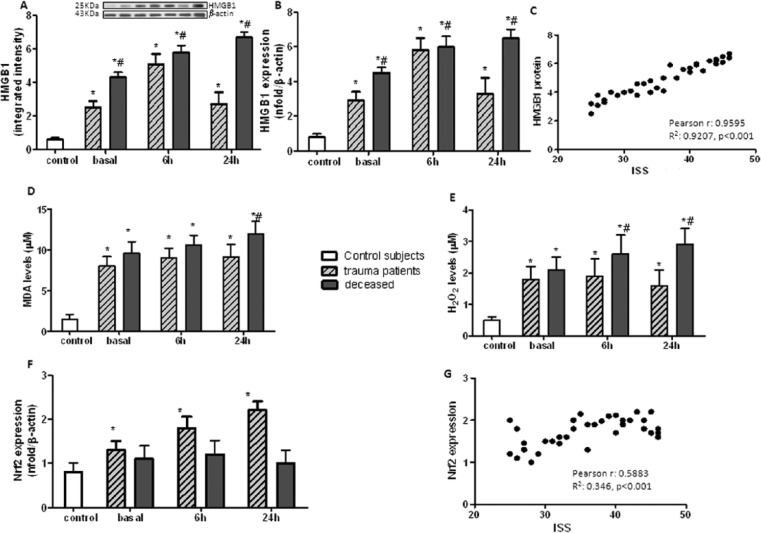
HMGB1 serum levels. HMGB1 protein levels in sera of multiple trauma patients at the same time-points, by using western blot analysis were first recorded. This densitometric analysis showed that protein expression of HMGB1 in serum was significantly affected immediately after trauma; it increased at 6 h and then decreased at 24 h after admission, compared to healthy participants (Figure 1a).
Figure 1.
(a) HMGB1 protein expression evaluated by western blot analysis in serum samples; (b) HMGB1 mRNA real-time PCR expression evaluated in blood collected from controls healthy participants (n = 15) or multiple trauma (n = 39) or deceased (n = 8) participants at hospital admittance (basal), 6 h and 24 h (after hospital admittance); (c) Pearson correlation between HMGB1 protein level and ISS, r = 0.9595, R2 = 0.9207, P <0.001; (d) Malonldialdehyde (MDA) serum levels (µM); (e) hydrogen peroxide (H2O2) serum levels (µM); and (f) Nrf2 mRNA real-time PCR expression evaluated in serum samples collected from controls (n = 15) or multiple trauma (n = 39) or deceased (n = 8) participants at hospital admittance (basal), 6 h and 24 h (after hospital admittance); (g) Pearson correlation between Nrf2 expression and ISS, r = 0.5883, R2 = 0.346, P <0.001. *P <0.01 vs. control healthy participants and #P <0.001 vs. trauma patients.
HMGB1 mRNA expression
An alike expression trend in HMGB1 mRNA, with a significant increase at 6 h after trauma, and a lower expression at 24 h, although remained significantly high during the time-course analysis, was recorded. This finding demonstrates that HMGB1 overexpression is an early event in multiple trauma patients. Conversely, on examining the expression levels of HMGB1 in deceased patients, a marked and progressive increase was observed at all time-points (Figure 1b).
HMGB1 expression levels were significantly correlated with ISS; patients with higher ISS showed higher levels either of HMGB1 protein (Pearson correlation r = 0.9595, R2 = 0.9207, P <0.001, Figure 1c) or mRNA (Pearson correlation r = 0.8752, R2 = 0.766, P <0.001). Kaplan–Meier survival analysis indicated that increased levels of HMGB1 was significantly associated with the overall poor survival of multiple trauma patients (Gehan–Breslow–Wilcoxon test, P <0.001).
Oxidative stress markers
The perturbation of redox homeostasis, immediately after traumatic injuries, caused MDA and hydrogen peroxide release, which remained elevated at 6 and 24 h after trauma. Interestingly, deceased patients showed the highest release of oxidative stress biomarkers compared to surviving ones (Figure 1d and 1e).
Nrf2 expression
A progressive and marked increase of Nrf2 expression was recorded in multiple trauma patients compared to healthy participants (Figure 1f), thus suggesting the activation of the Nrf2 pathway against the oxidative stress. On the other hand, in deceased patients, a significant reduction of Nrf2 expression compared to multiple trauma patients was observed. This condition indicated the failure of the defence mechanism in this patient’s subgroup. Nrf2 expression related to ISS showed a statistically significance result (Pearson correlation r = 0.5883, R2 = 0.346, P <0.001).
Discussion
Patients with primary serious multiple trauma are characterised by the presence of secondary complications, as severe inflammation, infection, biochemical and physiological imbalances, leading to a significant increase in morbidity and mortality.1 Identification of specific biomarkers might facilitate early identification of a patient subset with a poor prognosis due to an increased risk of developing post-trauma complications.2,5,9
Several inflammatory biomarkers have been studied as predictive markers of inflammatory response severity, among these HMGB1 seems to be a good candidate to become a reliable outcome prognostic marker for critically ill patients. Previous studies have demonstrated that circulating levels of HMGB1 rise in patients with traumatic haemorrhagic shock, sepsis and MODS, as well as in chest trauma.3,4,10
Recent findings suggest that activation of HMGB1 could be regulated by oxidative stress, which occurs early after trauma;11 causing a redox balance misregulation with an overproduction of reactive oxygen species (ROS), that sustains and amplifies the inflammatory state, leading to SIRS and MODS.5,6
Nrf2, being a key regulator of the cytoprotective response, plays a neuroprotective role in anti-oxidative, anti-apoptotic and anti-inflammatory responses; it induces the expression of a wide number of antioxidant molecules and reduces HMGB1 release in ischemia/reperfusion model and in other sterile inflammatory injuries.12
In this study, all patients with multiple trauma showed markedly high HMGB1 circulating levels in all time-points evaluated, and a significant association between HMGB1 expression, both mRNA and protein, and ISS, supporting the prognostic role of HMGB1 in multiple trauma patients, was found. Enhanced HMGB1 levels were associated with a poor prognosis and patients with highest levels died few days after trauma. The oxidative stress mediators were also found to be increased early after the traumatic injury; as recorded for HMGB1, the higher levels of oxidative stress mediators were observed in deceased patients. Furthermore, multiple traumatic injuries leaded to a significant increase in Nrf2 expression and it was demonstrated by the activation of Nrf2 pathway which limits the cell damage secondary to the oxidative stress. Interestingly, Nrf2 enhancement at 24 h was inversely related to the HMGB1 reduction, suggesting that this transcription factor could perform a protective role through the inhibition of HMGB1 release. Conversely, considering the deceased patients, a significant decrease of Nrf2 expression was observed indicating that, despite the intensive care, the defence mechanisms fail to counteract the oxidative stress.
In conclusion, the results of the present study suggest the role of HMGB1 as predictive biomarker of outcome in multiple trauma patients and hypothesizes that activation of Nrf2 might play a protective role, mainly both by reducing oxidative stress and by realising HMGB1. Results obtained have led us to hypothesize that HMGB1/Nrf2 measurement combination might represent a potential useful tool in the early identification of post-trauma complications, and an effective predictor of outcome in critical ill patients.
Acknowledgments
We wish to thank Mr Sam Palella, a native English speaker with an extensive experience on scientific papers, for the accurate revision of the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Gunn ML, Kool DR, Lehnert BE. (2015) Improving outcomes in the patient with polytrauma: A review of the role of whole-body computed tomography. Radiologic Clinics of North America 53: 639–656. [DOI] [PubMed] [Google Scholar]
- 2. Tsung A, Tohme S, Billiar TR. (2014) High-mobility group box-1 in sterile inflammation. Journal of Internal Medicine 276: 425–443. [DOI] [PubMed] [Google Scholar]
- 3. Peltz ED, Moore EE, Eckels PC, et al. (2009) HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock 32: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hatada T, Wada H, Nobori T, et al. (2005) Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thrombosis and Haemostasis 94: 975–979. [DOI] [PubMed] [Google Scholar]
- 5. Roth E, Manhart N, Wessner B. (2004) Assessing the antioxidative status in critically ill patients. Current Opinion in Clinical Nutrition & Metabolic Care 7: 161–168. [DOI] [PubMed] [Google Scholar]
- 6. Hohl A, Gullo Jda S, Silva CC, et al. (2012) Plasma levels of oxidative stress biomarkers and hospital mortality in severe head injury: A multivariate analysis. Journal of Critical Care 27: 523.e11–19. [DOI] [PubMed] [Google Scholar]
- 7. Tsung A, Klune JR, Zhang X, et al. (2007) HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. Journal of Experimental Medicine 204: 2913–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu XY, Wang HD, Xu JG, et al. (2015) Deletion of Nrf2 exacerbates oxidative stress after traumatic brain injury in mice. Cellular and Molecular Neurobiology 35: 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding K, Wang H, Xu J, et al. (2014) Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2-ARE signaling pathway as a potential mechanism. Free Radical Biology and Medicine 73: 1–11. [DOI] [PubMed] [Google Scholar]
- 10. Bitto A, Barone M, David A, et al. (2010) High mobility group box-1 expression correlates with poor outcome in lung injury patients. Pharmacological Research 61: 116–120. [DOI] [PubMed] [Google Scholar]
- 11. Tang D, Kang R, Zeh HJ, 3rd, et al. (2011) High-mobility group box 1, oxidative stress, and disease. Antioxidants & Redox Signaling 14: 1315–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel VP, Chu CT. (2011) Nuclear transport, oxidative stress, and neurodegeneration. International Journal of Clinical and Experimental Pathology 4: 215–229. [PMC free article] [PubMed] [Google Scholar]